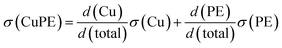Synthesis of stretchable hybrid copper films via nanoconfinement†
Xin
Ma
ac,
Donghao
Xie
ac,
Jiayi
Wang
ac,
Zekun
Wang
b,
Qiao
Gu
b,
Yonghong
Deng
*d and
Ping
Gao
 *bc
*bc
aAdvanced Materials Thrust, Interdisciplinary Office, The Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong 999077, China. E-mail: dxieaf@connect.ust.hk; jwangfw@connect.ust.hk
bDepartment of Chemical and Biological Engineering, The Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong, 999077, China. E-mail: zwangfg@connect.ust.hk; guqiao@ust.hk; kepgao@ust.hk
cAdvanced Materials Thrust, Function Hub, The Hong Kong University of Science and Technology (Guangzhou), Nansha District, Guangzhou, 511453, China
dDepartment of Materials Science and Engineering, Southern University of Science and Technology, Shenzhen 518055, China. E-mail: dengyh@sustech.edu.cn
First published on 22nd November 2023
Abstract
Flexible ultrathin copper films are desired for use as flexible electromagnetic shielding, smart clothing, and energy storage devices. The fabrication of such films can be realized through plasma assisted surface coating on flexible polymer thin films. Unfortunately, the use of plasma treatment leads to substantial damage to the polymer substrates, which is particularly serious for ultrathin films. Here, we report the fabrication of stretchable, ultrathin, yet ultrastrong hybrid copper films via simple nanoconfinement. The hybrid films are prepared by the conformal electroless deposition of copper nanoparticles on nanofibrils of ultrastrong ultrahigh molecular weight polyethylene (UHMWPE) membranes. These flyweight hybrid copper films (0.3 mg cm−2) possess a high mechanical strength of 390 MPa, 26% larger than pure copper foils; a low resistivity of 5 × 10−8 Ω m at a cyclic extensional strain of 1%; and macroscopic shape recovery after applying a biaxial extension strain of 10%. The shape recoverability and the high mechanical strength of the hybrid copper film were derived from the unique nanofibrous network of the ultrastrong UHMWPE substrate. As an application, we fabricated a lithium-ion battery using the CuPE as the current collector at the graphite anode and observed a near 100% and 10% increase in energy density concerning the total mass and total volume of the anode, respectively.
1. Introduction
With the rapid development of the flexible and wearable electronics industry, there is an increasing demand for lightweight electronic devices that conform to the curves of the body or solid objects.1,2 Flexible electronic devices require rechargeable batteries that are stable under repeated mechanical deformations under working circumstances.3–5 Furthermore, as stretching deformation induces the largest strain in the sample, a stretchable rechargeable battery will be flexible when tested by bending, twisting, and folding.4,6 Lithium-ion batteries are the primary power solutions for portable electronics and electric vehicles because of their high energy density, low self-discharge, and light weight.7,8 However, conventional lithium-ion batteries are inflexible primarily because of the electrode and current collector components that are inflexible.Owing to its high electrical conductivity (5.8 × 107 S m−1, only slightly lower than silver but of much lower cost) and high mechanical strengths, copper is the most commonly used current collector in the anode of lithium-ion batteries and provides both mechanical support and electron transport of the battery. However, conventional copper films are inflexible and heavy (8.0 mg cm−2), and efforts have been made to develop lightweight and flexible copper current collectors to satisfy the flexibility requirements.9,10 Several innovative designs have been reported to improve the flexibility of lithium-ion batteries.11–13
Literature reported strategies for flexible copper current collectors may be divided into three different categories: (a) synthesis of copper foils from copper nanowires;14,15 (b) surface cladding of thin copper films on flexible solid film substrates;9 (c) conformal coating of copper nanoparticles on flexible porous substrates.16–18 By rolling and sintering copper nanowires, flexible and porous light copper current collectors with mass area density down to 1.2 mg cm−2 have been reported, and the porous structures also exhibit a higher surface area for strong anode adhesion in addition to good electrical conductivity.14 Unfortunately, these delicate structures are difficult and costly for scalable implementation. Using fire-retardant modified polyimide films, flexible copper current collectors consisting of thin-film copper cladded sandwich structures with tensile strengths up to 45 MPa have been reported.9 However, these values are 15% that of pure copper foils. The low tensile strength is because of the low tensile strength of the polyimide substrate, which is further weakened by plasma treatment for mechanical interlocking with the surface coated copper. Indeed, most pristine polymers are intrinsically weaker than copper foils, and relatively thick substrate films with thickness greater than 8 μm had to be employed to satisfy the mechanical stability requirement. Lastly, deposition of copper nanoparticles on a porous substrate is both scalable and of low cost, and the surface cladded current collectors have also been tested in lithium-ion batteries, lithium metal batteries, and supercapacitors.10,14,16 However, all porous substrates reported so far are mechanically weak (≤10 MPa), enabling the hybrid current collector to achieve flexibility at the cost of heavier in weight and bulkier in volume; they are typically more than 80 μm thick and about 30 mg cm−2 in area density.10 Therefore, it is necessary to develop flexible ultrathin and robust copper current collectors.
In this study, we used a low-cost electroless deposition in conjunction with an electrodeposition approach to fabricate stretchable hybrid copper films with controlled copper layer thicknesses. Copper nanoparticles were conformally coated onto an ultrathin ultrahigh molecular weight polyethylene membrane that is nanofibrous and ultrastrong.19,20 The UHMWPE's high specific surface areas, which consist of 10 nm diameter nanofibrils, allowed for strong interfacial interactions between the copper nanoparticles and the substrate through van der Waals interactions. The hybrid copper film derived its high tensile strength and shape recoverability from the substrate UHMWPE, which is composed of an interlocked triangulated nanofibrous network structure through interpenetrating molecular entanglements. Additionally, its flexibility and large surface area will improve adhesion between active materials and the current collector,21 or have the potential to release lithium dendrites in anode free batteries.22
2. Experimental section
2.1 PE membrane preparation
The PE membranes were prepared using the methods developed in our group.19,20,23 Firstly, gel films were prepared using a HAAKE twin-screw extruder with raw PE material (GUR 4022, Mw ∼3.5 × 106 kg mol−1) and petrolatum (Protopet 1S). Then these gel films were further stretched with a spontaneous two-direction method. Finally the stretched PE membranes were fixed in carbon fiber frames with a square shape of 70 mm per side and extracted by n-hexane to remove petrolatum.2.2 Electroless deposition (ELD) for the preparation of surface coated CuPE membranes
DP-H catalyst from DuPont is dispersed homogeneously in an aqueous solution containing 1.72 mol L−1 NaCl and 0.274 mol L−1 HCl with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 in volume as the catalyst solution. The membranes were first immersed in catalyst solution for 5 min at 40 °C, and then washed with DI water to remove extra catalysts. Copper deposition solution (CIRCUPOSITTM 3350-1, DuPont) was also heated to 40 °C under rigorous agitation. The membranes with catalysts need to soak into copper deposition solution with fast shaking to avoid bubbles in the first minute and grow Cu for 5 min total. Finally, the membranes with Cu were dipped into a 0.1% benzotriazole (BTA) aqueous solution for 1 min to suppress oxidation.
50 in volume as the catalyst solution. The membranes were first immersed in catalyst solution for 5 min at 40 °C, and then washed with DI water to remove extra catalysts. Copper deposition solution (CIRCUPOSITTM 3350-1, DuPont) was also heated to 40 °C under rigorous agitation. The membranes with catalysts need to soak into copper deposition solution with fast shaking to avoid bubbles in the first minute and grow Cu for 5 min total. Finally, the membranes with Cu were dipped into a 0.1% benzotriazole (BTA) aqueous solution for 1 min to suppress oxidation.
The ELD CuPE was further attached with copper tape on both sides for further electrodeposition. 200 g CuSO4 was dissolved in 1 L DI water, and then 50 ppm Cl−, 20 ppm benzotriazole (BTA), 10 ppm 2,2′-bipyridine and 10 ppm sodium dodecyl sulphate were added. This solution was filled into a square tank and heated to 30 °C with stirring. ELD CuPE was fixed at the middle of the tank as the cathode, along with a two linked copper plate as the anode. The electrodeposition current density was 2 A dm−2.
2.3 Electrodes and full cells
Graphite (MA-EN-AN-02, Canrd) was chosen as the anode material. The standard slurry contains graphite, superconductive carbon, and binder LA-133 in a ratio of 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and water as solvent. This slurry was mixed by ball milling and coated with a thickness of 200 μm on CuPE and then dried at 110 °C for 12 h in a vacuum oven.
3 and water as solvent. This slurry was mixed by ball milling and coated with a thickness of 200 μm on CuPE and then dried at 110 °C for 12 h in a vacuum oven.
NMC 811, superconductive carbon and PVDF in a ratio of 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 with NMP solvent were mixed and coated on commercial Al foil as the cathode side.
6 with NMP solvent were mixed and coated on commercial Al foil as the cathode side.
The graphite electrode was cut into 40 × 30 mm and the NMC 811 electrode was 36 × 26 mm, along with a tab area of 10 mm in square. All the batteries were assembled in pouch bag cells covered with 152 μm Al plastic foil. The electrolyte was 1 M LiPF6 in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (v/v) ethylene carbonate/diethyl carbonate (EC/DEC), and polypropylene–polyethylene–polypropylene (Celgard 2325) was used as a separator.
7 (v/v) ethylene carbonate/diethyl carbonate (EC/DEC), and polypropylene–polyethylene–polypropylene (Celgard 2325) was used as a separator.
All the batteries were tested with electrochemical stations (Neware, Shenzhen China) at room temperature. The Li//graphite batteries were discharged and charged from 0.01 V to 3 V with 3-cycle formation progress at 0.1C. The NMC 811//graphite pouch bags were also activated with 3 cycles at 0.1C in the voltage range of 2.9–4.3 V. The rate test procedure is at the sequence of 0.1C, 0.2C, 0.5C, 1C and 2C. Then, the long cycling performance was tested between 2.9 V and 4.3 V at 0.5C after 3-cycle formation progress at 0.1C.
2.4 Materials characterization
Scanning electron microscopy (SEM) images were taken on a JEOL-7800F scanning electron microscope. All the samples are cut into a 5 mm square and tested without any further conductive coating due to CuPE's intrinsic conductivity. The cross-sectional SEM images were taken on sections prepared by focused ion beam milling using gallium ions on an FEI Helios G4 UX system with 25 pA current and 2 kV testing voltage. The tensile test was conducted on an Advanced Rheology Expansion System ARES with a normal load cell, which had a maximum of 20 N force with a resolution of 0.001 N. Rectangular-shaped samples were carefully cut in the direction of the second stretching direction of the membrane using a sharp scalpel blade. The width of the sample was 5 mm and its length was at least 10 mm. Paper frames with gaps of 10 mm were cut from paper. The sample strips were stuck to the paper frame using adhesive tape. The cross-sectional area of each sample was estimated by measuring the weight and area of the sample and from the known density of the solid material. The tensile test was conducted at a constant strain rate of 0.1% s−1 until fracture under ambient conditions. Sheet resistance was characterized by using a DMR-1C (Damint, Nanjing China) four-probe tester. Wide-angle X-ray diffraction (XRD) was performed on an X'pert Pro (PANalytical) diffractometer. All batteries were assembled in a glove box (Super 1220/750/900, Mikrouna, China) and tested using a Netware Battery Tester (Netware, Guangzhou, China) at room temperature.3. Results and discussion
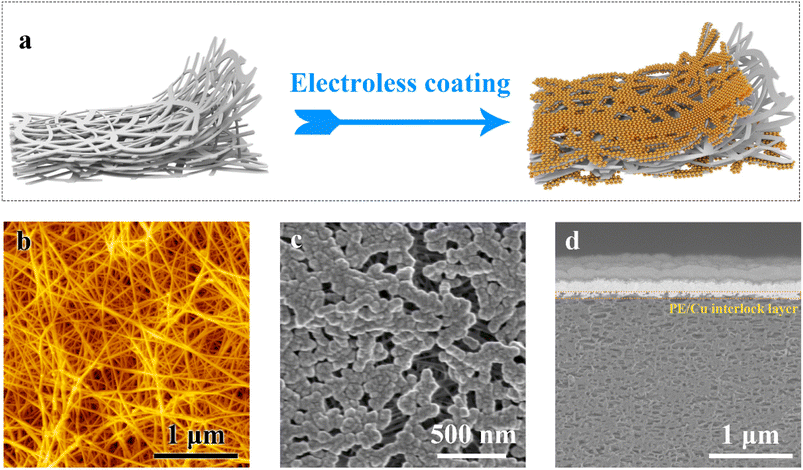
Fig. 1a depicts a schematic diagram illustrating the conformal growth of copper nanoparticles on the nanofibrils constituting the UHMWPE substrate during electroless deposition. The conformal growth of the nanoparticles on the UHMWPE surface results in a strong interfacial locking with the PE substrate. The strong interfacial interlocking allows the hybrid film to derive its stretchability and high mechanical strength from the stretchable and ultra strong UHMWPE substrate. Additionally, the interpenetrating network of the copper nanoparticles effectively enables the hybrid copper film to exhibit stretchable electrical conductivity.Fig. 1b–d demonstrate the conformal growth of the copper nanoparticle on the PE substrate. Here, the pristine UHMWPE displays an interpenetrating nanofibrous topology, with nanofibrils of diameters of the order of 10–20 nm in Fig. 1b. The initial growth of the copper nanoparticles closely maps the surface of the PE nanofibrils, resulting in the formation of a fibrous network structure, as evidenced by the scanning electron micrograph (SEM) in Fig. 1c. The interlocking of the copper nanoparticles with the PE substrate is revealed by the cross-sectional SEM in Fig. 1d. Here the copper layer thickness was about 150 nm on each side of the membrane surface based on the characterization of copper weight. It is evident that there exists an interlayer between the dense copper and the porous PE substrate, leading to an excellent combination of the metal and polymer. A close examination of the interface shows a core–shell microstructure, where the deposited copper nanoparticles wrap around the PE nanofibrils. We also observed similar interlocking layers in thinner membranes with thicknesses about 200 nm (solid content) of PE (Fig. S1, ESI†). Therefore, light CuPE with mass area density as low as 0.3 mg cm−2 is obtained, which is about 26 times lighter than 9 μm thick commercial copper current collectors. To our knowledge, this represents the lightest copper current collector reported to date.
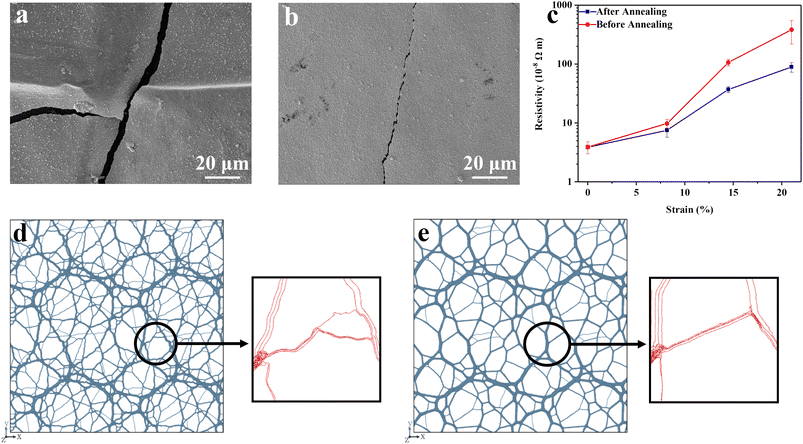
The stretchability of the hybrid copper film is demonstrated in Fig. S2–S4.† Fig. S2† shows the photograph of the hybrid film that was stretched biaxially at an aerial extensional strain of 21%. Upon relaxation to the undeformed state, the film becomes highly wrinkled as depicted in Fig. S3.† Microscopically, the copper layer exhibits wide cracks and local debonding from the PE substrate, see the SEM in Fig. 2a; the widths of these cracks are about ∼8 μm. These wrinkles, however, can slowly become smooth with time, and the shape recovery can be accelerated when the stretched film is subjected to constrained thermal annealing at 100 °C (Fig. S4†). Fig. 2b shows the photographs of the film after annealing. These photos demonstrate that no visible wrinkles were observable after being annealed for 10 min at 100 °C. Microscopically, the wrinkled film exhibited large cracks. Annealing resulted in crack healing, so that no notable debonding can be discernible. The recovery of imprinted cracks shows a clear healing effect. The fractures and debonding are analogous to those seen in metal glass coated polymer surfaces under extension,24 but cracks healing or rebonding with the substrate have not been reported, to the best of our knowledge.
The shape recoverability is also seen by the recoverable electrical conductivity in the hybrid film. In Fig. 2c, the CuPE shows a low resistivity of 3.9 × 10−8 Ω m before deformation, which is 2.3 times that of pure copper but only 45% as conductive as pure iron. The resistivity initially increases slowly with increasing extension strains until the areal strain reached 8%, and both the annealed and unannealed samples exhibit similar resistivities within this strain range. However, at strains larger than 8%, a more rapid increase in resistivity is observed. The increase is particularly more marked in the un-annealed sample, which also shows increasingly large standard deviations; at 21% strain (10% at each dimension), the resistivity values are 3.1 ± 1.65 × 10−6 Ω m and 8.9 ± 1.7 × 10−7 Ω m, respectively, for the unannealed and the annealed sample. These results further demonstrate that the conductivity recovery is accompanied by the shape recovery, and the sample shows reversible conductivities at extension strains up to 8%.
To further elucidate that the stretchability of the hybrid copper film is because of the substrate, we simulated the stretchability characteristics of the UHMWPE membrane using large-scale molecular dynamics simulation under LAMMPs. Fig. 2d and e exhibit the structural transformation of the PE membrane upon unloading of sequential biaxial planar extension strains of 10% along the orthogonal x- and y-axes, respectively, corresponding to an aerial extensional strain of 21%. The fibrils held between triangulated entanglements in the PE membrane become highly curved during the unloading (retraction) process. But these curved fibrils rapidly straighten as soon as the lateral contraction reached 10%; the cessation of contraction was imposed on the membrane by holding the lateral dimensions constant.
To elucidate the shape recovery mechanisms, we studied the chain conformation evolutions associated with the deformation process. Fig. S5† shows that the chains held between entanglements take two different kinds of conformations during and after retraction deformations. During the retraction process, the chains adopt primarily the high-energy state of gauche conformations (Fig. S6†). But, as soon as the retraction was arrested, the chains begin to adopt the lower energy state of trans-conformations, leading to a reduction in the system energy (Fig. S7†). To illustrate this more clearly, we captured the conformation changes in an animated video, see ESI Video (S1 & S2).† Therefore, the shape recovery process is thermodynamically driven to return to the lower energy state of the system.
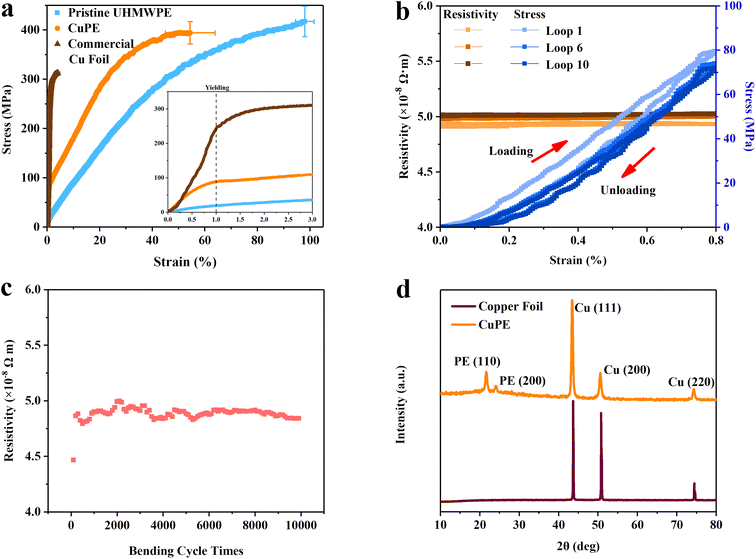
These ultralight and stretchable CuPE films are also mechanically robust. The tensile properties of the CuPE in terms of stress–strain relationships are plotted together with a commercial copper film current collector and the pristine PE membrane in Fig. 3a. The composite film is stronger than the pure pristine PE at the same strains and drastically more ductile than the ultrathin copper film; the ultimate tensile strengths of the CuPE are 394.4 ± 22.7 MPa, which are about 26% higher than those of pure copper foil (312.3 ± 2.5 MPa). The ductility of the CuPE is 15 times larger than that of the copper foil. There exists a yield in the CuPE film at about 1% strain, corresponding to that of the pure copper foil. In this elastic range of 0–1%, the strength of CuPE is nearly a linear sum of pure copper foil and the PE membrane, corresponding to a Voigt parallel coupling between the copper film and the PE substrate. The formula is shown below, where σ and d are the strength and the thickness, respectively.
To further elucidate the stretchability mechanism, we measured the electro-mechanical performance of the CuPE under looped uniaxial extension tests at a limiting strain of 0.8%. We selected this strain to avoid the yielding strain of the pure copper foil. Fig. 3b shows that at 0.8% strain, both the resistivity and the tensile stress in the CuPE show little hysteresis, and the average resistivity is 4.95 ± 0.05 × 10−8 Ω m, which is about the same as that of the undeformed film.
To evaluate CuPE as a current collector in lithium-ion batteries, we conducted AC impedance testing and compared it with commercial copper and aluminum collectors. Film samples were assembled under a clamping pressure of 50 MPa between stainless steel plates. The results, depicted in Fig. S8,† revealed that CuPE exhibits frequency-dependent impedance characteristics similar to the commercial collectors, with zero out-of-phase impedance values in the tested frequency range (10–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz). The in-phase impedance of CuPE (100 mΩ) falls between those of pure copper (60 mΩ) and pure aluminum (160 mΩ), suggesting that using CuPE as the anode current collector will not cause adverse battery overheating. These findings indicate that CuPE holds promise as a suitable alternative for current collectors in lithium-ion batteries, offering comparable impedance behavior to commercial counterparts and mitigating potential overheating concerns.
000 Hz). The in-phase impedance of CuPE (100 mΩ) falls between those of pure copper (60 mΩ) and pure aluminum (160 mΩ), suggesting that using CuPE as the anode current collector will not cause adverse battery overheating. These findings indicate that CuPE holds promise as a suitable alternative for current collectors in lithium-ion batteries, offering comparable impedance behavior to commercial counterparts and mitigating potential overheating concerns.
The bending performance of the film is demonstrated in Fig. 3c. This shows that the film shows a constant resistivity of 5 × 10−8 Ω m, for bending cycles up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 at a 1 mm bending radius. It is to be noted that the resistivity value of the CuPE is less than 0.17% graphite.25 This guarantees its effectiveness in conducting current from the graphite electrode in a lithium-ion battery if used as a current collector. Its high flexibility also makes it an ideal electromagnetic shielding material.
000 at a 1 mm bending radius. It is to be noted that the resistivity value of the CuPE is less than 0.17% graphite.25 This guarantees its effectiveness in conducting current from the graphite electrode in a lithium-ion battery if used as a current collector. Its high flexibility also makes it an ideal electromagnetic shielding material.
The high conductivity of the surface coated CuPE results from the high purity of the electroless deposited copper, which is revealed by X-ray diffraction (XRD) analysis. The peaks depicted in Fig. 3d show the reflections of Cu (111) (2θ = 43.31°), Cu (200) (2θ = 50.52°), and Cu (220) (2θ = 74.15°). And no reflection of oxides of copper can be found.
With the combined merits of ultralow mass area density (0.3 mg cm−2), low electrical resistivity (4 × 10−8 Ω m), high stretchability (8%), and high mechanical strengths (390 MPa), our newly prepared CuPE holds premise for use as an anode current collector for high energy density flexible lithium-ion batteries. Next, we will demonstrate that the CuPE is highly compatible with the graphite electrode and can impart its stretchability to the graphite electrode. We then applied the CuPE as a current collector in both a half and a full cell for a high mass and volumetric energy density lithium-ion battery.
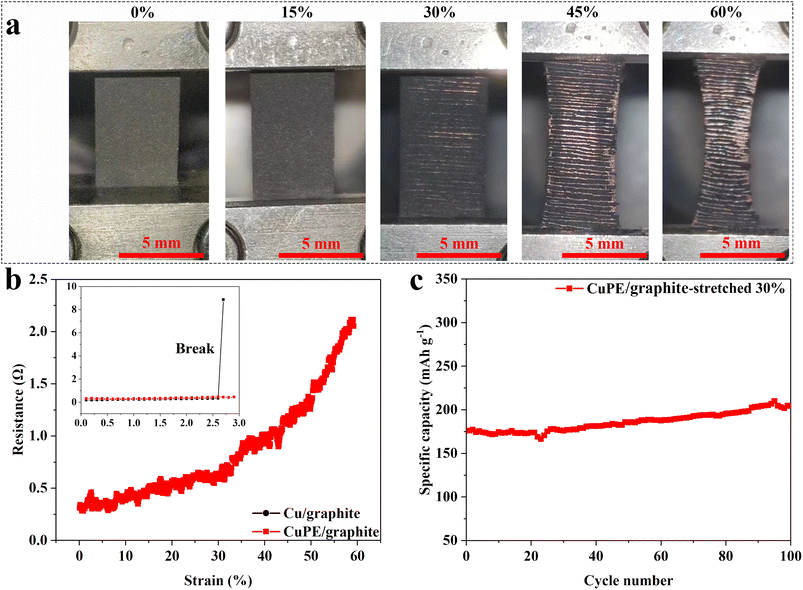
The enhanced adhesion of CuPE to the graphite anode electrode was demonstrated by its electro-mechanical performances at different extensional deformations. Graphite anodes at a coating thickness corresponding to 2 mA h cm−2 on CuPE and commercial copper foil were prepared. As shown in the photographic images depicted in Fig. 4a, no visual changes in graphite can be discerned at 15% on CuPE, and the values measured on the electrodes prepared from both the commercial copper and the CuPE are nearly identical before the former electrode failed (Fig. 4b and inset). On the other hand, the overall resistance increased rapidly after the total stretching strain was above 30%, and the overall resistance reached 2.2 Ω at a strain of 60%. In contrast, the electrodes with commercial copper foil broke at a strain of 2.7%, resulting in an open circuit state.
Qualitatively, we conducted tests to evaluate the shape recovery and adhesion performance of CuPE. This involved subjecting the graphite-coated anode on CuPE to rigorous mechanical folding and twisting distortions, as shown in ESI Video S3.† To provide a basis for comparison, we also prepared an identical electrode with graphite on commercial copper and subjected it to similar mechanical manipulation. Selected photographs depicting the state of the electrodes were captured and clipped, as illustrated in Fig. 5a and b.
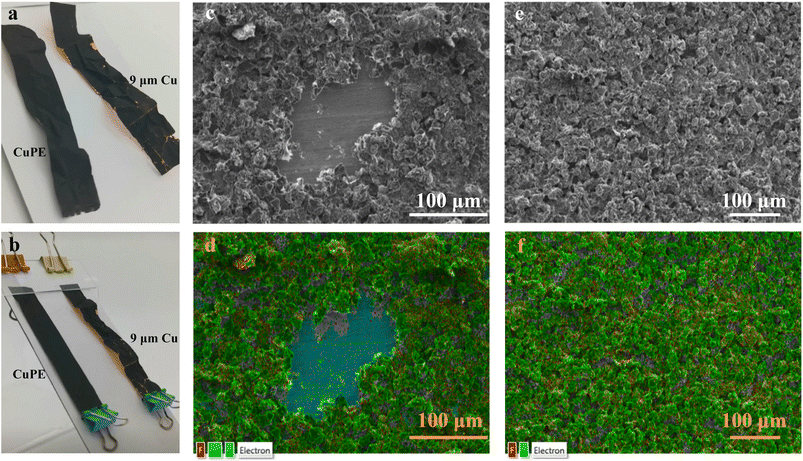
The electrode with graphite on commercial copper foil exhibited irreversible wrinkles, and the shiny reflections observed around the folded lines suggested that some graphite flakes had detached from the copper current collector. In contrast, the graphite on the CuPE current collector displayed no visible differences in surface reflection. Moreover, the graphite/CuPE electrode demonstrated remarkable shape recovery characteristics, akin to the hybrid CuPE. The rate of shape recovery was further enhanced by thermally annealing the electrode current collector assembly at 80 °C, as depicted in the photos in Fig. 5b.
Fig. 5c–f present SEM micrographs illustrating the graphite on copper foil and CuPE after undergoing mechanical distortions and subsequent shape recovery. In Fig. 5c, the graphite particles on the copper foil display severe distortions, with areas of bare copper exposed due to detachment of the electrode material. This detachment is confirmed by the energy-dispersive X-ray spectroscopy (EDS) analysis shown in Fig. 5d. In contrast, the particles on the CuPE surfaces (Fig. 5e) appear homogeneous and primarily consist of carbon (from the conductive additive graphite) and fluorine (from the binder polyvinylidene difluoride (PVDF)). This elemental composition is further supported by the elemental mapping obtained through EDS, as depicted in Fig. 5f.
To demonstrate the applicability of graphite on CuPE electrodes, even after undergoing severe distortions, we conducted performance tests in a lithium half-cell, comparing them to graphite/Cu electrodes. The results, presented in Fig. S9 & S10,† showcase the performance comparisons at various charge/discharge rates. Interestingly, cells assembled using electrodes prepared on pure copper foil exhibited only half the capacity compared to those prepared with CuPE. The flexible nature of the copper film allows it to better adapt to the volume changes in graphite by deforming itself, in contrast to Cu foil. As a result, it can recover more capacity, even surpassing the initial charge capacity at low current densities.
The data for half-cells assembled with graphite/CuPE electrodes after applying a 30% extension strain are depicted in Fig. 4c. Although these cells exhibited a lower capacity of 177–200 mA h g−1 at 1C, which is 26 to 35% lower than that of the undistorted electrodes (see Fig. 6a), the increasing capacity trend with cycle numbers suggests continuous activation of the cells over time. This indicates that the distortions in the graphite, even after undergoing a large extensional stretching strain of 30%, are partially reversible.
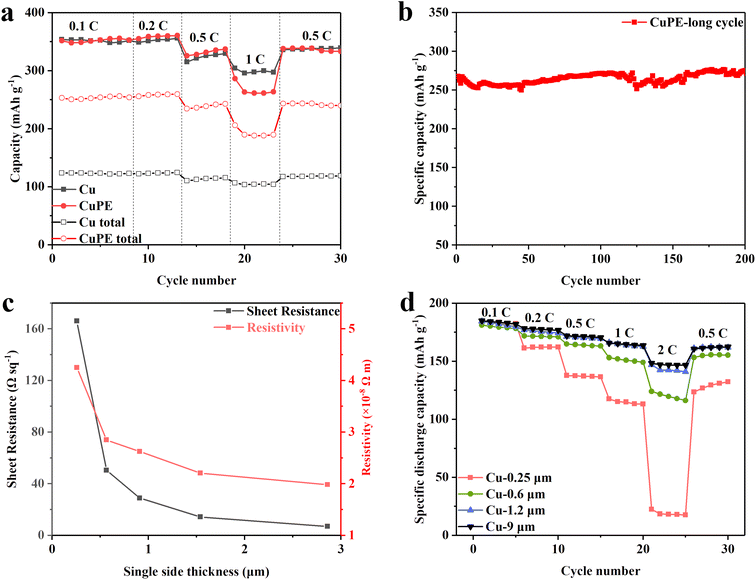
Half-cells comprising CuPE/graphite and Cu/graphite, loaded at a density of 2 mA h cm−2, were tested at room temperature (∼25 °C). The measured discharge capacity densities with respect to both the mass of the active material and the combined mass of the active material and current collector (CuPE or Cu) are plotted in Fig. 6a. The corresponding charge/discharge curves for the initial three cycles are presented in Fig. S11.† All half-cells underwent activation over three discharge/charge cycles using the sequence of 0.1C, 0.2C, 0.5C, 1C, and 0.5C rates. No significant differences in capacity or coulombic efficiencies (85% in the first cycle) were observed. These coulombic efficiencies are comparable to or higher than those reported in the literature for other lightweight current collectors, including those prepared using delicate nanostructures.14
The discharge capacities align well with previous findings in the literature. For example, at 0.1C, the discharge capacity is 355 mA h g−1, while at 0.5C, it is 325 mA h g−1. Considering that the application of a cell depends on its total mass and volume, employing lighter and thinner current collectors can lead to higher overall energy densities. In Fig. 6a, we estimated the discharge capacity density relative to the mass of the anode electrode and the total mass of the anode electrode plus the current collector. By using CuPE as the anode current collector, the total discharge capacity density increases by 80–108% compared to using 9 μm-thick Cu foil. When considering the total volume of the anode, the CuPE current collector yields a 10% higher energy density than the copper foil electrode. Replacing the anode current collector with CuPE results in substantial increases in overall mass and volume energy densities.
The stability of CuPE in half-cells at high current density was also tested, as shown in Fig. 6b. It maintains a consistent capacity of around 270 mA h g−1 over 200 cycles at 1C. However, it is necessary to demonstrate the stability of CuPE in a real full cell containing a high-voltage cathode, such as NCM materials, as the products of graphite-lithium half cells are limited to the 0.05–1.5 V range.
While the hybrid CuPE has demonstrated good performances in the half-cell tests, it is important to consider its performances in a full cell, particularly at high current densities to assess its potential for use in practical applications. Previously, an engineering commentary has suggested that the metal layer should thicker than 500 nm at large current, otherwise the heat generated during application might cause battery energy density degradation during use.26 To avoid such potential issues, we prepared thicker CuPE current collectors by increasing the copper layer thickness on the ultrathin CuPE prepared by electroless deposition (ELD) using a low-cost and fast electrodeposition method. Fig. 6c shows that both sheet resistance (R) and resistivity (ρ) decrease rapidly with increasing copper thickness, reaching the value of pure copper at 1.2 μm. Subsequently, these CuPE films at different thicknesses together with commercial copper foil were loaded with a graphite electrode and assembled into pouch bag full cells.
Full-cells of a NCM811 cathode at a loading density of 2.7 mA h cm−2, counter to CuPE/graphite and Cu/graphite at a loading density of 3 mA h cm−2, were tested at room temperature (∼25 °C). All full-cells were activated over three discharge/charge cycles following the rate sequence of 0.1C, 0.2C, 0.5C, 1C, 2C, 3C and 0.5C.14,15 We plotted the measured discharge capacity densities as a function of discharge rates and current collector thicknesses of CuPE together with the commercial copper foil in Fig. 6d. The associated galvanic charge/discharge curves are presented in Fig. S12–16.† Evidently, the copper layer thicknesses have a significant impact on the rate performances of these pouch cells, and a minimum critical copper layer thickness is required for the hybrid copper films to exhibit similar performances to the commercial copper foil at a desired discharge rate. For example, the CuPE films coated with 0.25 μm-thick copper on either side of the PE film showed increasingly poorer performances at increasing rates. It only showed comparable performances to pure copper at 0.1C, and becomes totally different at 2C. To satisfy the need of 2C, it is necessary to coat the PE with 1.2 μm of copper. Further increase in copper loading might be required at even higher discharge rates for applications in higher rates systems. Therefore, our results here demonstrate that the copper layer thickness should match with the discharge current required for a given situation. While such a constraint seems to limit the merit of the hybrid copper current collector, its combined merit of shape memory properties and flexibility cannot be matched by the commercial pure copper current collectors.
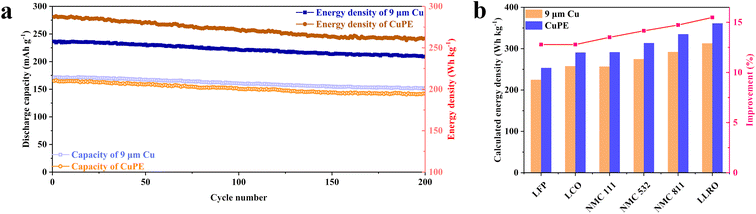
To demonstrate the merit of these newly prepared CuPE current collectors, we have chosen to test the long cycle performance of a 0.6 μm copper coated CuPE at 0.5C (1C = 180 mA h g−1, with a loading of 15 mg cm−2). The use of the CuPE in full cells is tested in cells prepared using CuPE/graphite or Cu/graphite (for reference) as the anode and NMC 811 on commercial Al foil as the cathode. We conducted room temperature measurement on these full cells over 200 charge/discharge cycles at a constant rate of 0.5C. For the sake of comparison with literature reports, we estimated the discharge capacity density using the mass of the electrode material only, and plotted the estimated discharge capacity density using opened symbols in Fig. 7a. To signify the advantages gained using the ultralight CuPE current collector, we computed the energy density using the method in ref. 5 and plotted the energy density in filled symbols. As expected from the half-cell tests, the discharge capacity of the full cells is almost indistinguishable at 0.5C when the capacity density was estimated without considering the mass contribution from inactive materials such as the current collectors and the separators. But the cell's discharge energy density depends strongly on the mass of the current collector. By simply replacing the anode current collector using the ultralight CuPE, we estimated an energy density increase by 8–11% in the first 200 discharge cycles; the energy density of the full cells assembled using CuPE is about 287 W h kg−1 in the first cycle at 0.5C, which is 11% higher than that using 9 μm commercial copper (220 W h kg−1), and the enhancement decreases at high cycle numbers, implying possible side reaction effects with the ultrathin current collector because of its high surface area.27 Coulombic efficiency (CE) of the whole battery is also plotted (Fig. S17 and S18†). The CE of CuPE is slightly lower than that of commercial copper, attributable to possibly more side reactions of nanostructures of the copper surface. Fortunately, after the 2nd cycle at 0.5C, the difference between commercial copper and CuPE becomes negligible, which demonstrates that CuPE has stable electrochemical properties after the formation progress. The galvanic charge and discharge curves of the 2nd, 52nd, 102nd and 152nd cycles at 0.5C (Fig. S19 and S20†) further demonstrate the similarity of commercial copper and CuPE.
To further illustrate the specific energy density enhancement with CuPE, we have computed the energy capacity increase by paring with different cathode materials. All of these cells were based on a capacity loading of 3 mA h cm−2 at specific parameters listed in Table S1.† The results presented in Fig. 7b show the calculated energy density and energy density enhancement for cells based on LCO, LFP, NMC111, NMC532, NMC811 and layered lithium-rich oxide (LLRO). Substitution with CuPE increases the energy density of all the batteries by more than 15%. Moreover, considering that the total thickness of our CuPE is only 3 μm in thickness, an increase in volumetric energy density of 3% is also expected.
4. Conclusions
We have successfully developed a CuPE film that possesses remarkable characteristics, including ultra-high strength (390 MPa), ultra-light weight (as low as 0.3 mg cm−2), flexibility, shape recoverability, and high conductivity. To the best of our knowledge, the hybrid copper film presented in this study demonstrates superior load-bearing performance compared to all other lightweight designs documented in the literature. Additionally, the electroless deposition method combined with electrode deposition employed in our research offers an efficient, scalable, and cost-effective approach for large-scale production, making the CuPE film highly suitable for industrial applications.The final CuPE film weighs only 10% and occupies 33% of the volume of a standard 9 μm commercial copper foil, while maintaining a consistent strength of 390 MPa. In terms of energy density relative to the total mass, the CuPE film outperforms commercial alternatives by more than 12.5%. When tested in NMC811//graphite batteries at a charge/discharge rate of 0.5C, the CuPE film exhibited stable cycling performance over 200 cycles, surpassing the performance of commercial current collectors. This design holds significant potential for enhancing the capacity of various energy storage systems, including lithium secondary batteries and supercapacitors. Furthermore, its unique combination of stretchability, shape memory properties, and outstanding performance makes it well-suited for applications in electromagnetic shielding.
In summary, our innovative CuPE film represents a major advancement in lightweight, high-strength materials for a range of practical applications. Its exceptional properties and compatibility with scalable manufacturing techniques position it as a promising solution for enhancing energy storage systems and electromagnetic shielding components.
Author contributions
Xin MA: methodology, validation, investigation, writing – original draft, and visualization. Donghao Xie: methodology, validation, investigation, and visualization. Jiayi Wang: methodology, formal analysis, and visualization. Zekun Wang: methodology and validation. Qiao Gu: methodology. Yonghong Deng: methodology and supervision. Ping Gao: conceptualization, methodology, writing – review & editing, supervision, project administration, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial support from the Guangzhou Municipal Government. We wish also to acknowledge the technical support from the Materials Characterization and Preparation Facility of HKUST (GZ) and HKUST (CWB). The financial support from the Research Institute of Tsinghua, Pearl River Delta with grant number RITPRD23EG01 is also appreciated.References
- T. R. Ray, J. Choi, A. J. Bandodkar, S. Krishnan, P. Gutruf, L. Tian, R. Ghaffari and J. A. Rogers, Chem. Rev., 2019, 119, 5461–5533 CrossRef CAS PubMed.
- M. U. A. Khan, R. Raad, F. Tubbal, P. I. Theoharis, S. Liu and J. Foroughi, Polymers, 2021, 13, 1–34 Search PubMed.
- Y. Khan, A. Thielens, S. Muin, J. Ting, C. Baumbauer and A. C. Arias, Adv. Mater., 2020, 32, 1–29 CrossRef PubMed.
- M. Park, J. Im, M. Shin, Y. Min, J. Park, H. Cho, S. Park, M. B. Shim, S. Jeon, D. Y. Chung, J. Bae, J. Park, U. Jeong and K. Kim, Nat. Nanotechnol., 2012, 7, 803–809 CrossRef CAS PubMed.
- Y. Zhu, Y. Deng, P. Yi, L. Peng, X. Lai and Z. Lin, Adv. Mater. Technol., 2019, 4, 1–22 Search PubMed.
- Q. Huang and Y. Zhu, Adv. Mater. Technol., 2019, 4, 1–41 Search PubMed.
- G. Chen, F. Zhang, Z. Zhou, J. Li and Y. Tang, Adv. Energy Mater., 2018, 8, 1801219 CrossRef.
- A. Chen, X. Guo, S. Yang, G. Liang, Q. Li, Z. Chen, Z. Huang, Q. Yang, C. Han and C. Zhi, Energy Environ. Sci., 2021, 14, 3599–3608 RSC.
- Y. Ye, L. Y. Chou, Y. Liu, H. Wang, H. K. Lee, W. Huang, J. Wan, K. Liu, G. Zhou, Y. Yang, A. Yang, X. Xiao, X. Gao, D. T. Boyle, H. Chen, W. Zhang, S. C. Kim and Y. Cui, Nat. Energy, 2020, 5, 786–793 CrossRef CAS.
- J. Chang, J. Shang, Y. Sun, L. K. Ono, D. Wang, Z. Ma, Q. Huang, D. Chen, G. Liu, Y. Cui, Y. Qi and Z. Zheng, Nat. Commun., 2018, 9, 1–11 CrossRef PubMed.
- V. Jabbari, V. Yurkiv, M. G. Rasul, M. Cheng, P. Griffin, F. Mashayek and R. Shahbazian-Yassar, Small, 2022, 18, 1–11 CrossRef PubMed.
- Z. Liu, Y. Dong, X. Qi, R. Wang, Z. Zhu, C. Yan, X. Jiao, S. Li, L. Qie, J. Li and Y. Huang, Energy Environ. Sci., 2022, 15, 5313–5323 RSC.
- S. Jin, Y. Jiang, H. Ji and Y. Yu, Adv. Mater., 2018, 30, 1802014 CrossRef PubMed.
- H. C. Chu and H. Y. Tuan, J. Power Sources, 2017, 346, 40–48 CrossRef CAS.
- L. L. Lu, J. Ge, J. N. Yang, S. M. Chen, H. Bin Yao, F. Zhou and S. H. Yu, Nano Lett., 2016, 16, 4431–4437 CrossRef CAS PubMed.
- Z. Wang, A. Malti, L. Ouyang, D. Tu, W. Tian, L. Wågberg and M. M. Hamedi, Small, 2018, 14, 1–8 Search PubMed.
- K. Wang, S. Luo, Y. Wu, X. He, F. Zhao, J. Wang, K. Jiang and S. Fan, Adv. Funct. Mater., 2013, 23, 846–853 CrossRef CAS.
- K. Wang, Y. Wu, H. Wu, Y. Luo, D. Wang, K. Jiang, Q. Li, Y. Li, S. Fan and J. Wang, J. Power Sources, 2017, 351, 160–168 CrossRef CAS.
- R. Li and P. Gao, Glob. Chall., 2017, 1, 1700020 CrossRef PubMed.
- J. Li, R. Li, Q. Gu, Q. Zhang, T. X. Yu and P. Gao, arXiv, 2019, preprint, arXiv:1901.07952, DOI:10.48550/arXiv.1901.07952.
- M. Wu, J. Y. Kim, O. B. Chae, W. Bin Jung, S. Choi, D. Y. Kim, J. Suk, I. Gereige, Y. Kang and H. T. Jung, ACS Appl. Mater. Interfaces, 2021, 13, 2576–2583 CrossRef CAS.
- X. Wang, W. Zeng, L. Hong, W. Xu, H. Yang, F. Wang, H. Duan, M. Tang and H. Jiang, Nat. Energy, 2018, 3, 227–235 CrossRef CAS.
- J. Feng, J. Wang, Q. Gu, W. Thitisomboon, D. Yao, Y. Deng and P. Gao, J. Mater. Chem. A, 2022, 10, 13969–13977 RSC.
- O. Glushko, C. Gammer, L. M. Weniger, H. Sheng, C. Mitterer and J. Eckert, Mater. Today Commun., 2021, 28, 102547 CrossRef CAS.
- Y. Chen, K. Fu, S. Zhu, W. Luo, Y. Wang, Y. Li, E. Hitz, Y. Yao, J. Dai, J. Wan, V. A. Danner, T. Li and L. Hu, Nano Lett., 2016, 16, 3616–3623 CrossRef CAS.
- R. Choudhury, J. Wild and Y. Yang, Joule, 2021, 5, 1301–1305 CrossRef.
- J. Shu, M. Shui, F. Huang, D. Xu, Y. Ren, L. Hou, J. Cui and J. Xu, Electrochim. Acta, 2011, 56, 3006–3014 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ta04958b |
| This journal is © The Royal Society of Chemistry 2024 |