Open Access Article
Open Access ArticleThermal approaches based on microwaves to recover lithium from spent lithium-ion batteries†
A.
Cornelio
,
A.
Zanoletti
,
M.
Scaglia
,
E.
Galli
,
D.
La Corte
,
G.
Biava
and
E.
Bontempi
 *
*
INSTM and Chemistry for Technologies Laboratory, Department of Mechanical and Industrial Engineering, University of Brescia, Via Branze, 38, 25123, Brescia, Italy. E-mail: elza.bontempi@unibs.it
First published on 15th July 2024
Abstract
During the energy transition, the demand for strategic metals has become a focal point due to their crucial roles in advancing cleaner energy technologies and sustainable practices. As a response to the potential supply vulnerabilities of critical raw materials, recycling has gained attention, despite some methods being more expensive than traditional mining. In this context, new technologies based on microwave radiation have been recently introduced to recover lithium from spent lithium-ion batteries. This study highlights the innovative results achieved through the application of microwave heating to lithium cobalt oxide (LCO) black mass, showing that mass increase can support the possibility of proposing the technology as a new sustainable approach. The possibility of coupling carbon materials with magnetic materials, available in the black mass (BM) results in a strategic approach to increasing the final temperature of microwave-absorbing materials. The process proves highly efficient in lithium recovery, with a treatment at 600 W for 5 minutes, reaching a value higher than 80%, while also eliminating some labour-intensive pre-treatment steps. The research sheds light on both the advantages and potential challenges associated with this ground-breaking technology.
Sustainability spotlightStrategic metals recovery from spent lithium-ion batteries is a critical step toward environmental sustainability and resource conservation. Our research introduces a new promising method to recover lithium from spent batteries. This process not only mitigates the ecological and economic impacts associated with mining and refining virgin materials but also fosters a circular economy. By enabling the reuse of valuable metals, we support the transition to renewable energy systems and contribute to lowering the cost of energy storage solutions. This research contributes directly to several UN Sustainable Development Goals: ensuring sustainable consumption and production patterns (SDG 12), building resilient infrastructure and fostering innovation (SDG 9), and supporting industry efforts to promote sustainable practices (SDG 11). Additionally, it underpins efforts to secure the availability and sustainable management of water and sanitation for all (SDG 6) by reducing the water-intensive processes of metal extraction and processing. |
1. Introduction
Literature underscores that the availability of strategic metals, for example lithium, will pose a significant long-term sustainability challenge for the transportation sector unless a combination of measures is implemented to address this issue.1 Among them, recycling is the most suitable option to avoid precious material loss. The advantages of recycling metals in lithium-ion batteries (LIBs) are not only limited to the mitigation of raw materials dependence and risks of shortage, but also include environmental and health benefits, and promotion of circular economy strategies.1 Recycling can prevent environmental and health problems associated with the disposal of spent batteries1 and helps to reduce the extraction of raw materials, such as cobalt, nickel, and lithium, which are limited resources and can be subject to supply chain disruptions.1 By recycling LIBs, valuable metals can be recovered and reused, reducing the need for new mining and extraction.2 This not only conserves natural resources but also minimizes the environmental impact of mining activities.3 However, because of the delayed impacts associated with material recycling, the recycling process is anticipated to have a substantial influence in addressing the long-term material cost challenges in Electric Vehicle (EV) development.4 Recycling is set to increase the adoption rate of EVs from 59% to 67% by the year 2060.4Then, recycling holds promise in tackling prolonged challenges related to critical material prices, thanks to anticipated technological advancements and economies of scale that will drive down recycling expenses. While recycling has the advantage of shortening supply chains and cutting down on logistical expenses, it remains more cost-effective to extract minerals through mining than to recycle them. Consequently, there is an urgent need to explore cost-efficient and sustainable methods for reclaiming valuable minerals that can compete with freshly mined ones.4
Moreover, this is not straightforward, partly owing to the intricacies associated with spent lithium-ion battery waste. For example, because the spent LIBs waste is made of complex mixtures of different materials, and the classification and sorting of batteries by cathode chemistry are extremely onerous, the automatization of the separation processes, preliminary for the recovery, is quite complex.5 Even if more than 95% in the efficiency of lithium extraction and recovery can be found, the proposed processes are generally addressed to specific cathodes.4 Additionally, recycled materials often have a lower market value compared to virgin resources.6 This discrepancy can be attributed to several factors, including the increase in mining activities, which are considered crucial for meeting environmental targets for greenhouse gas (GHG) reduction. Furthermore, there is an absence of explicit provisions for evaluating the environmental impacts of different mineral sources, such as extraction from geothermal brines, coal, unconventional sources, the ocean, spent electronics, and virgin mineral mining. This situation poses a significant threat to environmental protection.7 Therefore it is essential to make efforts towards establishing a sustainable battery recycling system and providing resources for the continuous development of new and advanced battery recycling technologies.
The primary objective of this paper is to introduce a novel approach, utilizing microwave-based non-conventional heating systems, for the treatment of spent LIBs, highlighting the potentialities of this technology. The efficacy of the process is demonstrated by the lithium recovery, which allows making evidence occurring of the carbothermic reactions, induced by thermal treatments. This is a significant contribution, also because existing literature suggests separating lithium recovery from the other metals but doing so through traditional thermal methods may present challenges.8 This study is particularly dedicated to waste samples, and there are limited comparable examples available due to the prevailing tendency in the literature to adhere to ideal samples, obtained by using virgin materials.
2. Pyrometallurgy recovery technologies
The prevailing recycling procedures for spent LIBs can be broken down into three key stages: pre-treatment, the metallurgical processing of recycling, and the creation of new materials. In the pre-treatment phase, the initial step involves discharging the spent LIBs to prevent short circuits. Subsequently, they are roughly disassembled into various components, such as cathodes, anodes, membranes, and metallic shells.5 However, in most cases, mechanical pre-treatment involves crushing the anodic and cathodic battery parts, as it can be challenging to separate these components, making more complicate the recovery of valuable materials. Thermal treatment is also commonly employed as a preferred method for removing binders and electrolytes during the pre-treatment process. Following pre-treatment, more advanced technologies are necessary to refine these mixed products through chemical or physical techniques. The present metallurgical processing methods predominantly encompass pyrometallurgical and hydrometallurgical technologies. Nonetheless, the extensive use of chemicals and high energy consumption, along with the associated high emissions during the metallurgical processes, can pose challenges in achieving environmentally friendly and efficient recycling of various metals from spent LIBs.5In particular, pyrometallurgical methods involve high-temperature procedures used to extract and recover valuable materials from battery waste. Essentially, the pyrometallurgical process entails exposing the battery waste to heat while utilizing a reducing agent, like carbon. The main objective is to transform metal oxides into either their elemental form or less complex metal oxides. This method has been proven to be especially efficient in the recovery of metals such as cobalt, nickel, and copper, even if some problems can be established with lithium recovery9 which is usually present in the slag, with other elements such as Al, Ca, and Si.
Carbothermal reduction of the lithium cobalt oxide (LiCoO2 – LCO), lithium manganese oxide (LiMn2O4 – LMO), and lithium nickel manganese oxide (LiNi0.5Mn1.5O4 – LNMO) can occur due to carbon present in the black mass (originating from the anodic part of the battery), which is available for the reaction, promoted by the heat treatment. Indeed, the anodic part of the LIBs is generally composed of graphite.
At high temperatures and in the presence of oxygen (derived from the cathodic material decomposition), carbon oxidizes and can form CO and/or CO2. The decomposition of LMO, LCO, and LNMO can produce nickel, manganese, and cobalt oxides, which may be also further reduced by C and CO at higher temperatures.10
The thermal analysis following the kinetics of the reactions shows that, generally, until about 600 °C cathode material seems to be stable (the weight loss of the black mass is quite limited), and it is more evident beyond 600 °C.11 Weight losses observed after 850 °C are generally associated with the formation of oxides of Mn and Co.11
Although pyrometallurgical techniques offer the potential for metal reclamation, they have certain limitations, mainly related to their energy demand.12 High-temperature processes require substantial energy input, leading to heightened environmental consequences and elevated expenses in comparison to other recycling approaches. Furthermore, pyrometallurgical methods might not be applicable for recovering all materials contained within lithium-ion batteries, and specific hazardous constituents within the battery waste could necessitate supplementary treatment and disposal.
In this frame, the advantages of microwave (MW) technology over traditional pyrometallurgical processes are commonly recognized.13
3. Microwave treatments and the hybrid-heating mechanism
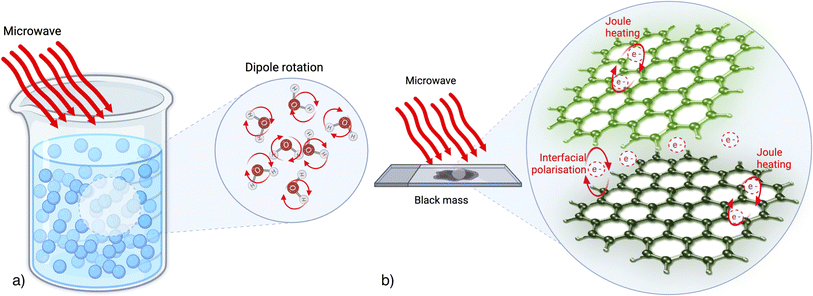
MW technologies have been recently applied in various ways as new methodologies to recycle metals in LIBs. One example is the MW-enhanced approach which uses a biodegradable and recyclable deep eutectic solvent to leach valuable metals from spent LIBs.14 The high electric field induced by MW heating increases the dipole moments on the battery surface, allowing for fast leaching of metals such as lithium and cobalt. MW was also used for sample leaching, in a closed vessel, to facilitate the dissolution of the metals.15 Another example consists of biomass pyrolysis supporting the recovery of LIB from biomass waste, with the result of reducing the decomposition temperature of the cathodic materials.16 Finally, a very recent example concerns the use of the microwave-assisted reduction roasting method to promote carbothermal reduction reactions.17,18 In particular, the use of a hybrid-heating mechanism has shown promising results in the direct recovery of lithium from spent batteries. In this case, Li was shown to be available as lithium oxide, then it is soluble in water.Nonetheless, even though certain research findings showcase the potential of MW technology for recycling metals from LIBs, efforts in this field remain relatively restricted. Specifically, the impacts of MW heating on the treatment of spent cathode materials are not yet comprehensively understood and necessitate further exploration. This is also because the black mass (BM), based on carbon materials with microwave absorption properties, brings both opportunities and challenges in providing new ways to recover strategic metals. Then a deep understanding of the MW heating-related phenomena must be realized. In particular, MW heating, with its distinctive internal heat mechanism, can mitigate the shadow effect during material heating, alter the heat transfer mode, and significantly reduce reaction times. Nevertheless, it's essential to emphasize that this heating mechanism differs from the process that takes place in solutions.
Fig. 1 illustrates the difference between the heating mechanisms in a water-based solution and a solid composed of carbon-based material. In the context of MW heating in a solution, the primary process involves the rotation of polar solvent molecules. Conversely, for electron-rich solids devoid of freely rotatable dipoles, such as carbon-based solids, the literature suggests that heat is generated through electron motion, manifesting as joule heating within the material's structure. This heat generation arises either due to collisions triggered by graphite's delocalized pi-electrons moving within a confined region and interacting with the oscillating field or as heat generated at grain/phase boundaries19 due to the interfacial polarization. Furthermore, the comprehensive mechanism behind the MW heating of solids remains incompletely understood. Therefore, its potential applied to BM treatments hasn't yet been highlighted in the literature.
The main parameters used to model MW heating are permittivity, permeability, and loss tangent. Permittivity (ε) refers to a material's ability to engage with the MW electric field. It comprises both a real and an imaginary component. The real part (ε′) signifies the extent to which the electric field permeates, often referred to as the dielectric constant or permittivity. The imaginary part (ε′′) signifies the energy retained within the material and is known as the electric loss factor.20 It dictates the material's capacity to transform electric energy into heat. Permeability, on the other hand, characterizes the material's aptitude to interact with the magnetic field. The substrate's reaction to converting microwave energy into thermal loss is denoted as the loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) and it is calculated as ε′′/ε'.17 The MW penetration depth (Dp) indicates the distance a wave travels before its energy diminishes to 1/e, and it is proportional to the MW wavelength and inversely proportional to (tan
δ) and it is calculated as ε′′/ε'.17 The MW penetration depth (Dp) indicates the distance a wave travels before its energy diminishes to 1/e, and it is proportional to the MW wavelength and inversely proportional to (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ)1/2. It can be calculated as reported in eqn (1):21
δ)1/2. It can be calculated as reported in eqn (1):21
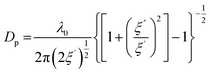 | (1) |
On exposure to MW, the electromagnetic loss in a material is converted into heat. Moreover, with the temperature rise, the material's dielectric properties can change. This can alter the MW loss, with the consequence of changes in the heating characteristics.22 As an example, alumina results transparent to MW up to 900 °C (resulting in high penetration depth of MW radiation), yet it starts to interact with microwaves at temperatures surpassing 1000 °C.20 This makes it necessary to investigate suitable materials to be used for MW adsorbing and/or heat insulation applications.
It was already shown that MW can penetrate the spent carbon cathode material (produced in the process of aluminum electrolysis) with a depth higher than 0.01 m,21 also reaching values higher than 0.3 m for cathodic materials and graphite10 and 0,2–1 m for anthracite23 and bituminous coal.24 In particular, as the dielectric properties of the materials depend on the temperature, it results that MW penetration depth also can be variable. It also depends on the material density24 and packing, making its evaluation extremely complicated for a mixture of different materials, such as waste made of several compounds and some contaminants, with variable chemistry.
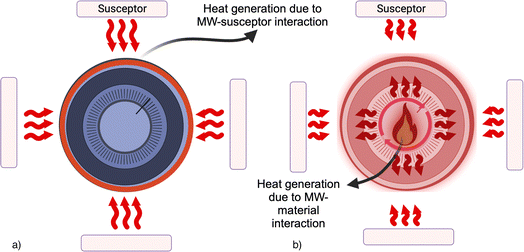
In the case of MW heating the use of a solid-state material that can be excited by MW radiation, such as carbon, allows a very efficient heating, because the target material absorbs the MW energy. This is the case of LIBs, where the cathodic and anodic (generally made in graphite) materials are both in black mass. Graphite has π-electrons, which are responsible for heat generation (see Fig. 1). For carbon-based materials combined with magnetic materials, these heating effects can be further enhanced. Magnetic materials can absorb microwaves through magnetic losses, including hysteresis, eddy currents, and relaxation losses.20 The interplay between the magnetic losses and the Joule heating in carbon can lead to efficient internal heating of the material. Finally, in MW processing of various materials, a susceptor can be also employed to amplify the MW-absorbing capabilities of the sample being treated. A susceptor material absorbs energy from the MW, converting it into heat energy that is then transferred to the processed material. For example, carbon can be used as a susceptor. For applications requiring reaching high temperatures, such as high melting point materials treatments, Si is often used. Conversely, charcoal powder is easily accessible at a significantly lower cost.20
A susceptor can be also external to the sample, but in its proximity: the heat will be subsequently conveyed to the sample through conventional modes of heat transfer.
The susceptor assisted microwave heating is generally defined as “hybrid heating” (see Fig. 2). This was recently proposed for BM treatment25 because graphite is generally used for low-temperature applications,26 then the presence of an external susceptor can be effective in the increasing of system temperature.
The effective and suitable configuration of a susceptor-assisted MW heating system needs to couple a material that can be excited by MW with a thermal insulation system, which must envelop it. Indeed, thermal insulation plays a fundamental role in any technology involving a thermal process by limiting heat dissipation, especially at high temperatures. A suitable thermal insulation for MW depends not only on the material thermal stability but also on its ability to remain transparent to MW radiation across the entire temperature range. Alumina is a commonly used insulation material due to its outstanding transparency to MW radiation and its strong resistance to thermal and wear-related challenges over a wide range of temperatures.
Concerning the drawbacks connected with thermal treatments, the main negative aspect is pollutants gas generation, including electrolyte components, oxygenated hydrocarbons, hydrocarbons, and others.27 The formation of different gas species is due to electrolyte volatilization, electrolyte degradation/decomposition, and pyrolysis of the organic separator and binder, followed by complex radical reactions among the species formed by the physicochemical reactions.
4. Experimental
LCO spent BM was provided by Spirit s.r.l., a company recycling battery located in Chiampo, Vicenza, Italy.The BM was obtained after conventional mechanical pretreatments to separate the battery case, the plastic components, and the electrolyte.
The sample was digested to evaluate the concentration of Li, as described in ref. 18. The composition results in 38.4 g kg−1 of Li, 288 g kg−1 of Co, 5.2 g kg−1 of Mn, 49 g kg−1 of Ni, and 4.8 g kg−1 of Cu. The amount of graphite in the BM is 19.2%.18
BM was treated at 600 W at 2.4 GHz for 5 minutes, increasing the sample mass, in each experiment, from 0.5 to 4.5 g (see Table 1 for the sample name with the corresponding mass), in a PYRO Advanced Microwave Muffle Furnace (Milestone s.r.l., Bergamo, Italy), which was equipped with a susceptor.25 The tests were repeated three times. The temperature of the samples, after the thermal treatment was provided by thermal imaging camera testo 890.
| Sample name | Sample mass (g) | Li (g kg−1) | Li recovery (%) |
|---|---|---|---|
| 0 | 0.5 | 21.76 ± 0.33 | 56.7 ± 1.2 |
| 1 | 1 | 24.47 ± 0.37 | 63.7 ± 1.4 |
| 2 | 2 | 23.80 ± 0.36 | 62.0 ± 1.4 |
| 3 | 3 | 20.74 ± 0.32 | 54.0 ± 1.2 |
| 4 | 4 | 20.14 ± 0.31 | 52.4 ± 1.1 |
| 5 | 4.5 | 34.19 ± 2.85 | 89.0 ± 7.4 |
The sample resulted in filling the sample holder to about 1 cm.
All the samples, after the MW treatments, were analysed by X-ray diffraction (XRD), using a Panalytical diffractometer using Cu Ka (1.5406 Å) radiation and operating at 40 kV and 40 mA. The patterns were acquired from 10° to 70° (2Θ). For the leaching test, BM was mixed with Milli-Q water in a heating plate at 80 °C for 30 min using a solid/liquid ratio of 40 g L−1.25 After this time the solution was filtered with a vacuum filtration by using a nylon membrane with 0.45 μm. Li+ concentration was evaluated by ion chromatography (IC), Metrohm 883 Compact IC plus (Metrohm AG, Herisau, Switzerland) and cation exchange column Metrosep C4-150/4.0 (Metrohm AG, Herisau, Switzerland).
5. Results and discussion
5.1 A mass and sample heating correlation
LiCoO2, in the presence of a carbon source, can decompose into LiO2 and Co (or CoO), by increasing the temperature, showing the most favourable condition at about 700 °C.28 Graphite is converted into CO and CO2. The reactions can be followed by analysing the sample mass loss, which is because CO2 removes solid carbon contained in the BM, to form a gas.However classical heating systems are more time-consuming in comparison to MW technologies, and the target temperature needs more time to be reached. To obtain an efficient heating system, it is fundamental to evaluate the MW penetration depth: if the penetration depth is not high, the heating efficiency may be low.
For an LCO BM, containing about 20% of graphite, the penetration depth of the microwave can be calculated based on (1).17 The results reported in Fig. S1† (see the ESI) show that till to about 400 °C, the MW penetration depth decreases with the increase of the temperature, due to the rise of the MW-absorbing properties of the BM. This means that the loss factor (ε′′) and the dielectric constant (ε′) increase.
Fig. S1† shows that for the range of temperatures reached by these experiments, the penetration depth of MW radiation ranges from 0.025 to 0.065 m.17 For a range of temperatures from 650 °C to 900 °C the penetration depth may also be higher than 0.06 m, depending on the BM density and dielectric parameters.
Concerning the samples investigated in this work, it is possible to conclude that all the samples were penetrated by MW radiation (see Fig. S1†), with the consequence that an increase in the sample mass subjected to the treatment produces an increase in its temperature, due to the increase of the material that is excited by MW.
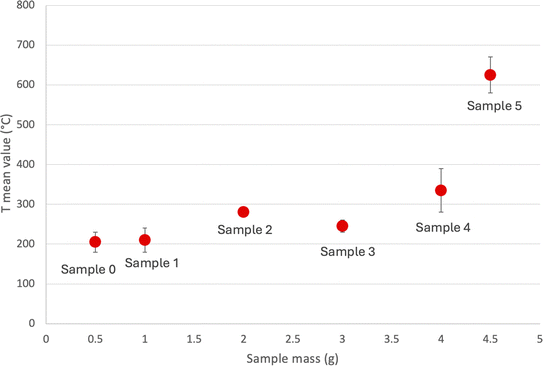
The temperature reached by the samples versus the different masses is reported in Fig. 3.
An increase in the mass undergoing treatment leads to a rise in sample temperature during MW excitation because there's more BM to absorb the MW energy. This specific effect hasn't been explored in spent LIBs, although it's documented in other contexts. According to the literature, this may be attributed to the fact that a larger mass in the same container will have a reduced surface area to volume ratio, thus lowering heat dissipation.29–31 Unlike conventional heating methods, where heat is applied externally, MW systems generate heat internally. As a result, it can be expected that the temperature of the material will rise. Therefore, the loss of heat from the sample becomes a significant factor that governs the heating process. In the present case, the rate of temperature increase is not linear, probably because of the changes in the permittivity (ε′), and the electric loss factor (ε′′) that after 600 °C start to increase significantly.
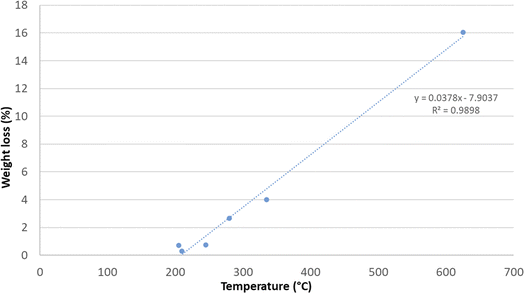
The mass loss (%) of samples treated in this work is shown in Fig. 4 and is plotted against the mean detected sample temperatures. The linear correlation observed in the data can be explained by the fact that an increase in temperature, due to a more prolonged treatment, more effectively promotes the oxidation of carbon. With the increase in the treatment time and the temperature rise, the carbon within the samples reacts more readily with oxygen to form carbon dioxide. This gas is released into the atmosphere, resulting in a carbon loss for the sample (weight loss). This process highlights the occurrence of carbothermic reactions, where the elevated temperatures facilitate the transformation of solid carbon into gaseous carbon dioxide. Thus, higher temperatures enhance the efficiency of carbon oxidation, leading to greater mass loss in the samples due to the emission of CO2 (as described in Section 2).
Fig. 5 shows the XRD patterns of LCO BM before (LCO_tq) and after MW treatments with the mass increased (sample 2 and sample 5, see Fig. 3). The results show that LCO powder (before MW treatment) is characterized by graphite and lithium cobalt oxide (LiCoO2). Some peaks of Ni were also detected. This is also confirmed by the chemical analysis reported in the experimental section, probably due to some other cathode contamination. Also, some peaks of CoO, Co3O4, and Li2O are present. The presence of unexpected phases may be due to several reasons: first, it is important to highlight that the BM used for this study is a real sample, provided by a recycling facility, then it also contains Mn and Ni probably due to contamination of other cathodic materials. In addition, the pre-treatment (performed at 800 °C) realised in the recycling facility, to remove the binder and the electrolyte, can have partially promoted some carbothermal reactions.17
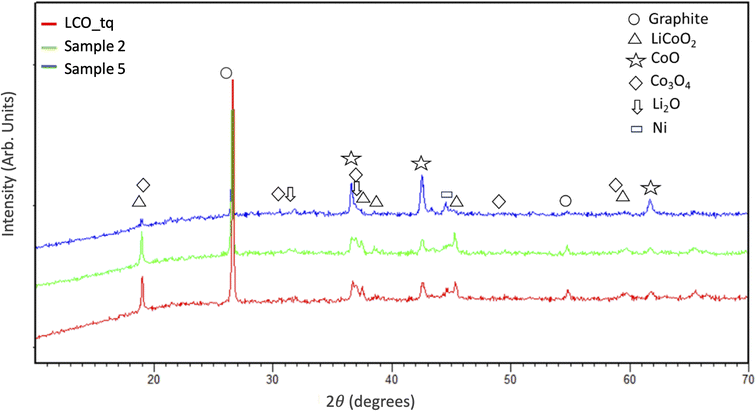 | ||
| Fig. 5 XRD patterns of LCO BM before (sample LCO_tq) and after MW treatment with the increase of black mass (sample 2 and sample 5). | ||
After MW treatment, XRD patterns show an evident decrease in graphite peaks and the reduction of LiCoO2 with the formation of CoO. In the case of a sample with a higher mass amount (sample 5), it is clear the increase in the CoO intensity. This means that the carbothermal reactions are promoted by the mass increase, which is related to the higher temperature rise (see Fig. 3).
These results suggest that the heating mechanism is completely different in comparison to conventional systems, where an increase in the mass needs a corresponding increase in the energy that must be transferred for the increase in the sample temperature.32
Carbon materials possess excellent electrical conductivity, which is crucial for MW absorption. When combined with magnetic materials, the interface between these two types of materials increases.29 This interface can act as a site for interfacial polarization, where the electric field can cause charges at the interface to oscillate, leading to energy dissipation as heat (see Fig. 1). LCO exhibits some magnetic properties, even though it is not typically classified as a magnetic material for applications relying on strong magnetic interactions, such as magnetic storage devices or magnetic structural components. However, cobalt oxides, which are formed by carbothermic reaction (see Fig. 3) are magnetic materials.33 These cobalt oxides can significantly contribute to the magnetic loss mechanisms essential for effective MW absorption, making them valuable as high-temperature MW-absorbing materials.20 When these materials interact with MW radiation, they absorb the electromagnetic energy and convert it into heat, which typically increases their temperature. Magnetic loss materials dissipate electromagnetic energy through hysteresis loss, natural resonance, eddy current loss, magnetic hysteresis loss, domain-wall resonance, and other forms.20 This effect was very recently demonstrated for cobalt oxide composite.34 In this work, this effect is proposed, for the first time, to explain the heating mechanism of the BM, induced by MW.
To assess the potential of this technology, the amount of lithium that can be recovered is quantified.
For a simplified recovery of strategic metals from spent LIBs it is suggested to separate Li from transition metals. This is possible due to the high water solubility of Li2O, which is formed after carbothermic reduction of LCO.35 Then after the MW treatment, the samples were subjected to water leaching.
Table 1 shows the amount (%) of Li recovered by water leaching. As expected, an increase in the recovered Li corresponds to a higher mass treated in the MW oven. This demonstrates that higher temperatures promote carbothermic reductions and that this treatment can allow to recovery high amount of the lithium found in spent LIBs in a few minutes.
5.2 Revision of the pyrometallurgy characteristics associated with battery recovery
Literature indicates that hydrometallurgical methods are better suited for recycling specific cathodic materials since they must be optimized for the material to be recovered. On the other hand, pyrometallurgical approaches are suitable for recycling different cathodic materials, also in a mixture, due to the presence of carbon in the BM, which supports carbothermic reactions.36However, traditional pyrometallurgical techniques employed in the recycling of LIBs have numerous drawbacks, and there is a prevailing idea that these processes might not be suitable for large-scale industrial implementation. Although they offer relative simplicity in execution, these methods are not completely investigated. In particular, new technologies involving the use of MW are still at low Technology Readiness Level (TRL).
Furthermore, literature also suggests that lithium is generally recycled in low quantities through pyrometallurgical methods, while these techniques exhibit their highest effectiveness in recovering particularly valuable metals like cobalt.8 It is preferred to extract Li, after the thermal treatment, by hydrometallurgy.37 As battery technology advances, the cobalt content in electrode materials is diminishing, while the utilization of nickel and manganese is on the rise. This shift makes pyrometallurgy less attractive.
However, the treatment proposed in this paper, obtained by using MW radiation, demonstrates that some drawbacks of the traditional pyrometallurgical procedures can be overcome.
The advantages and improvements of the new proposed technologies are reported in the following.
First, the dosage of a carbon source to have a carbothermic reaction is not necessary, because in the BM the graphite is already present, and it can be used as the reducing agent. This means that it is not mandatory to use onerous pre-treatment methods to separate the cathode and anode materials.
Second, the reactions can be carried out also in an ambient atmosphere, even if the rapid increase in the temperature of the samples must be monitored. Then inert atmosphere can be not necessary for the reactions.
In addition, Li can be recovered directly after the thermal process by water leaching and then precipitated as lithium carbonate (Li2CO3) by the carbonation of the water solution. After water leaching and Li recover, the other metals (Mn, Co, Ni, etc.) can be precipitated through hydrometallurgical processes, by using organic acids instead of inorganic ones25 Concerning the aspect connected with the management of gases generated by the reactions, it would be possible to equip the treatment apparatus with suitable air treatment systems, to recover and neutralize pollutant gases. For this aim, the organic gas species may be recovered as liquids or solids. For example, a dedicated condensation system, with a controlled temperature may be designed to recover the organic gaseous components via low-temperature volatilization.38 There is also the possibility of using an afterburner and/or an adsorbent.38 This must also be evaluated considering that it may allow to avoid some onerous thermal pre-treatments which are generally realized to remove plastics, electrolytes, and the binder. Finally, the possibility of recovering the carbon dioxide emitted during the carbothermic reactions, after its partial purification of some pollutants, to be reused for lithium carbonation seems quite attractive. A second way to face this drawback would be to improve the pre-treatments: the organic solvents, binders, and separators would be recovered with dedicated treatments, to improve the quality of the BM addressed to the treatment.
In summary, the proposed technology would also allow a simplification of the entire recovery process, by reducing some pre-treatment steps. However, this requires more dedicated studies, involving considerations about all the pre-treatment steps (considering LIBs collection, separation, sorting, and preliminary thermal processes).
6. Conclusions and outlook
This work outlines both the benefits and hurdles associated with a novel MW heating approach for lithium recovery from spent lithium-ion batteries. The possibility of coupling carbon materials with magnetic materials in the BM appears to result in a strategic approach to increasing the final temperature of MW-absorbing materials, by coupling dielectric loss with magnetic loss.The application of MW allows for the rapid elevation of temperature in the BM, facilitating carbothermic reactions that yield lithium oxide and enable lithium separate recovery through a water solution. Experimental results obtained from a spent LCO BM sample reveal an efficiency higher than 80% within just a 5 minutes treatment period. These findings support the proposition of this technology as a promising alternative to the traditional pyrometallurgical method and offer the opportunity to avoid several onerous BM pre-treatment steps. However, despite the evident advantages, full comprehension, and modelling of the mechanism of MW heating are still in infancy and some open issues need to be addressed in the future:
(i) The dielectric loss, and consequently the penetration depth of the BM has great importance for the energy conversion efficiency, nevertheless, BM is the result of a combination of the cathodic materials in graphite. Then, dedicated studies to promote the realization of a library containing the data of the loss capacity due to the different cathodic species mixed with graphite should be implemented;
(ii) All the advantages of MW processes involved in LIBs recovery are not completely considered and investigated. For example, additional thermal effects, defined as the localized superheating effects are still not investigated for this waste;39
(iii) The possibility of recovering carbon dioxide generated during the carbothermic reactions, to be used as reactants to produce lithium carbonate must be exploited;
(iv) The design and development of MW reactors operating in continuous mode for large batches of the process should be considered;
(v) The introduction of susceptors may allow for the expansion of the applicability of MW technology to a range of applications, including the treatment of materials that are typically highly transparent to MW, for example, some ceramics, and materials that are highly reflective to MW, such as metals. This opportunity must be better investigated for BM recovery.
In conclusion, given the early stage of LIBs recovery efforts, MW-heating presents a highly promising method to be explored and developed to propose a sustainable approach for strategic metals recovery.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Conceptualization, A. C., A. Z., M. S., and E. B.; methodology, A. C, A. Z., M. S., and E. B.; validation, A. C., A. Z., M. S., and E. B.; formal analysis, A. C., A. Z., M. S., D. L. C., G. B., and E. B.; investigation, A. C., A. Z., and M. S.; resources, E. B.; data curation, A. C., A. Z., M. S., and E. B.; writing—original draft preparation, A. C., A. Z., M. S., and E. B.; writing—review and editing, A. C., A. Z., M. S., and E. B. visualization, A. C., A. Z., M. S., G. B. and E. B. supervision E. B.; project administration, E. B.; funding acquisition, E. B. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was realized with the support of Fondazione CARIPLO to “Tech4Lib—Spent Lithium-ion battery recovery” (CUP D73C23000170007) and by InnovFin Equity, with financial backing of the European Union under Horizon 2020 Financial Instruments and the European Fund for Strategic Investments (EFSI) set up under the Investment Plan for Europe. The purpose of EFSI is to help support financing and implementing productive investments in the European Union and to ensure increased access to financing. Alessandra Zanoletti acknowledges financial support from the Next-GenerationEU (Italian PNRR – M4 C2, Invest 1.3 – D.D. 1551.11-10-2022, PE00000004) within the MICS (Made in Italy – Circular and Sustainable) Extended Partnership for her research fellowship. Matteo Scaglia acknowledges financial support for his grant from CSMT Gestione S.c.a.r.l. This manuscript reflects only the authors' views and opinions, neither the European Union nor the European Commission can be considered responsible for them. The authors thank Spirit s.r.l. company for the BM supply. Graphical abstract Created with https://www.biorender.com/ and IA support. Fig. 1 and 2 were realised with https://www.biorender.com/.References
- N. Vieceli, R. Casasola, G. Lombardo, B. Ebin and M. Petranikova, Hydrometallurgical recycling of EV lithium-ion batteries: Effects of incineration on the leaching efficiency of metals using sulfuric acid, Waste Manage., 2021, 125, 192–203, DOI:10.1016/j.wasman.2021.02.039.
- X. Chen, D. Kang, J. Li, T. Zhou and H. Ma, Gradient and facile extraction of valuable metals from spent lithium ion batteries for new cathode materials re-fabrication, J. Hazard. Mater., 2020, 389, 121887, DOI:10.1016/j.jhazmat.2019.121887.
- E. Mossali, N. Picone, L. Gentilini, O. Rodrìguez, J. M. Pérez and M. Colledani, Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments, J. Environ. Manage., 2020, 264, 110500, DOI:10.1016/j.jenvman.2020.110500.
- Q. Cheng, Z. Wang, Y. Wang, J. T. Li and H. Fu, Recent advances in preferentially selective Li recovery from spent lithium-ion batteries: A review, J. Environ. Chem. Eng., 2024, 12(3), 112903, DOI:10.1016/j.jece.2024.112903.
- X. Wang, G. Gaustad, C. W. Babbitt, C. Bailey, M. J. Ganter and B. J. Landi, Economic and environmental characterization of an evolving Li-ion battery waste stream, J. Environ. Manage., 2014, 135, 126–134, DOI:10.1016/j.jenvman.2014.01.021.
- P. Söderholm and T. Ekvall, Metal markets and recycling policies: impacts and challenges, Miner. Econ., 2020, 33, 257–272, DOI:10.1007/s13563-019-00184-5.
- J. N. Trost and J. B. Dunn, Assessing the feasibility of the Inflation Reduction Act's EV critical mineral targets, Nat. Sustain., 2023, 6, 639–643, DOI:10.1038/s41893-023-01079-8.
- Z. J. Baum, R. E. Bird, X. Yu and J. Ma, Lithium-Ion Battery Recycling–Overview of Techniques and Trends, ACS Energy Lett., 2022, 7, 712–719, DOI:10.1021/acsenergylett.1c02602.
- N. Li, J. Guo, Z. Chang, H. Dang, X. Zhao, S. Ali, W. Li, H. Zhou and C. Sun, Aqueous leaching of lithium from simulated pyrometallurgical slag by sodium sulfate roasting, RSC Adv., 2019, 9, 23908–23915, 10.1039/c9ra03754c.
- S. Pindar and N. Dhawan, Evaluation of in-situ microwave reduction for metal recovery from spent lithium-ion batteries, Sustainable Mater. Technol., 2020, 25, e00201, DOI:10.1016/j.susmat.2020.e00201.
- S. Pindar and N. Dhawan, Comparison of Microwave and Conventional Indigenous Carbothermal Reduction for Recycling of Discarded Lithium-Ion Batteries, Trans. Indian Inst. Met., 2020, 73, 2041–2051, DOI:10.1007/s12666-020-01956-2.
- A. Fahimi, A. Zanoletti, A. Cornelio, E. Mousa, G. Ye, P. Frontera, L. E. Depero and E. Bontempi, Sustainability Analysis of Processes to Recycle Discharged Lithium-Ion Batteries, Based on the ESCAPE Approach, Materials, 2022, 15, 8527, DOI:10.3390/ma15238527.
- Y. Fu, Y. He, Y. Yang, L. Qu, J. Li and R. Zhou, Microwave reduction enhanced leaching of valuable metals from spent lithium-ion batteries, J. Alloys Compd., 2020, 832, 154920, DOI:10.1016/j.jallcom.2020.154920.
- A. Zhu, X. Bian, W. Han, Y. Wen, K. Ye, G. Wang, J. Yan, D. Cao, K. Zhu and S. Wang, Microwave-ultra-fast recovery of valuable metals from spent lithium-ion batteries by deep eutectic solvents, Waste Manage., 2023, 156, 139–147, DOI:10.1016/j.wasman.2022.11.035.
- J. Lie and J. C. Liu, Closed-vessel microwave leaching of valuable metals from spent lithium-ion batteries (LIBs) using dual-function leaching agent: Ascorbic acid, Sep. Purif. Technol., 2021, 266, 118458, DOI:10.1016/j.seppur.2021.118458.
- Y. Zhao, B. Liu, L. Zhang and S. Guo, Microwave Pyrolysis of Macadamia Shells for Efficiently Recycling Lithium from Spent Lithium-ion Batteries, J. Hazard. Mater., 2020, 396, 122740, DOI:10.1016/j.jhazmat.2020.122740.
- Y. Zhao, B. Liu, L. Zhang and S. Guo, Microwave-absorbing properties of cathode material during reduction roasting for spent lithium-ion battery recycling, J. Hazard. Mater., 2020, 384, 121487, DOI:10.1016/j.jhazmat.2019.121487.
- M. Scaglia, A. Cornelio, A. Zanoletti, D. La Corte, G. Biava, I. Alessandri, A. Forestan, C. Alba, L. E. Depero and E. Bontempi, Microwave-Assisted Recovery of Spent LiCoO2 Battery from the Corresponding Black Mass, Batteries, 2023, 9, 536, DOI:10.3390/batteries9110536.
- T. Kim, J. Lee and K. H. Lee, Full graphitization of amorphous carbon by microwave heating, RSC Adv., 2016, 6, 24667–24674, 10.1039/c6ra01989g.
- Q. Xia, Z. Han, Z. Zhang, Z. Huang, X. Wang, J. Chang, Q. Chen and M. Chen, High temperature microwave absorbing materials, J. Mater. Chem. C, 2023, 11, 4552, 10.1039/d2tc04671g.
- Z. Zhu, L. Xu, Z. Han, J. Liu, L. Zhang, C. Yang, Z. Xu and P. Liu, Defluorination study of spent carbon cathode by microwave high-temperature roasting, J. Environ. Manage., 2022, 302, 114028, DOI:10.1016/j.jenvman.2021.114028.
- S. Tamang and S. Aravindan, Joining of dissimilar metals by microwave hybrid heating: 3D numerical simulation and experiment, Int. J. Therm. Sci., 2022, 172, 107281, DOI:10.1016/j.ijthermalsci.2021.107281.
- Z. Peng, X. Lin, X. Wu, J. Y. Hwang, B. G. Kim, Y. Zhang, G. Li and T. Jiang, Microwave absorption characteristics of anthracite during pyrolysis, Fuel Process. Technol., 2016, 150, 58–63, DOI:10.1016/j.fuproc.2016.04.036.
- Z. Peng, J. Y. Hwang, B. G. Kim, J. Mouris and R. Hutcheon, Microwave absorption capability of high volatile bituminous coal during pyrolysis, in Energy and Fuels, 2012, pp. 5146–5151, DOI:10.1021/ef300914f.
- A. Fahimi, I. Alessandri, A. Cornelio, P. Frontera, A. Malara, E. Mousa, G. Ye, B. Valentim and E. Bontempi, A microwave-enhanced method able to substitute traditional pyrometallurgy for the future of metals supply from spent lithium-ion batteries, Resour., Conserv. Recycl., 2023, 194, 106989, DOI:10.1016/j.resconrec.2023.106989.
- S. Rawat, R. Samyal, R. Bedi and A. kumar Bagha, Comparative performance of various susceptor materials and vertical cavity shapes for selective microwave hybrid heating (SMHH), Phys. Scr., 2022, 97, 125704, DOI:10.1088/1402-4896/ac9e7d.
- X. Hu, E. Mousa, L. Ånnhagen, Z. Musavi, M. Alemrajabi, B. Hall and G. Ye, Complex gas formation during combined mechanical and thermal treatments of spent lithium-ion-battery cells, J. Hazard. Mater., 2022, 431, 128541, DOI:10.1016/j.jhazmat.2022.128541.
- G. Lombardo, B. Ebin, M. R. J. St Foreman, B. M. Steenari and M. Petranikova, Chemical Transformations in Li-Ion Battery Electrode Materials by Carbothermic Reduction, ACS Sustain. Chem. Eng., 2019, 7, 13668–13679, DOI:10.1021/acssuschemeng.8b06540.
- M. Omran, T. Fabritius, E. P. Heikkinen and G. Chen, Dielectric properties and carbothermic reduction of zinc oxide and zinc ferrite by microwave heating, R. Soc. Open Sci., 2017, 4, 170710, DOI:10.1098/rsos.170710.
- R. K. Amankwah and C. A. Pickles, Microwave roasting of a carbonaceous sulphidic gold concentrate, Miner. Eng., 2009, 22, 1095–1101, DOI:10.1016/j.mineng.2009.02.012.
- C. A. Pickles, Microwave heating behaviour of nickeliferous limonitic laterite ores, Miner. Eng., 2004, 17, 775–784, DOI:10.1016/j.mineng.2004.01.007.
- R. R. Mishra and A. K. Sharma, Microwave-material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing, Composites, Part A, 2016, 81, 78–97, DOI:10.1016/j.compositesa.2015.10.035.
- A. Das, C. Balasubramanian, P. Orpe, G. M. Pugliese, A. Puri, A. Marcelli and N. L. Saini, Morphological, electronic, and magnetic properties of multicomponent cobalt oxide nanoparticles synthesized by high temperature arc plasma, Nanotechnology, 2022, 33, 095603, DOI:10.1088/1361-6528/ac30f5.
- A. Ahlawat, M. Bala, M. K. Nayak and S. Tyagi, Development of cobalt oxide and titanium carbide based composite for microwave absorption in X-band, Mater. Sci. Eng. B, 2024, 305, 117447, DOI:10.1016/j.mseb.2024.117447.
- Z. Yan, A. Sattar and Z. Li, Priority Lithium recovery from spent Li-ion batteries via carbothermal reduction with water leaching, Resour., Conserv. Recycl., 2023, 192, 106937, DOI:10.1016/j.resconrec.2023.106937.
- E. Fan, L. Li, Z. Wang, J. Lin, Y. Huang, Y. Yao, R. Chen and F. Wu, Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects, Chem. Rev., 2020, 120, 7020–7063, DOI:10.1021/acs.chemrev.9b00535.
- L. F. Zhou, D. Yang, T. Du, H. Gong and W. Bin Luo, The Current Process for the Recycling of Spent Lithium Ion Batteries, Front. Chem., 2020, 8, 578044, DOI:10.3389/fchem.2020.578044.
- X. Zhong, W. Liu, J. Han, F. Jiao, W. Qin, T. Liu and C. Zhao, Pyrolysis and physical separation for the recovery of spent LiFePO 4 batteries, Waste Manage., 2019, 89, 83–93, DOI:10.1016/j.wasman.2019.03.068.
- Z. Wang, C. Yu, H. Huang, W. Guo, J. Yu and J. Qiu, Carbon-enabled microwave chemistry: From interaction mechanisms to nanomaterial manufacturing, Nano Energy, 2021, 85, 106027, DOI:10.1016/j.nanoen.2021.106027.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00202d |
| This journal is © The Royal Society of Chemistry 2024 |