Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
What is better to enhance the solubility of hydrophobic compounds in aqueous solutions: eutectic solvents or ionic liquids?†
Olga
Ferreira
 *ab,
Liliana P.
Silva
*ab,
Liliana P.
Silva
 c,
Heloísa H. S.
Almeida
ab,
Jordana
Benfica
c,
Heloísa H. S.
Almeida
ab,
Jordana
Benfica
 c,
Dinis O.
Abranches
c,
Dinis O.
Abranches
 c,
Simão P.
Pinho
c,
Simão P.
Pinho
 ab and
João A. P.
Coutinho
ab and
João A. P.
Coutinho
 c
c
aCentro de Investigação de Montanha (CIMO), Instituto Politécnico de Bragança, Campus de Santa Apolónia, 5300-253 Bragança, Portugal. E-mail: oferreira@ipb.pt; Tel: + 351 273 303 087
bLaboratório para a Sustentabilidade e Tecnologia em Regiões de Montanha, Instituto Politécnico de Bragança, Campus de Santa Apolónia, 5300-253 Bragança, Portugal
cCICECO – Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, 3810-193 Aveiro, Portugal. E-mail: jcoutinho@ua.pt; Tel: +351 234 401 507
First published on 18th November 2024
Abstract
The solubilities of benzoic acid, (S)-hesperetin, and L-tryptophan in aqueous solutions of ionic liquids (choline glycolate and choline malonate) and the analogous eutectic solvents (choline chloride:glycolic acid and choline chloride:malonic acid) were studied. It is shown that while ionic liquids (IL) and eutectic solvents (ES) were able to increase the solubility of all compounds studied in aqueous solution, ionic liquids were much more efficient for neutral and acidic compounds, while eutectic solvents showed a better performance for the alkaline substances. The results reported here show that the solubility enhancement is related, in the first instance, to the pH of the aqueous solution, which is the dominant effect on the increase in solubility and the main parameter that must be taken into account when selecting a co-solvent to successfully achieve the solubilization of ionizable hydrophobic biomolecules in aqueous solution. In addition, a hydrotropy mechanism was identified when the pH effect was removed, supporting the idea that ionic liquids and eutectic solvents behave as hydrotropes in aqueous solutions. The results here reported show that rather than a focus on the type of solvents (IL vs. ES), the molecular mechanisms such as speciation and co-solvation/hydrotropy, which in some cases may have complementary and synergetic effects, are the parameters that must be addressed in the design or selection of the best solubility enhancer.
Sustainability spotlightAccording to the green chemistry principles, solvents should be chosen minimizing their hazard and risk and to be derived from renewable raw materials. In this context, ionic liquids (ILs) and eutectic solvents (ES) can be designed as advantageous alternatives to volatile organic compounds in many applications, aiming to fulfil, as much as possible, those guidelines, in line with UN goals 3 (ensure healthy lives and promote well-being for all at all ages) and 12 (ensure sustainable consumption and production patterns). Particularly, in this work, they have been studied as additives to water, still the greenest solvent, to increase the solubility of hydrophobic compounds. The results obtained contribute with additional criteria for the selection of solvents. |
Introduction
The study of green and sustainable solvents such as ionic liquids (IL) and eutectic solvents (ES) is a topic of great interest with impact in diverse scientific and technological areas.1–3 Ionic liquids are salts with a low melting temperature, resulting from disperse charges and ion asymmetry that leads to poor crystallization behavior. Unlike ionic liquids, which are pure substances, deep eutectic solvents (DES) are mixtures of compounds that interact strongly through hydrogen bonding, leading to the formation of a liquid phase.4 Many mixtures reported as deep eutectic solvents in the literature do not have a eutectic point temperature below that of an ideal liquid mixture,5 therefore, the more generic designation of eutectic solvent (ES), as opposed to deep eutectic solvent, is here adopted. Both IL and ES present interesting and unusual properties as solvents, such as their negligible (or very low) vapor pressure and can be tailored to have unique physical and chemical properties in terms of polarity, solvability, miscibility, and stability.2,6,7Water remains the greenest solvent due to its non-toxicity, availability, and stability,8,9 but its use as a solvent is often hindered by the low aqueous solubility of the compounds to be processed.10 This limitation can be addressed by pH control or by using additives such as surfactants, co-solvents, or hydrotropes, which enhance the aqueous solubility of hydrophobic compounds. As such, the use of green solvents as additives to water has attracted the interest of the scientific community in recent years, and aqueous solutions of IL and ES have already been shown to increase the aqueous solubility of biocompounds and drugs through different mechanisms.11–18
Although the advantages of ES and IL as co-solvents in aqueous solutions have been much praised by the scientific community, a clear comparison between their relative capacities as (co-)solvents has never been reported before. It is well established that ionic liquids can increase the aqueous solubility of hydrophobic substances through various mechanisms. Certain ionic liquids have been identified as excellent hydrotropes,11,14 in which both anion and cation can contribute synergistically to the hydrotropy.19 Ionic liquids have been successfully applied to increase the solubility of different molecules in aqueous solutions.11,14,16 Similar studies were carried out to evaluate the solubility of quercetin, benzoic acid, and phenolic acids using ES.12,15,20 However, the differences in the mechanisms controlling the solubility enhancement effects by either ES or IL are still poorly understood, being unclear which performs better, why, and the selection criteria to choose the best solubilizing agent. Unfortunately, the solubility data available in the literature are still too scarce, and the co-solvents used from the two families of green solvents are too different in nature to allow for a direct comparison between IL and ES.
The goal of this work is to carry out a fair comparison between the performances of IL and ES as aqueous solubility enhancers of poorly water-soluble compounds. To do that, two structurally similar IL and ES are used. Equimolar mixtures of cholinium chloride ([Ch]Cl) and one organic acid (malonic acid – MalA or glycolic acid – GlyA) were selected as eutectic solvents, while their corresponding ionic analogues (choline malonate and choline glycolate) were chosen as ionic liquids. Equimolar mixtures of ES are used (rather than other mole ratios) to mimic the stoichiometry of their ionic liquid counterparts. As model compounds for poorly water-soluble solutes, benzoic acid (a natural organic acid), (S)-hesperetin (a flavonoid), and L-tryptophan (an amino acid) were selected. The chemical structures of the eutectic solvents, ionic liquids and solutes studied in this work are depicted in Fig. 1.
Materials and methods
Chemicals
All compounds were used as received from suppliers and stored in a desiccator to avoid water contamination. Their purity and source details are listed in Table 1. Ultrapure water (resistivity 18.2 MΩ cm and total organic carbon below 5 μg dm−3) was used to perform the solubility experiments.| Compounds | pKaa | MM (g mol−1) | CAS number | Source | Purity (wt%) |
|---|---|---|---|---|---|
| a Data taken from ref. 21. | |||||
| Benzoic acid | 4.08 | 122.12 | 65-85-0 | Acros Organics | >99.0 |
| (S)-Hesperetin | 7.86; 9.33; 9.98 | 302.27 | 69097-99-0 | Merck | >95.0 |
| L-Tryptophan | 2.54; 9.40 | 204.22 | 73-22-3 | TCI | >98.5 |
| Malonic acid | 2.43; 5.92 | 104.07 | 141-82-2 | Fluka | >98.0 |
| Glycolic acid | 3.53; 14.78 | 76.05 | 79-14-1 | Sigma Aldrich | >99.0 |
| Choline bicarbonate | 104.17 | 78-73-9 | Sigma Aldrich | >80.0 | |
| Choline chloride | 139.62 | 67-48-1 | Acros Organics | >98.0 | |
| Diethyl ether | 74.12 | 60-29-7 | Panreac | >99.8 | |
Ionic liquids synthesis
The ionic liquids studied were synthesized by simple metathesis reactions adapting the procedure from the literature.22 Summarily, to synthesize the choline malonate, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio of choline bicarbonate (80% by weight in water) and malonic acid were mixed by slowly adding the acid to a choline bicarbonate aqueous solution at 303.2 K. The homogeneous reaction was carried at room temperature under an inert atmosphere with continuous stirring for 2 hours. For choline glycolate, choline bicarbonate was added dropwise to an aqueous solution of glycolic acid, with a molar excess of 1.1 equivalent, at 303.2 K and stirred for 2 hours. The solvent was then removed under reduced pressure (2 kPa). After, diethyl ether was added under vigorous stirring to precipitate the organic acid in excess. The mixture was left stirring for 1 h, and the organic acid in excess was then removed by filtration. In a final step, diethyl ether was evaporated initially under reduced pressure (2 kPa), and both ionic liquids were purified under high vacuum (0.1 Pa) and moderate temperature (303 K) for at least 48 h. The washing and removal of trace solvents were repeated until the chemical structure and the absence of impurities were confirmed by 1H and 13C NMR, and the water content of the IL was less than 1500 ppm in mass, measured using a Metrohm 831 Couple Karl Fischer, with the analyte Hydranal-Coulomat AG of Riedel-de Haen. The 1H and 13C NMR spectra were acquired with a Bruker AMX 300 NMR equipment operating at 300.13 and 75.47 MHz, and spectra are depicted in Fig. S1 and S2.†
1 mole ratio of choline bicarbonate (80% by weight in water) and malonic acid were mixed by slowly adding the acid to a choline bicarbonate aqueous solution at 303.2 K. The homogeneous reaction was carried at room temperature under an inert atmosphere with continuous stirring for 2 hours. For choline glycolate, choline bicarbonate was added dropwise to an aqueous solution of glycolic acid, with a molar excess of 1.1 equivalent, at 303.2 K and stirred for 2 hours. The solvent was then removed under reduced pressure (2 kPa). After, diethyl ether was added under vigorous stirring to precipitate the organic acid in excess. The mixture was left stirring for 1 h, and the organic acid in excess was then removed by filtration. In a final step, diethyl ether was evaporated initially under reduced pressure (2 kPa), and both ionic liquids were purified under high vacuum (0.1 Pa) and moderate temperature (303 K) for at least 48 h. The washing and removal of trace solvents were repeated until the chemical structure and the absence of impurities were confirmed by 1H and 13C NMR, and the water content of the IL was less than 1500 ppm in mass, measured using a Metrohm 831 Couple Karl Fischer, with the analyte Hydranal-Coulomat AG of Riedel-de Haen. The 1H and 13C NMR spectra were acquired with a Bruker AMX 300 NMR equipment operating at 300.13 and 75.47 MHz, and spectra are depicted in Fig. S1 and S2.†
Eutectic solvents preparation
Eutectic mixtures were prepared gravimetrically (±10−4 g) by mixing choline chloride and the organic acid in closed glass flasks in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, under constant agitation at (353.2 ± 0.5) K.23 The water content of the pure components and mixtures was also measured by Karl Fischer titration to ensure the correct preparation of the ES and their aqueous solutions.
1 molar ratio, under constant agitation at (353.2 ± 0.5) K.23 The water content of the pure components and mixtures was also measured by Karl Fischer titration to ensure the correct preparation of the ES and their aqueous solutions.
pH measurements
The pH of the solutions was measured using a Mettler Toledo U402-M3-S7/200 microelectrode. The instrument was calibrated with standard solutions of pH 4.0 and 7.0. The readings were performed in duplicate at the temperature of the solubility experiments. The pH measurements of the binary aqueous solutions of ionic liquids or eutectic solvents (without solute) were carried out at 303.2 K.Solubility measurements
The solubilities were determined by the isothermal analytical shake flask method, previously described in the literature.24 A small excess of solute was added to each aqueous solution until the solvent was saturated. The aqueous solutions of benzoic acid and (S)-hesperetin were equilibrated at (298.2 ± 0.5) K and of L-tryptophan at (303.2 ± 0.5) K, under constant stirring and an equilibrium time of at least 40 h, using an Eppendorf Thermomixer Comfort equipment. For higher viscosity solvents, the solutions were placed on magnetic stirring plates inside a thermostatic water bath for at least 40 h. After equilibration, all samples remained at rest for at least 12 h and were subsequently filtered to guarantee the separation of the undissolved solute from the liquid phase. Three samples of the liquid phase were carefully collected and diluted in ultra-pure water. The solutes concentration was quantified by UV spectroscopy using a T70 spectrometer, PG Instruments (benzoic acid and (S)-hesperetin assays) and a SHIMADZU UV-1700, Pharma-Spec spectrometer (L-tryptophan assays) at appropriate wavelengths (273 nm for benzoic acid, 289 nm for (S)-hesperetin, and 280 nm for L-tryptophan). Each data point presented in this work is an average of at least 2 independent measurements.Results and discussion
Solubility curves
As discussed in the introduction, in this work, the ability of two ionic liquids (choline glycolate, [Ch][Gly], and choline malonate, [Ch][Mal]), and two eutectic solvents (choline chloride:glycolic acid, [Ch]Cl:GlyA, and choline chloride:malonic acid, [Ch]Cl:MalA) to increase the aqueous solubilities of benzoic acid, (S)-hesperetin, and L-tryptophan is investigated.The solubility data, together with the respective standard deviations, are presented in ESI (Tables S1–S13†). The solubilities of the model compounds were first studied in pure water, and the results are compared to the literature data25–32 in Table S1.† For L-tryptophan and benzoic acid, the results are consistent with the literature. For hesperetin, our results are consistent with those of Srirangam and Majumdar,27 which are one order of magnitude higher than the results from two other groups.25,26 Regarding the solubilities of the aqueous solutions of IL and ES, the coefficient of variation (CV) of the solubilities is lower than 10% in about 90% of the systems. The maximum CV, higher than 20%, refer to two hesperetin-containing systems, in the lower solubility range (<0.05 g kg−1 solution).
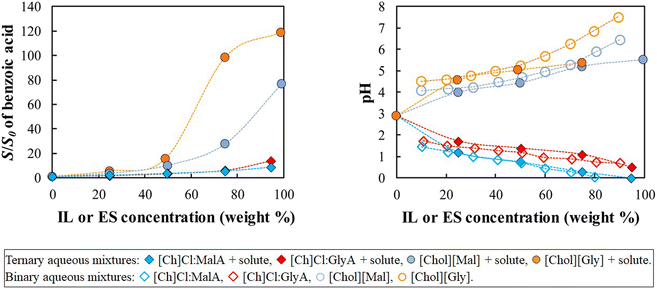
This section starts by examining the behavior of benzoic acid, the acidic solute. The relative solubilities (S/S0) in aqueous solutions of IL and ES are depicted in Fig. 2 (left), where S represents the solubility (mol L−1) of the solute in the aqueous solutions of ionic liquid or eutectic solvent and S0 is the solubility (mol L−1) in pure water. This is a common and convenient way of representing solubility enhancements. Fig. 2 (right) depicts the pH of the aqueous solutions of IL and ES in the presence or absence of the solute.
Fig. 2 shows that both IL and ES are able to increase the aqueous solubility of benzoic acid. [Chol][Gly] provided the best solubility enhancement (119-fold increase), followed by [Chol][Mal] (77-fold increase). Even though a solubility increase of the solute is observed for all cases, the magnitude and shape of the solubility curves are different, with ionic liquids providing larger solubility enhancements than the corresponding eutectic solvents, especially at higher concentrations.
Given the structural similarity of the IL and ES here studied, their significantly different effects on the solubility of benzoic acid are surprising. However, the pH of the aqueous solutions, a parameter often overlooked when discussing the use of ES and IL as solubilizers in the literature, provides an important clue. In the absence of solute, the pH of the aqueous ionic liquids ranges between 4.1 and 7.6, while the pH of the eutectic solvents is always lower than 2. Because acidic compounds dissolve better in solutions with a higher pH, the lower pH of the eutectic solvents is deleterious to the solubility enhancement of benzoic acid. This simple observation explains the differences between the eutectic solvents and the ionic liquids studied and suggests that the solutes are solubilized in aqueous IL solutions primarily through a pH effect that changes the speciation of the benzoic acid into benzoate.
Notwithstanding the previous paragraph, the eutectic solvents are still able to increase the solubility of the acidic solutes (14-fold increase, for [Ch]Cl:GlyA ES). This is surprising since the lower pH of the eutectic solvents, when compared to the aqueous solution of the pure solute, should lead to a decrease in solubility, rather than the enhancement depicted in Fig. 2. This suggests the existence of a second mechanism that competes with and reverses the adverse effect of the pH in the case of eutectic solvents.
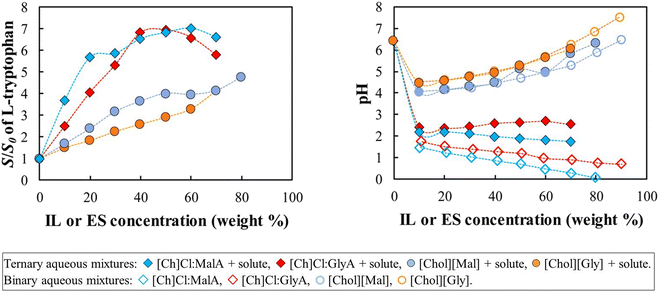
These results reveal the pH to be the primary solubilizing mechanism for the acidic solute, although a secondary mechanism, to be addressed later in this work, seems to be also playing a role. Following this line of thought, this tendency should be inverted when dealing with alkaline solutes, where eutectic solvents should be superior to ionic liquids. To test this hypothesis, the solubility enhancement of L-tryptophan, an ampholyte solute, containing both acidic and basic groups, was investigated.
The data depicted in Fig. 3 show that all IL and ES are able to increase the solubility of the amino acid in water. However, contrary to the case of the acidic solutes (Fig. 2), here eutectic solvents have an advantage over ionic liquids on the solubility enhancement. Tryptophan should be predominantly in the zwitterionic form in the pH region between its pKa values21 (2.54 and 9.40), so the major solubility enhancement due to the pH effect should be achieved outside that range. By analyzing the pH of the aqueous solutions, the eutectic systems (with pH < 2) should cause the highest increase in the solubility of L-tryptophan, as experimentally observed, due again to the speciation of the solute that, as previously observed for the benzoic acid, is the main driving force for the enhanced solubility observed. As suggested for the benzoic acid, another mechanism must be acting since although the IL do not affect the speciation of the L-tryptophan, they nevertheless contribute to an increase of its solubility in the zwitterionic form.
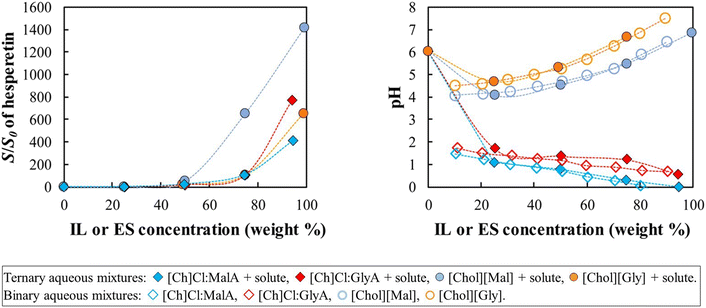
Finally, Fig. 4 shows the relative solubilities of (S)-hesperetin, at 298.2 K. The lowest acidic pKa of (S)-hesperetin is 7.86,32 so this molecule should be mostly in its neutral form in the pH range studied. The enhanced solubilities observed in this case cannot be attributed to speciation effects.
Surprisingly, in all cases, the addition of either IL or ES significantly increases the relative solubility of (S)-hesperetin. The highest increase is observed with choline malonate, about 1400-fold, which is significantly higher than the effect of adding the analogue eutectic solvent ([Ch]Cl:MalA) with “just” a 410-fold increase. Interestingly, the other IL and ES pair ([Ch]Cl:GlyA and [Chol][Gly]) presents solubility curves that are much more similar.
Solubilization mechanisms
The discussion in the previous section asserted pH to be the dominant parameter that controls the ability of IL and ES to enhance the solubility of acidic or basic solutes. However, a second mechanism must also be considered to account for (i) the ability of the acidic eutectic solvents to enhance the solubility of acidic solutes, (ii) the ability of the relatively pH-neutral ionic liquids to greatly enhance the solubility of a zwitterion, and (iii) the ability of both ES and IL to enhance the solubility of neutral solutes. In an attempt to disclose this second mechanism, we attempted to remove from the solubility enhancements previously presented the effect of the pH. To do so, the solubility of all solutes was predicted as a function of pH by the appropriate Henderson–Hasselbalch or derived relationships.21,33,34 The calculated solubility-pH profile, along with the results already discussed above, are depicted in Fig. 5.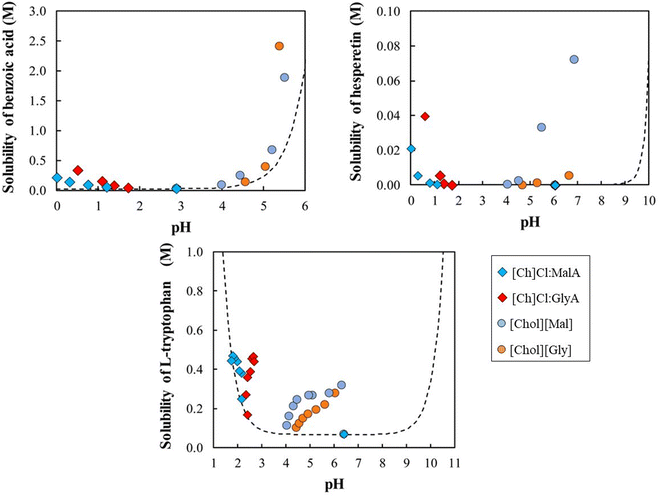 | ||
| Fig. 5 Solubility (M) profiles of benzoic acid (298.2 K), (S)-hesperetin (298.2 K) and L-tryptophan (303.2 K) in aqueous solutions of [Ch]Cl:MalA, [Ch]Cl:GlyA, [Chol][Mal] and [Chol][Gly]; --- the black dashed line represents the solubility-pH profile predicted by the appropriate Henderson–Hasselbalch or derived relationships, at 298 K.21,33,34 | ||
These results reveal several interesting phenomena. For the acidic solute (benzoic acid), the solubility enhancement provided by the ionic liquids is dominated by the pH variation. However, it is also clear that the solubility enhancement provided by the eutectic solvents is not explained by the change in pH and consequent protonation of the solute.
For the ampholyte solute (tryptophan), the solubility enhancement at lower pH provided by the eutectic solvents is partly explained by the effect of the pH, while the enhancement due to the presence of the ionic liquids is uncorrelated with the ionization state of tryptophan. Finally, for the hesperetin that is in its neutral form in the studied pH range, there is no apparent correlation between the solubility enhancement due to the pH change and the solubility enhancements due to the eutectic solvents or ionic liquids. All of this supports and corroborates the discussion of the previous section that while pH is an important mechanism of solubilization it is not the only mechanism in action on these systems.
Hydrotropy appears to be the solubilization mechanism that may explain the enhanced solubilization above the pH-induced effect observed in Fig. 5. Hydrotropy is a phenomenon by which the aqueous solubility of a hydrophobic compound is enhanced due to the presence of a hydrotrope, which is a small, amphiphilic molecule. The mechanism of hydrotropy is loosely based on the hydrophobic effect. When hydrotropes are present in solution, their apolar moieties tend to aggregate around hydrophobic solutes, while their polar moieties remain interacting with water. This minimizes contacts between solute and water and maximizes the extent of the hydrogen bond network of water.35,36 The claim that hydrotropy is responsible for the solubility enhancements of the solutes where the pH does not play a major role is supported by recent studies that show ionic liquids to be excellent hydrotropes for a large group of hydrophobic molecules.11,19,24
Fig. 6 clearly shows that the two solubilization mechanisms (pH and hydrotropy) are non-synergetic. In other words, the solubility enhancement due to hydrotropy is large when there is no pH effect, but is negligible when pH plays an important role, with the experimental data for the water/hydrotropes systems matching the data for the expected solubility-pH profile. This phenomenon is explained by the mechanism of hydrotropy. Since the pH impacts the solubility of the studied solutes by simple proton association/dissociation, whenever it influences the solubility, this means that the solute becomes charged. Hydrotropy, however, relies on the formation of solute-hydrotrope aggregates through apolar moieties to enhance the solubility of hydrophobic solutes. When the solute becomes charged due to a pH-induced speciation, the formation of these aggregates becomes less likely.
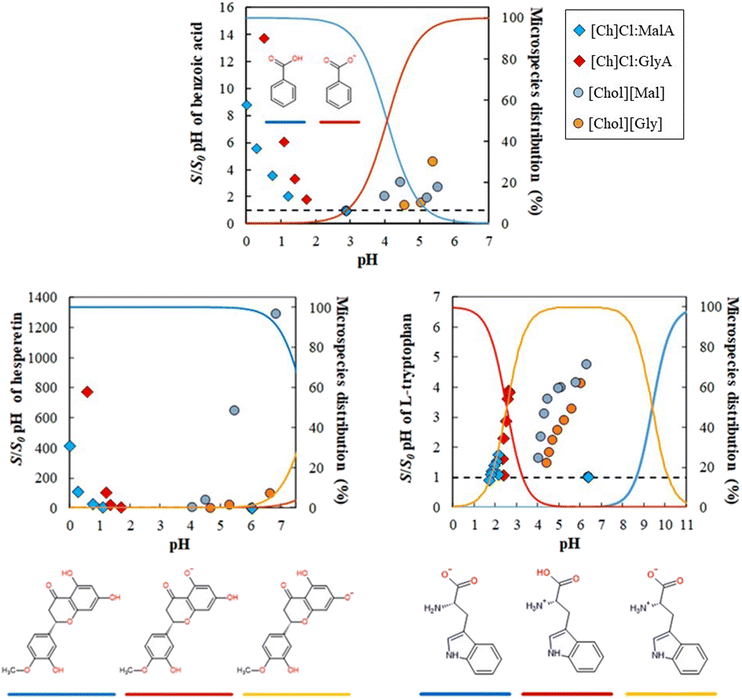 | ||
| Fig. 6 S/S0,pH enhancement measured in aqueous solutions of [Ch]Cl:MalA, [Ch]Cl:GlyA, [Chol][Mal] and [Chol][Gly]; ---- the black dashed line is a visual guide. The microspecies distributions in aqueous solutions were calculated using the Chemicalize software, at 298 K.21 | ||
Conclusions
In this work, the ability of ionic liquids and eutectic solvents to increase the solubility of hydrophobic solutes of different acidity such as benzoic acid, (S)-hesperetin, and L-tryptophan was investigated. Both the ionic liquids (cholinium malonate and cholinium glycolate) and the analogous eutectic solvents (choline chloride:malonic acid and choline chloride:glycolic acid) used in this study seem to induce significant increases in solubility. However, the results obtained show that different mechanisms influence the solubility depending on the nature of the solute and the solvent used. The pH-induced speciation was identified as being a dominant influence on the ability of ionic liquids and eutectic solvents to increase the solubility of acidic or alkaline solutes. Furthermore, hydrotropy has also been disclosed as being a second relevant mechanism of enhanced solubility. The claim that hydrotropy is responsible for increases in the solubility of solutes where pH does not play an important role is supported by the experimental data presented here for this set of solutes. Thus, the results presented here will pave the way for future work in studies on the solubility of water-IL and water-ES mixtures and provide additional criteria for the selection of solvents.Data availability
Supporting experimental data have been included in the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support through national funds FCT/MCTES (PIDDAC): CIMO, UIDB/00690/2020 (DOI: 10.54499/UIDB/00690/2020) and UIDP/00690/2020 (DOI: 10.54499/UIDP/00690/2020), SusTEC, LA/P/0007/2020 (DOI: 10.54499/LA/P/0007/2020) and CICECO-Aveiro Institute of Materials, UIDB/50011/2020 (DOI 10.54499/UIDB/50011/2020), UIDP/50011/2020 (DOI 10.54499/UIDP/50011/2020) & LA/P/0006/2020 (DOI 10.54499/LA/P/0006/2020). J.B.S. acknowledges FCT for her PhD grant 2020.05802.BD.References
- F. Pena-Pereira and J. Namieśnik, Ionic Liquids and Deep Eutectic Mixtures: Sustainable Solvents for Extraction Processes, ChemSusChem, 2014, 7(7), 1784–1800, DOI:10.1002/cssc.201301192.
- J. L. Shamshina, O. Zavgorodnya and R. D. Rogers, Ionic Liquids, in Encyclopedia of Analytical Science, Elsevier, 2019, pp. 218–225, DOI:10.1016/B978-0-12-409547-2.13931-9.
- F. Chemat, M. Abert Vian, A.-S. Fabiano-Tixier, M. Nutrizio, A. Režek Jambrak, P. E. S. Munekata, J. M. Lorenzo, F. J. Barba, A. Binello and G. Cravotto, A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products, Green Chem., 2020, 22(8), 2325–2353, 10.1039/C9GC03878G.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Deep Eutectic Solvents (DESs) and Their Applications, Chem. Rev., 2014, 114(21), 11060–11082, DOI:10.1021/cr300162p.
- M. A. R. Martins, S. P. Pinho and J. A. P. Coutinho, Insights into the Nature of Eutectic and Deep Eutectic Mixtures, J. Solution Chem., 2019, 48(7), 962–982, DOI:10.1007/s10953-018-0793-1.
- J. Pernak, A. Syguda, I. Mirska, A. Pernak, J. Nawrot, A. Pradzyńska, S. T. Griffin and R. D. Rogers, Choline-Derivative-Based Ionic Liquids, Chem.–Eur. J., 2007, 13(24), 6817–6827, DOI:10.1002/chem.200700285.
- T. P. Thuy Pham, C.-W. Cho and Y.-S. Yun, Environmental Fate and Toxicity of Ionic Liquids: A Review, Water Res., 2010, 44(2), 352–372, DOI:10.1016/j.watres.2009.09.030.
- M. Castro-Puyana, M. L. Marina and M. Plaza, Water as Green Extraction Solvent: Principles and Reasons for Its Use, Curr. Opin. Green Sustainable Chem., 2017, 5, 31–36, DOI:10.1016/j.cogsc.2017.03.009.
- K. Häckl and W. Kunz, Some Aspects of Green Solvents, C. R. Chim., 2018, 21(6), 572–580, DOI:10.1016/j.crci.2018.03.010.
- M. H. Zainal-Abidin, M. Hayyan, A. Hayyan and N. S. Jayakumar, New Horizons in the Extraction of Bioactive Compounds Using Deep Eutectic Solvents: A Review, Anal. Chim. Acta, 2017, 979, 1–23, DOI:10.1016/j.aca.2017.05.012.
- A. F. M. Cláudio, M. C. Neves, K. Shimizu, J. N. Canongia Lopes, M. G. Freire and J. A. P. Coutinho, The Magic of Aqueous Solutions of Ionic Liquids: Ionic Liquids as a Powerful Class of Catanionic Hydrotropes, Green Chem., 2015, 17(7), 3948–3963, 10.1039/c5gc00712g.
- B. Soares, A. J. D. Silvestre, P. C. Rodrigues Pinto, C. S. R. Freire and J. A. P. Coutinho, Hydrotropy and Cosolvency in Lignin Solubilization with Deep Eutectic Solvents, ACS Sustain. Chem. Eng., 2019, 7(14), 12485–12493, DOI:10.1021/acssuschemeng.9b02109.
- H. G. Morrison, C. C. Sun and S. Neervannan, Characterization of Thermal Behavior of Deep Eutectic Solvents and Their Potential as Drug Solubilization Vehicles, Int. J. Pharm., 2009, 378(1–2), 136–139, DOI:10.1016/j.ijpharm.2009.05.039.
- T. E. Sintra, D. O. Abranches, J. Benfica, B. P. Soares, S. P. M. Ventura and J. A. P. Coutinho, Cholinium-Based Ionic Liquids as Bioinspired Hydrotropes to Tackle Solubility Challenges in Drug Formulation, Eur. J. Pharm. Biopharm., 2021, 164, 86–92, DOI:10.1016/j.ejpb.2021.04.013.
- N. López, I. Delso, D. Matute, C. Lafuente and M. Artal, Characterization of Xylitol or Citric Acid:Choline Chloride:Water Mixtures: Structure, Thermophysical Properties, and Quercetin Solubility, Food Chem., 2020, 306, 125610, DOI:10.1016/j.foodchem.2019.125610.
- A. Xu, X. Guo, Y. Zhang, Z. Li and J. Wang, Efficient and Sustainable Solvents for Lignin Dissolution: Aqueous Choline Carboxylate Solutions, Green Chem., 2017, 19(17), 4067–4073, 10.1039/C7GC01886J.
- A. Zarei, R. Haghbakhsh and S. Raeissi, Overview and Thermodynamic Modelling of Deep Eutectic Solvents as Co-Solvents to Enhance Drug Solubilities in Water, Eur. J. Pharm. Biopharm., 2023, 193, 1–15, DOI:10.1016/j.ejpb.2023.10.007.
- C. Ma, A. Laaksonen, C. Liu, X. Lu and X. Ji, The Peculiar Effect of Water on Ionic Liquids and Deep Eutectic Solvents, Chem. Soc. Rev., 2018, 47(23), 8685–8720, 10.1039/C8CS00325D.
- D. O. Abranches, J. Benfica, B. P. Soares, A. M. Ferreira, T. E. Sintra, S. Shimizu and J. A. P. Coutinho, The Impact of the Counterion in the Performance of Ionic Hydrotropes, Chem. Commun., 2021, 57(23), 2951–2954, 10.1039/D0CC08092F.
- H. G. Morrison, C. C. Sun and S. Neervannan, Characterization of Thermal Behavior of Deep Eutectic Solvents and Their Potential as Drug Solubilization Vehicles, Int. J. Pharm., 2009, 378(1–2), 136–139, DOI:10.1016/j.ijpharm.2009.05.039.
- Chemicalize, http://Chemicalize.Com/, Developed by ChemAxon, (accessed on 19/04/2022).
- T. E. Sintra, Synthesis of More Benign Ionic Liquids for Specific Applications, 2017, http://hdl.handle.net/10773/22516.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Novel Solvent Properties of Choline Chloride/Urea Mixtures, Chem. Commun., 2003,(1), 70–71, 10.1039/b210714g.
- T. E. Sintra, K. Shimizu, S. P. M. Ventura, S. Shimizu, J. N. Canongia Lopes and J. A. P. Coutinho, Enhanced Dissolution of Ibuprofen Using Ionic Liquids as Catanionic Hydrotropes, Phys. Chem. Chem. Phys., 2018, 20(3), 2094–2103, 10.1039/c7cp07569c.
- L. Liu and J. Chen, Solubility of Hesperetin in Various Solvents from (288.2 to 323.2) K, J. Chem. Eng. Data, 2008, 53(7), 1649–1650, DOI:10.1021/je800078j.
- S. Tommasini, M. L. Calabrò, R. Stancanelli, P. Donato, C. Costa, S. Catania, V. Villari, P. Ficarra and R. Ficarra, The Inclusion Complexes of Hesperetin and Its 7-Rhamnoglucoside with (2-Hydroxypropyl)-β-Cyclodextrin, J. Pharm. Biomed. Anal., 2005, 39(3–4), 572–580, DOI:10.1016/j.jpba.2005.05.009.
- R. Srirangam and S. Majumdar, Passive Asymmetric Transport of Hesperetin across Isolated Rabbit Cornea, Int. J. Pharm., 2010, 394(1–2), 60–67, DOI:10.1016/j.ijpharm.2010.04.036.
- K. Sasahara and H. Uedaira, Solubility of Amino Acids in Aqueous Poly (Ethylene Glycol) Solutions, Colloid Polym. Sci., 1993, 271(11), 1035–1041, DOI:10.1007/BF00659292.
- W. Zhu, Y. Fan, Q. Xu, X. Liu, B. Heng, W. Yang and Y. Hu, Saturated Solubility and Thermodynamic Evaluation of L-Tryptophan in Eight Pure Solvents and Three Groups of Binary Mixed Solvents by the Gravimetric Method at T = 278.15–333.15 K, J. Chem. Eng. Data, 2019, 64(9), 4154–4168, DOI:10.1021/acs.jced.9b00562.
- H. B. Bull, K. Breese and C. A. Swenson, Solubilities of Amino Acids, Biopolymers, 1978, 17(4), 1091–1100, DOI:10.1002/bip.1978.360170421.
- H. Nogami and H. Umeyama, Effect of Third Component on Water Structure around Tryptophan in Aqueous Solution, Chem. Pharm. Bull., 1970, 18(2), 328–334, DOI:10.1248/cpb.18.328.
- S. H. Yalkowsky, Y. He and P. Jain, Handbook of Aqueous Solubility Data, New York, 2nd edn, 2010 Search PubMed.
- A. Avdeef, Solubility of Sparingly-Soluble Ionizable Drugs, Adv. Drug Delivery Rev., 2007, 59(7), 568–590, DOI:10.1016/j.addr.2007.05.008.
- E. Shoghi, E. Fuguet, E. Bosch and C. Ràfols, Solubility-PH Profiles of Some Acidic, Basic and Amphoteric Drugs, Eur. J. Pharm. Sci., 2013, 48(1–2), 291–300, DOI:10.1016/j.ejps.2012.10.028.
- S. Shimizu and N. Matubayasi, The Origin of Cooperative Solubilisation by Hydrotropes, Phys. Chem. Chem. Phys., 2016, 18(36), 25621–25628, 10.1039/c6cp04823d.
- D. O. Abranches, J. Benfica, B. P. Soares, A. Leal-Duaso, T. E. Sintra, E. Pires, S. P. Pinho, S. Shimizu and J. A. P. Coutinho, Unveiling the Mechanism of Hydrotropy: Evidence for Water-Mediated Aggregation of Hydrotropes around the Solute, Chem. Commun., 2020, 56(52), 7143–7146, 10.1039/d0cc03217d.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00379a |
| This journal is © The Royal Society of Chemistry 2024 |