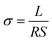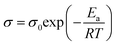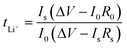Fluorinated carbon nitride with a hierarchical porous structure ameliorating PEO for high-voltage, high-rate solid lithium metal batteries†
Shuohan
Liu
a,
Jieqing
Shen
a,
Zhikai
Wang
a,
Wensheng
Tian
b,
Xiujun
Han
c,
Zhixin
Chen
d,
Hui
Pan
*a,
Lei
Wang
a,
Dongyu
Bian
a,
Cheng
Yang
*be and
Shenmin
Zhu
 *a
*a
aState Key Laboratory of Metal Matrix Composites, School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: smzhu@sjtu.edu.cn
bState Key Laboratory of Space Power-Sources Technology, Shanghai Institute of Space Power-Sources, No. 2965, Dongchuan Road, Minhang District, Shanghai 200245, China
cSchool of Materials Science and Engineering, Qilu University of Technology (Shandong Academy of Sciences), Jinan, Shandong Province 250353, China
dSchool of Mechanical, Materials & Mechatronics Engineering, University of Wollongong, Wollongong, NSW 2522, Australia
eSchool of Materials Science and Engineering, Harbin Institute of Technology, Harbin, Heilongjiang 150001, China
First published on 2nd November 2023
Abstract
Instability caused by lithium dendrites and low oxidation potentials hinders the commercialization of poly(ethylene oxide) (PEO) based all-solid-state lithium metal batteries (ASSLBs). Herein, fluorinated carbon nitride (FCN) with a hierarchical porous structure was designed and fabricated to modify a PEO-based electrolyte. The resultant FCN effectively suppressed the lithium dendrite growth in ASSLBs and elevated the oxidation potential of the PEO-based electrolyte to as high as 5.2 V. The fluorine and nitrogen in the FCN resulted in the formation of a LiF- and Li3N-rich composite solid electrolyte interface (SEI) that prevented continuous side reactions between the Li metal anode and the electrolyte. The produced hierarchical structure has abundant mesopores, which provided fluent channels for Li ion diffusion without extra resistance and greatly promoted the cycling and rate performance of the batteries. By using this porous FCN modified electrolyte, Li–Li symmetrical cells exhibited an ultra-long lifespan of more than 2500 h at a current density of 0.1 mA cm−2. The ASSLBs combined with a high-voltage ternary cathode can work stably at high rates of 2C and 5C with capacity retentions of 87.8% after 150 cycles and 92.1% after 50 cycles, respectively. This work presents a facile and effective strategy for the design of polymer based electrolytes for high-voltage, high-rate ASSLBs.
1 Introduction
The use of a solid lithium metal anode together with a high-voltage cathode can considerably increase the energy density of lithium batteries,1 and yet uncontrollable growth of lithium dendrites on the Li anode remains a huge obstacle.2 The use of solid-state electrolytes, instead of volatile organic liquid solvents, could effectively suppress the growth of lithium dendrites and significantly boost the battery safety.3 Inorganic solid electrolytes have high ionic conductivities being close to those of liquid electrolytes; however, limited interfacial compatibility with porous electrodes and poor machinability greatly impede their practical applications.4,5 Solid polymer electrolytes (SPEs) are superior in terms of interface stability and scalability.6 Among them, polyethylene oxide (PEO) has been the most widely used electrolyte matrix because of its low cost and excellent Li salt solubility.7 Unfortunately, due to their poor mechanical properties, narrow electrochemical window and low ion conductivity, PEO-based electrolytes still face great challenges in terms of lithium dendrite penetration and applicability in high-voltage, high-rate batteries.8,9The introduction of two-dimensional (2D) nanosheets with a large specific surface area can considerably lower the crystallinity and improve the mechanical properties of a PEO matrix.10 It is an effective mean to enhance the ion conductivity and address the lithium dendrite penetration problem of a PEO matrix electrolyte.11–13 Carbon nitride (g-C3N4) nanosheets as an typical 2D material show superior mechanical properties and thermal and chemical stabilities.14–19 Their triazine ring structure effectively minimizes the transport distance of Li ions when they are incorporated into polymer electrolytes.17,18 More importantly, g-C3N4 can react with a Li metal anode during cycling to form Li3N, which is regarded as a vital component of an ideal solid electrolyte interface (SEI) layer.14 However, a high content of Li3N alone in the SEI is incapable of completely addressing the lithium dendrite issue due to its low interface energy against Li metal.20
The integration of LiF and Li3N to construct a Li3N-rich and LiF-rich composite SEI would considerably inhibit the formation of lithium dendrites.20–25 For example, Li3PS4 electrolyte coated with a Li3N–LiF composite layer shows a significant suppression effect on Li dendrites;20 a Li3N–LiF interface layer formed in PEO/LiTFSI which is ameliorated by mesoporous LaxCoO3 − δ nanofibers allows for stable cycling even when it was matched with a LiNi0.8Mn0.1Co0.1O2 cathode.25 However, the preparation processes above need either strict fabrication conditions or using expensive rare earth elements, and thus they are unsuitable for large-scale commercial manufacturing.
Herein, we designed and produced a fluorinated carbon nitride (FCN) by using a gas fluorination technology, during which, a hierarchical porous structure was formed in the FCN. The resultant porous FCN having a morphology of nanosheets was employed to modify PEO-based electrolytes. The fluorine and nitrogen elements in the FCN benefit the formation of a LiF- and Li3N-rich composite SEI, which can significantly reduce interfacial resistance and boost uniform Li+ deposition. Furthermore, the highly reactive F2 gas fluorination created mesopores on g-C3N4. The mesopores combined with the micropores from the tris-triazine ring of g-C3N4 form a hierarchical porous structure (Fig. 1). When using normal 2D nanosheet materials such as GO, boron nitride, etc. as electrolyte fillers, transportation of Li ions within the composite electrolyte could only be realized by detouring the nanosheets. Meanwhile, the hierarchical porous structure of FCN allows Li ions to be transported through the FCN nanosheets, thus significantly shortening the Li-ion transport path (Fig. S1†).18,26 As expected, the Li‖LiFePO4 cell with 0.1FCN-PEO/LiTFSI electrolyte (0.1 is the mass fraction of the FCN in the PEO-based electrolytes) delivered a reversible capacity of 105.2 mA h g−1 with a low capacity fading rate (0.03% per cycle) after cycling at 1C for 1000 cycles. The Li‖LiNi0.5Co0.2Mn0.3O2 cell also delivered a highly stabilized capacity as high as 120.3 mA h g−1 even after 150 cycles at a high current density of 2C.
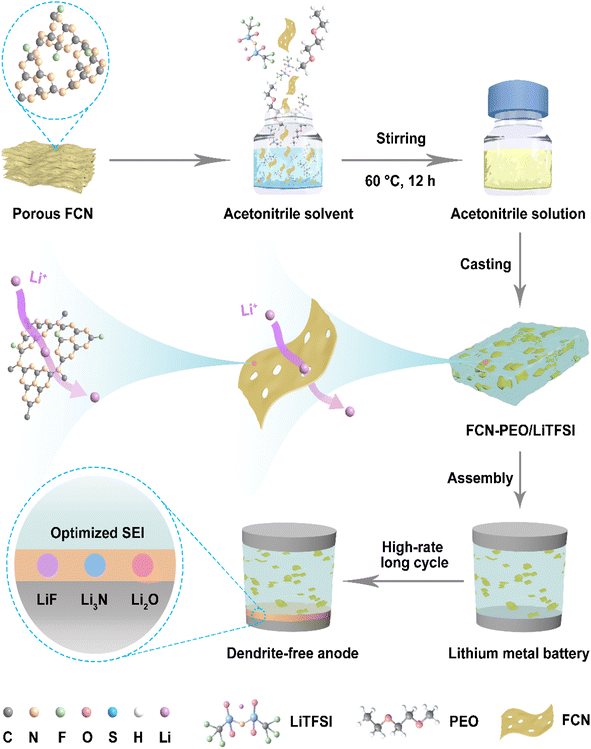 | ||
| Fig. 1 Schematic illustration of the material fabrication process and the mechanism of lithium dendrite-free batteries prepared by using FCN modified PEO based electrolytes. | ||
2 Experimental section
2.1 Preparation of bulk g-C3N4
The bulk g-C3N4 particles were prepared via a high temperature reaction. Typically, 10 g of melamine (Aladdin) was heated to 550 °C for 4 h in a N2 atmosphere at a heating rate of 5 °C min−1. Yellow powder was obtained. The product was fully ground for subsequent uses.2.2 Preparation of FCN
FCN was synthesized in a nickel reactor in a constant fluorination atmosphere. Firstly, 2 g of synthesized bulk g-C3N4 powder was placed in a nickel reactor. After the reactor was sealed, N2 was continuously injected into it for 1 h to exclude air. Then, a mixed gas of F2/N2 (20 vol% F2) was slowly introduced into the reactor until the pressure reached 0.1 MPa. Subsequently, the reactor was heated to 300 °C at 5 °C min−1 and held for 2 h. After the reaction was completed, the residual F2 and byproducts were blown out using N2 gas. The resulting FCN is light-yellow in color.2.3 Preparation of the PEO-based solid polymer electrolyte membrane
PEO (Aladdin, Mv = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and LiTFSI (Canrd New Energy Technology Co., Ltd) were vacuum-dried and stored in a glovebox (M. Braun) prior to use. The PEO-based polymer electrolyte membrane was prepared by solution casting. In a specific experiment, PEO and LiTFSI were dissolved in 30 ml acetonitrile (Aladdin) at a specific molar ratio (EO
000 g mol−1) and LiTFSI (Canrd New Energy Technology Co., Ltd) were vacuum-dried and stored in a glovebox (M. Braun) prior to use. The PEO-based polymer electrolyte membrane was prepared by solution casting. In a specific experiment, PEO and LiTFSI were dissolved in 30 ml acetonitrile (Aladdin) at a specific molar ratio (EO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li+ = 16
Li+ = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and stirred at 60 °C for 12 h to obtain a uniform transparent solution. Following that, FCN with mass fractions of 0%, 3%, 5%, 10%, 15% and 20% to PEO was added into the solution and stirred for 8 h to achieve a homogeneous light-yellow solution. The solution was then cast in a Teflon mold and dried for 24 h at 60 °C. The resulting films were punched into 19 mm discs for further use.
1) and stirred at 60 °C for 12 h to obtain a uniform transparent solution. Following that, FCN with mass fractions of 0%, 3%, 5%, 10%, 15% and 20% to PEO was added into the solution and stirred for 8 h to achieve a homogeneous light-yellow solution. The solution was then cast in a Teflon mold and dried for 24 h at 60 °C. The resulting films were punched into 19 mm discs for further use.
2.4 Preparation of the cathode
In order to achieve better compatibility between the cathode and electrolyte, cathodes were prepared using the PEO/LiTFSI electrolyte as the binder. A LFP cathode was prepared by mixing LFP active material (MTI), PEO/LiTFSI binder, and conductive carbon black (SP, MTI) in N-methyl pyrrolidone (NMP, Canrd) solvent with a mass ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and then coated on aluminum foil. After vacuum drying at 110 °C for 12 h, the obtained cathode loading was about 1 mg cm−2. A NCM523 cathode was prepared by the same method. The difference was that the mass ratio of NCM523 active material (MTI), PEO/LiTFSI, SP and PVDF (Aladdin) was 75
15 and then coated on aluminum foil. After vacuum drying at 110 °C for 12 h, the obtained cathode loading was about 1 mg cm−2. A NCM523 cathode was prepared by the same method. The difference was that the mass ratio of NCM523 active material (MTI), PEO/LiTFSI, SP and PVDF (Aladdin) was 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.
5.
2.5 Material characterization
The morphologies of the samples were examined on a scanning electron microscope (SEM, Hitachi S-4800). X-ray photoelectron spectroscopy (XPS) was performed on a Thermo Scientific K-Alpha. The specific surface area and pore size distribution of the samples were obtained by using an Autosorb-iQ2 system (Quantachrome). X-ray diffraction (XRD, Rigaku D/max 2550) and small-angle X-ray scattering (SAXS, BL19U2) were used to evaluate the crystallinity of the samples. Thermogravimetric analysis (TGA) and differential scanning calorimeter (DSC) curves of polymer electrolytes were obtained in a N2 environment on a TGA machine (Netzsch STA449F3) at a heating rate of 10 °C min−1. For the TGA measurements, the temperature range was set from ambient to 600 °C; for the DSC measurements, it was set from −60 to 150 °C. Tensile tests were performed on an Instron 5966 testing machine at an extension rate of 10 mm min−1. The Fourier transform infrared (FTIR) spectra of polymer electrolytes were obtained on a Thermo Scientific Nicolet 6700 instrument with a wavelength number in the range of 500–4000 cm−1. For ex situ XPS characterization and ex situ SEM measurements, the corresponding cells were carefully disassembled in an argon-filled glovebox, and the cycled Li foil was peeled off from the electrolyte film.2.6 Electrochemical measurements
Electrolyte membranes were incorporated into 2025 or 2032 type coin cells (MTI) to characterize electrochemical performance. The ionic conductivities of xFCN-PEO/LiTFSI samples were measured using electrochemical impedance spectroscopy (EIS) on a VMP3 multichannel electrochemical workstation (Bio Logic Science Instruments, France) at a frequency of 1 MHz to 0.1 Hz and an AC oscillation voltage of 5 mV. Prior to EIS measurements, the batteries were kept at each test temperature (from 30 to 80 °C) for 2 h to achieve thermal equilibrium. Ionic conductivity (σ) is calculated by using the formula:where L is the thickness of the solid electrolyte, R is the measured resistance from the EIS of SS‖solid electrolyte‖SS batteries at a given temperature, and S is the area of the SS electrode. The activation energy is calculated by using the Arrhenius equation:
where σ0 and Ea are the pre-exponential factor and the activation energy of ion transport respectively.
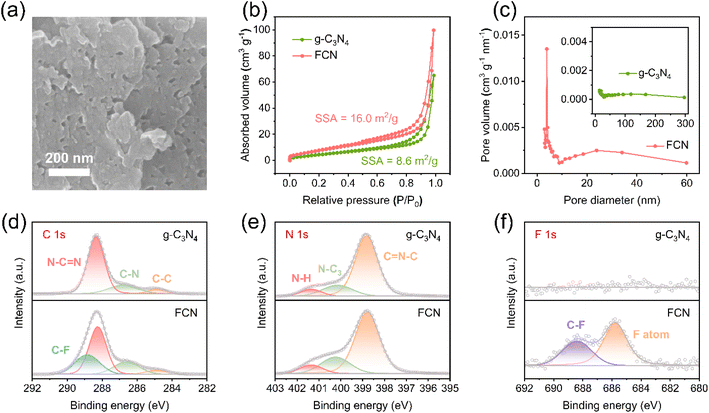
Li‖SS cells were assembled for the linear sweep voltammetry (LSV) tests, which were carried out at 60 °C to measure the electrochemical windows of 0.1FCN-PEO/LiTFSI and PEO/LiTFSI. LSV curves were examined from 0 to 6 V at a scan rate of 0.1 mV s−1. The lithium-ion transference number (tLi+) was determined via Li–Li symmetric cells from the EIS tests and direct current (DC) polarization. tLi+ is calculated by using the following formula:
The rate and cycling performances of Li‖LFP cells were tested on a battery test system (LAND CT 2001A) in a voltage range of 2.5–3.8 V at 60 °C. The constant current charge–discharge cycles of Li‖NCM523 cells were carried out at 60 °C and in the voltage range of 3.0–4.3 V. The EIS during the cycle was measured on a CHI 660E electrochemical workstation to investigate the evolution of interfacial impedance.
2.7 Calculation method
All structures were fully relaxed at the ωB97XD/6-31G(d, p) level of theory based on density functional theory (DFT) by using Gaussian 16 software.27,28 The binding energies were calculated in terms of electronic energies using the formula ΔE = Eab − Ea − Eb, where ab is the complex formed by molecules a and b.3 Results and discussion
3.1 Synthesis and physicochemical characterization of FCN
FCN with a light-yellow color was produced by melamine sintering, and then fluorinated at 300 °C for 1 h in a constant F2 gas environment (Fig. S2†). A scanning electron microscope (SEM) was used to characterize the morphology of the g-C3N4 and FCN. A densely stacked lamellar structure of the g-C3N4 (Fig. S3†) might impede the Li+ transportation in the polymer electrolyte. After the fluorination, bulk g-C3N4 transforms into porous, thin nanosheets (Fig. 2a). The presence of a large number of mesopores (about 10–50 nm) confirms the strong etching effect of F2 gas on g-C3N4. The porous structure of the FCN was further characterised by N2 adsorption–desorption. The measured Brunauer–Emmett–Teller (BET) specific surface area of the FCN (16.0 m2 g−1) is nearly twice that of the bulk and untreated g-C3N4 (8.6 m2 g−1) (Fig. 2b). In addition, the pore size distribution revealed by the Barrett–Joyner–Halenda (BJH) method confirms that the fluorination generated abundant mesopores (<4 nm and between 10 and 50 nm) on the FCN (Fig. 2c). Considering the micropores of the intrinsic triazine ring structure of g-C3N4, the FCN has a hierarchical porous structure.The X-ray diffraction (XRD) patterns of g-C3N4 and the FCN exhibit two peaks at 13.1° and 27.3° (Fig. S4†), corresponding to the lattice planes parallel to the c-axis and the periodic stacking of lamellae, respectively.29 Compared with the bulk g-C3N4, the FCN has a lower diffraction peak intensity at 27.3°, which suggests the exfoliation of the bulk g-C3N4 through the fluorination process.14 X-ray photoelectron spectroscopy (XPS) spectra given in Fig. S5† demonstrate the successful fluorination of the g-C3N4 with an obvious F 1s peak at 684.3 eV. Comparing the C 1s spectra of the g-C3N4 and FCN (Fig. 2d), besides the typical peaks of g-C3N4 (the adventitious carbon species at 284.8 eV, C–NHx at 286.3 eV and N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N at 288.4 eV), a new peak representing a C–F bond appears at 289.5 eV for the FCN. The distinctive peaks in the N 1s spectra of the g-C3N4 and FCN are comparable (Fig. 2e), indicating that F atoms prefer to interact with less electronegative C than N.30 The F 1s spectra of the FCN can be deconvoluted into covalent C–F bonds at 688 eV and physiosorbed or entrapped F atoms at 685.1 eV, whereas these peaks are not shown in the F 1s spectra of g-C3N4 (Fig. 2f). The results demonstrate the formation of 2D hierarchical porous FCN nanosheets.
N at 288.4 eV), a new peak representing a C–F bond appears at 289.5 eV for the FCN. The distinctive peaks in the N 1s spectra of the g-C3N4 and FCN are comparable (Fig. 2e), indicating that F atoms prefer to interact with less electronegative C than N.30 The F 1s spectra of the FCN can be deconvoluted into covalent C–F bonds at 688 eV and physiosorbed or entrapped F atoms at 685.1 eV, whereas these peaks are not shown in the F 1s spectra of g-C3N4 (Fig. 2f). The results demonstrate the formation of 2D hierarchical porous FCN nanosheets.
3.2 Physicochemical and electrochemical characterization of FCN-PEO/LiTFSI
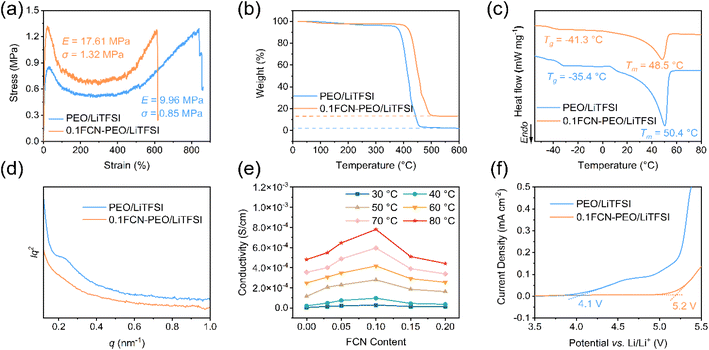
Samples of the mixtures of FCN, LiTFSI and PEO (xFCN-PEO/LiTFSI, x is the mass fraction of FCN/PEO and x = 0, 0.03, 0.05, 0.1, 0.15 and 0.2) were prepared using a solution casting method. FCN, PEO and LiTFSI were dissolved in acetonitrile (AN) and stirred until a homogeneous light-yellow solution was obtained. No significant sedimentation occurred after storing for 7 days (Fig. S6a†), indicating a homogeneous dispersion of the FCN in the PEO/AN solution. After removing the AN, xFCN-PEO/LiTFSI films were obtained and they have a light-yellow color (Fig. S6b†). The composite electrolyte films showed a good flexibility under bending (Fig. S6c†). The cross-sectional SEM images of the electrolytes with different FCN contents are shown in Fig. S7.† It is found that significant agglomeration occurs when the content of FCN exceeds 10 wt%, which is consistent with the EDS mapping results (Fig. S8†). Tensile tests show that the Young's modulus of 0.1FCN-PEO/LiTFSI is 17.61 MPa, nearly twice as high as that of PEO/LiTFSI (9.96 MPa) as shown in Fig. 3a. Due to the high Young's modulus, the 0.1FCN-PEO/LiTFSI electrolyte would provide a stronger barrier against lithium dendrite puncturing.12The PEO/LiTFSI and xFCN-PEO/LiTFSI films were characterized by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) and the results are given in Fig. 3b and c. It can be seen that the introduction of the FCN significantly increases the thermal stability of the PEO based electrolyte (Fig. 3b). When the temperature reaches 600 °C, the difference in the remaining mass of 0.1FCN-PEO/LiTFSI and PEO/LiTFSI matches well with the added FCN content. The FCN might affect the melting point (Tm), glass transition temperature (Tg) and crystallization of the PEO according to the DSC measurements (Fig. 3c). The Tm of PEO/LiTFSI is 50.4 °C and 48.5 °C for 0.1FCN-PEO/LiTFSI. The Tg of the PEO in 0.1FCN-PEO/LiTFSI falls to −41.3 °C from −35.4 °C observed in PEO/LiTFSI, which suggests that the FCN could elevate the mobility of the PEO chain segments and thus increase the ion conductivity of the composite electrolyte. The FCN can also effectively reduce the crystallinity of PEO according to the DSC results. The crystallinities of PEO/LiTFSI and 0.1FCN-PEO/LiTFSI were calculated to be 31.2% and 22.4%, respectively (Table S1†). This result is consistent with small angle X-ray scattering (SAXS) observations of these two electrolytes as shown in Fig. 3d. It can be seen that a long period peak corresponding to the lamellar structure of the PEO disappears in the 0.1FCN-PEO/LiTFSI composite.31
To evaluate the effect of the FCN content on the ionic conductivity of the composite electrolyte, electrochemical impedance spectroscopy (EIS) measurements using stainless steel (SS)‖xFCN-PEO/LiTFSI‖SS cells were performed at temperatures between 30 and 80 °C in the frequency range of 106–0.1 Hz. The ionic conductivities of xFCN-PEO/LiTFSI increase with increasing temperature as shown in Fig. 3e. The composite electrolyte containing 10 wt% FCN (0.1FCN-PEO/LiTFSI) exhibits a maximum ionic conductivity at all the testing temperatures. As the FCN content increases to more than 10 wt%, the ionic conductivity of the electrolyte decreases. This is probably due to FCN agglomeration, which occurs when the filler content exceeds 10 wt%. The agglomeration weakens the interactions of the FCN filler with PEO and LiTFSI. Arrhenius curves for the electrolytes with varying FCN filler contents are shown in Fig. S9.† At 50–80 °C and 30–50 °C, the activation energies of 0.1FCN-PEO/LiTFSI are 0.34 eV and 0.96 eV, respectively, which are significantly lower than those of PEO/LiTFSI.
The electrochemical window is directly related to PEO degradation on the cathode surface and the anion decomposition. It determines if an electrolyte can be used in a high-voltage battery.32 As shown in Fig. 3f, the oxidation potential of 0.1FCN-PEO/LiTFSI is up to 5.2 V, higher than 4.1 V of PEO/LiTFSI.
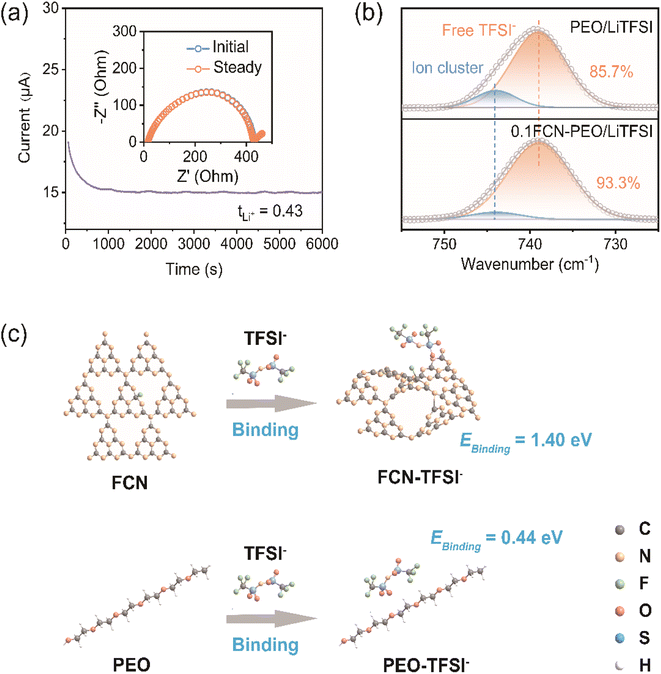
Fourier transform infrared spectroscopy (FTIR) was utilized to examine the interactions between the PEO, LiTFSI and FCN in the 0.1FCN-PEO/LiTFSI electrolyte and FTIR spectra of PEO/LiTFSI and 0.1FCN-PEO/LiTFSI are given in Fig. S10.† The peaks at 2889, 1467, and 1359 cm−1 represent the symmetric stretching, bending, and wagging vibrations of CH2, respectively. The peaks at 1281, 1099 and 961 cm−1 correspond to the asymmetric stretching, symmetric stretching and deformation vibrations of C–O–C, respectively, and the peak at 842 cm−1 is ascribed to the deformation vibration of CH2CH2O.18 The synergistic decrease in the intensity of these peaks implies considerable interactions between the FCN and PEO chains. After mixing with the FCN, the peaks at 1299 cm−1 (SO2 asymmetric stretching), 1193 cm−1 (CF3 asymmetric stretching), 763 cm−1 (S–N stretching and CF3 deformation), and 741 cm−1 (S–N–S stretching) clearly declined,33,34 which would be caused by the binding effect of the FCN and TSFI−. Therefore, the FTIR results tell us that the FCN has such a strong adsorption effect on the TFSI− anion that it can promote LiTFSI dissociation as well as increasing the lithium-ion transference number (tLi+).
Furthermore, the tLi+ of 0.1FCN-PEO/LiTFSI was measured to be 0.43 (Fig. 4a), much higher than 0.17 of PEO/LiTFSI as shown in Fig. S11.† It is due to the adsorption effect of the FCN on TFSI−, which can effectively prevent TFSI− migration. The improved tLi+ allows for a reduction in concentration polarization during cycling, which is critical for the suppression of lithium dendrites.35
The dissociation of LiTFSI to Li+ and TFSI− can be determined by FTIR in the wavenumber range from 725 to 755 cm−1. The free TFSI− anion is represented by the peak at about 740 cm−1, whereas the bonded Li-TFSI ion is represented by the peak at about 746 cm−1.36 Gaussian–Lorentzian fitting was employed to resolve the peaks, and the proportion of free anions in 0.1FCN-PEO/LiTFSI reaches 93.3%, higher than 85.7% of PEO/LiTFSI as shown in Fig. 4b. It indicates that FCN increased the dissociation of Li salts and released more free Li+.
To confirm the strong interaction of the FCN with the TFSI− anion, the binding energies of the TFSI− anion with the PEO chains and the TFSI− anion with the FCN were calculated according to density functional theory (DFT). As presented in Fig. 4c, the calculated binding energy of the FCN and TFSI− is 1.40 eV, much higher than the binding energy 0.44 eV of the PEO with TFSI−. Thus, TFSI− is more likely to bind to FCN, allowing for more free Li+ release.
3.3 Effect of FCN on stabilization of Li metal
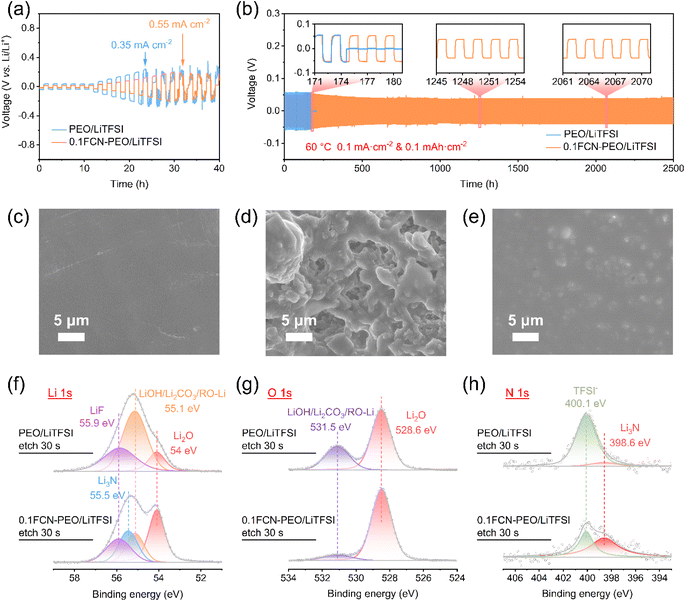
To evaluate the effect of the FCN on the stability of the electrolyte-Li metal interface, Li‖PEO/LiTFSI‖Li and Li‖0.1FCN-PEO/LiTFSI‖Li symmetric cells were assembled. Critical current density (CCD) is defined as the maximum current density that the cell can withstand before failure.37 It is usually used to evaluate the efficiency of the electrolyte to restrain lithium dendrites. As shown in Fig. 5a, the overpotential of Li‖0.1FCN-PEO/LiTFSI‖Li is smaller than that of Li‖PEO/LiTFSI‖Li at the same current density. When the current density is up to 0.35 mA cm−2, the CCD curve of Li‖PEO/LiTFSI‖Li undulates and a soft short circuit occurs. These phenomena are the typical adverse outcomes of a lithium dendrite penetration. However, the CCD of Li‖0.1FCN-PEO/LiTFSI‖Li can go up to 0.55 mA cm−2 before a soft short circuit occurs, suggesting that 0.1FCN-PEO/LiTFSI can more effectively inhibit the lithium dendrite growth compared with PEO/LiTFSI. Long-term cycling tests of the Li‖0.1FCN-PEO/LITFSI‖Li cell and Li‖PEO/LiTFSI‖Li cell were carried out at a current density of 0.1 mA cm−2 and the results are shown in Fig. 5b. The Li‖0.1FCN-PEO/LITFSI‖Li cell maintains a small polarization voltage of ∼40 mV over 2500 h, whereas the Li‖PEO/LiTFSI‖Li cell can only cycle for 175 h before a short circuit occurred.The morphologies of Li deposition can be directly observed by SEM on the cycled Li metal surfaces. The initial Li metal surface in the PEO/LiTFSI system was smooth (Fig. 5c), but after cycling it becomes rough and visible lithium dendrites can be observed, indicating inhomogeneous Li plating/stripping (Fig. 5d). The Li metal in 0.1FCN-PEO/LiTFSI shows an extremely flat morphology after long term cycling, and no significant lithium dendrites can be found (Fig. 5e). These results suggest that the FCN is conducive to a homogeneous lithium plating/stripping as well as the suppression of Li dendrite growth.
The composition of the SEI layer between the Li metal and the electrolyte has a major effect on its stability. To determine the role of the FCN in the SEI, we measured the components of the SEI layer on the Li metal surface using ex situ XPS. The Li 1s spectra of PEO/LiTFSI consist LiF at 55.9 eV, LiOH/Li2CO3/RO-Li at 55.1 eV, and Li2O at 54 eV.38 In contrast, the Li 1s spectra of 0.1FCN-PEO/LiTFSI exhibit a distinct Li3N peak at 55.5 eV (Fig. 5f).22 Moreover, the peak of LiOH/Li2CO3/RO-Li is significantly reduced (consistent with the O 1s spectrum at 531.5 eV), while the peak of Li2O increased (consistent with the O 1s spectrum at 528.6 eV) (Fig. 5g).38 This suggests that the FCN induces the formation of a LiF-, Li3N- and Li2O-rich composite SEI layer. LiF has a high interfacial energy against Li, which can effectively inhibit the generation of lithium dendrites.21 The high ionic conductivities of Li3N and Li2O can greatly lower the interfacial resistance.21,38 The synergistic effect of LiF, Li3N, and Li2O results in an unparalleled stability of Li metal. Residual Li2CO3, trapped TFSI− and its decomposition products are the main factors responsible for interfacial passivation in PEO/LiTFSI, which are further manifested by the large overpotential during the lithium plating/stripping.38 It can be clearly observed that the peaks of TFSI− (at 688.6 eV in F 1s and 400.5 eV in N 1s) and its lithiation product (Li2S at 161.1 eV in S 2p) in 0.1FCN-PEO/LiTFSI were significantly weakened or even disappeared (Fig. 5h, S12a and b†).38,39 This is due to the strong absorption effect of the FCN on TFSI−, which inhibits the aggregation and decomposition of TFSI− on the Li metal surface. This also results in no additional carbon source on the anode surface to convert the high ionic conductive Li2O to passivated Li2CO3, which facilitates the formation of a high ionic conductive SEI layer.
3.4 Performance of all-solid-state Li metal batteries
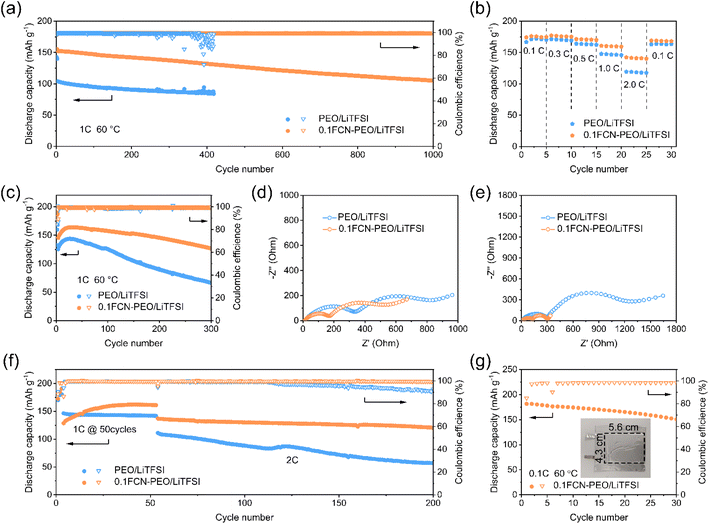
The synergistic effect of the FCN modified PEO-based electrolyte enables outstanding performance of ASSLBs. The performance of 0.1FCN-PEO/LiTFSI was verified by employing a LiFePO4 (LFP) cathode. The Li‖0.1FCN-PEO/LiTFSI‖LFP cell could cycle stably for more than 1000 cycles at 1C, and the Coulomb efficiency (CE) remained at nearly 100% (Fig. 6a). A capacity retention of 69% (105.2 mA h g−1) of the initial capacity (152.5 mA h g−1) was obtained after 1000 cycles. In comparison, the Li‖PEO/LiTFSI‖LFP cell delivered an initial capacity of only 104.1 mA h g−1, which rapidly declined to 87.1 mA h g−1 after 400 cycles. Meanwhile, the Li‖PEO/LiTFSI‖LFP cell showed a rapid fade in CE. This is consistent with the failure mechanism of the Li‖Li cells—a soft short circuit induced by lithium dendrites. During charge and discharge, the Li‖0.1FCN-PEO/LiTFSI‖LFP cell showed a low and stable overpotential (Fig. S13a†), indicating improved electrochemical stability. However, the overpotential of the Li‖PEO/LiTFSI‖LFP cell increased obviously with cycling time (Fig. S13b†). As shown in Fig. S14,† the 0.1FCN-PEO/LiTFSI-based Li‖LFP cells also exhibited excellent rate performance. The Li‖0.1FCN-PEO/LiTFSI‖LFP cell delivered a capacity of 127.7 mA h g−1 at 2C, which is 31.2% higher than that of the Li‖ PEO/LiTFSI‖LFP cell. When the current density returned to 0.1C, the capacity of the Li‖0.1FCN-PEO/LiTFSI‖LFP cell recovered to 164.64 mA h g−1, which is close to the theoretical specific capacity (170 mA h g−1).Considering the important role of a high-voltage cathode in boosting energy density, Li‖NCM523 cells were assembled for electrochemical performance tests at 3.0–4.3 V. In rate performance tests (Fig. 6b), the Li‖0.1FCN-PEO/LiTFSI‖NCM523 cell delivered high and stable discharge capacities of 175, 176.0, 170.7, 160.0, and 141.1 mA h g−1 at 0.1, 0.3, 0.5, 1, and 2C, respectively, significantly higher than those of the Li‖PEO/LiTFSI‖NCM523 cell (170.3, 170.3, 163.3, 146.8, and 118.4 mA h g−1 at 0.1, 0.3, 0.5, 1, and 2C, respectively). When the current density returned to 0.1C, the discharge capacity of the Li‖0.1FCN-PEO/LiTFSI‖NCM523 cell recovered to 96.3% of its original value, indicating an outstanding reversible stability. More importantly, the Li‖0.1FCN-PEO/LiTFSI‖NCM523 cell delivered a capacity of 86.7 mA h g−1 at 5C. After 50 cycles, the capacity retention still reached 92.1% (Fig. S15†).
As shown in Fig. 6c, the Li‖0.1FCN-PEO/LiTFSI‖NCM523 cell and Li‖PEO/LiTFSI‖NCM523 cell delivered initial reversible capacities of 145.3 mA h g−1 and 125.4 mA h g−1 at 1C, respectively. After 300 cycles, the Li‖0.1FCN-PEO/LiTFSI‖NCM523 cell still delivered a reversible capacity of 126.7 mA h g−1, while the Li‖PEO/LiTFSI‖NCM523 cell only has a capacity of 66.6 mA h g−1. The charge/discharge curves show an increased cycling stability of the 0.1FCN-PEO/LiTFSI-based cells than that of the PEO/LiTFSI -based cells (Fig. S16a and b†).
To further analyze the interfacial evolution during cycling, the Li‖NCM523 cells were evaluated by EIS. A high frequency semicircle in an EIS test represents the impedance of ions traveling through the SEI layer (RSEI), while a medium frequency semicircle reflects the charge transfer impedance between the electrode and the electrolyte (Rct).39 As shown in Fig. 6d, the RSEI and Rct of the 0.1FCN-PEO/LiTFSI system after 100 cycles were 140.4 and 560.2 Ω, respectively, which are much lower than those of PEO/LiTFSI (RSEI 324.2 Ω and Rct 629.7 Ω). After 300 cycles, the RSEI and Rct of 0.1FCN-PEO/LiTFSI decreased to 117.7 Ω and 172 Ω, respectively, due to the formation of a stable and highly ionic conductive SEI layer. However, the Rct of PEO/LiTFSI steeply increased to 1095 Ω, caused by a continuous thickening of a high impedance interface (Fig. 6e).
To further assess the superiority of 0.1FCN-PEO/LiTFSI, the testing current density was further increased to 2C after an initial 50 cycles of activation at a current density of 1C (Fig. 6f). The Li‖0.1FCN-PEO/LiTFSI‖NCM523 cell still delivered a reversible capacity of 120.3 mA h g−1 after 150 cycles. The CE of the Li‖PEO/LiTFSI‖NCM523 cell, however, began to decay after around 50 cycles, with a reversible capacity of only 56.9 mA h g−1. The cycle performance of the Li‖0.1FCN-PEO/LiTFSI‖NCM523 cell is superior to that of the majority of previously reported ASSLBs assembled with PEO-based electrolytes (Table S2†). The excellent battery performance further confirms that the FCN contributes to the rapid transport of Li+ as well as to the interfacial stability.
A flexible Li‖0.1FCN-PEO/LiTFSI‖NCM523 pouch cell was assembled to demonstrate its application potential. As shown in Fig. 6g, the CE and discharge specific capacity of the pouch cell after 30 cycles were 98.4% and 150.6 mA h g−1, respectively. The flexible pouch cell could power an LED device under bending, puncturing, and cutting situations (Fig. S17a–d†), which shows its exceptional safety. The superior performance of the 0.1FCN-PEO/LiTFSI electrolyte in the NCM523-based cells offers a prospective application of the electrolyte in high energy density devices.
4 Conclusion
In summary, hierarchical porous FCN nanosheets were developed by using a gas fluorination method and used to modify a PEO-based polymer electrolyte for ASSLBs. Gas fluorination not only introduces F in the FCN, but also creates abundant mesopores in the FCN. Thus, the obtained FCN nanosheets have a hierarchical porous structure and can effectively adsorb TFSI− anions and induce the formation of a LiF- and Li3N-rich composite SEI layer with a high ionic conductivity and high interface energy. The synergetic contributions from F and N in the FCN play a critical role in inhibiting the growth of lithium dendrites. In addition, the hierarchical porous structure of the FCN can provide ion transport channels and shorten the transport distance of Li+. As a result, 0.1FCN-PEO/LiTFSI achieved high ionic conductivity, a wide voltage window, and high mechanical properties at the same time. A Li–Li symmetrical cell assembled with 0.1FCN-PEO/LiTFSI achieved a sustained cycle of more than 2500 h without evident lithium dendrite formation. The Li‖0.1FCN-PEO/LiTFSI‖NCM523 cell delivered a reversible capacity of 120.3 mA h g−1 after 150 cycles at a current density of 2C, and a super high rate of 5C can be attained with a capacity of 86.7 mA h g−1. This strategy for the constructing of an FCN-reinforced polymer electrolyte offers a prospective approach to developing high-voltage, high-rate ASSLBs.Author contributions
Shuohan Liu: writing – original draft, formal analysis, investigations, data curation, and visualization. Jieqing Shen: formal analysis and data curation. Zhikai Wang: formal analysis. wensheng tian: formal analysis. Xiujun Han: software. Zhixin Chen: writing – review & editing. Hui Pan: conceptualization and formal analysis. Lei Wang: formal analysis. Dongyu Bian: formal analysis. Cheng Yang: formal analysis. Shenmin Zhu: writing – review & editing, conceptualization, funding acquisition, project administration, and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors kindly acknowledge the financial support from the National Natural Science Foundation of China (51672173 and U1733130); Shanghai Science and Technology Committee (21ZR1435700, 18520744700, and 18JC1410500); Shanghai Jiao Tong University Medical Engineering Cross Research Programme (YG2023ZD18). We also acknowledge the support from the Shanghai Synchrotron Radiation Facility (SSRF) during the measurements of small angle X-ray scattering at BL19U2.References
- J. Liu, X. Shen, J. Zhou, M. Wang, C. Niu, T. Qian and C. Yan, ACS Appl. Mater. Interfaces, 2019, 11, 45048–45056 CrossRef CAS PubMed.
- T. Li, X. Zhang, N. Yao, Y. Yao, L. Hou, X. Chen, M. Zhou, J. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2021, 60, 22683–22687 CrossRef CAS PubMed.
- H. Wang, Q. Wang, X. Cao, Y. He, K. Wu, J. Yang, H. Zhou, W. Liu and X. Sun, Adv. Mater., 2020, 32, 2001259 CrossRef CAS.
- N. Yang, Y. Cui, H. Su, J. Peng, Y. Shi, J. Niu and F. Wang, Angew. Chem., Int. Ed., 2023, e202304339 CAS.
- X. Miao, H. Wang, R. Sun, C. Wang, Z. Zhang, Z. Li and L. Yin, Energy Environ. Sci., 2020, 13, 3780–3822 RSC.
- J. Xiang, Y. Zhang, B. Zhang, L. Yuan, X. Liu, Z. Cheng, Y. Yang, X. Zhang, Z. Li, Y. Shen, J. Jiang and Y. Huang, Energy Environ. Sci., 2021, 14, 3510–3521 RSC.
- Z. Li, A. Li, H. Zhang, R. Lin, T. Jin, Q. Cheng, X. Xiao, W.-K. Lee, M. Ge, H. Zhang, A. Zangiabadi, I. Waluyo, A. Hunt, H. Zhai, J. J. Borovilas, P. Wang, X.-Q. Yang, X. Chuan and Y. Yang, Nano Energy, 2020, 72, 104655 CrossRef CAS.
- P. Zhai, Z. Yang, Y. Wei, X. Guo and Y. Gong, Adv. Energy Mater., 2022, 12, 2200967 CrossRef CAS.
- M. Lei, X. Wu, Y. Liu, K. Chen, J. Hu and C. Li, Nano Res., 2023, 1–9 CAS.
- V. Vijayakumar, M. Ghosh, K. Asokan, S. B. Sukumaran, S. Kurungot, J. Mindemark, D. Brandell, M. Winter and J. R. Nair, Adv. Energy Mater., 2023, 13, 2203326 CrossRef CAS.
- H. Xu, Y. Li, A. Zhou, N. Wu, S. Xin, Z. Li and J. B. Goodenough, Nano Lett., 2018, 18, 7414–7418 CrossRef CAS.
- P. Barai, K. Higa and V. Srinivasan, Phys. Chem. Chem. Phys., 2017, 19, 20493–20505 RSC.
- S. Liu, W. Liu, D. Ba, Y. Zhao, Y. Ye, Y. Li and J. Liu, Adv. Mater., 2023, 35, 2110423 CrossRef CAS.
- L. Chen, T. Gu, J. Ma, K. Yang, P. Shi, J. Biao, J. Mi, M. Liu, W. Lv and Y.-B. He, Nano Energy, 2022, 100, 107470 CrossRef CAS.
- J. Wei, X. Zheng, W. Lin, Y. Si, K. Ji, C. Wang and M. Chen, J. Alloys Compd., 2022, 909, 164825 CrossRef CAS.
- J. Yang, X. Wang, G. Zhang, A. Ma, W. Chen, L. Shao, C. Shen and K. Xie, Front. Chem., 2019, 7, 388 CrossRef CAS PubMed.
- Z. Sun, Y. Li, S. Zhang, L. Shi, H. Wu, H. Bu and S. Ding, J. Mater. Chem. A, 2019, 7, 11069–11076 RSC.
- J. Hu, K. Chen, Z. Yao and C. Li, Sci. Bull., 2021, 66, 694–707 CrossRef CAS PubMed.
- J. Hu, J. Tian and C. Li, ACS Appl. Mater. Interfaces, 2017, 9, 11615–11625 CrossRef CAS PubMed.
- X. Ji, S. Hou, P. Wang, X. He, N. Piao, J. Chen, X. Fan and C. Wang, Adv. Mater., 2020, 32, 2002741 CrossRef CAS.
- M. Wu, M. Li, Y. Jin, X. Chang, X. Zhao, Z. Gu, G. Liu and X. Yao, J. Energy Chem., 2023, 79, 272–278 CrossRef CAS.
- X. Zhang, G. Gao, W. Wang, J. Wang, L. Wang and T. Liu, ACS Appl. Mater. Interfaces, 2022, 14, 49811–49819 CrossRef CAS.
- J. Liu, M. Wu, X. Li, D. Wu, H. Wang, J. Huang and J. Ma, Adv. Energy Mater., 2023, 13, 2300084 CrossRef CAS.
- Z. Zhang, J. Wang, S. Zhang, H. Ying, Z. Zhuang, F. Ma, P. Huang, T. Yang, G. Han and W.-Q. Han, Energy Storage Mater., 2021, 43, 229–237 CrossRef.
- S. Liu, X. Shen, L. Wei, R. Wang, B. Ding, J. Yu and J. Yan, Energy Storage Mater., 2023, 59, 102773 CrossRef.
- J. Wang, Y. Liu, Q. Cai, A. Dong, D. Yang and D. Zhao, Adv. Mater., 2022, 34, 2107957 CrossRef CAS.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615 RSC.
- V. A. Rassolov, J. A. Pople, M. A. Ratner and T. L. Windus, J. Chem. Phys., 1998, 109, 1223–1229 CrossRef CAS.
- P. Niu, L. Zhang, G. Liu and H.-M. Cheng, Adv. Funct. Mater., 2012, 22, 4763–4770 CrossRef CAS.
- L. Sun, Y. Li and W. Feng, Nanomaterials, 2021, 12, 37 CrossRef.
- J. Shen, S. Liu, D. Bian, Z. Chen, H. Pan, C. Yang, W. Tian, Y. Li, L. Kong, H. Quan, D.-W. Wang and S. Zhu, Electrochim. Acta, 2023, 456, 142482 CrossRef CAS.
- S. Huo, L. Sheng, W. Xue, L. Wang, H. Xu, H. Zhang and X. He, InfoMat, 2023, 5, e12394 CrossRef CAS.
- S. Li, Z. Zhang, K. Yang and L. Yang, ChemElectroChem, 2018, 5, 328–334 CrossRef CAS.
- H. Chen, D. Adekoya, L. Hencz, J. Ma, S. Chen, C. Yan, H. Zhao, G. Cui and S. Zhang, Adv. Energy Mater., 2020, 10, 2000049 CrossRef CAS.
- Z. Hu, F. Ji, Y. Zhang, W. Guo, X. Jing, W. Bao, J. Qin, S. Huo, S. Li, Y. Zhang, W. Fan and H. Cheng, Chem. Eng. J., 2023, 468, 143857 CrossRef CAS.
- D. Lin, P. Y. Yuen, Y. Liu, W. Liu, N. Liu, R. H. Dauskardt and Y. Cui, Adv. Mater., 2018, 30, 1802661 CrossRef.
- Critical Current Density in Solid-State Lithium Metal Batteries: Mechanism, Influences, and Strategies - Lu - 2021 - Advanced Functional Materials, Wiley Online Library, https://onlinelibrary.wiley.com/doi/10.1002/adfm.202009925, (accessed March 23, 2023) Search PubMed.
- J. Hu, C. Lai, K. Chen, Q. Wu, Y. Gu, C. Wu and C. Li, Nat. Commun., 2022, 13, 7914 CrossRef CAS PubMed.
- H. M. Bintang, S. Lee, S. Shin, B. G. Kim, H.-G. Jung, D. Whang and H.-D. Lim, Chem. Eng. J., 2021, 424, 130524 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ta05495k |
| This journal is © The Royal Society of Chemistry 2024 |