Polycarbazole-SEBS-crosslinked AEMs based on two spacer polymers for high-performance AEMWE†
Kyungwhan
Min
ab,
Insu
Jeong
ab,
Hayoung
Kim
ab and
Tae-Hyun
Kim
 *ab
*ab
aOrganic Material Synthesis Laboratory, Department of Chemistry, Incheon National University, 119 Academy-ro, Yeonsu-gu, Incheon, 22012, Republic of Korea. E-mail: tkim@inu.ac.kr
bResearch Institute of Basic Science, Core Research Institute, Incheon National University, 119 Academy-ro, Yeonsu-gu, Incheon, 22012, Republic of Korea
First published on 27th November 2023
Abstract
Poly(aryl piperidinium) (PAP)-based anion exchange membranes (AEMs) are central to recent research due to their high ionic conductivity and chemical stability. PAP-AEM-based water electrolysis (AEMWE) systems exhibit remarkable cell performance. However, the absence of flexible structural units in PAP-based polymers produces brittle membranes, which have degraded mechanical properties. Well-developed ion channels do not form in PAP-based polymers due to the low flexibility of the main chain-type ion-conducting group. The present study aims to fabricate polycarbazole–SEBS-based crosslinked membranes (x-Car-SEBSs) with well-developed ion channels due to the highly flexible spacer-type ion conducting groups by crosslinking two spacer-type polymers, polycarbazole and SEBS, as novel AEM materials. The resulting x-Car-SEBS membranes also exhibit good mechanical properties (tensile strength of 17.2–22.3 MPa and elongation at break between 109.6% and 138.3%), driven by crosslinking SEBS, an elastic polymer component, with polycarbazole, a spacer-type PAP-based rigid polymer. The 40x-Car-SEBS membrane, with a 40% crosslinking degree, shows the most pronounced phase separation and achieves the highest ionic conductivity (153.16 mS cm−1 at 80 °C) and 840 hours of alkaline stability. Furthermore, 40x-Car-SEBS demonstrates superior AEMWE single-cell performance, achieving 1.25 mA cm−2 at 1.8 V, almost double that of FAA-3-50, a commercial membrane, and higher than previously developed main chain-type crosslinked AEMs and main chain–spacer-type crosslinked AEMs. Moreover, this membrane exhibits excellent cell durability, with a minimal voltage increase of 0.1 V after 100 hours.
Introduction
Hydrogen is widely used as an energy source in various industrial sectors, including steel production, chemical manufacturing, pharmaceuticals, energy generation, and transportation. In particular, hydrogen has gained widespread recognition in the energy industry as an efficient, eco-friendly energy source thanks to its high conversion efficiency from chemical to electrical energy, combined with its clean energy production process with no greenhouse gas emissions.1 However, as it currently stands, hydrogen is primarily produced through fossil fuel reforming, resulting in significant CO2 emissions that render its production process less environmentally favorable. Given the context, there is a growing demand for green hydrogen, which is generated without any CO2 emissions. Consequently, extensive research has increasingly focused on water electrolysis.2,3Among various water electrolysis methods, proton exchange membrane (PEM)-based water electrolysis (PEMWE) is known for highly efficient hydrogen production due to its exceptional proton conductivity. This system is also compact, so it uses relatively small devices. However, PEMWE operates under acidic conditions, necessitating the use of costly noble metal catalysts. Furthermore, using a perfluorinated polymer as a polymer electrolyte increases process costs and raises concerns about environmental regulations.4,5
Alkaline water electrolysis (AWE), in which an alkaline solution, such as KOH and NaOH, is used as an electrolyte while a porous membrane is employed as a separator, operates under high pH conditions (i.e., alkaline conditions), allowing the use of non-precious metal catalysts, such as iron and nickel, in addition to noble metal catalysts, such as ruthenium and platinum. This makes AWE a superior choice to PEMWE in terms of cost-effectiveness. However, the cell performance of AWE is inferior to that of PEMWE, and thus, AWE requires large-scale devices to achieve the desired performance. Moreover, using liquid electrolyte solutions in AWE can result in electrolyte leakage and difficulties when implementing high-voltage operations, unlike in PEMWE, where a polymer membrane is used as the electrolyte.6,7
Recently, extensive research has been aimed at developing anion exchange membrane (AEM)-based alkaline water electrolysis (AEMWE), which permits the use of non-precious metal catalysts, with a specific emphasis on AEMs as a key component. This system combines the advantages of both PEMWE and AEMs while employing an AEM both as an electrolyte and as a separator, thus enabling its operation in alkaline solutions. Thus, AEMWE combines the exceptional cost-effectiveness of AWE with the compact device structure and rapid start-up of PEMWE.
However, the low ionic conductivity of AEMs resulting from the limited ionic mobility of hydroxide ions (OH−) compared to protons, combined with their high reactivity, makes AEMs susceptible to degradation. Consequently, AEMWE typically exhibits low cell performance and durability.8
Against this backdrop, researchers have actively pursued the development of novel AEMs with improved ionic conductivity and alkaline (or chemical) stability to enhance the performance of AEMWE. For example, it has been accepted that polymer-based AEMs, when their morphology is enhanced through structural control, exhibit improved ion transport due to the formation of well-developed ion channels, and this enhanced morphology also contributes to improved chemical stability. A previous study reported that AEMs in which the polymer side chains were grafted with multi-cations as ion-conducting head groups exhibited improved ionic conductivity and more pronounced ion channels. This improvement was attributed to the increased number of ion-conducting head groups within each chain.9–11 Other strategies to achieve improved ionic conductivity through phase separation include the introduction of multiblock copolymers,12,13 the incorporation of fluorinated substituents,14–16 and the use of spacer-type ion-conducting head groups.11,17,18
Among these strategies, spacer-type AEMs, in which the main polymer backbone is linked to ion-conducting head groups through long alkyl chains, demonstrate well-developed continuous ion channels due to the robust connection between the ion-conducting head groups with enhanced flexibility compared to main chain-type AEMs, in which ion-conducting head groups are directly fixed on the polymer backbone. This unique configuration results in remarkable ionic conductivity and alkaline stability in spacer-type AEMs.17,19,20
The structure and type of the polymer backbone are known to significantly affect not only the ionic conductivity of AEMs but also their mechanical and chemical characteristics. Various polymer main backbones have been developed to implement novel high-performance AEMs. Among others, poly(aryl piperidinium) (PAP)-based AEMs have recently gained growing attention due to their excellent thermomechanical properties and the chemical stability achieved by the rigid polyarylene backbone.15,21,22 These PAP-based AEMs also exhibit high ion exchange capacity (IEC) values, resulting in enhanced ionic conductivity, as well as remarkable cell performance.
Yet, most of the recently developed PAP-based AEMs feature a main chain-type polymer structure in which piperidinium head groups are directly fixed to the polymer backbone. Therefore, much recent research has been directed toward developing AEMs based on spacer-type polymers, where ion-conducting head groups are separated from the polymer backbone. Polyfluorene14,23 and polycarbazole24,25 fall within this category, i.e., spacer-type polymers. Despite featuring a polyarylene structure, both polymers achieve excellent ionic conductivity even though their IEC values are lower than those of typical PAP-based AEMs. This achievement is attributed to the introduction of the spacer-type ion-conducting head groups.
Nevertheless, polyfluorene- and polycarbazole-based AEMs are also known for their low elasticity and hence high brittleness and, which result from the absence of flexible units within the polymer backbone, similar to typical PAP-based AEMs. Accordingly, when employed in water electrolysis cells subjected to repeated cycles of high-temperature operations (70–80 °C), these membranes may undergo mechanical failure, including pinhole formation, due to their intrinsic low elasticity. This may ultimately result in degraded cell performance and durability.26,27
In addition to polyarylene, aliphatic hydrocarbon-based polymers, such as poly(styrene-b-ethylene-co-butylene-b-styrene) (SEBS)28,29 and polynorbornene,30,31 have also garnered significant attention as promising AEM materials due to their highly chemically stable polymer backbones. Unlike polyarylene, these materials contain numerous flexible alkyl chains within their polymer backbones, enhancing elasticity and water uptake (WU). Generally, water within a membrane tends to hydrate with the hydroxide ions, preventing them from attacking the polymer backbone and ion-conducting head groups. This mechanism makes aliphatic hydrocarbon-based AEMs highly resistant to alkaline conditions.32,33 However, the high WU of aliphatic hydrocarbon-based polymers negatively affects the dimensional and mechanical stabilities of the resulting AEMs. Furthermore, excessively high WU may cause the so-called dilution effect, wherein ionic conductivity degrades rather than improves.34,35
In efforts to address these limitations, various strategies have been employed, including the incorporation of spacer-type ion-conducting head groups into SEBS for improved ionic conductivity, as well as the introduction of hydrophobic functional groups36 and crosslinks37 aimed at not only achieving microphase separation but also enhancing the physiochemical properties of the resulting membranes. In particular, SEBS-based crosslinked AEMs have advantageous water absorption and mechanical properties that can be adjusted as desired by tuning the degree of crosslinking.
The crosslinking of SEBS is primarily achieved by inducing crosslinking within the SEBS component and crosslinking SEBS with another polymer with a rigid structure. In the latter method, the chemical combination of two different polymers with distinctive mechanical properties through crosslinking produces a membrane that combines the excellent mechanical strength of the rigid polymer with the remarkable elasticity of SEBS. Crosslinked PPO-SEBS (xTQAn-PPO-SEBS)38 and crosslinked poly(m-terphenyl N-methyl piperidine)-SEBS (x-PmTP-SEBS)39 fall within this category. These membranes exhibit exceptional mechanical characteristics thanks to the introduction of crosslinking. However, PPO and poly(m-terphenyl) are main chain-type polymers, and thus, after crosslinking with SEBS, a spacer-type polymer, their ion-conducting head groups undergo limited rotational freedom or flexibility.
The present study aimed to develop high-performance crosslinked AEMs with high ionic conductivity and enhanced chemical and mechanical stability by crosslinking polycarbazole, one of the most widely used spacer-type polymers, with SEBS, another spacer-type polymer. Crosslinking polycarbazole and SEBS using ion-conducting head groups was expected to address the known mechanical weaknesses of the two spacer-type polymers while enhancing the flowability of the ion-conducting head groups. This, in turn, would result in the formation of well-developed ion channels, enhancing ionic conductivity and improving alkaline stability, ultimately contributing to enhanced cell durability (Fig. 1).
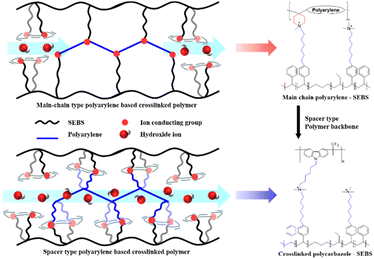 | ||
| Fig. 1 Illustrations and structure of main chain-type and spacer-type crosslinked polyarylene-SEBS membrane. | ||
Hence, crosslinked polycarbazole–SEBS (x-Car-SEBS) membranes with varying degrees of crosslinking were fabricated by adjusting the proportions of the polycarbazole and SEBS polymers. In addition, the effect of the degree of crosslinking on the physicochemical, morphological, and electrochemical properties of the resulting AEMs was investigated.
Experimental
Materials
9-(6-Bromohexyl)-9H-carbazole (99%) was purchased from KandY (Gyeongi-do, Republic of Korea). Trifluoromethanesulfonic acid (98%) and dimethylamine (ca. 10% in tetrahydrofuran, ca. 2 mol L−1) were purchased from TCI (Tokyo, Japan). Triethylsilane (98%) was purchased from Alfa Aesar (Haverhill, MA, USA). Aluminum chloride (99%), trimethylamine solution (45 wt% in H2O), 6-bromohexanoyl chloride (97%), and 1,1,1-trifluoroacetone (97%) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Poly(styrene-b-ethylene-co-butylene-b-styrene) (SEBS, A1535H) with 57 wt% styrene was obtained from Kraton (Houston, TX, USA). All chemicals were directly used without further purification.Fabrication of crosslinked polycarbazole-SEBS membranes with different degrees of crosslinking (x-Car-SEBS)
Dimethylamine-functionalized polycarbazole 2, bromohexyl SEBS 3, and HPLC-grade chloroform (15 mL) were placed in 20 mL vials, and the mixture was stirred until a homogenous solution was obtained. Polymers 2 and 3 were used (total: 0.9 g) as follows: dimethylamine-functionalized carbazole 2 was used at ratios of 30 mol%, 40 mol%, and 50 mol% relative to the bromohexyl SEBS 3. The polymer solution was heated at 40 °C for 6 hours to induce crosslinking. The solution was poured into a glass Petri dish through a cotton filter before it was allowed to dry at room temperature (r.t.) for 24 hours. The membranes were peeled from the Petri dish and immersed in deionized water (DI) water to remove residual solvent. Next, the membranes were soaked in trimethylamine (TMA) solution at 40 °C for 24 hours. Then, the membranes were washed with DI water to remove excess TMA. Finally, the membranes were immersed in 1 M KOH solution for at least 12 hours at room temperature to exchange Br− ions for OH− ions and washed with DI water more than three times to remove excess KOH before any measurements were performed. All the prepared solutions were purged with N2 gas before any measurements.Measurement and characterization
Polymer synthesis, characterization, and measurement methods are included in the ESI.†Results and discussion
Synthesis of dimethylamine-functionalized polycarbazole and bromohexyl SEBS
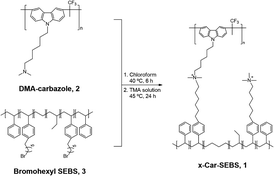
Crosslinked polycarbazole–SEBS (x-Car-SEBS, 1) was synthesized by crosslinking two precursor polymers: dimethylamine-functionalized polycarbazole (DMA-carbazole, 2) and bromohexyl SEBS 3 (Scheme 1).DMA-carbazole 2 was synthesized as follows. First, 9-(6-bromohexyl)-9H-carbazole and 1,1,1-trifluoroacetone were subjected to acid-catalyzed polymerization to synthesize polycarbazole 4. Subsequently, polycarbazole 4 was reacted with dimethylamine to introduce amine functional groups. As a result, DMA-carbazole 2 was obtained (Scheme S1†).
Next, bromohexyl SEBS 3 was obtained according to the synthetic route reported in a previous study. More specifically, bromohexanoyl functional groups were incorporated into SEBS, a commercial product, through Friedel–Crafts acylation, followed by the reduction of carbonyl groups (Scheme S1†).36,37
The structure of the precursor polymers was analyzed using 1H NMR spectroscopy. In the 1H NMR spectrum of polycarbazole 4, the relative integral ratio of aromatic hydrogen (H1), –CH2Br hydrogen (H9), and –CH3 hydrogen (H10) peaks was about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, confirming the successful synthesis (Fig. S1a†). After the dimethylamine treatment, the –CH2Br hydrogen peak (H9 in Fig. S1a†) disappeared, and a new N–CH3 hydrogen peak (H11 in Fig. S1b†) appeared. This demonstrates the replacement of the Br functional groups by DMA functional groups in a stoichiometric manner.
3, confirming the successful synthesis (Fig. S1a†). After the dimethylamine treatment, the –CH2Br hydrogen peak (H9 in Fig. S1a†) disappeared, and a new N–CH3 hydrogen peak (H11 in Fig. S1b†) appeared. This demonstrates the replacement of the Br functional groups by DMA functional groups in a stoichiometric manner.
Meanwhile, the synthesis of bromohexyl SEBS 3 was confirmed as follows: after pristine SEBS was subjected to Friedel–Crafts acylation, new peaks, including aromatic hydrogen peaks (H6,7) at 7.95–7.40 ppm and a –CH2Br hydrogen peak (H1), emerged, confirming the successful incorporation of the bromohexanoyl functional groups (Fig. S2a†). It was also determined that bromohexanoyl functional groups were introduced at a ratio of 70%, corresponding to a functionalization degree of 70%, based on the relative integral ratio of H6,7 and H9,10. As shown in Fig. S2c,† after the reduction process, the aromatic hydrogen peaks (H6,7 in Fig. S2b†) and the carbonyl alpha position hydrogen peak (H5 in Fig. S2b†) disappeared, while a new hydrogen peak (H5′ in Fig. S2c†) emerged. These changes provide solid evidence for the successful synthesis of bromohexyl SEBS.
Membrane fabrication and characterization
The x-Car-SEBS membrane 1 was obtained as follows: DMA-carbazole 2 and bromohexyl SEBS 3 were subjected to an in situ membrane casting process, followed by thermal crosslinking. Subsequently, the obtained membrane was treated with trimethylamine to convert any remaining unreacted Br functional groups into quaternary ammonium. By adjusting the proportion of the two precursor polymers 2 and 3, x-Car-SEBS membranes with varying degrees of crosslinking, 30%, 40%, and 50%, were fabricated. The obtained membranes were named 30x-Car-SEBS, 40x-Car-SEBS, and 50x-Car-SEBS, based on the degree of crosslinking. All membranes were transparent films with a thickness ranging from 40 to 45 μm (Fig. S3†).The gel fraction of the obtained x-Car-SEBS membranes was measured, and all three membranes exhibited a weight retention of 97% or higher after chloroform treatment. This observation confirms that the x-Car-SEBS membranes feature highly efficient crosslinking due to the reaction between DMA-carbazole and bromohexyl SEBS (Table S1†).
The structure of the x-Car-SEBS membranes with varying degrees of crosslinking was analyzed using FTIR spectroscopy. As the degree of crosslinking increased, the intensity of the C–H stretching peak at 3000–2800 cm−1 and the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending peak at 1600–1400 cm−1 attributed to bromohexyl SEBS decreased. In addition, the C–N stretching peak at 1200–1000 cm−1 attributed to DMA-carbazole increased in intensity (Fig. S4†).
C bending peak at 1600–1400 cm−1 attributed to bromohexyl SEBS decreased. In addition, the C–N stretching peak at 1200–1000 cm−1 attributed to DMA-carbazole increased in intensity (Fig. S4†).
Morphological analysis
Morphology is crucial in determining how ionic clusters form and develop in AEMs, affecting their hydroxide conductivity and AEMWE single-cell performance.11,14,40,41 In the present study, the formation of ionic clusters in the developed x-Car-SEBS membranes was analyzed by TEM.The TEM images in Fig. 2a–c show that the number of ionic clusters increases with increased crosslinking. This suggests that the ion-conducting head groups become more aligned as the crosslinking increases. However, 50x-Car-SEBS exhibited less pronounced phase separation due to an excessively high degree of crosslinking than 40x-Car-SEBS, resulting in the aggregation of ionic clusters. This phenomenon is frequently observed when crosslinking two different polymers with distinct properties.42,43 Therefore, the most pronounced phase separation was achieved in 40x-Car-SEBS, with crosslinking at 40%.
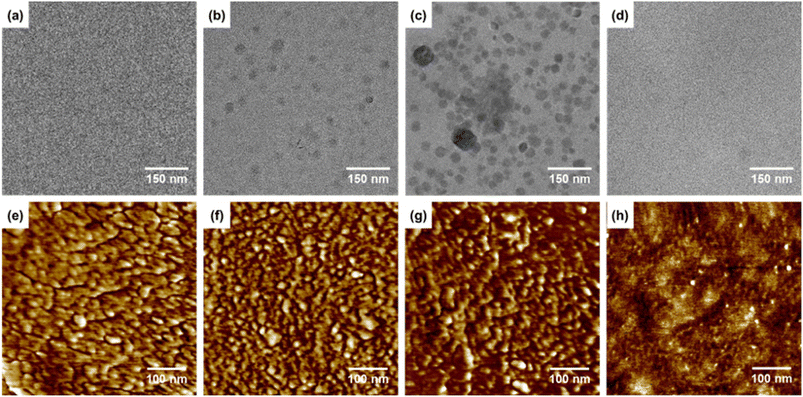 | ||
| Fig. 2 (a–d) TEM and (e–g) AFM images of 30x-Car-SEBS, 40x-Car-SEBS, 50x-Car-SEBS, and QA-SEBS membranes. | ||
Ion channel formation in the x-Car-SEBS membranes was further examined by performing AFM surface analysis. The ion channels observed in the TEM images were hydrophilic domains composed of ionic clusters. The AFM analysis confirmed that more ion channels developed with increasing crosslinking in the x-Car-SEBS membranes. Notably, 40x-Car-SEBS also exhibited the most pronounced phase separation, similar to the TEM analysis. Excessive ion channel development was observed in 50x-Car-SEBS, which had the highest degree of crosslinking (Fig. 2e–g). It has been reported that excessive ion channel development may attract too much water and cause a ‘dilution effect’ hindering ion transport.34,44
Moreover, the morphology of the crosslinked x-Car-SEBS membranes was compared with the non-crosslinked SEBS membrane (QA-SEBS). As shown in Fig. 2d and h, the QA-SEBS showed less well-developed phase separation with poor ionic cluster formation than the crosslinked x-Car-SEBS membranes. This result indicates that crosslinking of two spacer-type polymers successfully enhanced the microphase separation of the corresponding membranes.34,44
Ion exchange capacity (IEC), water uptake (WU), swellingratio (SR), and hydroxide ion conductivity
The IEC, which signifies the number of ion-conducting head groups within an AEM, is one critical factor that determines the WU, SR, and hydroxide conductivity of an AEM. However, excessive IEC results in an excessive increase in both WU and SR, degrading the ion-conducting efficiency. Therefore, it is critical to develop AEMs with suitable IEC values. To this end, in the present study, the IEC of the AEMs was varied by adjusting the proportion of the two precursor polymers, DMA-carbazole and bromohexyl SEBS, i.e., the degree of crosslinking.The theoretical IEC values of the x-Car-SEBS membranes with different degrees of crosslinking were estimated from the mole number ratio of Br functional groups within bromohexyl SEBS and DMA functional groups within DMA-carbazole determined based on the 1H NMR spectra. The theoretical IEC values of 30x-, 40x-, and 50x-Car-SEBS were 1.84, 1.71, and 1.61 meq. g−1, respectively (Table 1). The theoretical IEC values tended to decrease with an increased degree of crosslinking, and this tendency is attributed to the decreased content of bromohexyl SEBS, into which ion-conducting head groups are incorporated.
| IEC (meq. g−1) | Water uptake (%) | Swelling ratio (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| The. | Exp. | 20 °C | 80 °C | 20 °C (Δl) | 80 °C (Δl) | 20 °C (Δt) | 80 °C (Δt) | |
| 30x-Car-SEBS | 1.84 | 1.82 ± 0.02 | 65.7 | 83.9 | 20.9 | 28.6 | 30.2 | 38.5 |
| 40x-Car-SEBS | 1.71 | 1.70 ± 0.03 | 58.5 | 70.8 | 18.2 | 23.6 | 27.3 | 34.2 |
| 50x-Car-SEBS | 1.61 | 1.61 ± 0.01 | 25.9 | 50.0 | 11.4 | 14.5 | 20.0 | 29.5 |
Next, the experimental IEC values were measured using acid-base back titration. The experimental IEC values of 30x-, 40x-, and 50x-Car-SEBS were 1.82, 1.70, and 1.61 meq. g−1, respectively. In all three x-Car-SEBS membranes, the theoretical and experimental IEC values were comparable. This finding confirms that the crosslinking of the two polymers proceeded in a stoichiometric manner, successfully achieving the desired levels.
Afterward, the WU and SR values of the x-Car-SEBS membranes were measured at 20–80 °C to investigate the effect of the degree of crosslinking their water absorption properties (Fig. 3a, b and Table S2†). In all three x-Car-SEBS membranes, WU and SR values tended to decrease with increasing crosslinking. This tendency is attributed to the reduced IEC values that result from increasing the crosslinking. This translates into a decrease in ion-conducting head groups, resulting in reduced membrane hydrophilicity.
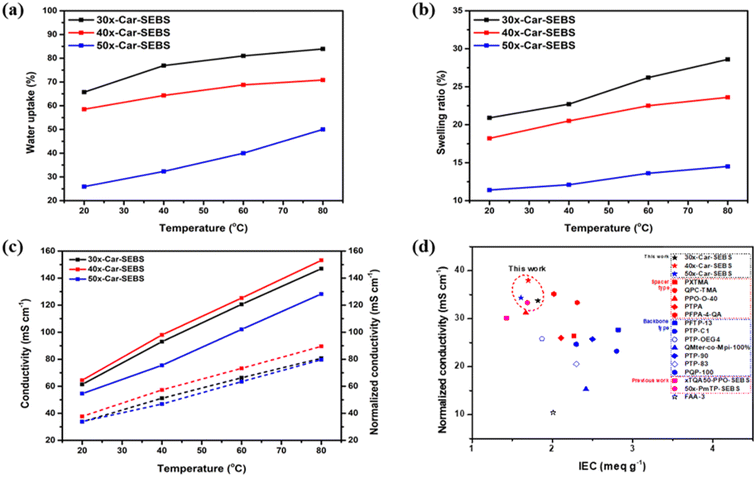 | ||
| Fig. 3 (a) Water uptake, (b) swelling ratio, and (c) hydroxide conductivity (solid line) and normalized conductivity (dashed line) of x-Car-SEBS membranes with different degrees of crosslinking with respect to temperature, and (d) comparison of normalized conductivity for the x-Car-SEBS membranes and other representative membranes from the literature.16–18,21,22,24,38,39,45–48 | ||
Additionally, the quaternary ammonium-functionalized SEBS (QA-SEBS) was also prepared following the procedure reported29,36 as a non-crosslinked control membrane, and the WU and SR of QA-SEBS were compared with the x-Car-SEBS membranes (Table S2†). The QA-SEBS membrane showed much higher WU and longer and thicker SR than x-Car-SEBS at all temperatures measured. This indicates that the dimensional stabilities of the crosslinked x-Car-SEBS membranes were much higher than their non-crosslinked counterpart, QA-SEBS.
Next, the hydroxide conductivity of each membrane was measured in the temperature range between 20 and 80 °C (Fig. 3c). The 40x-Car-SEBS membrane exhibited the highest hydroxide conductivity (153.16 mS cm−1 at 80 °C) despite having lower IEC and WU values than 30x-Car-SEBS across the entire temperature range (solid line in Fig. 3c). This improvement can be attributed to the enhanced morphological properties of 40x-Car-SEBS, which is characterized by well-developed ion channels, resulting in a more pronounced phase separation, as previously shown by the TEM and AFM analysis.
In addition, the normalized conductivities of the three x-Car-SEBS membranes were evaluated by dividing their ionic conductivities by the corresponding IEC values. The 40x-Car-SEBS membrane exhibited the highest normalized conductivity from 37.94 to 90.09 mS cm−1 at 20–80 °C (Fig. 3c and Table S3†). This confirmed the superiority of 40x-Car-SEBS in terms of ionic conductivity and ion-conducting efficiency. In contrast, 50x-Car-SEBS exhibited undesirable ion-conducting efficiency, which could be attributed to the aggregation of ionic clusters, as previously confirmed by the morphological analysis (Fig. 2d).
Moreover, the hydroxide conductivity and normalized conductivity of the crosslinked x-Car-SEBS membranes were compared with the non-crosslinked QA-SEBS membrane (Table S3†). Although the QA-SEBS has a higher IEC value, it showed lower and normalized conductivity than all three cross-linked x-Car-SEBS membranes. These results were ascribed to the well-developed microphase separation of x-Car-SEBS membranes caused by crosslinking of two spacer-type polymers.
Furthermore, the normalized conductivity values of the x-Car-SEBS membranes developed in this work were compared with those of spacer-type AEMs16–18,24,45 and main chain-type AEMs21,22,46–48 from the literature, as well as crosslinked AEMs including a previous study by the authors of the present study (Fig. 3d).38,39 As expected, the spacer-type AEMs exhibited higher normalized conductivity than the main chain-type AEMs, demonstrating their superior ion-conducting efficiency. The observed enhancement in spacer-type AEMs can be attributed to the long alkyl chains between the ion-conducting head groups and the polymer backbone. Meanwhile, despite their low IEC values, the x-Car-SEBS membranes exhibited even higher normalized conductivity than xTQA50-PPO-SEBS and 50x-PmTP-SEBS, which were fabricated by crosslinking main chain-type polymers with spacer-type SEBS.
These results can be attributed to the use of the two spacer-type polymers, DMA-carbazole and bromohexyl SEBS, in the fabrication of the x-Car-SEBS membranes, which enhance the alignment of ion-conducting head groups compared to main chain- and spacer-type AEMs. Additionally, the x-Car-SEBS membranes exhibit improved flowability, resulting in more efficient ion channel formation than the existing AEMs.
Mechanical and thermal properties
In the AEMWE system, an AEM serves as a medium to conduct hydroxide ions generated through catalytic reactions and as a physical barrier to isolate the cathode from the anode, preventing short circuits. Accordingly, membrane damage or deformation under operation conditions may degrade cell performance and durability and introduce safety concerns, such as the risk of short circuits. In this context, achieving the desired performance, durability, and safety of AEMWE requires the development of AEMs with mechanical and thermal properties suitable for the operating conditions of the WE cell.The mechanical properties of the x-Car-SEBS membranes were evaluated based on their stress–strain curves. As shown in Fig. 4a and Table S4,† the tensile strengths of 30x-Car-SEBS, 40x-Car-SEBS, and 50x-Car-SEBS in their Br− form fell within the range of 20.1 to 26.4 MPa, while their Young's moduli varied from 147.3 to 245.4 MPa. In summary, the trend of increasing mechanical strength with an increasing degree of crosslinking aligns with observations of the crosslinked membranes, where both Young's modulus and tensile strength increase with an increasing degree of crosslinking.43,49 Afterward, the mechanical properties of the x-Car-SEBS membranes in their OH− form, in which AEMWE operates, were further measured. The results showed that [x-Car-SEBS membranes][OH−] exhibited a similar trend to [x-Car-SEBS membranes][Br−] (Fig. 4a and Table S3†). In all x-Car-SEBS membranes, regardless of the degree of crosslinking, tensile strength was lower, while elongation at break was higher for OH− anions than Br− anions. This phenomenon can be attributed to the exchange of the counterions of the AEM with hydrophilic OH− ions, resulting in enhanced hydration.
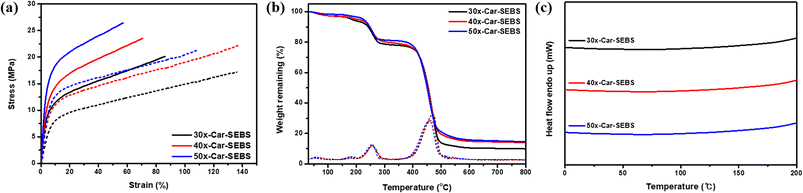 | ||
| Fig. 4 (a) Physical properties of Br− form (solid) and OH− form (dashed), (b) TGA graphs, and (c) DSC plots of x-Car-SEBS membranes with different degrees of crosslinking. | ||
Next, the tensile strength and elongation at break of the x-Car-SEBS membranes were compared with those of spacer-type AEMs,17,23,50 main chain-type AEMs,21,22,46–48 and crosslinked AEMs from the literature (Table S5†).38,39,51 Despite containing polycarbazole, a polyarylene-based polymer, the x-Car-SEBS membranes exhibited improved elongation at break (over 110%) compared to the other AEMs. The x-Car-SEBS membranes were superior to other AEMs in terms of elongation at break, even though the two groups exhibited comparable WU levels. This enhancement can be attributed to the long alkyl chains in the two crosslinked spacer-type polymers.52–55
As previously discussed, membranes used in cell operations require not only high tensile strength but also improved elasticity (preferably exceeding 100%) to prevent edge failure during prolonged cell operations under high-temperature and high-humidity conditions.26,27 Thus, the remarkable improvement in elongation at break observed in the x-Car-SEBS membranes was expected to enhance the durability of AEMWE cells. These findings suggest the possibility of effectively enhancing the mechanical properties of polyarylene-based AEMs, widely used for their performance in water electrolysis cells, by crosslinking them with SEBS, an elastic polymer component.
The thermal properties of the x-Car-SEBS membranes were examined using TGA and DSC as follows (Fig. 4b and c). In the TGA curves of all three x-Car-SEBS membranes, weight loss occurred in three distinct stages: the first loss was attributed to the evaporation of water within the membrane (below 120 °C), the second weight loss resulted from the degradation of ion-conducting groups (150–300 °C), and the third weight loss was due to polymer backbone degradation (over 350 °C). In the first and second stages, the weight loss was smaller when the degree of crosslinking was greater. This pattern was consistent with the WU and IEC measurements; both WU and IEC values decreased with increased crosslinking.
Meanwhile, in the DSC curves of all three membranes, the glass transition temperature (Tg) of SEBS, normally occurring at 40–70 °C, was not observed. The absence of Tg was attributed to the crosslinking of DMA-carbazole and SEBS. Simply put, the introduction of crosslinks enhanced the thermal stability of SEBS.
The TGA and DSC results indicated that the x-Car-SEBS membranes did not undergo the decomposition and structural degradation typically observed in polyarylene-based AEMs under the actual operating conditions of AEMWE cells, specifically between 60 to 80 °C. These findings confirm the significantly improved thermal properties of the three membranes, making them highly suitable for AEMWE cells.
Alkaline stability
Because AEMWE systems operate under alkaline conditions, unless AEMs demonstrate sufficient durability against alkalis, the resulting single-cell performance may deteriorate, ultimately resulting in reduced hydrogen production efficiency. Given the context, the chemical stability of an AEM plays a crucial role in determining the overall durability of AEMWE cells.The alkaline stability of the x-Car-SEBS membranes was assessed in a 2 M KOH solution at 80 °C over 840 hours. At intervals of 240 hours, the hydroxide conductivity was measured and used as an index for alkaline stability (Fig. 5a). All three x-Car-SEBS membranes retained a conductivity of 99% or higher compared to the initial levels even after 840 hours, demonstrating their remarkable alkaline stability. The enhanced alkaline stability of the x-Car-SEBS membranes can be attributed to the chemical crosslinking of SEBS and DMA-carbazole, which are different aryl ether-free polymers.
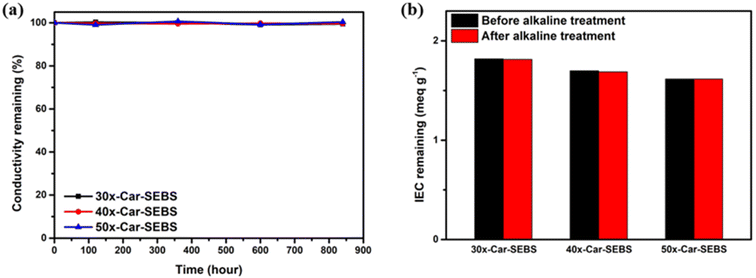 | ||
| Fig. 5 (a) Hydroxide ion conductivity and (b) IEC retention of x-Car-SEBS membranes with different degrees of crosslinking before and after alkaline treatment. | ||
After the alkaline stability tests, the x-Car-SEBS specimens were further analysed through IEC measurements and FTIR spectroscopy to assess whether the membranes had undergone chemical degradation. In all three membranes, the IEC values remained nearly unchanged even after the alkaline stability tests (Fig. 5b and Table S6†). Similarly, no new peaks emerged after the alkaline stability tests in the FTIR spectra of the three membranes (Fig. S5†).
Overall, the results of the IEC and FTIR analyses confirmed that the x-Car-SEBS membranes remained chemically stable, with no degradation of functional groups (or ion-conducting head groups) or polymer backbones, during immersion in a 2 M KOH solution at 80 °C for a total of 840 hours.
Next, the alkaline stability of the x-Car-SEBS membranes was compared with that of the other membranes, which are known for their excellent ionic conductivities, including spacer-type AEMs,16,17,24,45,50,56,57 main chain-type AEMs,21–23,47,48,58 and the main chain-spacer-type crosslinked AEMs previously developed by our group (Table S7†).38,39 As expected, spacer-type AEMs demonstrated superior alkaline stability to main chain-type AEMs because the former exhibited more pronounced phase separation resulting from the well-aligned hydrophilic ion-conducting head groups, as previously observed in the assessment of ion-conducting properties.
The x-Car-SEBS membranes, crosslinked membranes composed of two different spacer-type polymers, demonstrated even better alkaline stability than spacer-type and main chain-type AEMs, although the measurements were performed under harsher conditions. This significant improvement can be attributed to the introduction of crosslinks, which further enhance alkaline stability, a well-known mechanism.10,41,55 The x-Car-SEBS membranes were even superior to xTQA50-PPO-SEBS and 50x-PmTP-SEBS, which are crosslinked AEMs composed of main chain- and spacer-type polymers, in terms of alkaline stability, despite the measurements being conducted in more basic conditions and for longer durations. These results demonstrate the suitability of the x-Car-SEBS membranes for use in the real-world operating conditions of AEMWE cells, particularly in high-temperature alkaline environments.
AEMWE single-cell performance
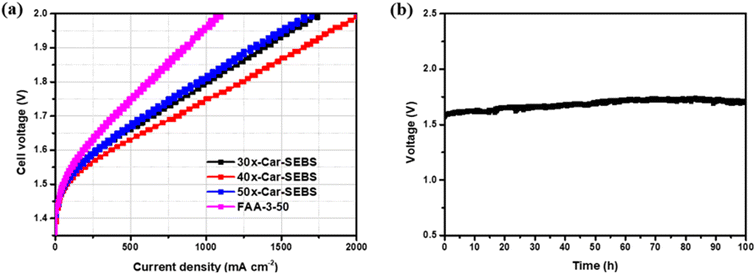
The x-Car-SEBS membranes, fabricated by crosslinking two distinctive spacer-type polymers, DMA-carbazole and bromohexyl SEBS, were subjected to AEMWE single-cell tests in a 1 M KOH solution at 70 °C. The AEMWE single-cell performance of the three membranes at 1.8 V was assessed, as shown in Fig. 6a. The current densities were 1022, 1249, and 980 mA cm−2 for 30x-Car-SEBS, 40x-Car-SEBS, and 50x-Car-SEBS, respectively. This pattern corresponds to that observed in the ion conductivity measurements of the x-Car-SEBS membranes, as presented in Table S3.† This finding suggests that employing AEMs with high ionic conductivity enhances the hydrogen production efficiency in AEMWE systems.In addition, the cell performance of the x-Car-SEBS membranes was compared with that of FAA-3-50, a commercial membrane. All three x-Car-SEBS membranes, regardless of the degree of crosslinking, exhibit significantly improved WE cell performance compared to FAA-3-50, which achieved a current density of 642 mA cm−2 at 1.8 V. Remarkably, 40x-Car-SEBS, exhibiting the best performance, demonstrated WE cell performance that was twice as high as that of FAA-3-50.
The AEMWE single-cell performance of the x-Car-SEBS membranes was compared with that of the other membranes, including spacer-type,23,24,50,56 main chain-type,22,47,48,51 and typical crosslinked AEMs,28,50,51,59 along with the main chain–spacer crosslinked AEMs previously developed the authors of the present study (Table S8†).
Given that AEMWE single-cell performance is affected by a wide range of factors, including the ion-conducting properties of the membranes and the types of ionomers and catalysts used for membrane electrode assembly (MEA) fabrication, as well as cell operating temperature and voltage, it is challenging to make direct comparisons among results reported by different researchers.60–62 However, the x-Car-SEBS membranes demonstrated single-cell performance superior or on a par with main chain- and spacer-type AEMs despite the use of FAA-3-SOLUT-10, a commercial ionomer known for its relatively low ionic conductivity. Notably, 40x-Car-SEBS, which demonstrated the best performance, exhibited cell performance approximately 50% higher than that achieved by the main chain–spacer crosslinked AEMs when measured at the same voltage.
This enhancement was attributed to the introduction of crosslinks, resulting in improved alignment among the ion-conducting head groups. Additionally, incorporating the two spacer-type polymers increased the flowability of the ion-conducting head groups while contributing to the formation of well-developed ion-conducting channels.
Finally, the cell durability of 40x-Car-SEBS, which demonstrated the best cell performance among the x-Car-SEBS membranes, was assessed using long-term durability tests (Fig. 6b). The measurements were performed in a 1 M KOH solution at 70 °C with a constant current of 0.2 A cm−2. After 100 hours, the cell voltage of 40x-Car-SEBS was 1.698 V, with a minimal average increasing rate of approximately 1.03 mV h−1. Simply put, their operation remained stable throughout the entire 100 hours duration.
After the durability tests, the 40x-Car-SEBS specimens were further assessed through IEC measurements and FTIR spectroscopy to determine whether the membrane had experienced chemical degradation (Fig. S6†). As shown in Fig. S6a,† 40x-Car-SEBS exhibited comparable IEC values before and after the durability tests, 1.70 ± 0.03 and 1.70 ± 0.04 meq. g−1, respectively. Similarly, as shown in Fig. S6b,† there was no noticeable difference in their FTIR spectra before and after the durability tests. These results confirm that 40x-Car-SEBS exhibits enhanced chemical stability, making it well-suited to withstanding the AEMWE durability tests. Consequently, the minor cell performance degradation observed in the membrane after 100 hours of durability tests may be ascribed to factors beyond chemical degradation, including electrochemical catalysts, compatibility with ionomers, electrolyte type, and membrane–catalyst interfacial compatibility.60–62
Additionally, the AEMWE cell durability of the 40x-Car-SEBS membrane was evaluated at 0.5 A cm−2 (Fig. S7a†). The 40x-Car-SEBS membrane showed a 4.40 mV h−1 voltage increase for 40 h, and the AEMWE cell performance at 2.0 V was reduced to 1.50 A cm−2 after the durability test (Fig. S7b†). This is a decrease of 20% compared to the WE cell data before the durability test (1.90 A cm−2).
The ohmic resistance of 40x-Car-SEBS was then measured after the durability tests at 0.5 A cm−2 using in situ EIS, and the results were compared with the ohmic resistance before the durability test. A similar resistance of 15.1 mΩ was found after the durability test compared to the resistance before the durability test (15.5 mΩ). This strongly indicates that the 40x-Car-SEBS membrane did not degrade during the durability test even under 0.5 A cm−2 at 70 °C. The reduction in AEMWE cell performance over time was likely caused not by the degradation of the membrane but rather other factors, such as a degradation of the catalyst and ionomer or defects at the membrane–catalyst interface.
Conclusions
The present study developed a series of x-Car-SEBS membranes by chemically crosslinking two spacer-type polymers with distinctive properties. Their ionic conductivity, morphology, and cell performance were assessed while varying the proportion of the two polymers. Crosslinking the two polymers made the developed x-Car-SEBS suitable for the membrane-forming process. In addition, the obtained membranes exhibited remarkable dimensional stability (SR of 14.5–28.6% at 80 °C), exceptional mechanical properties (tensile strength of 17.2–22.3 MPa and elongation at break of 109.6–138.3%), and improved thermal stability.The x-Car-SEBS membranes demonstrated more pronounced phase separation than the main chain–spacer crosslinked AEMs previously developed by us. This was attributed to the flowability of the ion-conducting head groups achieved by the intrinsic structure of the spacer-type polymers, coupled with the enhanced alignment of ion-conducting head groups resulting from the introduction of crosslinks. Notably, the results of TEM and AFM analyses confirmed that the 40x-Car-SEBS membrane with a degree of crosslinking of 40% exhibited the most well-distributed ionic clusters and ion channels.
The x-Car-SEBS membranes demonstrated exceptional alkaline stability because of their significantly enhanced morphology, with over 99% retention in conductivity and IEC after immersion in a 2 M KOH solution at 80 °C for 840 hours. Remarkably, 40x-Car-SEBS, which displayed the most pronounced phase separation, exhibited excellent ion-conducting performance. It achieved a hydroxide conductivity of 153.16 mS cm−1 at 80 °C despite its low IEC value compared to main chain- and spacer-type AEMs.
All three x-Car-SEBS membranes exhibited enhanced AEMWE single-cell performance (30x-Car-SEBS: 1022 mA cm−2, 40x-Car-SEBS: 1249 mA cm−2, and 50x-Car-SEBS: 980 mA cm−2 @ 1.8 V). The 40x-Car-SEBS membrane, which exhibited the best performance, achieved cell performance approximately twice that of FAA-3-50, a commercial AEM. Furthermore, 40x-Car-SEBS was confirmed to be highly durable through AEMWE durability tests conducted at a constant current for 100 hours. The membrane achieved a minimal voltage increasing rate of 1.03 mV h−1 throughout a 100 hours period.
As applied in this study, the method of crosslinking two different spacer-type polymers proves to be an effective approach in addressing the limitations of AEMs based on main chain-type polymers, which have recently been drawing increasing research attention, i.e., their insufficient ionic conductivity, alkaline stability, and film-forming ability. Furthermore, the developed method is expected to contribute to developing high-performance, high-durability AEMWE systems.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a grant from the Industrial Strategic Technology Development Program (20015599) financed by the Ministry of Trade, Industry, and Energy (MOTIE, Republic of Korea). Part of this work was also supported by the Core Research Institute (CRI) Program, the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1A6A1A06015181).Notes and references
- A. Nicita, G. Maggio, A. P. F. Andaloro and G. Squadrito, Int. J. Hydrogen Energy, 2020, 45, 11395–11408 CrossRef CAS.
- T. Terlouw, C. Bauer, R. McKenna and M. Mazzotti, Energy Environ. Sci., 2022, 15, 3583–3602 RSC.
- C. Lamy, Int. J. Hydrogen Energy, 2016, 41, 15415–15425 CrossRef CAS.
- X. Sun, K. Xu, C. Fleischer, X. Liu, M. Grandcolas, R. Strandbakke, T. S. Bjørheim, T. Norby and A. Chatzitakis, Catalysts, 2018, 8, 657–697 CrossRef.
- M. Di Virgilio, A. Basso Peressut, V. Arosio, A. Arrigoni, S. Latorrata and G. Dotelli, Clean Technol., 2023, 5, 74–93 CrossRef.
- K. Zeng and D. Zhang, Prog. Energy Combust. Sci., 2010, 36, 307–326 CrossRef CAS.
- X. Hu, M. Liu, Y. Huang, L. Liu and N. Li, J. Membr. Sci., 2022, 663, 121005 CrossRef CAS.
- N. Chen and Y. M. Lee, Prog. Polym. Sci., 2021, 113, 101345 CrossRef CAS.
- J. Qian, C. Wang, X. Zhang, J. Hu, X. Zhao, J. Li and Q. Ren, J. Power Sources, 2023, 564, 232877 CrossRef CAS.
- T. Zhao, C. Long, Z. Wang and H. Zhu, ACS Appl. Energy Mater., 2021, 4, 14476–14487 CrossRef CAS.
- J. Zhang, W. Yu, X. Liang, K. Zhang, H. Wang, X. Ge, C. Wei, W. Song, Z. Ge, L. Wu and T. Xu, ACS Appl. Energy Mater., 2021, 4, 9701–9711 CrossRef CAS.
- Y. Pan, K. Jiang, X. Sun, S. Ma, Y. M. So, H. Ma, X. Yan, N. Zhang and G. He, J. Membr. Sci., 2021, 630, 119290 CrossRef CAS.
- S. Gong, L. Bai, L. Li, N. A. Qaisrani, L. Ma, G. He and F. Zhang, Int. J. Hydrogen Energy, 2021, 46, 2269–2281 CrossRef CAS.
- T. Kimura, A. Matsumoto, J. Inukai and K. Miyatake, ACS Appl. Energy Mater., 2020, 3, 469–477 CrossRef CAS.
- J. Wang, Y. Zhao, B. P. Setzler, S. Rojas-Carbonell, C. Ben Yehuda, A. Amel, M. Page, L. Wang, K. Hu, L. Shi, S. Gottesfeld, B. Xu and Y. Yan, Nat. Energy, 2019, 4, 392–398 CrossRef CAS.
- T. Wang, Y. Zhao, S. Wang, S. Cheng, S. Yang, H. Wei and Y. Ding, J. Power Sources, 2023, 557, 232590 CrossRef CAS.
- L. Liu, W. Ma, J. Zhang, Z. Liu, D. Chu, R. Shao, S. Chen, X. Chu and N. Li, J. Membr. Sci., 2023, 678, 121663 CrossRef CAS.
- D. Pan, S. Chen and P. Jannasch, ACS Macro Lett., 2023, 12, 20–25 CrossRef CAS PubMed.
- Q. Liu, W. Ma, L. Tian, J. Li, L. Yang, F. Wang, Z. Wang, J. Li, Z. Wang and H. Zhu, J. Power Sources, 2022, 551, 232105 CrossRef CAS.
- L. Li, J. Wang, M. Hussain, L. Ma, N. A. Qaisrani, S. Ma, L. Bai, X. Yan, X. Deng, G. He and F. Zhang, J. Membr. Sci., 2021, 624, 119088 CrossRef CAS.
- L. Liu, L. Bai, Z. Liu, S. Miao, J. Pan, L. Shen, Y. Shi and N. Li, J. Membr. Sci., 2023, 665, 121135 CrossRef CAS.
- M. Liu, X. Hu, B. Hu, L. Liu and N. Li, J. Membr. Sci., 2022, 642, 119966 CrossRef CAS.
- H. Lim, I. Jeong, J. Choi, G. Shin, J. Kim, T. H. Kim and T. Park, Appl. Surf. Sci., 2023, 610, 155601 CrossRef CAS.
- M. S. Cha, J. E. Park, S. Kim, S. H. Han, S. H. Shin, S. H. Yang, T. H. Kim, D. M. Yu, S. So, Y. T. Hong, S. J. Yoon, S. G. Oh, S. Y. Kang, O. H. Kim, H. S. Park, B. Bae, Y. E. Sung, Y. H. Cho and J. Y. Lee, Energy Environ. Sci., 2020, 13, 3633–3645 RSC.
- J. Li, Q. Liu, L. Tian, W. Ma, F. Wang, Z. Wang and H. Zhu, Int. J. Hydrogen Energy, 2022, 47, 32262–32272 CrossRef CAS.
- W. E. Mustain, M. Chatenet, M. Page and Y. S. Kim, Energy Environ. Sci., 2020, 13, 2805–2838 RSC.
- D. Qiu, L. Peng, X. Lai, M. Ni and W. Lehnert, Renewable Sustainable Energy Rev., 2019, 113, 109289 CrossRef CAS.
- Z. Xu, V. Wilke, J. J. Chmielarz, M. Tobias, V. Atanasov, A. S. Gago and K. A. Friedrich, J. Membr. Sci., 2023, 670, 121302 CrossRef CAS.
- A. Z. Al Munsur, I. Hossain, S. Y. Nam, J. E. Chae and T. H. Kim, J. Membr. Sci., 2020, 599, 117829 CrossRef CAS.
- D. Cao, F. Yang, W. Sheng, Y. Zhou, X. Zhou, Y. Lu, F. Nie, N. Li, L. Pan and Y. Li, J. Membr. Sci., 2022, 641, 119938 CrossRef CAS.
- S. Huang, X. He, C. Cheng, F. Zhang, Y. Guo and D. Chen, Int. J. Hydrogen Energy, 2020, 45, 13068–13079 CrossRef CAS.
- D. R. Dekel, M. Amar, S. Willdorf, M. Kosa, S. Dhara and C. E. Diesendruck, Chem. Mater., 2017, 29, 4425–4431 CrossRef CAS.
- Y. Zhang, W. Chen, X. Yan, F. Zhang, X. Wang, X. Wu, B. Pang, J. Wang and G. He, J. Membr. Sci., 2020, 598, 117650 CrossRef CAS.
- J. S. Olsson, T. H. Pham and P. Jannasch, J. Membr. Sci., 2019, 578, 183–195 CrossRef CAS.
- N. A. Qaisrani, L. Ma, M. Hussain, J. Liu, L. Li, R. Zhou, Y. Jia, F. Zhang and G. He, ACS Appl. Mater. Interfaces, 2020, 12, 3510–3521 CrossRef CAS PubMed.
- A. Z. Al Munsur, J. Lee, J. E. Chae, H. J. Kim, C. H. Park, S. Y. Nam and T. H. Kim, J. Membr. Sci., 2022, 643, 120029 CrossRef CAS.
- A. Z. Al Munsur, I. Hossain, S. Y. Nam, J. E. Chae and T. H. Kim, Int. J. Hydrogen Energy, 2020, 45, 15658–15671 CrossRef CAS.
- S. Sung, J. E. Chae, K. Min, H. J. Kim, S. Y. Nam and T. H. Kim, J. Mater. Chem. A, 2021, 9, 1062–1079 RSC.
- K. Min, J. E. Chae, Y. Lee, H. J. Kim and T. H. Kim, J. Membr. Sci., 2022, 653, 120487 CrossRef CAS.
- A. N. Lai, Y. Z. Zhuo, P. C. Hu, J. W. Zheng, S. F. Zhou and L. Zhang, Int. J. Hydrogen Energy, 2020, 45, 1998–2008 CrossRef CAS.
- C. Long, Z. Wang and H. Zhu, Int. J. Hydrogen Energy, 2021, 46, 18524–18533 CrossRef CAS.
- W. Chen, M. Mandal, G. Huang, X. Wu, G. He and P. A. Kohl, ACS Appl. Energy Mater., 2019, 2, 2458–2468 CrossRef CAS.
- A. N. Lai, D. Guo, C. X. Lin, Q. G. Zhang, A. M. Zhu, M. L. Ye and Q. L. Liu, J. Power Sources, 2016, 327, 56–66 CrossRef CAS.
- C. Hu, Q. Zhang, C. Lin, Z. Lin, L. Li, F. Soyekwo, A. Zhu and Q. Liu, J. Mater. Chem. A, 2018, 6, 13302–13311 RSC.
- R. Ma, Y. Kang, T. Wang, T. Jiang, H. yan Yin, C. Liu, H. Wei and Y. Ding, J. Membr. Sci., 2023, 678, 121667 CrossRef CAS.
- N. Chen, H. H. Wang, S. P. Kim, H. M. Kim, W. H. Lee, C. Hu, J. Y. Bae, E. S. Sim, Y. C. Chung, J. H. Jang, S. J. Yoo, Y. Zhuang and Y. M. Lee, Nat. Commun., 2021, 12, 2367 CrossRef CAS PubMed.
- X. Yan, X. Yang, X. Su, L. Gao, J. Zhao, L. Hu, M. Di, T. Li, X. Ruan and G. He, J. Power Sources, 2020, 480, 228805 CrossRef CAS.
- X. Hu, Y. Huang, L. Liu, Q. Ju, X. Zhou, X. Qiao, Z. Zheng and N. Li, J. Membr. Sci., 2021, 621, 118964 CrossRef CAS.
- N. Chen, C. Hu, H. H. Wang, J. H. Park, H. M. Kim and Y. M. Lee, J. Membr. Sci., 2021, 638, 119685 CrossRef CAS.
- M. Guo, T. Ban, Y. Wang, X. Wang and X. Zhu, J. Colloid Interface Sci., 2023, 638, 349–362 CrossRef CAS PubMed.
- N. Chen, S. Y. Paek, J. Y. Lee, J. H. Park, S. Y. Lee and Y. M. Lee, Energy Environ. Sci., 2021, 14, 6338–6348 RSC.
- A. K. Singh, P. Sharma, K. Singh and V. K. Shahi, J. Power Sources, 2022, 520, 230856 CrossRef CAS.
- J. Liao, X. Yu, Q. Chen, X. Gao, H. Ruan, J. Shen and C. Gao, J. Membr. Sci., 2020, 599, 117818 CrossRef CAS.
- Y. Xu, J. Yang, N. Ye, M. Teng and R. He, Eur. Polym. J., 2015, 73, 116–126 CrossRef CAS.
- J. J. Wang, W. T. Gao, Y. S. L. Choo, Z. H. Cai, Q. G. Zhang, A. M. Zhu and Q. L. Liu, J. Colloid Interface Sci., 2023, 629, 377–387 CrossRef CAS PubMed.
- X. Chu, Y. Shi, L. Liu, Y. Huang and N. Li, J. Mater. Chem. A, 2019, 7, 7717–7727 RSC.
- N. Yu, J. Dong, H. Li, T. Wang and J. Yang, Eur. Polym. J., 2021, 154, 110528 CrossRef CAS.
- N. Chen, C. Hu, H. H. Wang, S. P. Kim, H. M. Kim, W. H. Lee, J. Y. Bae, J. H. Park and Y. M. Lee, Angew. Chem., Int. Ed., 2021, 60, 7710–7718 CrossRef CAS PubMed.
- W. Lu, Z. Yang, H. Huang, F. Wei, W. Li, Y. Yu, Y. Gao, Y. Zhou and G. Zhang, Ind. Eng. Chem. Res., 2020, 59, 21077–21087 CrossRef CAS.
- Q. Xu, L. Zhang, J. Zhang, J. Wang, Y. Hu, H. Jiang and C. Li, EnergyChem, 2022, 4, 100087 CrossRef CAS.
- N. Du, C. Roy, R. Peach, M. Turnbull, S. Thiele and C. Bock, Chem. Rev., 2022, 122, 11830–11895 CrossRef CAS PubMed.
- P. Shirvanian, A. Loh, S. Sluijter and X. Li, Electrochem. Commun., 2021, 132, 107140 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of 1H NMR and FT-IR spectra of synthesized compounds, ion conductivity, cell data, SEM image and data tables. See DOI: https://doi.org/10.1039/d3ta05984g |
| This journal is © The Royal Society of Chemistry 2024 |