Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rapid screening of toxicity to thermotolerant yeasts: inhibition of growth and fermentation by ionic liquids and zwitterions†
Mayu
Shibata‡
a,
Ayumi
Hachisu‡
a,
Souta
Uemori
a,
Hitomi
Tobe
a,
Kazuaki
Ninomiya
b and
Kosuke
Kuroda
 *ac
*ac
aFaculty of Biological Science and Technology, Graduate School of Natural Science and Technology, Kanazawa University, Kanazawa 920-1192, Japan. E-mail: kkuroda@staff.kanazawa-u.ac.jp
bInstitute for Frontier Science Initiative, Kanazawa University, Kanazawa 920-1192, Japan
cNanoMaterials Research Institute, Kanazawa University, Kakuma-machi, Kanazawa 920-1192, Japan
First published on 6th August 2024
Abstract
For extremely efficient bioethanol production, simultaneous pretreatment, saccharification, and fermentation in the same reaction pot (called a one-pot process) is necessary. Thermotolerant yeast Kluyveromyces marxianus can ferment at around 50 °C and is thus suitable for this process. We have developed cellulose-dissolving zwitterionic liquids, which are suitable pretreatment solvents to enable a one-pot process. On the other hand, there are no studies of the toxicity to yeasts including Kluyveromyces marxianus. We here studied the toxicity after establishing the screening methods applicable to high temperature. The zwitterion was confirmed to be low-toxic in most cases, compared to the most famous cellulose-dissolving ionic liquid. We further subjected two natural zwitterions, trimethylglycine and L-carnitine, to the same screening. Trimethylglycine, especially, was low-toxic, while it does not dissolve cellulose. The inhibition of growth and fermentation depended on the ion species, concentration, microorganism species, and temperature.
Sustainability spotlightInedible cellulosic biomass-derived ethanol is a solution for the competition between food and energy, which is a problematic issue in starch-derived bioethanol. However, cellulosic bioethanol has not been practically industrialised for decades. One of the reasons which prevent its industrialisation is the production energy cost. An ideal bioethanol production process includes simultaneous pretreatment, saccharification, and fermentation at 50 °C; which requires biocompatible cellulose solvents and thermotolerant yeasts in addition to cellulase. This study revealed the relation between a biocompatible cellulose solvent (zwitterionic liquid) and a thermotolerant yeast (Kluyveromyces marxianus), contributing to achieving SDGs 2 (zero hunger), 7 (affordable and clean energy), and 13 (climate action). |
Introduction
Ethanol from cellulosic biomass is a potential alternative to fossil fuels.1 However, the recalcitrant and complex structure of cellulosic biomass makes chemical/biological conversion difficult.2 Therefore, pretreatment is necessary to relax the structure. A typical pretreatment method is dissolution in solvents, which requires high temperature (low temperature in some cases) and/or high pressure; in other words, it demands a high energy cost.3–6 Because the heat of combustion of ethanol is only approximately 60% that of petroleum, the high energy cost is a critical issue for industrialisation.Although ionic liquids have high cost, they dissolve cellulose at ambient temperature and pressure, thus reducing the energy cost of bioethanol production.7–12 For the industrialisation of cellulosic bioethanol, successive pretreatment, saccharification, and fermentation in a single vessel are required to reduce energy costs further.13–16 However, typical cellulose-dissolving ionic liquids, except for few exceptions,17,18 are highly toxic to microorganisms, and this successive process is not feasible with these ionic liquids.
A well-known mechanism of ionic liquid toxicity is the disruption of cell membranes, as detailed below:17,19–22 (1) the cations of ionic liquids are attracted by electrostatic interactions with the phospholipids of the cell membrane. (2) The hydrophobic alkyl chains of the cations are inserted into the cell membrane via hydrophobic interactions. Based on these mechanisms, in 2017, we designed a low-toxicity zwitterionic liquid lacking hydrophobic alkyl chains (OE2imC3C; Fig. 1).13,19,23–28 OE2imC3C dissolves cellulose because of its highly polar anion. Successive bioethanol production was achieved with OE2imC3C; however, the reduction in energy cost was still insufficient. The application of simultaneous saccharification and fermentation is the next potential means to reduce energy costs further.29 Simultaneous saccharification and fermentation are generally performed at around 30 °C using Saccharomyces cerevisiae; however, this is inefficient because the optimum temperature for cellulase is around 50 °C. The heat-resistant yeast Kluyveromyces marxianus can ferment at 40–45 °C, allowing efficient simultaneous saccharification and fermentation.30,31 Until now, fermentation in OE2imC3C has been studied only with recombinant Escherichia coli KO11 (optimum temperature: 37 °C),13 and the effects of OE2imC3C on other microorganisms have not been investigated. Therefore, this study examined the effects of OE2imC3C on the growth and fermentation of K. marxianus at around 40–50 °C. The relationship between temperature and toxicity was also examined since K. marxianus can be cultured even at 30 °C, which means it can be grown over a relatively wide temperature range. To the best of our knowledge, the relationship between the toxicity of ionic/zwitterionic liquids and the growth and fermentation temperatures has not yet been investigated.
In studies using ionic/zwitterionic liquids, the number of samples is often very large as there may be several conditions, such as ion species, ion concentration, incubation time, and temperature. Therefore, it is important to establish a method for rapidly evaluating growth and fermentation. Therefore, establishing a rapid evaluation method applicable to high-temperature cultures is also one of the purpose of this study. High temperatures induce evaporation of water and ethanol from the media, causing critical problems for analyses. Screening methods at high temperature, which have not been well-established, were developed in this study.
Experimental
Materials
K. marxianus DMKU3-1042 and NBRC1777 were purchased from the National Institute of Technology and Evaluation Biological Resource Center (NBRC). OE2imC3C was synthesised as previously reported, with minor modifications.13,32 The modification is changing the starting material from benzenesulfonyl chloride to p-toluenesulfonyl chloride. Trimethylglycine and L-carnitine were purchased from Tokyo Chemical Industry Co., Ltd. (Chuo-ku, Tokyo, Japan) and used as received. [C2mim]OAc was purchased from Iolitech GmbH (Heilbronn, Germany) and was used as received. D (+)-Glucose, meat peptone, and yeast extract (dried yeast extract) were purchased from Nacalai Tesque, Inc. (Nakagyo-ku, Kyoto, Japan) and used as components of the YPD media.Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min) and inoculated into OE2imC3C/YPD (OE2imC3C concentration: 0.01, 0.05, 0.1, 0.5, and 1 M). The initial OD600 was 20, measured using a cuvette with 1 cm path length. A high initial OD was set so that glucose was not used for growth. The inoculated medium (1 mL) was incubated in 2 mL cryotubes (TR7001, NIPPON Genetics Co., Ltd., Tokyo, Japan), used to prevent the evaporation of ethanol, at various temperatures for 3 h using a rotator (ASONE, ACR-100) at 10 rpm. The ethanol concentrations were analysed using high-performance liquid chromatography (HPLC). 100 μL of solution was taken and filtered through a small-dead-volume syringe filter (Millex®-LG, SLLGH04NK, Merck) for HPLC analysis. Glass inserts (TORAST vial insert, GLCTV-I01; Shimadzu GLC, Ltd.) were placed in the HPLC vials. The sample injection volume for HPLC was 20 μL. HPLC analyses were conducted under the following conditions: refractive index detector (Shimadzu Co.); column oven, 70 °C; mobile phase, 4.25 mM H2SO4; flow rate, 0.7 mL min−1. Coregel ION-300 column (Tokyo Chemical Industry Co., Ltd). This experiment was conducted three times to ensure reproducibility.
000 rpm, 5 min) and inoculated into OE2imC3C/YPD (OE2imC3C concentration: 0.01, 0.05, 0.1, 0.5, and 1 M). The initial OD600 was 20, measured using a cuvette with 1 cm path length. A high initial OD was set so that glucose was not used for growth. The inoculated medium (1 mL) was incubated in 2 mL cryotubes (TR7001, NIPPON Genetics Co., Ltd., Tokyo, Japan), used to prevent the evaporation of ethanol, at various temperatures for 3 h using a rotator (ASONE, ACR-100) at 10 rpm. The ethanol concentrations were analysed using high-performance liquid chromatography (HPLC). 100 μL of solution was taken and filtered through a small-dead-volume syringe filter (Millex®-LG, SLLGH04NK, Merck) for HPLC analysis. Glass inserts (TORAST vial insert, GLCTV-I01; Shimadzu GLC, Ltd.) were placed in the HPLC vials. The sample injection volume for HPLC was 20 μL. HPLC analyses were conducted under the following conditions: refractive index detector (Shimadzu Co.); column oven, 70 °C; mobile phase, 4.25 mM H2SO4; flow rate, 0.7 mL min−1. Coregel ION-300 column (Tokyo Chemical Industry Co., Ltd). This experiment was conducted three times to ensure reproducibility.
Results & discussion
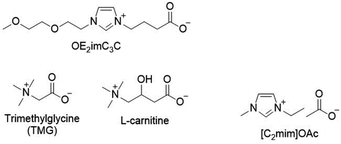
In this study, we employed K. marxianus and S. cerevisiae. K. marxianus has excellent thermostability, and S. cerevisiae has an optimum temperature of around 30 °C and critical growth inhibition occurring below 40 °C.30 In contrast, K. marxianus DMKU3-1042 is capable of growing at least at 49 °C and fermenting at 45 °C.30In addition to OE2imC3C, trimethylglycine (TMG) and L-carnitine (Fig. 1) were examined as references in this study, whereas they have been reported to have low biomass pretreatment potential.25 TMG and L-carnitine are natural zwitterions containing ammonium cations. A typical cellulose-dissolving ionic liquid ([C2mim]OAc) was also used as a reference control (Fig. 1).
Growth inhibition of K. marxianus by OE2imC3C
The culture was performed in a 96-well plate incubator under agitation. However, the medium evaporates quickly when cultured at high temperatures. Therefore, an air-permeable but non-moisture-permeable sheet was used. Furthermore, we placed wet paper towels in the incubator, wetted them again every 2 hours when incubated at 50 °C or higher, and poured 100 μL of water into unused wells. The outermost wells were not used because the water in these wells is prone to evaporation (the effects are shown in Fig. S1†).
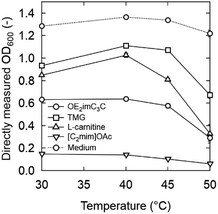
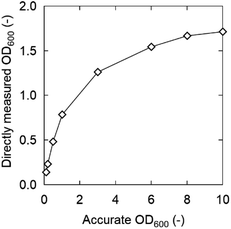
The OD600 is generally proportional to the density of bacteria at low densities, but the sensitivity of spectrophotometers becomes poor at high densities and is not proportional to the bacterial density. Therefore, initially the OD600 was properly diluted to a range proportional to the bacterial density and measured. The correct OD600 value was calculated by multiplying it with the dilution factor (described below as “accurate OD600”). On the other hand, in this study, OD600 measured directly in 96-well plates without dilution (defined as “directly measured OD600”) is preferable to rapidly screen a large number of samples. We assumed that there is a difference between “directly measured OD600” and “accurate OD600” at high OD600, and the relation is shown in Fig. 2.
 | ||
| Fig. 2 Relation between directly measured and accurate OD600 values measured in 96-well plates. Strain: K. marxianus DMKU3-1042. | ||
In K. marxianus, these values did not match above an accurate OD600 of 0.5 (directly measured OD600 = 0.34 at an accurate OD600 of 0.5), whereas they were still similar when the accurate OD600 was 0.2 (directly measured OD600 = 0.17). The slope became very small, especially when the OD600 exceeded 1.3. However, the directly measured OD600 increased slightly even at a highly accurate OD600, indicating that the 96-well plate can be used for qualitative/semi-quantitative evaluation. Therefore, the directly measured OD600 values were adequate for rapid screening.
When K. marxianus was cultured without OE2imC3C at an initial OD600 of 0.2, growth was complete within 6 h at appropriate temperatures (Fig. S2†). Since the general method requires incubation for at least 24 h,13 the duration of the experiment was highly conserved. This also means that midnight sampling and repeated wetting of paper towels (at temperatures above 50 °C) can be avoided, which are also significant advantages.
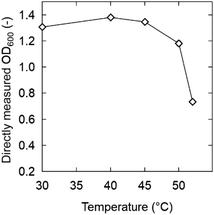 | ||
| Fig. 3 OD600 of the media without zwitterion/ionic liquid after 6 h of incubation. The time courses of OD600 at specific temperatures are shown in Fig. S3.† Strain: K. marxianus DMKU3-1042. | ||
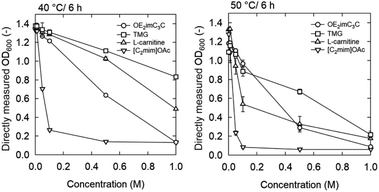 | ||
| Fig. 4 OD600 after 6 h incubation in media supplemented with OE2imC3C, TMG, L-carnitine, or [C2mim]OAc at 40 and 50 °C. Strain: K. marxianus DMKU3-1042. Initial OD600: 0.2. The figures for 30 and 45 °C are shown in Fig. S4.† | ||
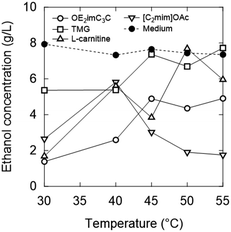
Natural ammonium-based zwitterions, TMG and L-carnitine, were less toxic than OE2imC3C (Fig. 4; 40 °C). The growth of K. marxianus was inhibited depending on the concentration of the zwitterions; notably, K. marxianus grew even in 1.0 M TMG and L-carnitine solutions. In particular, TMG showed very low toxicity.
We further investigated the reasons behind the lower toxicities of TMG and L-carnitine. A significant pH effect on growth was not observed (Fig. S3, Table S1†). The molecular weights of OE2imC3C and TMG/L-carnitine are different (OE2imC3C: 256, TMG: 117 and L-carnitine: 161), and the difference in molecular weight might have an effect. Similar trends in the three zwitterions were observed when the abscissa concentration was g L−1 instead of the molar concentration, which might indicate that the unit of g L−1 is important in the toxicity (Fig. S4†). To explore the scientific meaning of the unit of g L−1, the ion-to-water molar ratio was calculated (Table S2†). When ions are present at high concentrations, the zwitterion/water molar ratio changes even at the same molar concentration, depending on the molecular weight. We here hypothesised that the consequent difference, the amount of free water, was reflected in the toxicity. However, the molar ratios of zwitterion/water in the mixtures were similar. Even at 1.0 M, the zwitterion/water molar ratio was approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40–1
40–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, which did not seem to indicate the lack of free water. Furthermore, in the case of E. coli, the EC50 values of OE2imC3C (Mw: 256) and a smaller zwitterion (Mw: 168, an analogous zwitterion with a methyl cation side chain) were 158 and 141 g L−1,24 respectively, suggesting that the molecular weight itself is not a critical factor and the observation of similar trends in the g L−1 unit may be coincidental.
50, which did not seem to indicate the lack of free water. Furthermore, in the case of E. coli, the EC50 values of OE2imC3C (Mw: 256) and a smaller zwitterion (Mw: 168, an analogous zwitterion with a methyl cation side chain) were 158 and 141 g L−1,24 respectively, suggesting that the molecular weight itself is not a critical factor and the observation of similar trends in the g L−1 unit may be coincidental.
Biologically, TMG and L-carnitine, which are natural zwitterions, are known to be biocompatible solutes that act as osmolytes in microorganisms.35,36 Some yeasts can take up TMG,37 and they may alleviate osmotic pressure through the uptake. In other words, the difference between non-uptakable OE2imC3C and uptakable TMG and carnitine is assumed to be a possible reason for growth inhibition although further investigation is needed.
To interpret it chemically in molecular design, we studied which is more important, being natural or ammonium-based. A similar artificial ammonium-based zwitterion (N2,2,2C3C, Fig. S5†) was synthesised and subjected to the same experiments. The results indicated that the presence of an ammonium cation was important for developing a less toxic zwitterion (Fig. S5†). In summary, ammonium-based zwitterions, including TMG and carnitine, were found to be superior zwitterions in terms of toxicity; however, TMG and carnitine showed no cellulose solubility and had a lower pretreatment capacity for biomass compared to OE2imC3C. The search for ammonium-based ZIs with superior pretreatment capacities will be an important design guideline in the future.
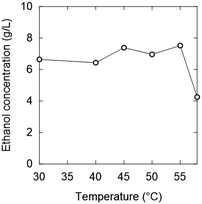
Considering simultaneous saccharification and fermentation, the effect of zwitterions at 50 °C is also important (Fig. 4). OE2imC3C was confirmed to be less toxic than [C2mim]OAc; growth was observed at OE2imC3C concentrations below 0.5 M. The effect of OE2imC3C concentration on growth at 50 °C was greater than that at 40 °C. The effects of OE2imC3C concentration were similar between 30 and 45 °C (Fig. S6†) but the effect of TMG and carnitine was the mildest at 40 °C. These results are summarized in Fig. 5 and indicate that temperature and growth inhibition are related and that the inhibition is minimal at around the optimum temperature.
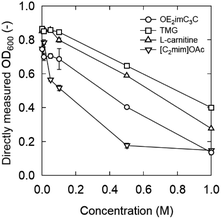 | ||
| Fig. 6 OD600 after 6 h incubation in media supplemented with OE2imC3C, TMG, L-carnitine, and [C2mim]OAc at 30 °C. Strain: S. cerevisiae BY4741. Initial OD600: 0.2. | ||
Fermentation inhibition by OE2imC3C against K. marxianus
To reduce the sample amount, which is important when using non-commercial or expensive compounds, the sample volume was set to 1 mL. Each time, 100 μL of solution was taken and filtered through a small-dead-volume syringe filter for HPLC analysis. The HPLC sample volume was significantly reduced by placing glass inserts (TORAST vial insert, GLCTV-I01) in the HPLC vials. The same company sells another type of glass insert (GLCTV-I02), which has 60% volume of GLCTV-I01 (0.25 vs. 0.15 mL). However, even when GLCTV-I02 was used, the amount of solution required for HPLC analysis was almost the same. Therefore, we decided not to use it because it was four times expensive (reuse by washing is difficult because it is too fine). The sample injection volume for HPLC was 20 μL.
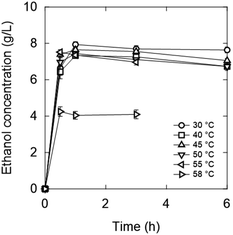
The initial OD600 value was set to 20. A higher initial OD600 value allows fermentation to be completed rapidly, which is favourable for screening. As a result, ethanol was produced within 1 h at most of the tested temperatures (Fig. 8). Fermentation, which typically takes 12–72 hours,13 can be reduced to 1 h. Furthermore, increasing the initial OD600 increases the yield because sugars are not used for yeast growth.38
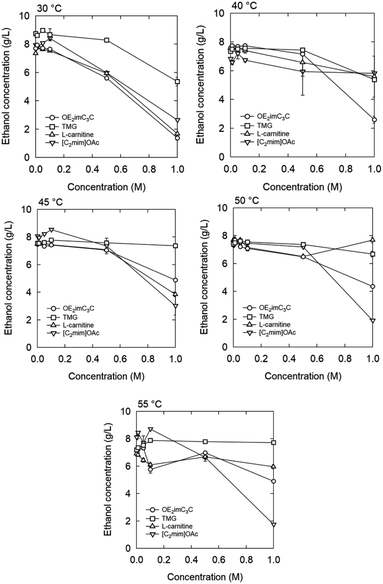 | ||
| Fig. 10 Ethanol concentration after 1 h fermentation with zwitterion/ionic liquid. Strain: K. marxianus DMKU3-1042. | ||
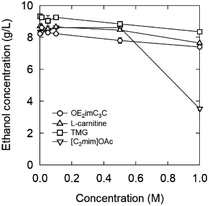
[C2mim]OAc also decreased the ethanol concentration in a concentration-dependent manner at a concentration of 0.5 M or higher. Specifically, when 0.5 and 1.0 M were added, the ethanol concentration decreased by 25% and 67%, respectively. Under our experimental conditions, [C2mim]OAc and OE2imC3C inhibited fermentation to the same extent. In the ethanol fermentation by E. coli KO11, [C2mim]OAc showed very strong inhibition;13,24 therefore, the microbial species may have an influence. However, the most plausible reason for the difference is based on the initial OD600. An initial OD600 of 1 was applied to E. coli KO11, whereas 20 was adopted in this study. Bacterial cell density is also known to affect growth inhibition by drugs.39 Additionally, since fermentation was completed within 30 minutes to an hour due to the high initial OD600, it is possible that fermentation was completed before toxicity appeared. Thus, there is still room for improving the screening method, such as lowering the initial OD600 value. In other words, these results, however, indicate that even a high concentration of [C2mim]OAc is applicable under specific conditions. We believe this finding is important because [C2mim]OAc has a high biomass processing ability and is currently the most popular ionic liquid, but its toxicity is a problem.11–13,24,40,41
When [C2mim]OAc was used, the ethanol concentration increased slightly below 0.1 M, compared to the case of 0 M. This phenomenon is known as hormesis and is commonly observed when antibiotics are added below the minimal inhibitory concentration.42,43
Inhibition by L-carnitine was comparable to that by OE2imC3C, but fermentation was less inhibited by TMG than OE2imC3C. In particular, almost no inhibition was observed at 0.5 M TMG. Although the trends in fermentation differed from those in proliferation, TMG had the lowest toxicity in both assays.
The temperature-dependence was investigated. At 40 °C or higher, no clear fermentation inhibition was observed with 0.5 M OE2imC3C, and OE2imC3C resistance was improved compared to that at 30 °C. Similar results were obtained for the other zwitterions. On the other hand, there are some exceptions. Focusing on 40 °C and 1 M, fermentation inhibition by OE2imC3C was comparable to that at 30 °C, but the inhibitory effect of [C2mim]OAc was weaker than that at 30 °C, and the strength of toxicity was reversed (described later).
55 °C was the highest temperature adequate for fermentation (Fig. 10). In the 0.5 and 1.0 M OE2imC3C groups, ethanol production was suppressed by 12% and 41%, respectively, compared with that in the 0 M solution. The toxicity seems less effective at 55 °C in the high concentration range. Fermentation was less inhibited by TMG than OE2imC3C, and L-carnitine inhibited fermentation to the same extent as OE2imC3C.
To clarify the relationship between the temperature and ion concentration during fermentation inhibition, the ethanol concentration in the 1.0 M solutions at each temperature is plotted in Fig. 11. When OE2imC3C was added, higher ethanol concentrations were obtained at 40 °C than at 30 °C, and even higher ethanol concentrations were obtained at 45–55 °C. This tendency was not much different for TMG and L-carnitine. In contrast, [C2mim]OAc exhibited a different trend. The ethanol concentration increased at 40 °C, compared to 30 °C, and decreased at higher temperatures. The increase in ethanol concentration at 40 °C may be due to hormesis. Hormesis was observed during growth and fermentation when low concentrations of [C2mim]OAc were used. A similar phenomenon was observed in some points in the zwitterionic solutions at higher concentrations. There are some points still unknown, but this experimental method is developed for rapid screening. To study the inhibition mechanisms in detail, we need to conduct time-consuming experiments, which will be conducted in the future.
Conclusions
A rapid screening method was established for growth inhibition of K. marxianus applicable to 30–50 °C. Using 96-well plates enabled direct measurement of OD600 with a 100 μL sample volume. Although the evaporation of culture media was a problematic issue especially at 50 °C, it was avoided by covering with sheets, putting wet towels and so on. OE2imC3C was less toxic for yeast growth than [C2mim]OAc. Growth inhibition was increased at 50 °C, compared to the optimal growth temperature of 40 °C. Natural zwitterions, TMG and L-carnitine were further less toxic. These trends were similar in two K. marxianus strains and S. cerevisiae.A rapid screening method for fermentation inhibition was also established at 30–55 °C. Using pressure-durable cryotubes enabled downscaling the sample volume to 1 mL, simultaneous testing over 18 samples, preventing the evaporation of media and ethanol. Using small-dead-volume syringe filters and glass inserts was also important for the downscaling. The trends in fermentation inhibition by zwitterions/ionic liquid were somewhat complicated. A possible reason for the complication is the very rapid assay completion within 1 h and there is room to improve. The rough toxicity trend was TMG < L-carnitine < OE2imC3C < [C2mim]OAc but the order changed depending on the concentration/temperature. S. cerevisiae was more resistant to OE2imC3C during fermentation than K. marxianus.
Data availability
All data are available in the main text or the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was also partly supported by KAKENHI (23H03824 from the Japan Society for the Promotion of Science), Kanazawa University SAKIGAKE project 2020/2022, Super Highway (for K. K., from Japan Science and Technology Agency), and the National BioResource Project (for K. K., MEXT, Japan).Notes and references
- J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. F. Jr., J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef
.
- E. Melro, L. Alves, F. E. Antunes and B. Medronho, J. Mol. Liq., 2018, 265, 578–584 CrossRef CAS
.
- N. Tamai, H. Aono, D. Tatsumi and T. Matsumoto, J. Soc. Rheol., 2003, 31, 119–130 CrossRef CAS
.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS
.
- M. W. Frey, L. Li, M. Xiao and T. Gould, Cellulose, 2006, 13, 147–155 CrossRef CAS
.
- K. Zhang, Z. Pei and D. Wang, Bioresour. Technol., 2016, 199, 21–33 CrossRef CAS PubMed
.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4934–5252 CrossRef
.
- Y. Fukaya, K. Hayashi, M. Wada and H. Ohno, Green Chem., 2007, 10, 44–46 RSC
.
- I. Kilpeläinen, H. Xie, A. King, M. Granstrom, S. Heikkinen and D. S. Argyropoulos, J. Agric. Food Chem., 2007, 55, 8825–9324 CrossRef PubMed
.
- C. Li, B. Knierim, C. Manisseri, R. Arora, H. V. Scheller, M. Auer, K. P. Vogel, B. A. Simmons and S. Singh, Bioresour. Technol., 2010, 101, 4900–4906 CrossRef CAS PubMed
.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC
.
- A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 537–848 RSC
.
- K. Kuroda, H. Satria, K. Miyamura, Y. Tsuge, K. Ninomiya and K. Takahashi, J. Am. Chem. Soc., 2017, 139, 16052–16055 CrossRef CAS PubMed
.
- C. Yu, B. A. Simmons, S. W. Singer, M. P. Thelen and J. S. VanderGheynst, Appl. Microbiol. Biotechnol., 2016, 100, 10237–10249 CrossRef CAS PubMed
.
- L. Das, E. C. Achinivu, C. A. Barcelos, E. Sundstrom, B. Amer, E. E. K. Baidoo, B. A. Simmons, N. Sun and J. M. Gladden, ACS Sustain. Chem. Eng., 2021, 9, 4422–4432 CrossRef CAS
.
- C. A. Barcelos, A. M. Oka, J. Yan, L. Das, E. C. Achinivu, H. Magurudeniya, J. Dong, S. Akdemir, N. R. Baral, C. Yan, C. D. Scown, D. Tanjore, N. Sun, B. A. Simmons, J. Gladden and E. Sundstrom, ACS Sustain. Chem. Eng., 2021, 9, 4042–4053 CrossRef CAS
.
- K. Kuroda, Bioethanol fermentation in the presence of ionic liquids: mini review, New J. Chem., 2024, 48, 10341–10346 RSC
.
- K. Ninomiya, C. Ogino, M. Ishizaki, M. Yasuda, N. Shimizu and K. Takahashi, Biochem. Eng. J., 2015, 103, 198–204 CrossRef CAS
.
- K. Kuroda, New J. Chem., 2022, 46, 20047–20052 RSC
.
- R. J. Bingham and P. Ballone, J. Phys. Chem. B, 2012, 116, 11205–11216 CrossRef CAS PubMed
.
- B. Jing, N. Lan, J. Qiu and Y. Zhu, J. Phys. Chem. B, 2016, 120, 2781–2789 CrossRef CAS
.
- G. S. Lim, J. Zidar, D. W. Cheong, S. Jaenicke and M. Klähn, J. Phys. Chem. B, 2014, 118, 10444–10459 CrossRef CAS
.
- K. Kuroda, T. Komori, K. Ishibashi, T. Uto, I. Kobayashi, R. Kadokawa, Y. Kato, K. Ninomiya, K. Takahashi and E. Hirata, Commun. Chem., 2020, 3, 163 CrossRef CAS PubMed
.
- T. Komori, H. Satria, K. Miyamura, A. Ito, M. Kamiya, A. Sumino, T. Onishi, K. Ninomiya, K. Takahashi, J. L. Anderson, T. Uto and K. Kuroda, ACS Sustain. Chem. Eng., 2021, 9, 11825–11836 CrossRef CAS
.
- A. Hachisu, H. Tobe, K. Ninomiya, K. Takahashi and K. Kuroda, ACS Sustain. Chem. Eng., 2022, 10, 6919–6924 CrossRef CAS
.
- K. Kuroda, T. Komori, K. Ishibashi, T. Uto, I. Kobayashi, R. Kadokawa, Y. Kato, K. Ninomiya, K. Takahashi and E. Hirata, Commun. Chem., 2020, 3, 163 CrossRef CAS PubMed
.
- S. Jadhav, V. Ganvir, M. K. Singh and K. Shanmuganathan, Cellulose, 2022, 30, 87–109 CrossRef
.
- G. Huet, M. Araya-Farias, R. Alayoubi, S. Laclef, B. Bouvier, I. Gosselin, C. Cézard, R. Roulard, M. Courty, C. Hadad, E. Husson, C. Sarazin and A. Nguyen Van Nhien, Green Chem., 2020, 22, 2935–2946 RSC
.
- J. Sun, N. V. S. N. M. Konda, R. Parthasarathi, T. Dutta, M. Valiev, F. Xu, B. A. Simmons and S. Singh, Green Chem., 2017, 19, 3152–3163 RSC
.
- S. Nonklang, B. M. Abdel-Banat, K. Cha-aim, N. Moonjai, H. Hoshida, S. Limtong, M. Yamada and R. Akada, Appl. Environ. Microbiol., 2008, 74, 7514–7521 CrossRef CAS
.
- B. M. Abdel-Banat, H. Hoshida, A. Ano, S. Nonklang and R. Akada, Appl. Microbiol. Biotechnol., 2010, 85, 861–867 CrossRef CAS
.
- G. Sharma, Y. Kato, A. Hachisu, K. Ishibashi, K. Ninomiya, K. Takahashi, E. Hirata and K. Kuroda, Cellulose, 2022, 29, 3017–3024 CrossRef CAS
.
- C. Baker Brachmann, A. Davies, G. J. Cost, E. Caputo, J. Li, P. Hieter and J. D. Boeke, Yeast, 1998, 14, 115–132 CrossRef
.
- Q. Dickinson, S. Bottoms, L. Hinchman, S. McIlwain, S. Li, C. L. Myers, C. Boone, J. J. Coon, A. Hebert, T. K. Sato, R. Landick and J. S. Piotrowski, Microb. Cell Fact., 2016, 15, 17 CrossRef PubMed
.
- B. Perroud and D. L. Rudulier, J. Bacteriol., 1985, 161, 392 CrossRef
.
- C. F. Schuster, L. E. Bellows, T. Tosi, I. Campeotto, R. M. Corrigan, P. Freemont and A. Gründling, Am. Assoc. Adv. Sci., 2016, 9, ra81 Search PubMed
.
- T. Linder, Antonie van Leeuwenhoek, 2020, 113, 437–445 CrossRef CAS PubMed
.
- M. G. Acedos, V. E. Santos and F. Garcia-Ochoa, Biotechnol. Prog., 2018, 34, 1073–1080 CrossRef CAS PubMed
.
- J. Karslake, J. Maltas, P. Brumm and K. B. Wood, PLoS Comput. Biol., 2016, 12, e1005098 CrossRef PubMed
.
- N. Sun, M. Rahman, Y. Qin, M. L. Maxim, H. Rodríguez and R. D. Rogers, Green Chem., 2009, 11, 646–655 RSC
.
- S. K. Ruokonen, C. Sanwald, A. Robciuc, S. Hietala, A. H. Rantamaki, J. Witos, A. W. T. King, M. Lammerhofer and S. K. Wiedmer, Chemistry, 2018, 24, 2669–2680 CrossRef CAS
.
- J. Davies, G. B. Spiegelman and G. Yim, Curr. Opin. Microbiol., 2006, 9, 445–453 CrossRef CAS PubMed
.
- Y. Imai, S. Sato, Y. Tanaka, K. Ochi and T. Hosaka, Appl. Environ. Microbiol., 2015, 81, 3869–3879 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00239c |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2024 |