 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mapping the end-of-life of chemicals for circular economy opportunities†
Taylor
Uekert
 *
*
Strategic Energy Analysis Center, National Renewable Energy Laboratory, Golden, Colorado 80401, USA. E-mail: taylor.uekert@nrel.gov
First published on 15th October 2024
Abstract
This work presents a material flow analysis of fourteen organic and inorganic chemicals in the United States, tracking their lifecycle from production through to intermediate conversions, end-products, and end-of-life (EoL) disposal on an annual basis. We show that only 10% of the 158 million metric tons (Mt) of chemicals produced each year are recycled, resulting in an estimated 40–100 Mt of wasted greenhouse gas emissions and the loss of 6000 years of healthy human life from toxic emissions each year. Aggressive recycling scenarios could reduce wasted GHG emissions by up to 60%, but additional circularity interventions related to reduction and redesign will be needed to further guide the chemical industry toward a more sustainable future.
Sustainability spotlightThe chemical sector is an energy- and emissions-intensive industry that is nevertheless crucial for modern life. A circular economy in which chemicals are kept in use has been proposed as one approach to minimize the environmental impacts of this sector, but the lack of data on the end-of-life (EoL) of chemical products makes it challenging to identify appropriate circularity interventions. By estimating the EoL of chemicals in the United States and the corresponding greenhouse gas and toxic emissions without and with circularity strategies, this work is in alignment with the United Nations' Sustainable Development Goals for climate action and responsible consumption and production. |
Introduction
The chemical sector plays an integral role in modern life, supplying the building blocks for common products such as plastics, fertilizers, glass, cleaning agents, and more.1 An estimated 96% of all manufactured goods are linked to the chemical industry, which has synthesized between 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 400
000 and 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 new molecules or mixtures.2,3 Global chemical production uses extractive feedstocks (fossil fuels and raw minerals) and energy-intensive synthesis and separation processes, resulting in the consumption of 10% of global energy and the release of 5% of global greenhouse gas (GHG) emissions.2,4 Ocean acidification and biosphere integrity, both of which are linked to GHG emissions, are also affected by the chemical sector.5 Furthermore, the release of toxic chemicals to the environment during manufacturing or use (e.g., mining, agriculture) can lead to harmful human exposure, often in low-income communities.3,6
000 new molecules or mixtures.2,3 Global chemical production uses extractive feedstocks (fossil fuels and raw minerals) and energy-intensive synthesis and separation processes, resulting in the consumption of 10% of global energy and the release of 5% of global greenhouse gas (GHG) emissions.2,4 Ocean acidification and biosphere integrity, both of which are linked to GHG emissions, are also affected by the chemical sector.5 Furthermore, the release of toxic chemicals to the environment during manufacturing or use (e.g., mining, agriculture) can lead to harmful human exposure, often in low-income communities.3,6
The circular economy, in which resources are kept in use rather than permitted to become waste, has been proposed as one strategy to curb the environmental impacts of the chemical industry.1,7–9 Circularity could reduce reliance on non-renewable resources through the use of waste feedstocks, as well as reduce overall manufacturing demand through the reuse or recycling of end-products that contain chemicals.8 A high circularity scenario has been shown to have the potential to reduce global chemicals demand by 23–33%.1 Many circularity efforts have focused on recycling of plastic,1,10,11 which accounts for approximately 40% of chemical end-products by weight.12 However, the diversity of chemistries both in plastics and in the sector more broadly makes it challenging to develop a unified circular economy approach.7
Understanding the end-of-life (EoL) of chemicals is a crucial first step towards identifying circular economy opportunities for the chemical sector. While material flow analysis (MFA) has been used to track quantities of chemicals being produced and used for various end-products, previous studies typically have not followed the chemicals to EoL,12 or they included EoL for only a subset of chemical products such as plastics.13–18 Meanwhile, EoL data are usually reported by end-use (e.g., plastics, food, losses) rather than primary chemical.19–21 Data mining and machine learning have been used to estimate aggregated chemical EoL as well as to predict EoL based on chemical structure,22–24 but these studies lack the granularity to form a complete picture of individual chemicals' life cycles.
Here, we present an MFA of chemicals in the United States (U.S.) from synthesis to EoL. Starting from the production of fourteen platform organic and inorganic chemicals – ethylene, propylene, butylenes, butadiene, benzene, toluene, xylenes, ethanol, methanol, ammonia, chlorine, sodium carbonate, sodium hydroxide, and sulfuric acid – we track their application to 30 end-products and their disposal to landfill, energy recovery, recycling, wastewater treatment, and the environment. We use carbon footprint and toxicity data to estimate the GHG and toxic emissions associated with chemical waste, as well as how these emissions could change with future circularity scenarios. Overall, this work provides a snapshot of the complete life cycle of key commodity chemicals in the U.S. to help guide the transition towards a more sustainable chemical sector.
Methods
Scope
The MFA spans platform chemical production, conversion to intermediates, conversion into end-products, and disposal in the U.S. It does not include extraction of fossil fuel or mineral feedstocks. Here, platform chemicals include organic and inorganic chemicals that are directly produced from fossil fuel or mineral feedstocks, the starting point for multiple chemical intermediates and end-products, and produced in the U.S. in high volumes of greater than 1 million metric tons (Mt) per year: (1) ethylene, (2) propylene, (3) butylenes, (4) butadiene, (5) benzene, (6) toluene, (7) xylenes, (8) ethanol, (9) methanol, (10) ammonia, (11) chlorine, (12) sodium carbonate, (13) sodium hydroxide, and (14) sulfuric acid.25,26 Intermediate chemicals are classified in tiers: Tier 1 intermediates require one reaction from a platform chemical, Tier 2 intermediates require one reaction from a Tier 1 intermediate, and so on. Losses from chemical reactions were tracked, as were additional inputs such as oxygen, water, and carbon monoxide and side products such as water, carbon dioxide, and hydrochloric acid (see “Mass balances”). Import and export data were included when available.The platform and intermediate chemicals were linked to end-products, which are considered materials or objects that can be directly used by a consumer. Here, we tracked thirty end-product categories: thermoplastic polymers including (1) high density polyethylene (HDPE), (2) low density and linear low density polyethylene (LDPE/LLDPE), (3) polypropylene (PP), (4) polyvinyl chloride (PVC), (5) polyethylene terephthalate (PET), (6) polystyrene (PS), (7) nylon-6 and nylon-6,6, and (8) polycarbonate (PC); thermoset polymers including (9) rubbers such as styrene–butadiene rubber, nitrile rubber, butyl rubber, polyisobutylene, silicone rubbers, and methyl isobutyl ketone (MIBK) additives, (10) polyurethanes (PUR), and (11) other polymers such as polymethyl methacrylate (PMMA), polyacetal resins, and fluoropolymers; (12) plastic and paper additives; formulated products including (13) cleaning agents, (14) wood laminate, (15) paints and surface coatings, (16) solvents, (17) adhesives, sealants, and functional fluids such as antifreeze and refrigerants, (18) health and personal care products, and (19) cigarettes; (20) gasoline; agriculture including (21) food, (22) animal feed, and (23) other crops for industrial use or specialty processing; (24) explosives; industrial use including (25) pulp and paper treatment, (26) water treatment, (27) mining and metals processing, and (28) other industrial applications such as catalysts, adsorbents, carbon fiber, and cement processing; (29) glass; and (30) aluminum metal. Chemicals that did not fit the above categories were classified as “other untracked products”. In general, other non-chemical materials that contribute to the end-products, such as the crude oil in gasoline or the biomass in food, were not included in the overall mass flows. The two exceptions are aluminum metal and glass, for which tracking the other components (aluminum and silica/lime, respectively) enabled verification against known production quantities.
The end-products were linked to nine EoL categories: (1) longer lifetime – end-products that continue to be used after one year, also known as material stocks; (2) recycling or reuse – including closed-loop recycling as well as down-cycling to lower-value products and composting; (3) landfill; (4) combustion with energy recovery for use in vehicles, equipment, or the electricity grid; (5) wastewater treatment; (6) uncontrolled emission to land; (7) uncontrolled emission to air; (8) uncontrolled emission to water; and (9) other, which could include waste exports, transfers, underground injection, storage, surface impoundment, or other unknown disposal mechanisms. It should be noted that while the longer lifetime end-products will eventually be disposed to landfill, recycling, or other EoL pathways, tracking these flows would require a stocks and flows model that was beyond the scope of the current study.
Data collection
Production and consumption data for all chemicals were sourced from market reports, academic literature, the U.S. Environmental Protection Agency (EPA), and the U.S. Geological Survey (USGS). EoL data for chemical losses were sourced from the U.S. EPA Toxics Release Inventory (TRI).27 EoL data for all other products were sourced from market reports, academic literature, and the U.S. EPA. If EoL data were unavailable, approximations were made based on expert judgement. The target year for this analysis was 2021, but in some cases, data could only be obtained for (or had to be supplemented by) 2015, 2018, or 2019. Where multiple data points were available, averages were used. Imports and exports were estimated by subtracting consumption from production values. All sources and assumptions are provided in Tables S1 and S2 in the ESI.†Mass balances
Stoichiometry was used to establish mass flows between platform and intermediate chemicals, as well as to estimate quantities of other reagents and side products (Table S3†). Reaction yields were taken from literature data28,29 or, if unavailable, approximated based on the quantity of intermediate chemical known to be produced. Any unreacted chemical was treated as a loss and assumed to undergo the EoL documented in the U.S. TRI. All flows are reported on a Mt basis.Environmental impacts
The GHG emissions associated with the production of individual chemicals were sourced from ecoinvent version 3.9.1 with the ReCiPe Hierarchist midpoint methodology, Carbon Minds, and the Material Flows through Industry (MFI) tool, all in kilogram of carbon dioxide equivalent per kilogram of product (kg CO2 eq.per kg, Table S4†).30–32 The GHG factors were multiplied by the total quantity of end-products originating from each platform chemical that are sent to landfill, wastewater treatment, or emitted to the environment in order to estimate total “wasted” GHG emissions. It is important to note that while the MFA covers all stages of a chemical's life cycle, these GHG emissions are for primary chemical production only and do not include the impacts of further conversion into intermediates and end-products. The toxicities associated with the production of individual chemicals listed in the U.S. TRI were sourced from ecoinvent version 3.9.1 with the ReCiPe Hierarchist endpoint methodology in disability adjusted life years (DALYs, Table S5†). One DALY represents the loss of one year of full health. These toxicity factors were multiplied by the total quantity of TRI-listed chemicals in end-products that are sent to landfill, wastewater treatment, or emitted to the environment in order to estimate the total impact of toxic waste.Circularity scenarios
Two circularity scenarios were developed to explore the potential impact of various circular economy interventions on chemical EoL. The “circularity targets” scenario was based on the U.S. EPA's 2030 Recycling Goal as well as the Food Loss and Waste Reduction Goal, which aim to increase the municipal solid waste recycling rate to 50% and halve food waste, respectively.33,34 The Recycling Goal was assumed to be applicable to glass, HDPE, LDPE/LLDPE, nylon-6/-6,6, PC, PET, PP, PUR, PVC, rubbers, and other polymers (Table S6†). The “optimistic circularity” scenario applied maximal recycling rates to antifreeze, solvents, paints and surface coatings, glass, HDPE, LDPE/LLDPE, nylon-6/-6,6, PET, PP, PUR, and rubbers, based on the highest reported recycling yields (Table S6†). It also assumed that food waste could be further halved and that the overall composting rate could meet the maximum reported for the food manufacturing sector (Table S6†). The adjusted EoL distributions were used to re-estimate the quantity of chemicals sent to the nine EoL categories.Results and discussion
The flow of platform chemicals from production to EoL in the U.S. is shown in the Sankey diagram in Fig. 1. For visual clarity, some of the chemicals are grouped into olefins (ethylene, propylene, butylenes, and butadiene), aromatics (benzene, toluene, and xylenes), alcohols (ethanol and methanol), and chlor-alkali (sodium hydroxide and chlorine). See Fig. S1–S6† for more detailed diagrams and ESI Data† for raw data. Of the 204 Mt of platform chemicals produced or imported in the U.S. each year and the 62 Mt of other inputs, approximately 124 Mt (47%) undergo conversion to Tier 1 intermediates, followed by 53 Mt, 8.0 Mt and 0.5 Mt to Tier 2, Tier 3, and Tier 4 intermediates, respectively. The platform and intermediate chemicals are converted to end-products, of which the largest applications include (1) gasoline additives at 49 Mt, (2) HDPE and LDPE at 19 Mt (part of the thermoplastic polymers category in Fig. 1), (3) other crops at 8.9 Mt (agriculture) (4) PP at 7.4 Mt (thermoplastic polymers), (5) PVC at 7.1 Mt (thermoplastic polymers), (6) animal feed at 5.2 Mt (agriculture), (7) PET at 4.9 Mt (thermoplastic polymers), (8) cleaning agents at 3.3 Mt (formulated products), (9) food at 3.3 Mt (agriculture), and (10) water treatment at 2.8 Mt (industrial use) (Table S7†). An estimated 25 Mt of end-products are kept in longer lifetime applications, with 15 Mt recycled or reused, 30 Mt landfilled, 13 Mt sent to wastewater treatment, 56 Mt combusted with energy recovery, 3.3 Mt emitted to air, 8.4 Mt emitted to land, 0.09 Mt emitted to water, and 7.3 Mt treated by other methods (Table S8†).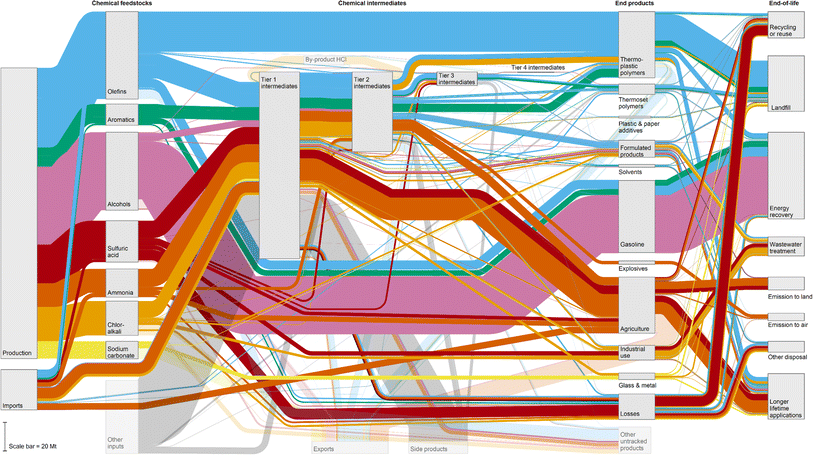 | ||
| Fig. 1 Sankey diagram representing the flow of chemicals in the U.S. in million metric tons from production through to intermediates, final products, and disposal. “Olefins” include ethylene, propylene, butylenes, and butadiene; “aromatics” include benzene, toluene, and xylenes; “alcohols” include ethanol and methanol; “chlor-alkali” include sodium hydroxide and chlorine; “other inputs” include feedstocks beyond those listed in the study, such as oxygen, water, hydrogen, or carbon monoxide; “side products” include by-products that are generated as a result of chemical reaction stoichiometry, such as carbon dioxide or water; “formulated products” include cleaning agents, paints and coatings, adhesives, functional fluids, health and personal care, cigarettes, and wood laminates; “industrial use” includes pulp and paper treatment, water treatment, mining, and other industrial use. Emissions to water are combined with wastewater treatment. Raw data are available in the ESI Data† (“overall” tab). | ||
Overall, these values come to total EoL rates of 16% longer lifetime application, 10% recycling or reuse, 35% combustion with energy recovery, 19% landfill, 8% wastewater treatment, 2% emission to air, 5% emission to land, and 5% other disposal (Fig. 2A). These estimates could vary by up to ±40% (e.g., the recycling rate could range from 6% to 14%). This is primarily due to uncertainty around disposal data and assumptions, as production and consumption data tend to have lower standard deviations of less than ±10%. Gasoline additives account for one-third of total end-products and are assumed to exclusively undergo energy recovery, while losses account for 11% of total end-products and are predominantly recycled. Thus, removing these two end-products shows a decrease in combustion with energy recovery (to 6%) and recycling (to 7%) as well as an increase in landfilling (to 32%) and longer lifetime applications (to 28%, Fig. 2B). A previous study that used data mining to estimate EoL of 640 industrial chemical releases (not all chemical products) in the U.S. suggested rates of 21% recycling, 19% sewerage, 18% landfill, 11% energy recovery, 10% destruction, 10% other treatment, and 11% other disposal,22 which is in near alignment with this work.
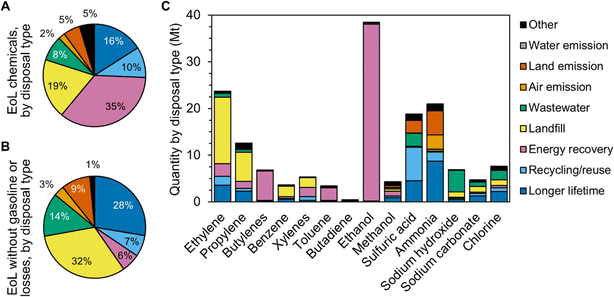 | ||
| Fig. 2 Breakdown of the proportion of chemicals reaching EoL in the U.S. by disposal type (A) across all chemicals and (B) when gasoline products and losses from the chemical production process are not considered. (C) Overview of EoL in the U.S. by chemical class and disposal type in million metric tons; these data include all end-products (i.e., gasoline and losses are not removed). Raw data are available in Table S8.† | ||
EoL differs greatly by chemical class (Fig. 2C, Table S8†). Approximately 88% of ethylene-based chemicals are used for plastics including HDPE, LDPE, PVC (ethylene > ethylene dichloride > vinyl chloride), PET (ethylene > ethylene oxide > ethylene glycol), and PS (ethylene > ethylbenzene > styrene).35 Ethylene end-products are predominantly sent to landfill (60%) and combustion with energy recovery (11%). Only 8% of ethylene end-products are estimated to be recycled, which aligns with reported plastic recycling rates in the U.S. of 1–15%.19 15% of ethylene end-products remain in longer lifetime applications such as PVC (applications in durable piping),36 adhesives, and paints. Approximately two-thirds of xylene-based chemicals (terephthalic acid) are also used in PET, resulting in overall recycling and landfill rates for this chemical class of 15% and 39%, respectively. Similarly, 71% of propylene-based chemicals are used in plastics including PP, PC (propylene > cumene > phenol > Bisphenol A), and nylon-6/-6,6 (propylene > acrylonitrile > adiponitrile > hexamethylene diamine).35 Overall these propylene end-products are landfilled (50%), incinerated with energy recovery (12%), and recycled (5%), with other applications such as paints, adhesives, or wood laminates kept in longer lifetime applications (19%). Benzene-based chemicals are also primarily used in PS (benzene > ethylbenzene > styrene), PUR (benzene > aniline > methylene diphenyl diisocyanate), and nylon-6/-6,6 (benzene > cyclohexanol/cyclohexanone > caprolactam).35 These plastics are challenging to recycle and result in EoL rates for benzene end-products of 62% landfilling, 10% combustion with energy recovery, 3% recycling, and 17% longer lifetime applications (PS insulation and PUR cushioning).36 Nearly all butadiene-based chemicals are used in rubbers for tires, seals, brake pads, and more.36 The vulcanization of rubbers yields cross-linked structures with embedded sulfur, making direct recycling challenging and leading to an estimated 39% of butadiene end-products being landfilled, 23% incinerated with energy recovery, and 23% reused in asphalt or playground floors.37 Butylenes, ethanol, toluene, and approximately one-third of xylenes are used as gasoline additives and are therefore predominately combusted with energy recovery, where the energy recovery is used to power vehicles and equipment. Methanol-based chemicals find a variety of applications, including 23% to formaldehyde for wood laminates (longer lifetime applications), 18% to biodiesel (energy recovery), 16% to cellulose acetates for cigarettes (primarily littered, which is captured here as emissions to land),38 13% to solvents (mostly recycled or incinerated with energy recovery), and 5% to cleaning agents and pharmaceuticals (wastewater treatment).27 This diversity of end-products leads to a diversity of EoL; methanol end-products are sent to 21% longer lifetime applications, 23% combustion with energy recovery, 12% landfill, 9% recycling, 10% land emissions, and 7% wastewater treatment.
Of the inorganic chemicals, an estimated 80% of ammonia-based chemicals are used in fertilizer for the growth of animal feed, food, and other crops. 41% of ammonia end-products are therefore kept in longer lifetime applications (i.e. crops), while 10% are recycled by composting or manure application, 15% are emitted to air by volatilization, and 24% are emitted to land (which may further leach into waterways). Similarly, approximately one-third of sulfuric acid ends up in fertilizers via phosphoric acid, resulting in 24% longer lifetime applications and 14% emission to land. Applications in mining (to leach copper from the ground) and pulp and paper treatment lead to an additional 15% of sulfuric acid-based chemicals being sent to wastewater treatment. Furthermore, nearly half of sulfuric acid is used in excess during the conversion to intermediates; most of these losses are recycled, and sulfuric acid-based chemicals therefore have an overall recycling rate of 38%. Sodium hydroxide-based chemicals are predominantly used for pulp and paper treatment (31%), aluminum ore purification for aluminum metal production (23%), water treatment (18%), and cleaning agents (14%). Approximately 68% of sodium hydroxide end-products are thus sent to wastewater treatment, with smaller fractions to landfill, longer lifetime applications, and recycling. Three-quarters of chlorine-based chemicals are similarly linked to pulp and paper treatment, water treatment, and cleaning agents, resulting in a 26% overall wastewater treatment rate. Chlorine-based chemicals are also used in PVC, PC, paints, and paper and plastic additives, which contribute to longer lifetime, landfilling, and recycling rates of 29%, 16%, and 11%, respectively. Lastly, the EoL of sodium carbonate-based chemicals is related to glass (landfill, longer lifetime, and recycling) and cleaning agents (wastewater treatment), yielding 27% longer lifetime, 26% landfill, 21% wastewater treatment, and 12% recycling.
The environmental implications of the estimated 158 Mt of chemical end-products reaching EoL each year in the U.S. are substantial. Approximately 52 Mt of end-products (33%) are wasted in EoL routes that cannot recover their material or energy value (i.e., landfill, wastewater treatment, or emission to air, land, or water). Note that this calculation does not include chlorine and sodium hydroxide-based chemicals that are directly used to treat water. The quantity of wasted end-products corresponds to 40–104 Mt of “wasted” GHG emissions from producing the corresponding platform chemicals (Fig. 3A, Table S4†), approximately 22–56% of U.S. chemical sector annual emissions35 or 0.6–1.6% of total annual U.S. emissions.39 Although these end-products have served a function before reaching EoL, the term “wasted” is used here because their irreversible disposal necessitates new chemical production, and the resulting GHG emissions represent an avoidable environmental burden that could be reduced through a circular economy. The range in GHG emissions is due to the different chemical manufacturing pathways and accounting methods assumed in ecoinvent, Carbon Minds, and MFI.40 Most wasted GHG emissions in this study are due to the production of ethylene and propylene (low per-kilogram emissions but high EoL volumes) as well as ammonia and sodium hydroxide (lower EoL volumes but higher per-kilogram emissions, Fig. 3A, Table S4†). Given that these chemical classes are linked to plastics (ethylene and propylene, to landfill), fertilizers (ammonia, to land and air emissions), and wastewater-related applications (sodium hydroxide) as discussed above, 54–67% of the wasted GHG emissions are associated with end-products that are landfilled, 18–21% with those that are sent to wastewater treatment, and 13–28% with those that are emitted to the environment by volatization, leaks, or other improper disposal (Fig. 3B).
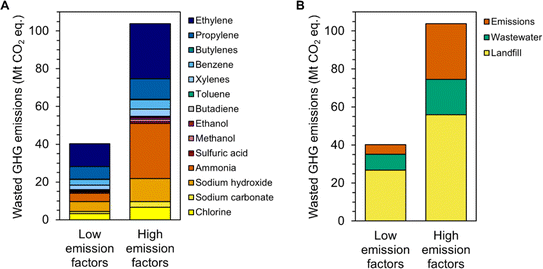 | ||
| Fig. 3 Quantity of greenhouse gas emissions associated with chemical production that are “wasted” each year due to the landfilling, wastewater treatment, or emission to air, land, or water of the final products, by (A) chemical class and (B) disposal type. The low and high emission factors refer to the lower and upper bounds of greenhouse gas emissions associated with the production of each chemical, as sourced from ecoinvent, Carbon Minds, and the Material Flows through Industry tool. Raw data are available in Table S4.† | ||
Furthermore, approximately 6 Mt of wasted end-products are toxic chemicals listed in the U.S. EPA's TRI, accounting for 17% of the 35 Mt of hazardous waste generated in the U.S. each year.41 Nearly 60% of toxic chemicals in this study are sent to wastewater treatment (Fig. 4A) due to their application in cleaning agents (ethoxylates from the ethylene class), mining (sulfuric acid), and health care (isopropanol from the propylene class and acetic anhydride from the methanol class, Fig. 4B, Table S5†). This ratio approaches that of hazardous waste treatment reported by the U.S. EPA, which is 80% wastewater and 20% non-wastewater.41 While wastewater treatment can remove many of these toxic chemicals, a fraction of pollutants will nevertheless be released from the process due to imperfect removal efficiencies.42 The 17% of toxic end-products sent to landfill in this study include functional fluids (ethylene glycol antifreeze from the ethylene class), paper and plastic additives (acrylate esters from the propylene class), paints (acrylate esters and methyl methacrylate from the propylene class, among others), and wood laminates (formaldehyde from the methanol class and phenol from the propylene class). The 22% of toxic end-products emitted to the environment primarily comprises ammonia that is volatilized or leached during application to crops. Total human toxicity associated with these wasted chemicals is 5820 DALYs (Fig. 4C, Table S5†); with an average life expectancy of 70 years,43 the DALYs corresponds to 83 lost lives per year in the U.S.
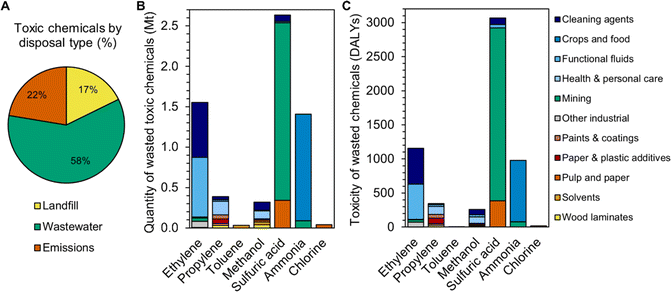 | ||
| Fig. 4 (A) Breakdown of the proportion of final chemical products that are listed in the U.S. EPA's Toxics Release Inventory (TRI) by disposal type. (B) Quantity of toxic chemical products that are landfilled, sent to wastewater treatment, or emitted to air, land, or water each year, by chemical class and product type, in million metric tons. Xylenes also generate toxic end-products (phthalic anhydride in paints), but they are not included here due to their low EoL quantity of 0.002 Mt. (C) Toxicity of wasted TRI-listed chemicals, by chemical class and product type, in disability-adjusted life years. Raw data are available in Table S5.† | ||
This analysis suggests that three major end-product categories require interventions to reduce waste and environmental impacts: plastics, crops, and chemicals that are sent to wastewater treatment including cleaning agents and mining waste. Several circular economy strategies could be applied to the relevant chemicals and end-products to reduce EoL waste and emissions (Fig. 5A). Circular economy strategies are typically shown as a hierarchy, from the most linear approaches (R9 Recover) to the most circular approaches (R0 Refuse).44 The R9 Recovery strategy could integrate anaerobic digestion into wastewater treatment plants to generate biogas from EoL chemicals that can then be burned for energy.45 R8 Recycle could be applied to a variety of plastics, glass, paints, solvents, and functional fluids such as antifreeze, refrigerants, and lubricants. Food waste could be used as a nutrient source for fertilizers and animal feed, addressing R7 Repurpose. R3 Reuse could apply to durable glass, wood laminates, and some plastic products, pending sufficient product redesign and implementation of return or collection schemes. The use of all chemicals could be minimized via R2 Reduce, and single-use packaging systems could be converted into reusable or other circular systems for R1 Rethink. Lastly, for R0 Refuse, cleaning agents and mining practices could be re-designed with non-toxic chemicals, unnecessary plastic and paper additives could be eliminated, and gasoline for transportation could be replaced with electricity or alternative fuels.
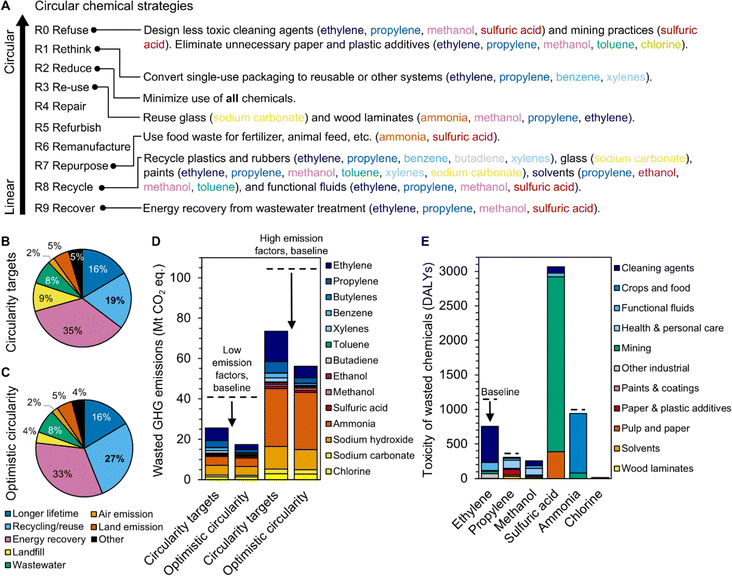 | ||
| Fig. 5 (A) Overview of potential circular economy strategies for the chemicals included in this study. Breakdown of chemical EoL by disposal type for the (B) circularity targets scenario and (C) optimistic circularity scenario. (D) Reduction in greenhouse gas emissions associated with chemicals that are “wasted” each year (landfill, wastewater treatment, or emission to air, land, or water) upon implementation of the circularity scenarios. The low and high emission factors refer to the lower and upper bounds of greenhouse gas emissions associated with the production of each chemical, as sourced from ecoinvent, Carbon Minds, and the Material Flows through Industry tool. (E) Reduction in toxicity associated with chemicals that are “wasted” each year upon implementation of the optimistic circularity scenario. The circularity targets scenario is based on U.S. EPA targets to increase recycling of municipal solid waste to 50% and reduce food waste by 50%. The optimistic circularity scenario is based on maximal recycling applicability and yields that are discussed in Table S6.† Raw data are available in Tables S8 and S9.† | ||
To understand the effect of some of these circularity interventions on chemical EoL, we developed two circularity scenarios: circularity targets (less aggressive, based on U.S. EPA recycling and food waste reduction targets) and optimistic circularity (more aggressive, based on optimal recycling rates, see Methods and Table S6†). The circularity targets scenario could increase the overall chemicals recycling rate from 10% to 19% and decrease the GHG emissions associated with wasted end-products by 29–36% relative to business as usual (Fig. 5B and D, Tables S8 and S9†). The optimistic circularity scenario could increase the overall chemicals recycling rate to 27% and reduce GHG emissions by 46–57% (Fig. 5C and D, Tables S8 and S9†). The analyzed circularity scenarios primarily affect chemical classes such as ethylene and propylene that are predominantly landfilled and contribute significantly to GHG emissions but less so to human toxicity. The optimistic circularity scenario does include improved recycling rates for antifreeze (ethylene glycol from the ethylene class), paints (E-series glycol ethers from the ethylene class; acrylate esters, P-series glycol ethers, methyl methacrylate, and epichlorohydrin from the propylene class), and food (ammonia), thereby reducing overall toxicity by 9% to 5350 DALYs or 76 lost lives per year in the U.S. (Fig. 5E). However, the incorporation of additional circularity innovations such as those discussed above would be necessary to reduce toxic emissions associated with end-products like fertilizers, cleaning agents, and sulfuric acid in mining.
Although the use of a portfolio of circularity strategies could promote a circular economy for all platform chemicals explored in this work, the existence of key barriers such as cost, consumer acceptance, infrastructure limitations, and end-product design requirements should be noted. For example, cleaning agents that are designed to be non-toxic (strategy R0) must still meet performance criteria such as cleaning time and effectiveness.46 Plastic recycling (strategy R8), meanwhile, tends to be limited by the collection stage; increasing access to recycling programs and expanding sorting infrastructure will require both economic and behavioral investment.47–49 Furthermore, circular economy strategies could introduce rebound effects, wherein the benefits of a circular product are negated by higher consumer demand.50
Conclusions
This work established material flows for fourteen platform chemicals from production through to EoL in the U.S. We showed that only 10% of the chemicals produced each year are recycled, with recycling rates varying from 0–40% depending on the chemical class. Furthermore, we identified three major end-product categories that contribute not only to EoL mass but also wasted GHG emissions and human toxicity impacts: plastics, fertilizers, and wastewater treatment end-products such as mining materials and cleaning agents. A variety of circular economy interventions ranging from improving recycling to refusing toxic cleaning agents and paper and plastic additives could help minimize the environmental impacts of the chemical sector, approaching a 60% reduction in wasted GHG emissions. While our analysis captures a single time point of the U.S. chemical industry on a national level, future work could model temporally dynamic flows and explore the geospatial distribution of chemical disposal and toxic emissions.Diversity and inclusion statement
Recent work in several fields of science has identified a bias in citation practices such that papers from women and other minority scholars are under-cited relative to the number of papers in the field. The author recognizes this bias; in this work, an estimated 38% of the cited papers are from female first authors and 32% from female corresponding authors.Data availability
The data supporting this article have been included in the ESI and ESI Data.†Conflicts of interest
The author declares no competing financial interest.Acknowledgements
This work was authored by the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC, for the U.S. Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. The views expressed in the article do not necessarily represent the view of the DOE or the U.S. Government. The U.S. Government retains and the publisher, by accepting the article for publication, acknowledges that the U.S. Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for U.S. Government purposes. The author thanks Birdie Carpenter, Dan Bilello, Emily Newes, Greg Avery, Nicholas Rorrer, and Nivedita Biyani for their feedback on this manuscript.References
- F. Meng, A. Wagner, A. B. Kremer, D. Kanazawa, J. J. Leung, P. Goult, M. Guan, S. Herrmann, E. Speelman, P. Sauter, S. Lingeswaran, M. M. Stuchtey, K. Hansen, E. Masanet, A. C. Serrenho, N. Ishii, Y. Kikuchi and J. M. Cullen, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2218294120 Search PubMed.
- P. Gabrielli, L. Rosa, M. Gazzani, R. Meys, A. Bardow, M. Mazzotti and G. Sansavini, One Earth, 2023, 6, 682–704 Search PubMed.
- R. Naidu, B. Biswas, I. R. Willett, J. Cribb, B. Kumar Singh, C. Paul Nathanail, F. Coulon, K. T. Semple, K. C. Jones, A. Barclay and R. John Aitken, Environ. Int., 2021, 156, 106616 Search PubMed.
- A. Kätelhön, R. Meys, S. Deutz, S. Suh and A. Bardow, Proc. Natl. Acad. Sci. U. S. A., 2019, 166, 11187–11194 Search PubMed.
- I. Barnosell and C. Pozo, Sustain. Prod. Consum., 2024, 44, 188–207 Search PubMed.
- J. Johnston and L. Cushing, Curr. Environ. Health Rep., 2020, 7, 48–57 Search PubMed.
- M. Royle and E. A. Gibson, Chem, 2023, 9, 543–546 Search PubMed.
- T. Keijer, V. Bakker and J. C. Slootweg, Nat. Chem., 2019, 11, 190–195 Search PubMed.
- H. Van Der Veen and M. G. Kapadia, Transitioning to a Circular Economy through Chemical and Waste Management, New York, 2022 Search PubMed.
- R. Meys, F. Frick, S. Westhues, A. Sternberg, J. Klankermayer and A. Bardow, Resour., Conserv. Recycl., 2020, 162, 105010 Search PubMed.
- T. Uekert, A. Singh, J. S. DesVeaux, T. Ghosh, A. Bhatt, G. Yadav, S. Afzal, J. Walzberg, K. M. Knauer, S. R. Nicholson, G. T. Beckham and A. C. Carpenter, ACS Sustainable Chem. Eng., 2023, 11, 965–978 Search PubMed.
- P. G. Levi and J. M. Cullen, Environ. Sci. Technol., 2018, 52, 1725–1734 Search PubMed.
- T. P. Hendrickson, B. Bose, N. Vora, T. Huntington, S. L. Nordahl, B. A. Helms and C. D. Scown, One Earth, 2024, 7, 520–531 Search PubMed.
- J. Di, B. K. Reck, A. Miatto and T. E. Graedel, Resour., Conserv. Recycl., 2021, 167, 105440 Search PubMed.
- U. S. Chaudhari, A. T. Johnson, B. K. Reck, R. M. Handler, V. S. Thompson, D. S. Hartley, W. Young, D. Watkins and D. Shonnard, ACS Sustainable Chem. Eng., 2022, 10, 13145–13155 Search PubMed.
- C. Picuno, A. Alassali, Z. K. Chong and K. Kuchta, Resour., Conserv. Recycl., 2021, 169, 105515 Search PubMed.
- N. Emami, T. M. Baynes, T. Kaushik, M. Singh, S. Bhattacharjya, K. Locock and H. Schandl, J. Mater. Cycles Waste Manage., 2024, 1–12 Search PubMed.
- W. T. Hsu, T. Domenech and W. McDowall, Clean. Environ. Syst., 2021, 2, 100004 Search PubMed.
- A. Milbrandt, K. Coney, A. Badgett and G. T. Beckham, Resour., Conserv. Recycl., 2022, 183, 106363 Search PubMed.
- A. Milbrandt, J. Zuboy, K. Coney and A. Badgett, Waste Manag. Bull., 2024, 2, 21–28 Search PubMed.
- National Overview: Facts and Figures on Materials, Wastes and Recycling, US EPA, https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials, accessed 22 November 2021 Search PubMed.
- J. D. Hernandez-Betancur, G. J. Ruiz-Mercado and M. Martin, Resour., Conserv. Recycl., 2023, 196, 107031 Search PubMed.
- J. D. Hernandez-Betancur, G. J. Ruiz-Mercado and M. Martin, ACS Sustainable Chem. Eng., 2023, 11, 3594–3602 Search PubMed.
- J. D. Hernandez-Betancur, M. Martin and G. J. Ruiz-Mercado, Resour., Conserv. Recycl., 2022, 178, 106040 Search PubMed.
- Chemical Production Data, US EPA, https://www.epa.gov/chemical-data-reporting/chemical-production-data, accessed 26 June 2024 Search PubMed.
- Chemicals Value Chain Decarbonization: Integrated Solutions for a Complex Challenge, Department of Energy, https://www.energy.gov/eere/iedo/articles/chemicals-value-chain-decarbonization-integrated-solutions-complex-challenge, accessed 26 June 2024 Search PubMed.
- Toxics Release Inventory (TRI) Program, US EPA, https://www.epa.gov/toxics-release-inventory-tri-program, accessed 18 January 2024 Search PubMed.
- Ullmann's Encyclopedia of Industrial Chemistry, Wiley, 2003 Search PubMed.
- S&P Global, Chemical Economics Handbook (CEH), 2023 Search PubMed.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, Int. J. Life Cycle Assess., 2016, 21, 1218–1230 Search PubMed.
- Carbon Minds, https://www.carbon-minds.com/, accessed 26 June 2024 Search PubMed.
- R. J. Hanes and A. Carpenter, Environ. Syst. Decis., 2017, 37, 6–12 Search PubMed.
- U.S. National Recycling Goal, US EPA, https://www.epa.gov/circulareconomy/us-national-recycling-goal, accessed 19 June 2024 Search PubMed.
- United States 2030 Food Loss and Waste Reduction Goal, US EPA, https://www.epa.gov/sustainable-management-food/united-states-2030-food-loss-and-waste-reduction-goal, accessed 26 June 2024 Search PubMed.
- S. R. Nicholson, N. A. Rorrer, T. Uekert, G. Avery, A. C. Carpenter and G. T. Beckham, ACS Sustainable Chem. Eng., 2023, 11, 2198–2208 Search PubMed.
- M. F. Ashby, in Materials and the Environment, Butterworth-Heinemann, 2013, pp. 459–595 Search PubMed.
- Z. Xiao, A. Pramanik, A. K. Basak, C. Prakash and S. Shankar, Clean. Mater., 2022, 5, 100115 Search PubMed.
- T. E. Novotny and E. Slaughter, Curr. Environ. Health Rep., 2014, 1, 208–216 Search PubMed.
- Inventory of U.S. Greenhouse Gas Emissions and Sinks, US EPA, https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks, accessed 27 June 2024 Search PubMed.
- L. Cullen, F. Meng, R. Lupton and J. M. Cullen, Nat. Chem. Eng., 2024, 1, 311–322 Search PubMed.
- RCRAInfo Web – Explore Our Data, https://rcrapublic.epa.gov/rcra-public-web/action/posts/2, accessed 26 August 2024 Search PubMed.
- M. Ahmed, M. O. Mavukkandy, A. Giwa, M. Elektorowicz, E. Katsou, O. Khelifi, V. Naddeo and S. W. Hasan, npj Clean Water, 2022, 5, 1–25 Search PubMed.
- Life Expectancy – Our World in Data, https://ourworldindata.org/life-expectancy, accessed 27 June 2024 Search PubMed.
- S. Upasani, J. Walzberg, D. Ravikumar, A. Carpenter, G. Heath, U. Gracida-Alvarez, T. Benavides, H. Xu, T. Hawkins, D. Desantis and J. Cresko, Mapping the Opportunity Space to Model the Circular Economy Using Tools Funded by the DOE Office of Energy Efficiency and Renewable Energy, 2022 Search PubMed.
- U. Ghimire, G. Sarpong and V. G. Gude, ACS Omega, 2021, 6, 11794–11803 Search PubMed.
- Identifying Greener Cleaning Products, US EPA, https://www.epa.gov/greenerproducts/identifying-greener-cleaning-products, accessed 8 October 2024 Search PubMed.
- M. Appel, A. Francis, A. Payne, A. Tanimoto and S. Mouw, State of Recycling: the Present and Future of Residential Recycling in the U.S. 2024, 2024 Search PubMed.
- M. Roosen, N. Mys, K. Kleinhans, I. S. Lase, S. Huysveld, M. Brouwer, E. U. Thoden van Velzen, K. M. Van Geem, J. Dewulf, K. Ragaert, A. Dumoulin and S. de Meester, Resour., Conserv. Recycl., 2022, 178, 106025 Search PubMed.
- J. Walzberg, S. Sethuraman, T. Ghosh, T. Uekert and A. Carpenter, Energy Res. Social Sci., 2023, 100, 103116 Search PubMed.
- M. Ferrante, M. Vitti, F. Facchini and C. Sassanelli, J. Cleaner Prod., 2024, 456, 142399 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00517a |
| This journal is © The Royal Society of Chemistry 2024 |
