 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemical advances in transforming pollutants into new materials
Tales da Silva
Daitx
 *
*
Instituto de Química, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil. E-mail: tales.daitx@ufrgs.br
First published on 18th November 2024
The quick technological advancement and the need to meet ever-increasing consumer demands means there is a predatory global pursuit for natural resources. One of the most representative milestones of this characteristic is known as “Earth Overshoot Day”, a time frame annually reached in August since 2005 and probably breaking the July barrier in the coming years. This date, reached in 2023 on August 2nd, represents the day when the demand for natural resources exceeds the planet’s capacity to produce or renew these resources over 365 days.1 It’s as if we borrowed annual resources from the planet which will never be replaced. Considering the current demand for natural resources, it is estimated that approximately 1.7 planets Earth would be needed to meet the annual need for them. Furthermore, a large part of our daily practices leads to the transformation of these resources into by-products that further harm our ecosystem, such as the infamous energy processes and processes from the agro-industrial sector.
According to data supported by the Global Carbon Projects science team,2 carbon dioxide (CO2) emissions from the burning of fossil fuels in 2023 reached the mark of 36.8 billion tons, breaking the annual emissions record presented at COP27, Conference of the Organization United Nations (UN) on climate change. In addition to this primary source, there is also the release of CO2 from other ways, such as fires and livestock farming, common in countries with a strong agro-industrial sector, such as Brazil. As shown by data from the Climate Observatory, a network of environmental entities from Brazilian civil society formed with the aim of discussing the problem of global warming specifically in the Brazilian context, agricultural processes are quite relevant in this context. With its vast area of cultivable land, the excessive use of pesticides and fertilizers to support the high agro-industrial demands causes various gases to be released into the environment, which come mainly from volatilization processes. In general, it is estimated that up to 90% of all agrochemicals used in Brazil are lost through different processes, which leads to greater quantities of these species being applied to the crops and further escalating the release of these gases.3,4
In order to mitigate these effects and finally begin to “pay off the debt” with planet Earth, different sustainable paths can be traced, with chemistry presenting a vast toolbox to propose and employ new alternatives, in addition to improving existing methodologies. Looking from this perspective, combining procedures that transform gases released through the processing and burning of fuels into other compounds, and using these compounds in the development of new materials is a very interesting and viable alternative. Thus, it would be possible to retain CO2 from burning processes, transform it into a value-added compound, such as a monomer, and then convert it into different materials that can be used in the agricultural sector. These materials can act as agrochemical supports and hydrogels, releasing active compounds in a slow and/or controlled manner and being able to provide moisture to a plant for a prolonged time (Fig. 1). This strategy, besides capturing harmful gases from the air, enables strategic application of pesticides to the soil, also reducing future emissions associated with agriculture.
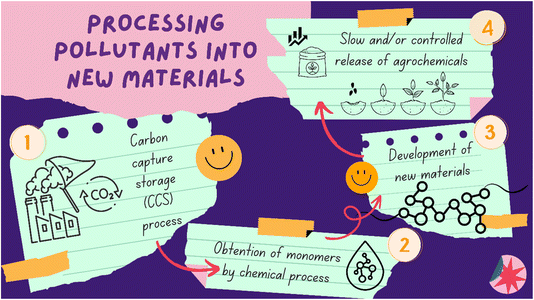 | ||
| Fig. 1 Proposed cycle for transforming pollutants into new materials to mitigate the greenhouse effect. | ||
What enables this possibility are the already existing technologies, which are constantly being developed in the most diverse academic and industrial research groups. For example, companies in the petrochemical sector already promote the transformation of CO2 into value-added products through new technologies, thus ensuring that the gas is not released into the atmosphere and that it is fixed in the form of different compounds, such as oxalic acid.5 However, the range of products to be generated from these transformation processes is very wide, and different synthetic protocols can be applied to obtain a library of compounds to be used in the development of new polymeric supports.
These supports can have a very positive impact on the environment, as they can be used in the development of new less polluting agricultural methods. The slow and/or controlled release devices produced with these materials can trap active agents within their structure, meaning that they are not easily lost to the environment via the action of natural processes, such as volatilization or leaching. Since component losses are minimized, smaller dosages need to be applied to a specific crop, resulting in a lower environmental impact.6–8 Furthermore, the ability of the polymer chains to modulate their release rate of agrochemicals to the external environment leads to less exposure of food to these harmful compounds. Thus, not only does the environment benefit from lower releases of pollutants, but also generates greater food security.
For example, studies show that thermoplastic polymer matrices based on biodegradable polymers can lead to a decrease in the release rate of active compounds by around 90% over 30 days of exposure to a cultivation environment, causing a fertilizer to act on different stages of a plant’s growth, and minimizing its exposure to a large load of chemical agents.6,7 In the same way, hydrogels with high water absorption and retention capacity have also been identified as control agents in the release of active compounds, especially nitrogenous compounds, such as urea and ammonia, which have a potential impact on the greenhouse effect. In this case, not only a reduction in the quantity and rate of release of these agents is achieved, but also prolonged soil humidification becomes possible, a very relevant artifice especially for environments with restricted water resources.9
All these classes of materials can be derived directly from monomers from carbon capture and storage (CCS) processes originating from gases that cause the greenhouse effect, and they can also be combined with other raw materials of natural origin, making these materials even more sustainable. However, the development of this type of technology and especially its large-scale application still faces very relevant socioeconomic barriers, especially in developing countries. In Brazil, the sector represents 5.9% of the Gross Domestic Product (GDP), being the third sector that most contributes to the generation of wealth, playing a strategic role in generating food for the population on a local and global scale through exports. However, the lack of public policies that enable the development of new research by promoting the development of new technologies in the sector and provide tax incentives for the application and dissemination of these created products means that there is still a long way to go.
Even though the country is not one of the main contributors to greenhouse gas emissions from energy generation, if all of humanity had the same Brazilian consumption pattern, Earth Overshoot Day would be reached on August 12, a period relatively short in relation to the milestone reached on a global scale. This is mainly due to the strong agroindustry sector, which yearns for new, more sustainable, and lower-impact alternatives. In fact, what exists today are individual and isolated practices, far from the collective point of view that the cause requires.
In short, chemistry plays a leading role in the decarbonization of the planet, being able to act not only in capturing CO2, but also using it as a tool that can help on other fronts. Technological innovations in the materials sector must act in conjunction with socioeconomic policies to catalyze not only the development of new proposals and creation of materials, but also encourage the application of these technologies more quickly in our daily lives, making the impact of the agricultural sector, one of the main drains of natural resources on planet Earth, as small as possible.
Statement on use of AI
The author declares that the essay was not written using any type of AI and AI-assisted technologies.Conflicts of interest
There are no conflicts to declare.References
- Earth Overshoot Day, https://overshoot.footprintnetwork.org/ (accessed 29th March 2024) Search PubMed.
- Fossil CO2 emissions at record high in 2023, https://globalcarbonbudget.org/fossil-co2-emissions-at-record-high-in-2023/ (accessed 29th March 2024) Search PubMed.
- Perdas de agrotóxicos, https://www.embrapa.br/en/agencia-de-informacao-tecnologica/tematicas/agricultura-e-meio-ambiente/qualidade/residuos/perdas-de-agrotoxicos (accessed 29th March 2024) Search PubMed.
- A. Sharma, V. Kumar, B. Shahzad, M. Tanveer, G. P. S. Sidhu and N. Handa, et al. , SN Appl. Sci., 2019, 1, 1446, DOI:10.1007/s42452-019-1485-1.
- J. H. H. Meurs, WO Pat., WO2016124646A1, 2016 Search PubMed.
- T. S. Daitx, M. Giovanela, L. N. Carli and R. S. Mauler, Polym. Adv. Technol., 2019, 30, 631–639, DOI:10.1002/pat.4499.
- T. S. Daitx, V. S. Lima, M. Gryczak, C. L. Petzhold, L. N. Carli and R. S. Mauler, Polym. Adv. Technol., 2020, 31, 2579–2587, DOI:10.1002/pat.4985.
- A. Sikder, A. K. Pearce, S. J. Parkinson, R. Napier and R. K. O’Reilly, ACS Appl. Polym. Mater., 2021, 3, 1203–1217, DOI:10.1021/acsapm.0c00982.
- C. Ding, S. Zhang, X. Fu, T. Liu, L. Shao and M. Fei, et al. , J. Mater. Chem. A, 2021, 9, 24613–24621, 10.1039/D1TA05442B.
| This journal is © The Royal Society of Chemistry 2024 |

