Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Liquid polybutadiene reinforced inverse vulcanised polymers†
Veronica
Hanna
*a,
Michael
Graysmark
b,
Helen
Willcock
 b and
Tom
Hasell
b and
Tom
Hasell
 *a
*a
aDepartment of Chemistry, University of Liverpool, Liverpool, L69 7ZD, UK. E-mail: t.hasell@liverpool.ac.uk; sgvhanna@liverpool.ac.uk
bDepartment of Materials, Loughborough University, Loughborough, LE11 3TU, UK
First published on 12th December 2023
Abstract
Inverse vulcanisation uses waste sulfur to produce high sulfur content polymers (>50 wt%) with vitrimer characteristics. The ability to be recycled due to the dynamic S–S bonds make sulfur polymers materials of interest, however, their mechanical properties require further improvement. Improving the impact resistance of these materials is of interest because several sulfur polymers have been reported to be highly brittle, limiting their applications in construction. Synthesis of high sulfur content polymers (50 wt% S) containing liquid polybutadiene (LPBD) at loadings of 10–30 wt% was found to increase impact resistance from 3.39 MPa (0 wt% LPBD) to 30 MPa (30 wt% LPBD) while allowing the polymer to remain recyclable at least 3 times.
Introduction
Rubbers are ubiquitous to widespread applications throughout modern society – from tyres to shoe soles, owing to the process of vulcanisation pioneered by Charles Goodyear in 1839.1–3 Vulcanisation of rubber uses sulfur and heat to crosslink the polymer chains, hardening the rubber to produce a thermosetting polymer. As vulcanised rubber cannot be remoulded through melt processing,4 recycling requires the rubber to be ground before the addition of sulfur, cement, or a binder such as latex, liquid polybutadienes, or moisture curable urethanes.5 As a result, accumulation of waste rubber in landfills has increased drastically in recent years.3 On the other hand, inverse vulcanisation generally produces vitrimers, as a result of the high sulfur content instilling the benefits of the reversible S–S bonds (Scheme 1).6,7 Vitrimers are covalently crosslinked networks similar to thermosets, and retain similar structures and properties to them under lower temperatures, but are defined by reversible bond-exchange reactions at higher temperatures that allow them to flow like viscoelastic liquids, permitting re-processability.8 The cleavage and reformation of S–S bonds within a crosslinked network therefore allows for recycling of the neat polymer through melt processing, increasing the ease of recycling.9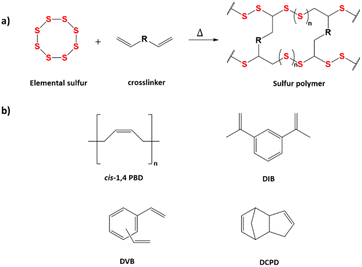 | ||
| Scheme 1 (a) General scheme for inverse vulcanisation reaction showing crosslink formation between polysulfide chains; (b) crosslinker monomers used in this study. | ||
As inverse vulcanised polymers, also known as “sulfur polymers”, contain a high sulfur loading (>50 wt%), they can potentially help reduce the large sulfur surplus that results from the production of >70 million tonnes of sulfur annually as a by-product of oil refinement.10,11 Currently, only a small fraction of sulfur is consumed for uses such as the production of sulfuric acid,12 vulcanisation of rubber,3 and fertilisers,13 which results in a significant quantity to remain as excess. Like various conventional polymers, many neat inverse vulcanised polymers benefit from additives for mechanical performance enhancement, as seen in previous work on the incorporation of fillers.14,15 Sulfur polymers with higher crosslinking densities have greater shape retention and are generally stronger, but often brittle making them susceptible to fracture, requiring the addition of plasticising monomers such as vegetable oils.16 For example, a polymer of 50 wt% sulfur and dicyclopentadiene (S-DCPD) has been reported by Smith et al. to produce brittle glassy polymers which required the addition of canola oil for an increase in strain which made tensile testing of the specimens possible.16,17 As this is the case with other crosslinkers such as divinylbenzene (DVB) and 1,3-diisopropenylbenzene (DIB), both capable of stabilising high sulfur loadings, increasing the toughness of such polymers would be advantageous for potential applications in construction, such as composites or concrete.18–20
One well established way to increase the toughness of a glassy polymer by the addition of rubbery particles to a glassy matrix, is the use of polybutadiene (PBD) in polystyrene (PS), known as high impact polystyrene (HIPS), to afford a heterogenous material with a glassy PS matrix and rubbery PBD particles.21 The rubber phase of HIPS consists of a lower crosslinking density capable of increased energy absorption vital to increasing impact strength.22 As a result of the rubbery phase, HIPS enhances craze initiation allowing for deformation, resulting in the absorption of impact energy before the formation of large cracks can occur.23 Another type of HIPS incorporates core–shell rubber into the PS matrix.24 The core–shell rubber used, often possesses a soft centre and a harder outer-shell designed to be compatible with the host polymer matrix. Some examples include a crosslinked polymethylmethacrylate (PMMA) or PS outer shell with a natural rubber core, or crosslinked PMMA outer shell with a crosslinked polybutylacrylate (PBA) core.25
Like HIPS, another example of increasing toughness of polymers is the use of liquid rubber in epoxy resins, often used due to the ease of introducing it into the polymer matrix.26 Unlike PS, cured epoxy resins are crosslinked, making them of particular interest as it can be assumed that crosslinked sulfur polymers may have similar fracture mechanisms to epoxy, hence a similar toughening mechanism would likely bring about a greater increase in toughness. The direct addition of liquid polybutadiene (LPBD) into the reaction mixture would also allow for the continuation of solvent-less synthesis of high sulfur content polymers. Functionalised liquid rubbers such as carboxyl terminated liquid nitrile-butadiene rubber (CTBN), hydroxyl-terminated liquid nitrile-butadiene rubber (HTBN), and amine-terminated liquid nitrile-butadiene rubber (ATBN) are often used in epoxy resins.26–28 In this study, only LPBD will be focussed on as there are different microstructures (cis, trans, vinyl) of PBD and different weight average molecular weights (Mws) to test if they have a significant effect on the mechanical properties. The brittle sulfur polymers previously mentioned will be used to investigate the use of LPBD to increase the toughness and so increase impact strength while still preserving their recyclability. The use of LPBD, and data on the impact strength of sulfur polymers has not been previously reported, making this study the first.
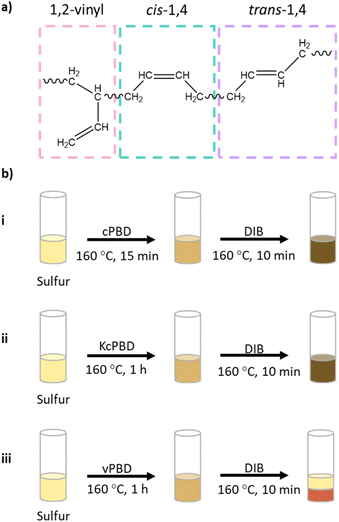
Results and discussion
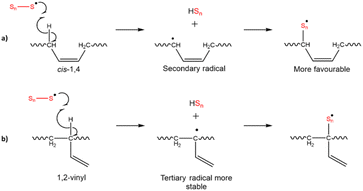
A brittle sulfur polymer, S-DIB (that has a flexural displacement at break of 0.46 mm), was chosen for the addition of LPBD at loadings 10, 20, and 30 wt% to observe the changes in mechanical properties. S-DIB usually produces polymers with a Tg above room temperature, with 50S-DIB attaining a glass transition temperature (Tg) of 33.5 °C in this study. The brittle nature and relatively low strength of S-DIB (flexural strength of 3.93 MPa) meant that it was an ideal matrix for demonstrating the toughening of sulfur polymers with LPBD (Table S3†). Three different LPBD samples (cPBD, KcPBD, vPBD) were tested as the different microstructure contents (cis, trans, vinyl) and Mw were predicted to influence the reaction (Fig. 1). Both cPBD and KcPBD are high cis-1,4 content LPBD samples of different Mw, and vPBD is a high 1,2-vinyl content LPBD. Use of KcPBD, but a grade of higher Mw (grade LBR-305), has been reported by Lim et al. to toughen poly(lactic acid) (PLA) by increasing its strain from 2.2% to approximately 38% while maintaining its tensile strength (46.6 MPa) close to the tensile strength of PLA (50.3 MPa).29 As KcPBD displayed the ability to increase toughness, a grade of lower Mw (grade LBR-302) was chosen as another cis-1,4 LPBD to cPBD. vPBD was then chosen as it had the lowest viscosity amongst the high 1,2-vinyl LPBD samples available.At first, the reaction progression of vPBD with sulfur seemed similar to cPBD experiments, until reaction with DIB resulting in significant separation of the reaction mixture into a red solid, likely S-DIB, and yellow liquid (Fig. S1†). This behaviour was displayed for loadings above 10 wt% PBD. To test if a higher percentage of PBD could be added, zinc dimethylcarbamate was tested as a catalyst, successfully allowing for loadings above 10 wt% without separation. However, reaction of DIB accelerated significantly in the presence of the catalyst, resulting in rapid vitrification. The resultant polymer was heterogenous in appearance with yellow-brown and black areas suggested unreacted sulfur had precipitated out (Fig. S2†). As the reaction behaviour observed both with and without a catalyst were difficult to control, reactions with vPBD were discontinued. Lower reactivity of the 1,2-vinyl group could be due to hydrogen abstraction alpha to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond resulting in a more stable radical on a tertiary carbon compared to a radical on a secondary carbon in cPBD (Scheme 2).30 cPBD has two carbons alpha to the C
C double bond resulting in a more stable radical on a tertiary carbon compared to a radical on a secondary carbon in cPBD (Scheme 2).30 cPBD has two carbons alpha to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond (whereas vPBD only has one), providing twice the number of sites for reaction with monomers compared to vPBD. This could be why irregular reaction behaviour is observed after the addition of DIB, as the reaction sites of vPBD could have become saturated by sulfur, preventing further reaction with DIB.31 Also, as the vinyl group is a pendant group, it is likely the cause for a higher viscosity compared to cPBD of a higher Mw, this increase in viscosity could also be a factor affecting miscibility.
C double bond (whereas vPBD only has one), providing twice the number of sites for reaction with monomers compared to vPBD. This could be why irregular reaction behaviour is observed after the addition of DIB, as the reaction sites of vPBD could have become saturated by sulfur, preventing further reaction with DIB.31 Also, as the vinyl group is a pendant group, it is likely the cause for a higher viscosity compared to cPBD of a higher Mw, this increase in viscosity could also be a factor affecting miscibility.
Other than the microstructure of the LPBD, the stage at which the LPBD was added to the reaction made a significant difference on the physical properties of the resultant polymer. Sufficient reaction of elemental sulfur with LPBD before addition of DIB was vital to achieving a polymer with physical properties different to S-DIB. Hence, LPBD was left to react with elemental sulfur until an increase in mixture viscosity was achieved before the addition of DIB. Reaction of elemental sulfur with DIB before the addition of LPBD afforded an orange opaque prepolymer with a similar brittleness to S-DIB after curing (Fig. S3†). However, a dark brown opaque prepolymer cured to form a polymer with increased toughness was synthesised when LPBD reacted before DIB (Fig. S3†). The reason for this difference in colour is unknown, however it shows that there could be a difference in the structure of the two polymers.
To confirm the importance of the addition of a crosslinker such as DIB in most of these S-PBD polymer systems, synthesis of S-PBD was carried out for all three LPBD samples. Synthesis of 50S-cPBD, produced a phase separated polymer with a rubbery centre and brittle phase on the exterior. This indicates that addition of a vinyl crosslinker such as DIB provides a more homogenous sample of greater stiffness. 50S-vPBD was also mostly rubbery and quite inhomogeneous in appearance. However, KcPBD (Mw = 5690 g mol−1) was observed to react effectively with exclusively sulfur, surprisingly affording a stiff thermoset polymer whilst cPBD has a higher Mw of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 413 g mol−1 on the other hand. Mechanical tests were not carried out on the S-KcPBD thermoset polymer as they could not be remoulded. This meant that the addition of DIB to S-KcPBD allowed for the synthesis of a recyclable polymer if the sulfur loading remained at 50 wt%.
413 g mol−1 on the other hand. Mechanical tests were not carried out on the S-KcPBD thermoset polymer as they could not be remoulded. This meant that the addition of DIB to S-KcPBD allowed for the synthesis of a recyclable polymer if the sulfur loading remained at 50 wt%.
The wt% of sulfur heavily determined the recyclability of the polymers, therefore different wt% of sulfur were initially tested. The sulfur loading had to be adjusted to provide recyclable polymers, while maintaining a high loading of PBD for higher strength. The lowest sulfur loading tested was 35 wt%, and resulted in a thermoset polymer, hence a sulfur loading of 50 wt% was important for recyclability. An increase in sulfur loading to 60 wt% increased the ease in processability, however the reaction was difficult to control as the viscosity significantly increased, and most samples had undergone auto-acceleration whilst curing in the oven. This meant that 50 wt% sulfur was ideal for the reaction to progress smoothly, produce recyclable samples, and allow for a high enough LPBD loading to cause a significant change in mechanical performance.
The polymers synthesised for mechanical testing, were characterised by FTIR, DSC, TGA, and elemental analysis (see ESI†). FTIR of cPBD confirms the presence of mainly cis-1,4 and trans-1,4 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds, with a very low intensity peak for the 1,2-vinyl group (Fig. 2b). In the spectrum for S-cPBD, the peak for cis-1,4 C
C double bonds, with a very low intensity peak for the 1,2-vinyl group (Fig. 2b). In the spectrum for S-cPBD, the peak for cis-1,4 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C significantly reduces in intensity indicating reaction with sulfur. There is also some decrease in intensity of the trans-1,4 C
C significantly reduces in intensity indicating reaction with sulfur. There is also some decrease in intensity of the trans-1,4 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak at 965 cm−1 in S-cPBD compared to neat cPBD demonstrating some reaction of the trans-1,4 C
C peak at 965 cm−1 in S-cPBD compared to neat cPBD demonstrating some reaction of the trans-1,4 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. An almost complete consumption of the cis-1,4 C
C bond. An almost complete consumption of the cis-1,4 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in S-cPBD indicates the cis-1,4 C
C bond in S-cPBD indicates the cis-1,4 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is favourable. KcPBD produces a high intensity peak for trans-1,4 C
C bond is favourable. KcPBD produces a high intensity peak for trans-1,4 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 964 cm−1, weaker peaks for 1,2-vinyl C
C at 964 cm−1, weaker peaks for 1,2-vinyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 993 and 909 cm−1, and a low intensity peak at 724 cm−1 for the cis-1,4 C
C at 993 and 909 cm−1, and a low intensity peak at 724 cm−1 for the cis-1,4 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. vPBD has higher intensity 1,2-vinyl C
C bond. vPBD has higher intensity 1,2-vinyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds compared to trans-1,4 and cis-1,4 C
C bonds compared to trans-1,4 and cis-1,4 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds as expected (Fig. 2c). There was not a decrease in the intensity of the peaks resulting from 1,2-vinyl C
C bonds as expected (Fig. 2c). There was not a decrease in the intensity of the peaks resulting from 1,2-vinyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the S-vPBD polymer compared to neat vPBD suggesting lower reactivity of the 1,2-vinly C
C bonds in the S-vPBD polymer compared to neat vPBD suggesting lower reactivity of the 1,2-vinly C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond compared to cis-1,4 C
C bond compared to cis-1,4 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (Fig. 2d).
C (Fig. 2d).
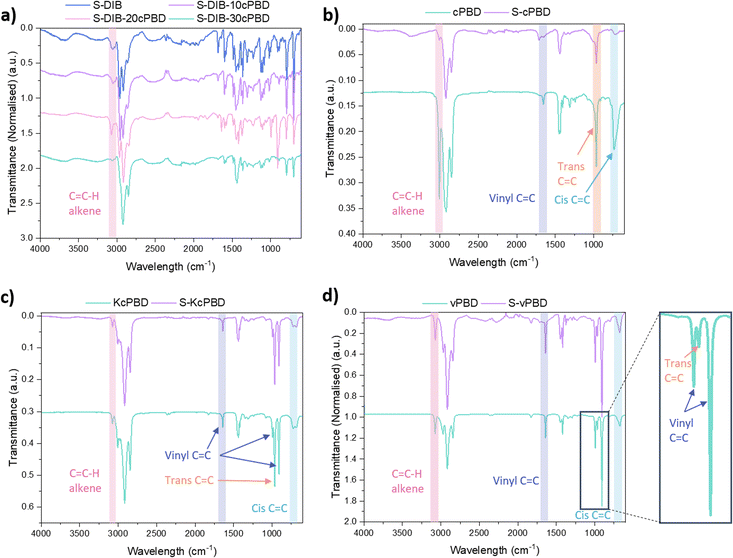 | ||
| Fig. 2 FTIR spectra of (a) S-DIB with cPBD loadings 10–30 wt%; (b) cPBD and S-cPBD; (c) KcPBD and S-KcPBD; (d) vPBD and S-vPBD. S at 50 wt% for all samples. | ||
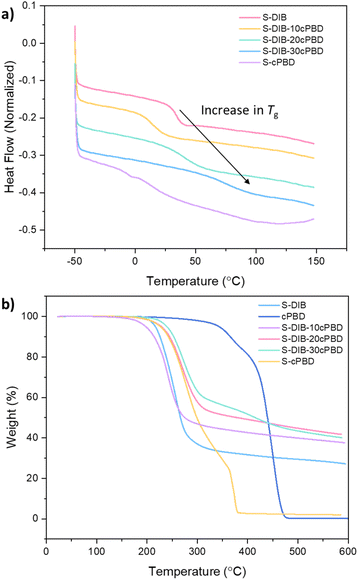
Significant changes in thermal properties of the sulfur polymers were observed upon addition of LPBD, which resulted in a general increase in Tg and LPBD loading increased. Both 20 and 30 wt% LPBD resulted in an increase in Tg, from 34 °C to 42, and 78 °C, respectively. Whilst 10 wt% loading of LPBD decreased the Tg to 16 °C resulting in some flexibility in the sample (Fig. 3a). S-cPBD does not show as pronounced of a Tg at −4.1 °C compared to the other samples, followed by a slope in the thermogram that could be a minor component of an inhomogeneous sample consisting of a broad molecular weight distribution, which is likely a result of the absence of DIB. The plasticisation effect of LPBD at 10 wt% could be due to a lower loading of LPBD being more disperse throughout the polymer matrix, therefore, fewer chains are crosslinked or entangled with each other. Increasing the LPBD content would likely result in more extensive crosslinking and chain entanglement, as seen from a substantial increase in pre-polymer viscosity as the reaction progressed. TGA showed an increase in onset temperature from 221 °C to 247 °C for both 20 and 30 wt% LPBD loadings, showing more resistance to thermal decomposition (Fig. 3b). A higher char residue is achieved for S-DIB-cPBD compared to S-cPBD suggesting that the aromatic nature of DIB has a considerable effect on the thermal properties of the polymer. All S-DIB samples loaded with cPBD have a similar percentage of char residue which supports that it is likely the type of crosslink, and not the number of crosslinks, which has this effect.32 Neat S-DIB has a comparatively higher char residue than S-cPBD, although the degradation temperature is lower, also indicating that the inclusion of DIB is likely the cause of the increasing char residue.
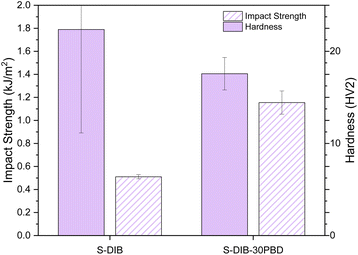
Studies on impact resistance and hardness were carried out on S-DIB and S-DIB-30cPBD. Charpy impact tests were carried out on unnotched samples as S-DIB was too brittle to be notched. The impact strength doubles with a 30 wt% loading of LPBD from 0.511 to 1.15 kJ m−2, successfully increasing the toughness (Fig. 4). As expected, there is no significant difference in the hardness of the two samples. A large error in the hardness data is seen for S-DIB, which could be an indicator of inhomogeneity in the sample exacerbated by the small area being tested and the close to room temperature Tg of the sample.
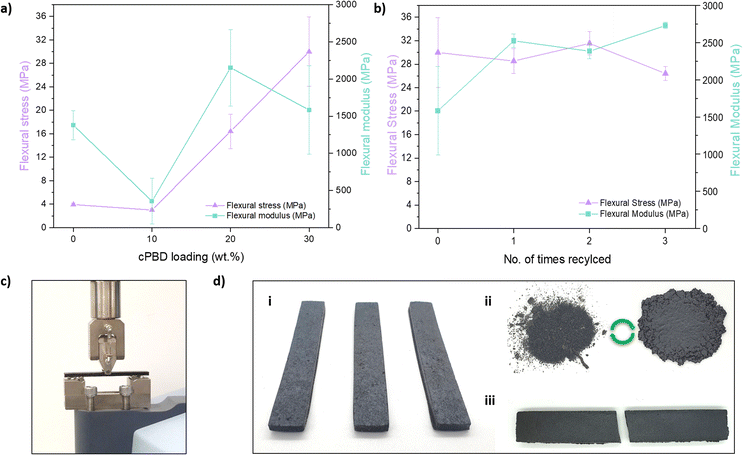
To observe the effects of the addition of L-PBD to the flexural properties of the sulfur polymers, 3-point bending tests were carried out at 25 °C on the samples synthesised from cPBD at 10–30 wt% loadings. As the cPBD loading in S-DIB increased, the flexural strength and modulus generally increased, except 50S-DIB-10cPBD attained a lower flexural strength (3 MPa) compared to neat 50S-DIB (4 MPa) as its Tg is below room temperature, making it more flexible (Fig. 5a). Unlike 50S-DIB-10cPBD, a significant increase in flexural strength is seen for 50S-DIB-20cPBD (12 MPa, 318% increase) and 50S-DIB-30cPBD (30 MPa, 663% increase) (Fig. 5a). 30 wt% loading of cPBD has allowed sulfur polymer a step closer to the flexural strength of conventional polymers, such as polypropylene (41–55 MPa).33 3-point bending tests were also carried out on S-DCPD and S-DVB to provide a comparison to other sulfur polymers of different physical properties (Fig. S6†). S-DVB has been reported to have a high crosslinking density, achieving a flexural strength of 16 MPa, slightly higher than the reported value for 20 wt% loading of cPBD. However, addition of 30 wt% cPBD outperforms S-DVB, achieving a significantly higher flexural strength. As S-DCPD is very brittle a lower flexural strength of 7 MPa was expected and would likely be another polymer matrix that could benefit from the addition of LPBD.
As such changes in properties were made with addition of cPBD, it was important to test the recyclability of the samples to ensure they remained recyclable like other inverse vulcanised polymers. The ability to be recycled would allow them to differ from typical vulcanised rubber as well as their ability to stabilise high sulfur loadings (Fig. S4†). Their high stiffness (Table S3†) also makes them unique to vulcanised rubber and elastomers, such as styrene butadiene rubber which is capable of a high strain of 450–600%.34 For recycling studies, samples were ground into a fine powder and hot-pressed at 150 °C into specimens for 3-point bending tests. Recycling studies were done on 50S-DIB-30PBD as it contained the highest loading of cPBD, therefore, this sample was expected to be the most difficult to recycle out of the polymers produced. Recycling was successful all three times, obtaining similar flexural strength and modulus to the original specimens (Fig. 5b). A smaller error bar was achieved for the recycled samples as processing became easier when in powder form, creating more even specimens for 3-point bending tests.
The cross-section of the 3-point bending test specimens were examined using SEM, showing an increase in roughness as the cPBD loading increases. The specimens in this case were broken by bending by hand (a vice had to be used for help with 20 wt% and 30 wt% cPBD loadings) to achieve smaller specimen sizes. This meant the surfaces imaged are not the result of the fracture during the 3-point bending tests, but by use of a similar bending motion. The micrograph of S-DIB shows a relatively flat surface with small polymer particles likely due to its susceptibility to fragment more (Fig. 6a). S-DIB-10cPBD also shows an overall flat surface but with a rough texture that is consistent throughout the cross-section, and the absence of isolated particles (Fig. 6b). S-DIB-20cPBD (Fig. 6c) and S-DIB-30cPBD (Fig. 6d) have rougher textures with branching of stress lines showing the dissipation of energy and so the ability to withstand greater loads. The increased roughness of the surface when increasing the LPBD loading from 20 wt% to 30 wt%, supports the findings of increased flexural stress.
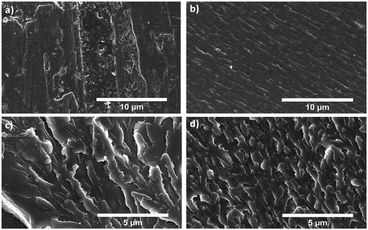 | ||
| Fig. 6 SEM micrographs of (a) S-DIB cross-section; (b) S-DIB-10cPBD cross-section; (c) S-DIB-20cPD cross-section; (d) S-DIB-30cPBD cross-section. | ||
Conclusions
Using a similar idea to the toughening of PS and epoxy resins, impact strength was successfully increased by the addition of LPBD in sulfur polymers, with 30 wt% loading achieving double the impact strength at 1.16 kJ m−2. The flexural strength, however, increased more significantly with 30 wt% LPBD loading resulting in a flexural strength of 30 MPa, approximately 6× greater than that of S-DIB, 5× greater than S-DCPD, and double the flexural strength of S-DVB. Also, the Tg of samples containing 20 and 30 wt% loading increased, along with their decomposition temperatures.Unlike vulcanised rubber, the recyclability of these sulfur polymers was retained with the addition of LPBD, and a sulfur content of 50 wt% was attained. This increase in impact resistance and flexural strength, while retaining recyclability may increase the range of possible practical applications of inverse vulcanised polymers – which could enable alleviation of excess industrial sulfur, while allowing for a more sustainable circular economy of crosslinked polymer components. Future work could include further tuning the properties of these materials with the use of alternative crosslinkers, incorporation of plasticisers, and/or fillers.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Materials Innovation Factory team for their assistance with DSC, FTIR, SEC and SEM. We also thank Joseph Dale for helpful discussions. T. H. is supported by a Royal Society University Research Fellowship.Notes and references
- J. L. Valentín, P. Posadas, A. Fernández-Torres, M. A. Malmierca, L. González, W. Chassé and K. Saalwächter, Macromolecules, 2010, 43, 4210–4222 CrossRef.
- B. Adhikari, D. De and S. Maiti, Prog. Polym. Sci., 2000, 25, 909–948 CrossRef CAS.
- J. E. Mark, B. Erman and M. Roland, The Science and Technology of Rubber, 2013 Search PubMed.
- J. Kim, P. Saha, S. Thomas, J. Haponiuk and M. Aswathi, Rubber Recycling: Challenges and Developments, Royal Society of Chemistry, 2018 Search PubMed.
- M. Myhre, S. Saiwari, W. Dierkes and J. Noordermeer, Rubber Chem. Technol., 2012, 85, 408–449 CrossRef CAS.
- P. Yan, W. Zhao, B. Zhang, L. Jiang, S. Petcher, J. A. Smith, D. J. Parker, A. I. Cooper, J. Lei and T. Hasell, Angew. Chem., Int. Ed., 2020, 59, 13371–13378 CrossRef CAS PubMed.
- J. J. Griebel, N. A. Nguyen, S. Namnabat, L. E. Anderson, R. S. Glass, R. A. Norwood, M. E. Mackay, K. Char and J. Pyun, ACS Macro Lett., 2015, 4, 862–866 CrossRef CAS PubMed.
- B. Krishnakumar, R. V. S. P. Sanka, W. H. Binder, V. Parthasarthy, S. Rana and N. Karak, Chem. Eng. J., 2020, 385, 123820 CrossRef CAS.
- N. A. Lundquist, A. D. Tikoalu, M. J. H. Worthington, R. Shapter, S. J. Tonkin, F. Stojcevski, M. Mann, C. T. Gibson, J. R. Gascooke, A. Karton, L. C. Henderson, L. J. Esdaile and J. M. Chalker, Chem.–Eur. J., 2020, 26, 10035–10044 CrossRef CAS PubMed.
- A. M. O. Mohamed and M. El-Gamal, Sulfur Concrete for the Construction Industry: A Sustainable Development Approach, J. Ross Pub., 2010 Search PubMed.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, Á. Somogyi, P. Theato, M. E. Mackay, Y.-E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518–524 CrossRef CAS PubMed.
- L. R. Bacon, J. Franklin Inst., 1936, 222, 111–112 CrossRef.
- C. C. Boswell and D. K. Friesen, Fert. Res., 1993, 35, 127–149 CrossRef CAS.
- J. Murphy, Additives for Plastics Handbook, Elsevier Science, 2001 Search PubMed.
- V. Hanna, P. Yan, S. Petcher and T. Hasell, Polym. Chem., 2022, 13, 3930–3937 RSC.
- J. A. Smith, S. J. Green, S. Petcher, D. J. Parker, B. Zhang, M. J. H. Worthington, X. Wu, C. A. Kelly, T. Baker, C. T. Gibson, J. A. Campbell, D. A. Lewis, M. J. Jenkins, H. Willcock, J. M. Chalker and T. Hasell, Chem.–Eur. J., 2019, 25, 10433–10440 CrossRef CAS PubMed.
- D. J. Parker, H. A. Jones, S. Petcher, L. Cervini, J. M. Griffin, R. Akhtar and T. Hasell, J. Mater. Chem. A, 2017, 5, 11682–11692 RSC.
- I. Bu Najmah, N. A. Lundquist, M. K. Stanfield, F. Stojcevski, J. A. Campbell, L. J. Esdaile, C. T. Gibson, D. A. Lewis, L. C. Henderson, T. Hasell and J. M. Chalker, ChemSusChem, 2021, 14, 2352–2359 CrossRef CAS PubMed.
- T. Gupta, R. K. Sharma and S. Chaudhary, Int. J. Impact Eng., 2015, 83, 76–87 CrossRef.
- S. Diez, A. Hoefling, P. Theato and W. Pauer, Polymers, 2017, 9, 59 CrossRef PubMed.
- C. Arends, Polymer Toughening, Taylor & Francis, 1996 Search PubMed.
- J. Rovere, C. A. Correa, V. G. Grassi and M. F. D. Pizzol, J. Mater. Sci., 2008, 43, 952–959 CrossRef CAS.
- A. S. Argon, R. E. Cohen and T. M. Mower, Mater. Sci. Eng., A, 1994, 176, 79–90 CrossRef CAS.
- F. Soriano-Corral, G. Morales, P. Acuña, E. Díaz-Barriga, B. Arellano, C. Vargas and O. De la Paz, Macromol. Symp., 2013, 325–326, 177–183 CrossRef CAS.
- M. Schneider, T. Pith and M. Lambla, J. Mater. Sci., 1997, 32, 6331–6342 CrossRef CAS.
- C. Wang, Q. Sun, K. Lei, C. Chen, L. Yao and Z. Peng, Polymers, 2020, 12, 433 CrossRef CAS PubMed.
- N. Chikhi, S. Fellahi and M. Bakar, Eur. Polym. J., 2002, 38, 251–264 CrossRef CAS.
- D. Ratna and A. K. Banthia, Macromol. Res., 2004, 12, 11–21 CrossRef CAS.
- S. W. Lim, M. C. Choi, J. H. Jeong, E. Y. Park and C. S. Ha, Adv. Mater. Res., 2018, 7, 149–162 Search PubMed.
- X. Wu, J. A. Smith, S. Petcher, B. Zhang, D. J. Parker, J. M. Griffin and T. Hasell, Nat. Commun., 2019, 10, 647 CrossRef PubMed.
- Y.-S. Lai and Y.-L. Liu, Macromol. Rapid Commun., 2023, 44, 2300014 CrossRef CAS PubMed.
- F. M. Uhl, G. F. Levchik, S. V. Levchik, C. Dick, J. J. Liggat, C. E. Snape and C. A. Wilkie, Polym. Degrad. Stab., 2001, 71, 317–325 CrossRef CAS.
- M. P. Stevens, Polymer Chemistry: An Introduction, Oxford University Press, 1999 Search PubMed.
- J. E. Mark, Physical Properties of Polymers Handbook, Springer, New York, 2nd edn, 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ta05470e |
| This journal is © The Royal Society of Chemistry 2024 |