Open Access Article
Open Access ArticleEco-friendly amidation of oxidized carbon black by dry ball milling†
Aida
Kiani
,
Marco
Palumbo
and
Maria Rosaria
Acocella
 *
*
Dipartimento di Chimica e Biologia and INSTM Research Unit, Università degli Studi di Salerno, Via Giovanni Paolo II 132, Fisciano, 84084, Italy. E-mail: macocella@unisa.it
First published on 19th July 2024
Abstract
An unprecedented amidation of oxidized carbon black (oCB) by a mechanochemical approach is reported. The reaction proceeds in the presence of octadecylamine (ODA) in a short time, providing the corresponding adduct (oCB/ODA) with a high degree of functionalization. Hummers and ball milling procedures were used to obtain oxidized carbon black in solution and solvent-free conditions, respectively. Although the oxidation procedure used for oCB synthesis can deeply affect the degree of amidation because of the different nature of the functional groups on the carbon surface, the resulting powders were both functionalized with amine. The corresponding adducts showed a strong inversion of polarity, going from the high dispersibility in the water solution of the starting material to the high hydrophobic behaviour due to the alkyl chains bonded on the surface. Since the mechanochemical approach respects important green metrics, the procedure is highly sustainable.
Sustainability spotlightCarbon materials are widely used in many applications such as catalysts, energy storage and conversion, supercapacitors, and water remediation and mainly in polymer composites because of their exceptional influence on polymer properties. Specifically, carbon black is extensively used as a cost-effective and readily available carbon material whose physical properties can be deeply modified by introducing new functional groups, which makes it versatile for all possible purposes. Although the introduction of new functional groups as well as different molecules bonded onto the carbon surface is a well-known strategy, all the syntheses reported until now have been performed in organic solvents and under very harsh conditions, failing to meet environmental sustainability expectations. In this context, we envisioned the development of our procedure that, by using a mechanochemical approach, is able to provide covalent functionalization on the carbon surface for generating new materials for hydrophobic coating. By using a long chain alkylamine, octadecylamine (ODA), the ball milling action was shown to be effective at achieving a highly functionalized adduct under solvent-free conditions. Typical adducts display a strong inversion of polarity, with the starting material being highly dispersible in water and the adducts showing high hydrophobic behavior. By respecting important green metrics, we demonstrate new perspectives for developing new materials using a mechanochemical approach that appears to be a safe strategy for honoring sustainability requirements. |
Introduction
Carbon materials such as graphite, graphene, carbon nanotubes, and carbon black are widely used in many applications such as catalysts,1–5 energy storage6–8 and conversion,9,10 supercapacitors,11 and water remediation12 and mainly in composites of polymers because of their exceptional influence on polymer properties.13–19 The ability to improve the physical and mechanical properties of polymer matrices has made these materials attractive and the subject of many research studies.Among them, carbon black (CB) is extensively used because it is waste from the incomplete combustion of petroleum derivatives and is cheap and readily available. Additionally, interest in this material has grown due to the ease of introducing new functional groups on the surface, which makes it versatile for all possible purposes.
To chemically modify the carbon surface, it is essential to introduce oxygen functional groups to make a further step of modification possible.
Carbon black has been functionalized by introducing oxygen-containing groups through several methods, e.g., Staudenmaier,20 Hofmann,21,22 Hummers,23 and sulfonitric treatment.24 However, most of them require expensive and toxic reagents and very harsh conditions to obtain a high oxidation degree, which fails to meet environmental sustainability expectations.
A high degree of oxidation has recently been achieved by using planetary ball milling under solvent-free conditions at room temperature with carbon black,25 pointing out the new perspectives for carbon materials to be chemically modified using the mechanochemical approach.
Based on green parameters (e.g., the E-factor, the EcoScale, energy demand, and CO2 production), this procedure is more environmentally friendly than previous H2O2 (ref. 26 and 27) or ozone oxidation28,29 methods.
As recently reported,30 further manipulations of oxidized carbon black (oCB) can be achieved using the mechanochemical approach by starting from oCB in the presence of a phosphonium salt. This sustainable functionalization is able to generate a new filler with flame retardant and/or antimicrobial properties.
The mechanochemical functionalization provides the adduct via cation exchange under solvent-free conditions in a short time, exploiting the acidic functionalities present on the surface.
Accordingly, the energy that comes from the collision of the balls during milling can facilitate the formation of covalent bonds, as well as ion exchange, allowing oxygen and cations to be introduced.
The choice of milling parameters, such as the speeding rate, ball-to-powder ratio, reaction time, and the nature of the balls, can profoundly affect the success of both procedures.
The need to functionalize the carbon surface derives from the possibility of deeply modifying the physical properties depending on the application required.
The introduction of long alkyl chains by using alkylamines, for instance, can increase the hydrophobic character of the carbon material, making it completely water-repellent.31,32
This modification can be obtained through the amidation reaction of oxidized carbon materials, i.e., graphite oxide, oxidized nanotubes, and oxidized carbon black, characterized by the presence of carboxyl groups on the surface and the amine residue of the chosen alkylamine. The reaction usually takes place in an organic solvent using coupling agents33,34 or under solvent-free conditions in a glass reactor, increasing the temperature to 180 °C and using high amine loading to promote amide conversion.35
This work aims to show the effectiveness of the ball milling action on carbon materials to obtain covalent functionalization on the surface by using amine.
As a matter of fact, many reports of covalent bond formation are disclosed for organic molecule synthesis36,37 induced by mechanochemical action, but, to the best of our knowledge, no examples are reported for the covalent amine functionalization of carbon black by ball milling.
Materials and methods
Carbon black samples (CB) of grade N110, containing 99.8% carbon and with BET surface area 130 m2 g−1, were purchased from Cabot Company (USA). Sulfuric acid, sodium nitrate, potassium permanganate, and octadecyl amine (ODA) were purchased from Sigma-Aldrich. All reagents were used as received, without purification.Oxidation of carbon black via Hummers' method
Oxidized carbon black (oCB) was prepared using Hummers' method. 12 mL of sulfuric acid and 0.25 g of sodium nitrate were introduced into a 1000 mL three-neck round-bottomed flask immersed in an ice bath and 0.5 g of carbon sample was added, with magnetic stirring. Once uniform dispersion was achieved, 1.5 g of potassium permanganate was added very slowly to minimize the risk of explosion. The reaction mixture was then heated to 35 °C and stirred for 24 hours. Afterward, 70 mL of deionized water was gradually added to the resulting black and dark green slurry, followed by the gradual addition of 0.5 mL of hydrogen peroxide 30 wt%; the obtained sample was poured into 700 mL of deionized water and then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min with a Neya16 centrifuge. The oCB powder was first washed twice with 10 mL of a 5 wt% HCl aqueous solution and subsequently washed with 50 mL of deionized water. Finally, the powder was dried at 60 °C for 12 h. About 0.4 g of the oCB sample was obtained.
000 rpm for 15 min with a Neya16 centrifuge. The oCB powder was first washed twice with 10 mL of a 5 wt% HCl aqueous solution and subsequently washed with 50 mL of deionized water. Finally, the powder was dried at 60 °C for 12 h. About 0.4 g of the oCB sample was obtained.
Preparation of oCB via ball milling
The ball-milling experiments were conducted at room temperature on a planetary ball mill Pulverisette 7 Premium (Fritsch GmbH, Germany).The milling conditions were configured as detailed in Table 1. Each procedure utilized 8 silicon nitride balls with a diameter of 10 mm within an 80 mL silicon nitride jar. Rotational frequencies were fixed at 500 rpm for 11 h. An Easy-GTM system (Fritsch GmbH, Germany) was used to measure the temperature of the milling beakers.
| Ball-to-powder weight ratio | 150 |
| Rotation frequency [min−1] | 500 |
| Ball size [mm] | 10 |
| Total balls weight [g] | 15 |
| Milling tool material | Silicon nitride |
| Beaker volume [cm3] | 80 |
| CB weight [mg] | 100 |
| Sample notation | oCBBM |
Preparation of oCBH/ODA and oCBBM/ODA via ball milling
The experiment was performed in an 80 mL silicon nitride jar with 8 silicon nitride balls and 300 rpm rotational frequencies by using a 1/1 mass ratio of oCBH or oCBBM and ODA for 2 h. The resulting powders were extensively washed only with ethanol (90 mL) to remove the excess amine, and the amine was recovered for reuse after evaporation of ethanol. The products were dried at 60 °C for 12 h in an oven and were recovered as black powders.To investigate the imine formation, the second part of the prepared oCBBM/ODA was washed extensively first with water and then with ethanol (90 mL) to remove the possible free amine coming from the ketone deprotection. The resulting powder was dried at 60 °C for 12 h in the oven.
Results and discussion
Oxidized carbon black (oCB) samples derived from the Hummers oxidation (oCBH) and ball milling oxidation (oCBBM) were chosen as reference materials to evaluate the best method of oxidation to proceed before amidation. The different natures of the functional groups present on the carbon surface can deeply affect the amidation reaction to provide the best performance.Therefore, first, the characterization of oCBH and oCBBM was performed using FTIR, XRD, BET, and elemental analysis.
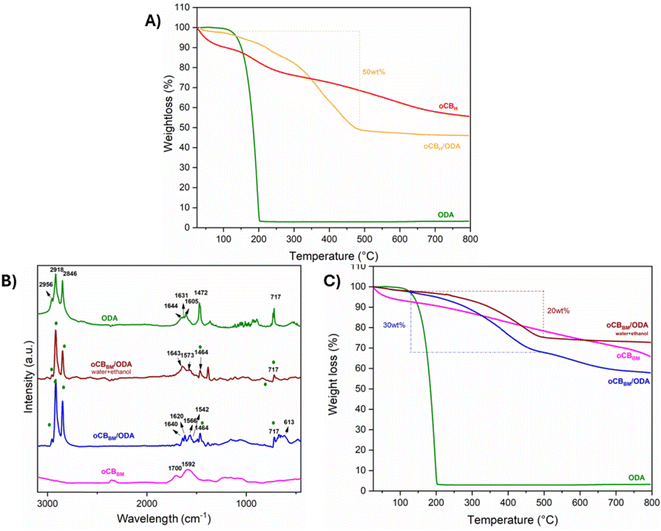
The FTIR evidences some important differences concerning the nature of the carbonyl group present on the surface (Fig. 1A). Specifically, while the region between 1300 and 1000 cm−1 is quite similar, with a comparable distribution of ethers, vinyl ethers, and alcohols for both oxidation procedures, the nature of the carbonyl group is deeply affected. In particular, the Hummers oxidation promotes the formation of carboxyl acid with characteristic vibration at 1715 cm−1, while the ball milling oxidation promotes the possible formation of unsaturated ketones and lactones25 with typical vibration at 1700 cm−1.
 | ||
| Fig. 1 FTIR (A) and X-ray diffraction patterns (B) of CB (black), oCBH (red), oCBBM (pink), oCBH/ODA (yellow), oCBBM/ODA (blue), and ODA (green). | ||
Therefore, the fragmentation and exfoliation during the milling drive the carbon material to react with the air oxygen and provide new oxygen functional groups whose nature can be different from that of the oxidation in solution. As a result, changing the oxidation procedure makes it possible to control the functional groups on the carbon surface.
The different degrees of oxidation were evaluated by elemental analysis, which showed a slightly higher O/C ratio of 0.44 for the oCB obtained by Hummer's oxidation (Table 2), as confirmed by the TGA analysis (Fig. 2A).
| Entry | Sample | N (%) | C (%) | H (%) | O (%) | O/C | H/C | N/C |
|---|---|---|---|---|---|---|---|---|
| a The sample was washed with water and then with ethanol. | ||||||||
| 1 | oCBH | 0.3 | 68 | 1.5 | 30.2 | 0.44 | 0.02 | 0.004 |
| 2 | oCBBM | 1.1 | 71 | 0.5 | 27 | 0.38 | 0.007 | 0.015 |
| 3 | oCBH/ODA | 3.0 | 79.7 | 6.3 | 11.0 | 0.14 | 0.08 | 0.04 |
| 4 | oCBBM/ODA | 2.8 | 89 | 5.6 | 2.6 | 0.029 | 0.06 | 0.031 |
| 5 | oCBBM/ODAa | 2.3 | 76.0 | 5.0 | 16.7 | 0.22 | 0.07 | 0.030 |
The increasing disorder induced by the milling action on the already amorphous carbon is evidenced by the X-ray diffraction pattern (Fig. 1B). The amorphous halo centered about at 2θ = 24° is broadened because of the milling treatment that also provides oCBBM with a higher surface area (291 m2 g−1) than oCBH (125 m2 g−1).
Based on recent reports about the possibility of performing amidation by the mechanochemical approach under solvent-free conditions with small molecules,36,37 we decided to evaluate the ability first of oCBH and then of oCBBM to react with octadecyl amine (ODA) in planetary milling and under solvent-free conditions.
The experiment was performed in an 80 mL silicon nitride jar with 8 silicon nitride balls at a 300 rpm speeding rate by using a 1/1 mass ratio of the oCBH or oCBBM and ODA.
After 2 h of milling and extensive washing with ethanol to remove the excess amine, the product was recovered as a black powder and characterized by FTIR, TGA, XRD, and elemental analysis.
As reported in Fig. 1A, interesting changes were found for the oCBH/ODA adduct. The FTIR spectrum of oCBH/ODA evidences the vibrations at 2953, 2917, 2846, and 717 cm−1 related to the –CH2 stretching of the octadecyl chain and N–H wagging, respectively. Moreover, a sensible decrease of the carbonyl stretching at 1715 cm−1, the appearance of a shoulder at 1526 cm−1, possibly due to the C–N amide stretching,38 and the blue shift from 1475 cm−1 in the free amine to 1462 cm−1 in the oCBH/ODA, confirm the occurrence of the amidation. It is worth noting that the presence of epoxide on oxidized carbon cannot exclude possible amine functionalization by epoxy ring opening.
Additional characterization was performed on the resulting powder to reveal the possible free amine after the reaction. As shown in Fig. 1B, the X-ray diffraction pattern of the product does not evidence any crystalline peak due to the free amine, confirming the complete removal of excess ODA.
As reported in a precedent paper39 alkyl amines can provide hydrogen bonds of neutral or protonated amines, as well as ionically bound protonated amines on the oxidized graphitic surface. Therefore, additional washing steps under acidic conditions (pH = 4.5 and then 2) were performed on the derived oCBH/ODA adduct. FTIR and EA confirmed no variation in the peak's intensity and the elemental composition, respectively (see the ESI†).
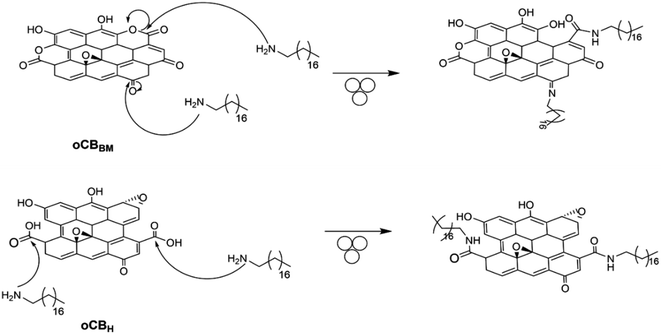
More surprisingly, interesting results were also obtained starting from oCBBM. Although ball milling oxidation does not provide carboxyl groups on the carbon surface, it does provide ketones and lactones, which are useful functional groups that react with amines. Specifically, while lactones are suitable for the amidation reaction, ketones are instead able to provide imines (Scheme 1).
The different nature of the functional groups on oCBBM is also evident from FTIR after the reaction. As reported in Fig. 1A, a more complex pattern is recorded for oCBBM/ODA. Specifically, the 1700 cm−1 band related to the carbonyl groups disappears and a vibration band at 1620 cm−1 comes to light possibly due to imine formation,40 as well as a vibration at 1640 cm−1 and a shoulder at 1542 cm−1 related to the amide. Again, the vibrations at 2953, 2917, 2846, and 717 cm−1 related to the –CH2 stretching of the octadecyl chain and N–H wagging, respectively, and the blue shift from 1475 to 1464 cm−1 are clearly observed also for oCBBM/ODA.
The X-ray diffraction pattern of the adduct also shows a broadened halo centered at 2θ = 22° due to the additional milling treatment, and no crystalline peaks are observed, confirming that there is no excess of free amine.
More interesting information was derived from the elemental analysis (Table 2). Comparing the nitrogen content of the starting materials, oCBH and oCBBM, with the resulting adducts, a higher uptake of nitrogen is recorded for oCBH/ODA (entry 3 vs. 1, Table 2) than oCBBM/ODA (entry 4 vs. 2, Table 2). The slightly higher oxidation degree, as well as the content of carboxyl groups, implicate higher functionalization through the amidation reaction.
Furthermore, the oxygen content is strongly reduced from 27% of the starting oCBBM to 2.6% in oCBBM/ODA, possibly due to the formation of imines.
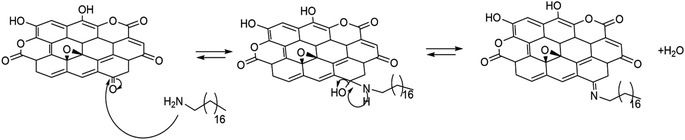
Being an equilibrium reaction with water as a side product, the reaction can be driven into complete imine formation, exploiting the ability of the carbonaceous material to act as a scavenger of water.41 Conversely, in the presence of water, the equilibrium is shifted to the reagent with the formation of ketones again.
Based on the typical mechanism of imine formation (Scheme 2), it becomes evident that in the presence of water, the imine should be removed, and only the amide provided by the mechanochemical reaction with lactones should still be present in oCBBM/ODA.
Therefore, oCBBM/ODA was extensively washed with water and then with ethanol to remove the possible free amine coming from the ketone deprotection. After drying at 60 °C, the resulting powder was analyzed by FTIR, EA, and TGA.
As shown in Fig. 2B, a dramatic change in the FTIR spectra is detected after the water treatment. In particular, the vibration at 1643 and 1541 cm−1 related to carbonyl and C–N stretching of amide, respectively, are evident, as are the bands at 2953, 2917, 2846, and 717 cm−1 typical of the starting amine, while 1620 stretching related to the imine disappears. Additionally a broad band in the 1680–1700 cm−1 region appears to be possibly related to the ketone deprotection.
The elemental analysis agrees with the FTIR results, showing a reduction of the nitrogen content and an enhanced percentage of oxygen due to the deprotection of ketone functionalities (entry 5, Table 2).
In addition, thermogravimetric analysis confirms that all the samples considered display different levels of functionalization. As reported in Fig. 2A, oCBH/ODA shows a 50% weight loss, much higher than the 30% observed for oCBBM/ODA (Fig. 2B). After water treatment, the oCBBM/ODA adduct shows a further reduction in weight loss to 20 wt% due to the removal of imine. It has to be noted that in all cases, the weight losses are in good agreement with the amount of nitrogen detected by the EA (see the ESI†).
Dispersibility tests
As previously reported,31,32 amine functionalization deeply changes the polarity of oCB, making it strongly hydrophobic because of the presence of long alkyl chains.To evaluate the polarity changes, dispersibility tests were conducted in the presence of water, ethyl acetate, and toluene chosen as reference solvents based on their polarity parameters42 of 10, 4.4, and 2.3, respectively.
The surface modification by the amidation reaction caused a profound variation in the polarity of the oCB powders (Fig. 3).
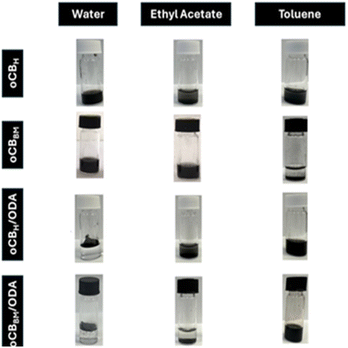 | ||
| Fig. 3 oCBH, oCBBM, oCBH/ODA, and oCBBM/ODA suspension in water, ethyl acetate, and toluene solution 5 mg mL−1 after 5 min of sonication and 30 min of storage. | ||
This is particularly evident when comparing the solubility in water of both the powders, oCBH and oCBBM, with the corresponding oCB/ODA adducts. Specifically, very hydrophobic materials were provided by the mechanochemical functionalization of oCBH and oCBBM that strongly repel water and are stratified on the surface, generating a hydrophobic coating. The behaviour is different in ethyl acetate: oCBH/ODA still results in a dispersible sediment in the solvent due to its residual polar functional groups (i.e., alcohols, ethers, etc.), while oCBBM/ODA precipitates as a non-dispersible sediment due to the conversion of ketones and lactones of the starting oCBBM into imines and amides with the corresponding reduction of oxygen content (2.6%, see Table 2 entry 4).
As expected, both the oCB/ODA adducts are well dispersed in toluene as an apolar solvent.
Sustainability evaluation of the process: green metrics
To show the effectiveness and greener character of the mechanochemical approach, a direct comparison with the less impactful procedure reported until now for the amidation of the carbon material, i.e., graphite oxide, under solvent-free conditions33 is proposed here.As a matter of fact, while the reaction by using a coupling agent in DMF or thionyl chloride and DMF under heating is clearly not sustainable, the amidation procedure under solvent-free conditions seems to be relatively eco-friendly. Taking into account the condition to operate under vacuum, the total heating time, and the high weight ratio between the oxidized carbon material and ODA, 1 to 5, respectively, reported in ref. 33, the evaluation of the green parameters was performed considering the atom efficiency (AE), the reagent mass efficiency (RME), the process mass intensity (PMI), the EcoScale, energy demand, and CO2 production associated with the process (greenhouse gas equivalents).
Atom economy is a measure of the conversion efficiency of a chemical process, which is extremely useful for predicting how much waste the process will generate. Conversely, RME takes yields and the molar quantities of reactants into account, evidencing the reagent excess used in the reaction. It is a realistic metric to illustrate how far from ‘green’ we are currently operating our processes. PMI is defined as “the total mass of materials used to produce a specified mass of product”.43 Therefore, a value close to 1 means that all the reagents are converted into the product, optimizing resource utilization. The E-factor is, instead, the effective amount of waste created.
In contrast, the EcoScale is a semiquantitative tool for selecting organic preparations that assigns penalty points based on a maximum value of 100 considering yield, cost, safety, and purification conditions.44 This tool is particularly useful when the procedure moves from the laboratory to the industrial scale.
As part of the assessment, the energy required for each process needs to be included since energy consumption generates primarily waste, such as carbon dioxide.
The comparison between the amidation by heating and the mechanochemical process starting from oCBH and oCBBM is reported in Table 3 (for all the calculations, see the ESI†).
| Procedure | AE (%) | RME (%) | PMI | E | EcoScalea (%) | Energy demand (kW) | CO2 productionb (kg) |
|---|---|---|---|---|---|---|---|
| a The EcoScale was evaluated based on the classification reported in ref. 43. b The CO2 production was evaluated based on the energy demand in kW h−1 for each process and converted into CO2 following the conversion factor reported by EPA (2020) AVERT, US Environmental Protection Agency, Washington, DC. | |||||||
| Heating, solvent-free | 97 | 46 | 2.16 | 1.16 | 91.5 | 2.4 | 1 |
| Ball milling oCBH | 97 | 65 | 1.54 | 0.5 | 95.5 | 2.4 | 1 |
| Ball milling oCBBM | 94 | 57.5 | 1.7 | 0.7 | 95.5 | 2.4 | 1 |
Based on the Heidolph data sheet, the energy required for keeping the reaction at 180 °C for 3 h was calculated using 0.800 kW, as well as the ball mill's 1.2 kW h−1 energy consumption. It is worth noting that, although the energy demand, the CO2 production, and AE are similar, the solvent-free reaction induced by heating shows an RME lower than both the milling procedures due to the excess of the amine reagent with respect to the carbon material used (5 to 1 by weight).
As a result, the PMI value was closer to one for both oCBH and oCBBM reactions with amine by ball milling procedures and the corresponding E factors were lower than those for the heating/under vacuum treatment, providing a more eco-friendly procedure with a higher EcoScale value.
Therefore, even assuming a higher efficiency for the literature procedure, the RME, PMI, E, and EcoScale values make the ball milling procedure more environmentally sustainable.
To get a comprehensive evaluation of the green metrics, not only the waste generated during the process but also the one provided by the reagent synthesis should also be evaluated.
Hence, although the green metrics for the oCBH amidation seem to be better than those of the corresponding reaction with oCBBM, the oxidation process for oCBH is based on the Hummers oxidation, characterized by toxic solvents and very harsh conditions. Therefore it is easy to understand that oCBBM, which is an oxidized material obtained from a highly sustainable milling process, achieves the best eco-friendly performances for providing functionalized carbon materials.
Conclusions
The mechanochemical functionalization of oCB in the presence of octadecyl amine (ODA) under solvent-free conditions has been reported to proceed efficiently.The study was performed, first comparing different oxidation methods for the carbon material, Hummers, and ball milling procedures to evidence the effect of the different nature of the functional groups in the amidation reaction step.
Although the oxidation procedures used for oCB synthesis can affect the degree of amidation because of the different nature of the functional groups present on the carbon surface, the resulting powders were both functionalized with amine. Specifically, the presence of carboxyl groups on the oCB by Hummers oxidation promotes amide formation with a degree of functionalization of 50 wt%, while the ketones and lactones of the oCB by milling oxidation provide imines and amides, respectively, with a degree of functionalization of 30 wt%.
Irrespective of the different functionalization degrees, the corresponding adducts showed both an inversion of polarity as long alkyl chains were introduced, which increased hydrophobicity and provided new fillers for water-repellent coatings.
Finally, to show the effectiveness and greener character of the mechanochemical approach, a direct comparison with the less impactful procedure reported until now for the amidation of carbon materials under solvent-free conditions was performed, also evaluating the impact of the synthetic procedure for the starting oCB.
Since the Hummers oxidation process for oCB involves toxic solvents and very harsh conditions, it is evident that the mechanochemical approach from the oCB synthesis to the amidation reaction achieves the best sustainability performance.
Data availability
The data supporting this article have been included as part of the ESI.† The data that support the findings of this study are available from the corresponding author, M. R. A., upon reasonable request.Author contributions
M. R. A. contributed to the conception and design of the study, A. K. performed the experimental analysis and characterization, M. P. performed part of the experimental analysis, and M. R. A. and A. K. wrote the first draft of the manuscript. All authors contributed to the manuscript revision and read and approved the submitted version.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support of Ministero dell’Università e della Ricerca (MUR) is acknowledged.References
- M. R. Acocella and G. Guerra, ChemCatChem, 2018, 10, 2350–2359 CrossRef CAS.
- C. Su and K. P. Loh, Acc. Chem. Res., 2013, 46, 2275–2285 CrossRef CAS PubMed.
- M. R. Acocella, A. Vittore, M. Maggio, G. Guerra, L. Giannini and L. Tadiello, Polymers, 2019, 11, 1330 CrossRef PubMed.
- M. R. Acocella, M. Maggio, C. Ambrosio, N. Aprea and G. Guerra, ACS Omega, 2017, 2, 7862–7867 CrossRef CAS PubMed.
- R. Villano, M. R. Acocella and G. Guerra, ChemistrySelect, 2017, 2, 10559–10564 CrossRef CAS.
- C. Liu, X. Liu, J. Tan, Q. Wang, H. Wen and C. Zhang, J. Power Sources, 2017, 342, 157–164 CrossRef CAS.
- Y. Zhou, L. Liu, Y. Shen, L. Wu, L. Yu, F. Liang and J. Xi, Chem. Commun., 2017, 53, 7565–7568 RSC.
- S. Liang, C. Liang, Y. Xia, H. Xu, H. Huang, X. Tao, Y. Gan and W. Zhang, J. Power Sources, 2016, 306, 200–207 CrossRef CAS.
- S. Zhuang, E. S. Lee, L. Lei, B. B. Nunna, L. Kuang and W. Zhang, Int. J. Energy Res., 2016, 40, 2136–2149 CrossRef CAS.
- G. Wang, J. Zhang and S. Hou, Mater. Res. Bull., 2016, 454–458 CrossRef CAS.
- C. Schneidermann, N. Jäckel, S. Oswald, L. Giebeler, V. Presser and L. Borchardt, ChemSusChem, 2017, 10, 2416–2424 CrossRef CAS PubMed.
- Y. Huang, J. Tang, L. Gai, Y. Gong, H. Guan, R. He and H. Lyu, Chem. Eng. J., 2017, 319, 229–239 CrossRef CAS.
- S. Xu, M. Wen, J. Li, S. Guo, M. Wang, Q. Du, J. Shen, Y. Zhang and S. Jiang, Polymer, 2008, 49, 4861–4870 CrossRef CAS.
- L. D'Urso, M. Acocella, G. Guerra, V. Iozzino, F. De Santis and R. Pantani, Polymers, 2018, 10, 139 CrossRef PubMed.
- L. D′Urso, M. R. Acocella, F. De Santis, G. Guerra and R. Pantani, Polymer, 2022, 256, 125237 CrossRef.
- P. Karami, S. S. Khasraghi, M. Hashemi, S. Rabiei and A. Shojaei, Adv. Colloid Interface Sci., 2019, 269, 122–151 CrossRef CAS PubMed.
- M. H. Islam, S. Afroj, M. A. Uddin, D. V. Andreeva, K. S. Novoselov and N. Karim, Adv. Funct. Mater., 2022, 32, 2205723 CrossRef CAS.
- Y. Fan, G. D. Fowler and M. Zhao, J. Cleaner Prod., 2020, 247, 119115 CrossRef CAS.
- L. B. Tunnicliffe and J. J. C. Busfield, in Designing of Elastomer Nanocomposites: from Theory to Applications, ed. K. W. Stöckelhuber, A. Das and M. Klüppel, Springer International Publishing, Cham, 2017, pp. 71–102 Search PubMed.
- L. Staudenmaier, Ber. Dtsch. Chem. Ges., 1898, 31, 1481–1487 CrossRef CAS.
- U. Hofmann and E. König, Z. Anorg. Allg. Chem., 1937, 234, 311–336 CrossRef CAS.
- U. Hofmann and R. Holst, Ber. Dtsch. Chem. Ges., 1939, 72, 754–771 CrossRef.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- S. Gómez, N. M. Rendtorff, E. F. Aglietti, Y. Sakka and G. Suárez, Appl. Surf. Sci., 2016, 379, 264–269 CrossRef.
- A. Kiani, M. R. Acocella, V. Granata, E. Mazzotta, C. Malitesta and G. Guerra, ACS Sustainable Chem. Eng., 2022, 10, 16019–16026 CrossRef CAS.
- J. C. Curtis, R. L. Taylor and G. A. Joyce, Hydrogen peroxide oxidation of Carbon Black, WO pat., 1998032804A1, 1998 Search PubMed.
- C. Y. Liu and W. T. Cheng, Surf. Interface Anal., 2019, 51, 316–325 CrossRef CAS.
- G. Lota, P. Krawczyk, K. Lota, A. Sierczyńska, Ł. Kolanowski, M. Baraniak and T. Buchwald, J. Solid State Electrochem., 2016, 20, 2857–2864 CrossRef CAS.
- I. Sutherland, E. Sheng, R. H. Bradley and P. K. Freakley, J. Mater. Sci., 1996, 31, 5651–5655 CrossRef CAS.
- A. Kiani, N. Sozio and M. Rosaria Acocella, Mol. Syst. Des. Eng., 2023, 8, 942–949 RSC.
- H. Wang, Q. Zhao, K. Zhang, F. Wang, J. Zhi and C.-X. Shan, Langmuir, 2022, 38, 11304–11313 CrossRef CAS PubMed.
- G. Achagri, Y. Essamlali, O. Amadine, M. Majdoub, A. Chakir and M. Zahouily, RSC Adv., 2020, 10, 24941–24950 RSC.
- One-step nondestructive functionalization of graphene oxide paper with amines - RSC Advances (RSC Publishing), https://rsc.66557.net/en/content/articlelanding/2018/ra/c8ra00986d, accessed 19 March 2024.
- S. Kang, A. R. Jones, J. S. Moore, S. R. White and N. R. Sottos, Adv. Funct. Mater., 2014, 24, 2947–2956 CrossRef CAS.
- N. Alzate-Carvajal, E. V. Basiuk, V. Meza-Laguna, I. Puente-Lee, M. H. Farías, N. Bogdanchikova and V. A. Basiuk, RSC Adv., 2016, 6, 113596–113610 RSC.
- T.-X. Metro, J. Bonnamour, T. Reidon, J. Sarpoulet, J. Martinez and F. Lamaty, Chem. Commun., 2012, 48, 11781–11783 RSC.
- W. I. Nicholson, F. Barreteau, J. A. Leitch, R. Payne, I. Priestley, E. Godineau, C. Battilocchio and D. L. Browne, Angew. Chem., Int. Ed., 2021, 60, 21868–21874 CrossRef CAS PubMed.
- S. Rani, M. Kumar, R. Kumar, D. Kumar, S. Sharma and G. Singh, Mater. Res. Bull., 2014, 60, 143–149 CrossRef CAS.
- Y. Matsuo, T. Miyabe, T. Fukutsuka and Y. Sugie, Carbon, 2007, 45, 1005–1012 CrossRef CAS.
- L. R. Knöpke, N. Nemati, A. Köckritz, A. Brückner and U. Bentrup, ChemCatChem, 2010, 2, 273–280 CrossRef.
- D. Luciana, M. R. Acocella, F. De Santis, G. Guerra and R. Pantani, Polymer, 2022, 256, 125237 CrossRef.
- L. R. Snyder, J. Chromatogr. Sci., 1978, 16, 223–234 CAS.
- C. Jimenez-Gonzalez, C. S. Ponder, Q. B. Broxterman and J. B. Manley, Org. Process Res. Dev., 2011, 15, 912–917 CrossRef CAS.
- K. Van Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem., 2006, 2(3) DOI:10.1186/1860-5397-2-3.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00216d |
| This journal is © The Royal Society of Chemistry 2024 |