Open Access Article
Open Access ArticleIncreased hydrophilicity of lignin-derivable vs. bisphenol-based polysulfones for potential water filtration applications†
Jignesh S.
Mahajan‡
 ab,
Hoda
Shokrollahzadeh Behbahani‡
ab,
Hoda
Shokrollahzadeh Behbahani‡
 c,
Matthew D.
Green
c,
Matthew D.
Green
 *c,
LaShanda T. J.
Korley
*c,
LaShanda T. J.
Korley
 *abd and
Thomas H.
Epps
III
*abd and
Thomas H.
Epps
III
 *abd
*abd
aDepartment of Materials Science and Engineering, University of Delaware, Newark, Delaware 19716, USA. E-mail: lkorley@udel.edu
bCenter for Research in Soft matter and Polymers, University of Delaware, Newark, Delaware 19716, USA
cDepartment of Chemical Engineering, School for Engineering of Matter, Transport and Energy, Arizona State University, Tempe, Arizona 85287, USA. E-mail: mdgreen8@asu.edu
dDepartment of Chemical and Biomolecular Engineering, University of Delaware, Newark, Delaware 19716, USA. E-mail: thepps@udel.edu
First published on 18th September 2024
Abstract
The functionality inherent in lignin-derivable aromatics (e.g., polar methoxy groups) can provide a potential opportunity to improve the hydrophilicity of polysulfones (PSfs) without the need for the additional processing steps and harsh reagents/conditions that are typically used in conventional PSf modifications. As determined herein, lignin-derivable PSfs without any post-polymerization modification exhibited higher hydrophilicity than comparable petroleum-based PSfs (commercial/laboratory-synthesized) and also demonstrated similar hydrophilicity to functionalized BPA-PSfs reported in the literature. Importantly, the lignin-derivable PSfs displayed improved thermal properties relative to functionalized BPA-PSfs in the literature, and the thermal properties of these bio-derivable PSfs were close to those of common non-functionalized PSfs. In particular, the glass transition temperature (Tg) and degradation temperature of 5% weight loss (Td5%) of lignin-derivable PSfs (Tg ∼165–170 °C, Td5% ∼400–425 °C) were significantly higher than those of typical functionalized BPA-PSfs in the literature (Tg ∼110–160 °C, Td5% ∼240–260 °C) and close to those of unmodified, commercial/laboratory-synthesized BPA-/bisphenol F-PSfs (Tg ∼180–185 °C, Td5% ∼420–510 °C).
Sustainability spotlightCommercial bisphenol A-polysulfones (BPA-PSfs) are hydrophobic in nature, and post-polymerization modification is often required to increase the hydrophilicity of commercial PSfs for water filtration applications. Such PSf modification adds extra processing steps and often requires harsh reaction conditions/reagents, and these extra functionalization steps also have a detrimental effect on application-specific thermal properties. Herein, bio-derivable PSfs were synthesized using potentially safer, lignin-derivable bisguaiacols. Notably, the lignin-derivable PSfs, without any post-polymerization modification, demonstrated similar hydrophilicity to functionalized BPA-PSfs reported in the literature, and they also exhibited improved thermal properties relative to functionalized BPA-PSfs. Moreover, the thermal properties of bio-derivable PSfs remained close to those of commercial BPA-PSfs. Overall, this work is aligned with the UN's Sustainable Development Goal 12 (responsible consumption and production of chemicals). |
Poly(arylene ether sulfone)s, or polysulfones (PSfs), are a class of engineering polymers widely used in food and beverage processing materials, including water filtration membranes, due to their high-temperature stability, resistance to hydrolysis, and chemical inertness.1–3 PSfs are hydrophobic in nature, and often post-polymerization functionalization (e.g., chlorination,4 sulfonation,5 incorporation of hydrophilic zwitterionic groups6,7) is necessary to increase PSf hydrophilicity,8,9 which also improves water permeability and fouling resistance through the formation of a hydration layer on the PSf membrane.8 This extra functionalization, however, requires harsh reaction conditions/reagents/toxic solvents, such as concentrated sulfuric acid or chlorinated solvents.4,5 Additionally, these functionalized PSfs often have significantly reduced thermal properties (i.e., glass transition temperature [Tg] and degradation temperature) in comparison to those of commercial PSfs, as the functionalization modifies the main-chain repeat unit of the polymer (e.g., the introduction of long aliphatic chains and/or thermally less stable linkages/functional groups).10 Moreover, commercial PSfs are primarily derived from bisphenol A (BPA), a suspected endocrine disruptor.11,12 A variety of commercial BPA alternatives, such as bisphenol F (BPF), bisphenol S, and tetramethyl BPA, have emerged; however, these alternatives may still have endocrine disruption potential.11,12 These bisphenols (unreacted and/or oligomers) can leach from polymeric products and potentially enter the human body. An additional concern is that BPA and its commercial analogues are non-renewable.13,14 Altogether, PSfs with increased hydrophilicity vs. unmodified BPA-based counterparts, while maintaining thermal properties, are highly desirable.
Lignin is the most abundant source of renewable aromatic chemicals and serves as a platform for the development of an array of sustainable polymers.15–25 Polymers made from bulk lignin typically have inconsistent thermomechanical properties due to structural heterogeneity and limited reactivity of lignin;16,26 however, bulk lignin can be deconstructed into substituted phenols with several inherent functional groups (e.g., methoxy groups) to mitigate this issue.16,26–28 These substituted phenols have been recently used to generate BPA mimics for epoxy–amine resins,29 isocyanate-free polyurethanes,30,31 and polycarbonates32 with highly tunable thermomechanical properties. Additionally, several studies have suggested that these materials have reduced toxicity concerns (e.g., estrogenic activity, genotoxicity, oxidative DNA damage) relative to their BPA-/BPF-based counterparts.11,33,34
Herein, lignin-derivable PSfs were synthesized from these bio-derivable bisguaiacols. The water contact angle (θw), water vapor uptake, and thermal properties of these bisguaiacol-based PSfs were benchmarked against laboratory-synthesized BPA-/BPF-based PSfs, commercial BPA-PSf (Udel® PSU, Solvay),2 and quaternary ammonium-functionalized BPA-PSfs reported in the literature.10 It was envisioned that the polar methoxy groups on lignin-derivable bisguaiacols would improve the hydrophilicity of resultant polymers without the need for any post-polymerization functionalization.35 Additionally, the thermal properties (Tg and degradation temperature) of these lignin-derivable PSfs were expected to be comparable to those of the unmodified, commercial PSfs due to their structural similarity.35
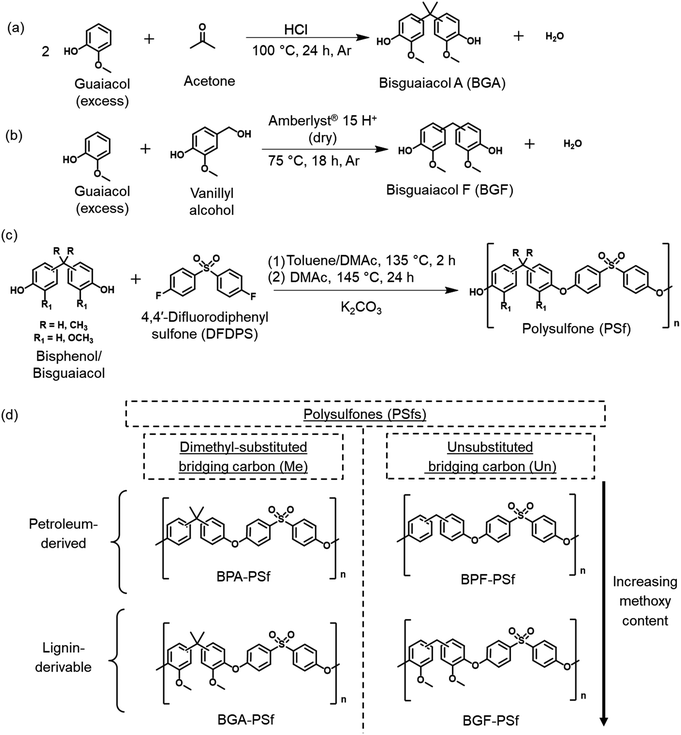
Lignin-derivable bisguaiacol A (BGA) and bisguaiacol F (BGF) were synthesized as analogues to BPA and BPF to achieve the above objective. BGA was synthesized via the electrophilic aromatic condensation between guaiacol and acetone.30 The reaction was conducted in an excess of guaiacol to minimize by-product formation from the self-condensation of acetone (Fig. 1a).31,35,36 BGA contains a dimethyl-substituted bridging carbon (i.e., isopropylene bridge) between the two aromatic moieties and more closely resembles BPA.30 BGF was synthesized via the electrophilic aromatic substitution between guaiacol and vanillyl alcohol (Fig. 1b), and the direct condensation approach eliminated the need for formaldehyde, which is prominent in BPF production.29,37 BGF has an unsubstituted bridging carbon (i.e., methylene bridge), which makes it more structurally similar to BPF.29,37 Detailed BGA/BGF synthesis and purification procedures, along with characterization information (Fig. S1 and S2†), can be found in the ESI.†
PSfs were subsequently synthesized via standard step-growth polymerization38 (Fig. 1c) by reacting the respective bisphenol/bisguaiacol with 4,4′-difluorodiphenyl sulfone. This process involved a nucleophilic aromatic substitution (SnAr) reaction, in which the bisphenolate salts generated through deprotonation using potassium carbonate reacted with the dihalide (4,4′-difluorodiphenyl sulfone) in a polar aprotic solvent, specifically, N,N-dimethylacetamide (DMAc). The detailed synthetic procedures, polymer isolation/purification protocols, nuclear magnetic resonance spectra (Fig. S3–S10†), and size exclusion chromatography data (Fig. S11†) are provided in the ESI.† The molecular structure of the petroleum-derived PSfs and their lignin-derivable analogues are presented in Fig. 1d.
The hydrophilicity of these bio-derivable PSfs was studied by measuring/estimating θw, water vapor uptake, and polymer polarity (Fig. 2 and Table 1). The typical θw for commercial BPA-PSf was ∼90°,2,10 which was similar to our laboratory-synthesized BPA-PSf. θw for lignin-derivable BGF-PSf was ∼75°, which was comparable to that of functionalized BPA-PSfs reported in the literature.10 Moreover, θw decreased (i.e., the hydrophilicity of the PSfs increased) with increasing methoxy content, likely due to the polar nature of methoxy groups in lignin-derivable PSfs (Fig. 2a). For instance, the laboratory-synthesized BPA-PSf (with 0 methoxy groups) had a θw of ∼90°. In contrast, its lignin-derivable analogue, BGA-PSf (with 2 methoxy groups), had a θw of ∼81°. Similarly, BPF-PSf (with 0 methoxy groups) had a θw of ∼84°, whereas its lignin-derivable counterpart, BGF-PSf (with 2 methoxy groups), had a θw of ∼75°. Hence, the methoxy moieties on lignin-aromatics appear to improve the hydrophilicity of PSfs.
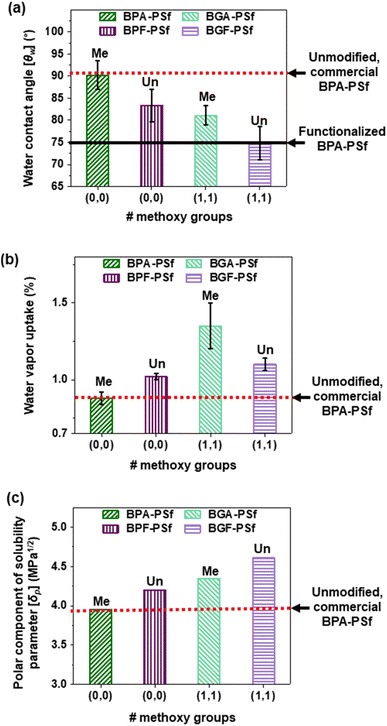 | ||
| Fig. 2 (a) θw, (b) water vapor uptake, and (c) δp values as a function of methoxy groups on the aromatic rings for laboratory-synthesized BPA/BGA/BPF/BGF-PSfs. The bar label indicates the bridging-carbon substitution type for the PSfs. The red dashed line represents a reported value (θw ∼90°, water vapor uptake ∼0.8%, polar component of solubility parameter ∼3.9) for unmodified, commercial BPA-PSf [Udel® PSU, Solvay],2 whereas the black solid line in panel (a) indicates an average reported value in the literature (θw ∼75°) for functionalized BPA-PSf.10 | ||
| Polymer | Methoxy groups | θ w | T g | T d5% | Char contentb | |
|---|---|---|---|---|---|---|
| a Chemical structures of the polymers are provided in Fig. 4. b Determined by thermogravimetric analysis from 40 °C to 800 °C at 10 °C min−1 in an N2 atmosphere. c Quaternary ammonium-functionalized BPA-PSfs with varying mole fractions (25, 50, 75 mol%) of ammonium charge content, which were synthesized in four steps (as reported in the literature).10 | ||||||
| Commercial PSf (Solvay2) | BPA-PSf | 0 | 91 ± 2° | 185 °C | 510 °C | 31% |
| Petroleum-based PSfs (laboratory-synthesized) | BPA-PSf | 0 | 90 ± 3° | 185 °C | 510 °C | 31% |
| BPF-PSf | 0 | 84 ± 3° | 179 °C | 420 °C | 44% | |
| Functionalized PSfs (from literature10)c | BPA-PSf | 0 | 77 ± 3° | 164 °C | 240 °C | 28% |
| BPA-PSf | 0 | 71 ± 2° | 127 °C | 260 °C | 30% | |
| BPA-PSf | 0 | 75 ± 6° | 112 °C | 255 °C | 21% | |
| Lignin-derivable PSfs (laboratory-synthesized) | BGA-PSf | 2 | 81 ± 2° | 166 °C | 425 °C | 25% |
| BGF-PSf | 2 | 74 ± 3° | 172 °C | 400 °C | 46% | |
The water vapor uptake of PSfs also increased with the number of methoxy groups (Fig. 2b). For example, laboratory-synthesized BPA-PSf (with 0 methoxy groups) exhibited a water vapor uptake of ∼0.9% (comparable to that of commercial BPA-PSf, ∼0.8%),2 whereas BGA-PSf (with 2 methoxy groups) had a water vapor uptake of ∼1.3%. This improvement was statistically significant (paired T-test, p-value = 0.048), indicating ∼50% higher water vapor uptake for BGA-PSf vs. BPA-PSf. BPF-PSf (with 0 methoxy groups) had a water vapor uptake of ∼1.0% and BGF-PSf (with 2 methoxy groups) had a water vapor uptake of ∼1.1%. This increase in water vapor uptake did not reach statistical significance (paired T-test, p-value = 0.080). Nonetheless, these lignin-derivable PSfs, which do not require the harsh reagents or conditions typically associated with PSf modifications, yield materials with improved water vapor uptake within the experimental constraints of dense film matrices.6,10
The methoxy groups on the aromatic rings in the lignin-derivable PSfs also increased the polymer polarity, consequently improving PSf hydrophilicity. This increase in the polar properties was determined via (1) solubility parameter calculations [using the Hoftyzer and van Krevelen method],39 (2) surface energy estimations [using the extended Fowkes method],40 and (3) octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) predications [using ChemAxon 2024].41 For the solubility parameter calculations, δ is the total solubility parameter, δd indicates the square root of energy density associated with dispersion forces, δp represents the square root of energy density associated with dipolar intermolecular forces, and δh is the square root of energy density associated with hydrogen-bonding interactions between molecules.39 Notably, δp increased with the number of methoxy groups on the PSfs (Fig. 2c and Table S1†), which strongly suggested that the methoxy groups were responsible for the improved polarity of lignin-derivable PSfs vs. BPA-/BPF-based analogues. These findings were supported by the subsequent analysis of surface energy measurements (details in ESI [Section 3.7]†); an increase in the polar component was noted with increasing methoxy content, directly attributable to the polarity introduced by the methoxy groups (Table S2†). Lastly, the log
P) predications [using ChemAxon 2024].41 For the solubility parameter calculations, δ is the total solubility parameter, δd indicates the square root of energy density associated with dispersion forces, δp represents the square root of energy density associated with dipolar intermolecular forces, and δh is the square root of energy density associated with hydrogen-bonding interactions between molecules.39 Notably, δp increased with the number of methoxy groups on the PSfs (Fig. 2c and Table S1†), which strongly suggested that the methoxy groups were responsible for the improved polarity of lignin-derivable PSfs vs. BPA-/BPF-based analogues. These findings were supported by the subsequent analysis of surface energy measurements (details in ESI [Section 3.7]†); an increase in the polar component was noted with increasing methoxy content, directly attributable to the polarity introduced by the methoxy groups (Table S2†). Lastly, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values reflected the hydrophilicity of the bisphenols/PSfs, and more negative log
P values reflected the hydrophilicity of the bisphenols/PSfs, and more negative log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values indicated reduced hydrophilicity.42,43 The log
P values indicated reduced hydrophilicity.42,43 The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values decreased with increasing methoxy content on bisphenols (Table S3†), which again suggested that the methoxy groups accounted for the higher hydrophilicity in the bio-derivable systems. Overall, the methoxy moieties offered improved polarity vs. their non-methoxy-containing, petroleum-derived counterparts.
P values decreased with increasing methoxy content on bisphenols (Table S3†), which again suggested that the methoxy groups accounted for the higher hydrophilicity in the bio-derivable systems. Overall, the methoxy moieties offered improved polarity vs. their non-methoxy-containing, petroleum-derived counterparts.
Lignin-derivable PSfs offer a sustainable alternative to traditional BPA-/BPF-derived PSfs for potential water filtration applications (e.g., support for reverse osmosis membranes, microfiltration membranes). As determined herein, methoxy-containing, bisguaiacol-derived PSfs demonstrated improved hydrophilicity relative to neat/unmodified BPA-/BPF-PSfs. This increased hydrophilicity can lead to improved water permeability and filter efficiency, which can result in more effective contaminant removal and reduced fouling of the membranes.5 For instance, in reverse osmosis processes, despite nearing the theoretical minimum energy requirements for water separation, overall energy demands remain high due to membrane limitations and the need for extensive pretreatment.44 The performance of such membranes can be improved by increasing their hydrophilicity, which can reduce total energy consumption/operational costs and make desalination processes more energy efficient.45 In addition to performance enhancement, filtration membranes generated using lignin-aromatics can lead to potential improvement in life-cycle assessment, which can reduce environmental impacts across the product lifetime.46
It is worth noting that the Tgs of these lignin-derivable PSfs were close to those of petroleum-derived (commercial/laboratory-synthesized) benchmarks, and these Tgs were much higher than those of functionalized BPA-PSfs reported in the literature (Fig. 3a and Table 1). The Tg values for unmodified, commercial and laboratory-synthesized BPA-PSf were identical (∼185 °C),2 and the Tg value for laboratory-synthesized BPF-PSf was ∼179 °C. The Tgs of our lignin-derivable BGA-PSf and BGF-PSf were 166 °C and 172 °C, respectively, whereas the Tgs of several functionalized BPA-PSfs reported in the literature were ∼112 °C, 127 °C, and 164 °C (Fig. 3a).10 Importantly, in lignin-derivable PSfs, the polymer repeat unit was retained in contrast to the functionalized PSfs in the literature, and thus, the Tgs of lignin-derivable BGA-/BGF-PSfs were close to those of unmodified, commercial and laboratory-synthesized BPA-/BPF-PSfs. The Tgs of lignin-derivable BGA-/BGF-PSfs were slightly lower than those of BPA-/BPF-PSfs, which may be due to less efficient chain packing caused by methoxy groups.29,31 Notably, the Tg values for lignin-derivable PSfs are more than sufficient for potential applications, such as water filtration membranes, microwaveable dishes, and hemodialysis membranes.6,7
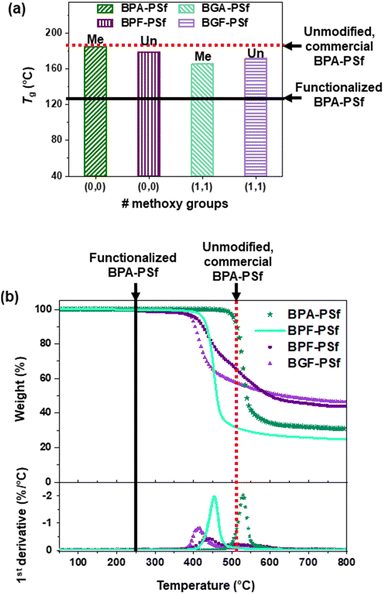 | ||
| Fig. 3 (a) Tg for laboratory-synthesized BPA/BGA/BPF/BGF-PSfs. (b) Representative thermogravimetric analysis traces and the respective first-derivative curves of sample mass remaining versus temperature for laboratory-synthesized BPA/BGA/BPF/BGF-PSfs obtained at a heating rate of 10 °C min−1 in an N2 atmosphere. The red dashed line represents a reported value (Tg ∼185 °C, Td5% ∼510 °C) for unmodified, commercial BPA-PSf [Udel® PSU, Solvay],2 whereas the black solid line indicates an average reported value in the literature (Tg ∼130 °C, Td5% ∼250 °C) for functionalized BPA-PSf.10 | ||
The thermal degradation temperatures of these bisguaiacol-based PSfs also were in the range of high-performance thermoplastics. The Td5% (i.e., temperature of 5% weight loss in an N2 atmosphere) values for commercial and laboratory-synthesized BPA-PSf were equivalent (∼510 °C),2 and the Td5% value for BPF-PSf was ∼420 °C. The Td5% values for our laboratory-synthesized BGA-PSf and BGF-PSf were 425 °C and 400 °C, respectively (Fig. 3b and Table 1), whereas Td5% values for several functionalized BPA-PSfs reported in the literature were ∼240 °C, 255 °C, and 260 °C.10 It is hypothesized that the lignin-derivable PSfs, were more thermally stable than the functionalized PSfs reported in the literature, likely due to the retention of the polymer repeat unit in contrast to the functionalized PSfs. Additionally, all PSfs exhibited a char content of ∼25–45% (Table 1) as these materials typically undergo pyrolysis under an inert atmosphere that leads to char formation rather than oxidative degradation.47 Overall, the bisguaiacol-based PSfs exhibited significantly higher thermal stability than those of functionalized BPA-PSfs in the literature and similar thermal/char behavior to unmodified materials.
Conclusions
This work highlights the importance of the inherent functionality (methoxy groups) of lignin-aromatics in improving the hydrophilicity of PSfs. The bisguaiacol-based PSfs without any post-polymerization functionalization exhibited equivalent hydrophilicity (θw ∼75°) to functionalized PSfs in the literature and higher hydrophilicity than petroleum-based, unmodified, commercial/laboratory-synthesized PSfs (θw ∼90°). Notably, θw decreased (i.e., the hydrophilicity of the PSfs increased) with increasing methoxy content due to the polar nature of the methoxy groups in lignin-derivable PSfs. Furthermore, these bisguaiacol-based PSfs (Tg ∼165–170 °C, Td5% ∼400–425 °C) had improved thermal properties relative to functionalized PSfs in the literature (Tg ∼110–160 °C, Td5% ∼240–260 °C). The thermal properties of lignin-derivable PSfs were close to those of commercial PSfs (Tg ∼180–185 °C, Td5% ∼420–510 °C), which was likely due to the retention of the polymer repeat unit. Together, these bisguaiacol-based PSfs may serve as potential alternatives to BPA-/BPF-based PSfs for water filtration applications.Abbreviations
| BGA | bisguaiacol A |
| BGF | bisguaiacol F |
| BPA | bisphenol A |
| BPF | bisphenol F |
| DMAc | N,N-dimethylacetamide |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P | octanol–water partition coefficient |
| Me | dimethyl-substituted bridging carbon |
| T d5% | degradation temperature of 5% weight loss |
| T g | glass transition temperature |
| PSf | polysulfone |
| Un | unsubstituted bridging carbon |
| δ | total solubility parameter |
| δ d | square root of energy density associated with dispersion forces |
| δ p | square root of energy density associated with dipolar intermolecular forces |
| δ h | square root of energy density associated with hydrogen-bonding interactions between molecules |
| θ w | water contact angle |
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Conceptualization: J. S. M., H. S. B., M. D. G., L. T. J. K., and T. H. E. Investigation and formal analysis: J. S. M. and H. S. B. Writing (original draft) J. S. M. and H. S. B. Writing (review and editing): J. S. M., H. S. B., M. D. G., L. T. J. K., and T. H. E. Funding acquisition, supervision, and project administration: M. D. G., L. T. J. K., and T. H. E.Conflicts of interest
M. D. G. is co-founder of NuAria, LLC and owns 50% equity interest in the company. The work of this manuscript is not directly related to the activities of the company.Acknowledgements
J. S. M., L. T. J. K., and T. H. E. are grateful for financial support from the Army Research Office under Cooperative Agreement Number W911NF-22-2-0257 (for monomer synthesis and characterization). J. S. M., L. T. J. K., and T. H. E. also thank the National Science Foundation (NSF) under the award NSF DMR POL 2004682, for partially supporting the initial experiments. The authors (J. S. M., L. T. J. K., and T. H. E.) acknowledge the UD Mass Spectrometry facility for the use of the mass spectrometer, along with the UD NMR laboratory for the use of the NMR spectrometer, partially supported by the Delaware COBRE program, with a grant from the National Institute of General Medical Sciences – NIGMS (5 P30 GM110758-02) from the National Institutes of Health (NIH). M. D. G. and H. S. B. gratefully acknowledge funding from the following sponsors (for polymer synthesis and characterization): the U.S. Department of Energy (DOE) through the Office of Basic Energy Sciences (BES) award number DE-SC0023343, the Advanced Research Projects Agency-Energy (ARPA-E) award number DE-AR0001103, Research Corporation for Science Advancement (Scialog), and the NSF Chemical, Bioengineering, Environmental and Transport systems division (CBET) award number 1836719. The authors also acknowledge Dr Yi Yang, Ermias Dheressa, and Taysha Telenar for experimental assistance. The views and opinions of authors expressed herein do not necessarily state or reflect those of the U. S. Government or any agency thereof. The Table of Contents figure was created with https://www.biorender.com/.References
- BASF, Ultrason® E, S, P, (PESU, PSU, PPSU), https://www.basf.com/cn/documents/en/chinaplas/UltrasonESPproductbrochureEN.pdf, accessed June 13, 2024.
- Solvay, Udel® PSU, https://www.solvay.com/sites/g/files/srpend221/files/2018-08/Udel-PSU-Design-Guide_EN-v5.0_0_0.pdf, accessed June 13, 2024.
- G. O. Jones, A. Yuen, R. J. Wojtecki, J. L. Hedrick and J. M. García, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 7722–7726 CrossRef CAS PubMed
.
- T. Xiang, Y. Xie, R. Wang, M.-B. Wu, S.-D. Sun and C.-S. Zhao, Mater. Lett., 2014, 137, 192–195 CrossRef CAS
.
- Z. Xu, J. Liao, H. Tang and N. Li, J. Membr. Sci., 2018, 548, 481–489 CrossRef CAS
.
- Y. Yang, H. S. Behbahani, B. F. Morgan, F. L. Beyer, A. Hocken and M. D. Green, Polymer, 2023, 264, 125522 CrossRef CAS
.
- E. R. Thomas, J. S. Lee, H. Shokrollahzadeh Behbahani, A. Nazari, Y. Li, Y. Yang, M. D. Green and M. L. Lind, ACS Omega, 2023, 8, 18462–18471 CrossRef CAS PubMed
.
- Y. Yang, T. L. Ramos, J. Heo and M. D. Green, J. Membr. Sci., 2018, 561, 69–78 CrossRef CAS
.
- L. Tang, K. J. T. Livi and K. L. Chen, Environ. Sci. Technol. Lett., 2015, 2, 59–65 CrossRef CAS
.
- H. Shokrollahzadeh Behbahani, H. Mithaiwala, H. L. Marques, W. Wang, B. D. Freeman and M. D. Green, Macromolecules, 2023, 56, 6470–6481 CrossRef CAS
.
- A. Amitrano, J. S. Mahajan, L. T. J. Korley and T. H. Epps, III, RSC Adv., 2021, 11, 22149–22158 RSC
.
- Y. Peng, K. H. Nicastro, T. H. Epps, III and C. Wu, J. Agric. Food Chem., 2018, 66, 11775–11783 CrossRef CAS PubMed
.
- J. S. Mahajan, R. M. O’Dea, J. B. Norris, L. T. J. Korley and T. H. Epps, III, ACS Sustain. Chem. Eng., 2020, 8, 15072–15096 CrossRef CAS
.
- G. F. Bass and T. H. Epps, III, Polym. Chem., 2021, 12, 4130–4158 RSC
.
- L. Trullemans, S. F. Koelewijn, I. Scodeller, T. Hendrickx, P. van Puyvelde and B. F. Sels, Polym. Chem., 2021, 12, 5870–5901 RSC
.
- W. Schutyser, T. Renders, S. van den Bosch, S. F. Koelewijn, G. T. Beckham and B. F. Sels, Chem. Soc. Rev., 2018, 47, 852–908 RSC
.
- M. M. Abu-Omar, K. Barta, G. T. Beckham, J. S. Luterbacher, J. Ralph, R. Rinaldi, Y. Román-Leshkov, J. S. M. Samec, B. F. Sels and F. Wang, Energy Environ. Sci., 2021, 14, 262–292 RSC
.
- R. M. Cywar, N. A. Rorrer, C. B. Hoyt, G. T. Beckham and E. Y. X. Chen, Nat. Rev. Mater., 2022, 7, 83–103 CrossRef CAS
.
- E. M. Maines, M. K. Porwal, C. J. Ellison and T. M. Reineke, Green Chem., 2021, 23, 6863–6897 RSC
.
- F. M. Haque, J. S. A. Ishibashi, C. A. L. Lidston, H. Shao, F. S. Bates, A. B. Chang, G. W. Coates, C. J. Cramer, P.
J. Dauenhauer, W. R. Dichtel, C. J. Ellison, E. A. Gormong, L. S. Hamachi, T. R. Hoye, M. Jin, J. A. Kalow, H. J. Kim, G. Kumar, C. J. LaSalle, S. Liffland, B. M. Lipinski, Y. Pang, R. Parveen, X. Peng, Y. Popowski, E. A. Prebihalo, Y. Reddi, T. M. Reineke, D. T. Sheppard, J. L. Swartz, W. B. Tolman, B. Vlaisavljevich, J. Wissinger, S. Xu and M. A. Hillmyer, Chem. Rev., 2022, 122, 6322–6373 CrossRef CAS PubMed
.
- C. Gioia, G. Lo Re, M. Lawoko and L. Berglund, J. Am. Chem. Soc., 2018, 140, 4054–4061 CrossRef CAS PubMed
.
- Y. Xu, K. Odelius and M. Hakkarainen, ACS Sustain. Chem. Eng., 2019, 7, 13456–13463 CrossRef CAS
.
- J. L. Fredricks, M. Parker, P. Grandgeorge, A. M. Jimenez, E. Law, M. Nelsen and E. Roumeli, MRS Commun., 2022, 12, 394–402 CrossRef CAS
.
- P. B. V. Scholten, J. Cai and R. T. Mathers, Macromol. Rapid Commun., 2021, 42, 2000745 CrossRef CAS PubMed
.
- G. Yang, B. J. Rohde, H. Tesefay and M. L. Robertson, ACS Sustain. Chem. Eng., 2016, 4, 6524–6533 CrossRef CAS
.
- Z. Sun, B. Fridrich, A. de Santi, S. Elangovan and K. Barta, Chem. Rev., 2018, 118, 614–678 CrossRef CAS PubMed
.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 1246843 CrossRef PubMed
.
- S. P. S. Chundawat, G. T. Beckham, M. E. Himmel and B. E. Dale, Annu. Rev. Chem. Biomol. Eng., 2011, 2, 121–145 CrossRef CAS PubMed
.
- K. H. Nicastro, C. J. Kloxin and T. H. Epps, III, ACS Sustain. Chem. Eng., 2018, 6, 14812–14819 CrossRef CAS
.
- J. S. Mahajan, Z. R. Hinton, E. Nombera Bueno, T. H. Epps, III and L. T. J. Korley, Mater. Adv., 2024, 5, 3950–3964 RSC
.
- S. V. Mhatre, J. S. Mahajan, T. H. Epps, III and L. T. J. Korley, Mater. Adv., 2023, 4, 110–121 RSC
.
- S. F. Koelewijn, S. van den Bosch, T. Renders, W. Schutyser, B. Lagrain, M. Smet, J. Thomas, W. Dehaen, P. van Puyvelde, H. Witters and B. F. Sels, Green Chem., 2017, 19, 2561–2570 RSC
.
- X. Zhang, J. S. Mahajan, L. T. J. Korley, T. H. Epps, III and C. Wu, Mutat. Res. Genet. Toxicol. Environ. Mutagen., 2023, 885, 1–9 CrossRef PubMed
.
- X. Zhang, J. S. Mahajan, J. Zhang, L. T. J. Korley, T. H. Epps, III and C. Wu, Food Chem. Toxicol., 2024, 190, 114787 CrossRef CAS PubMed
.
-
T. H. Epps, III, L. T. J. Korley, M. D. Green, J. S. Mahajan and H. S. Behbahani, US Pat. Application, 17/616245, 2022
.
- G. D. Yadav and N. Kirthivasan, Appl. Catal., A, 1997, 154, 29–53 CrossRef CAS
.
- E. D. Hernandez, A. W. Bassett, J. M. Sadler, J. J. La Scala and J. F. Stanzione, ACS Sustain. Chem. Eng., 2016, 4, 4328–4339 CrossRef CAS
.
- R. Viswanathan, B. C. Johnson and J. E. McGrath, Polymer, 1984, 25, 1827–1836 CrossRef CAS
.
-
D. W. van Krevelen and K. Te Nijenhuis, Properties of Polymers, Elsevier, Amsterdam, 4th edn, 2009, pp. 189–227 Search PubMed
.
- F. M. Fowkes, Ind. Eng. Chem. Res., 1964, 56, 40–52 CrossRef CAS
.
- ChemAxon, Software solutions and services for chemistry and biology, https://chemaxon.com/, accessed May 8, 2024.
- J. C. Foster, I. Akar, M. C. Grocott, A. K. Pearce, R. T. Mathers and R. K. O'Reilly, ACS Macro Lett., 2020, 9, 1700–1707 CrossRef CAS PubMed
.
- G. Tse and S. I. Sandler, J. Chem. Eng. Data, 1994, 39, 354–357 CrossRef CAS
.
- M. Elimelech and W. A. Phillip, Science, 2011, 333, 712–717 CrossRef CAS
.
- L. N. Nthunya, M. F. Bopape, O. T. Mahlangu, B. B. Mamba, B. van der Bruggen, C. A. Quist-Jensen and H. Richards, J. Environ. Manage., 2022, 301, 113922 CrossRef CAS
.
- C. Moretti, B. Corona, R. Hoefnagels, I. Vural-Gürsel, R. Gosselink and M. Junginger, Sci. Total Environ., 2021, 770, 144656 CrossRef CAS
.
- X.-G. Li and M.-R. Huang, React. Funct. Polym., 1999, 42, 59–64 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00314d |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2024 |