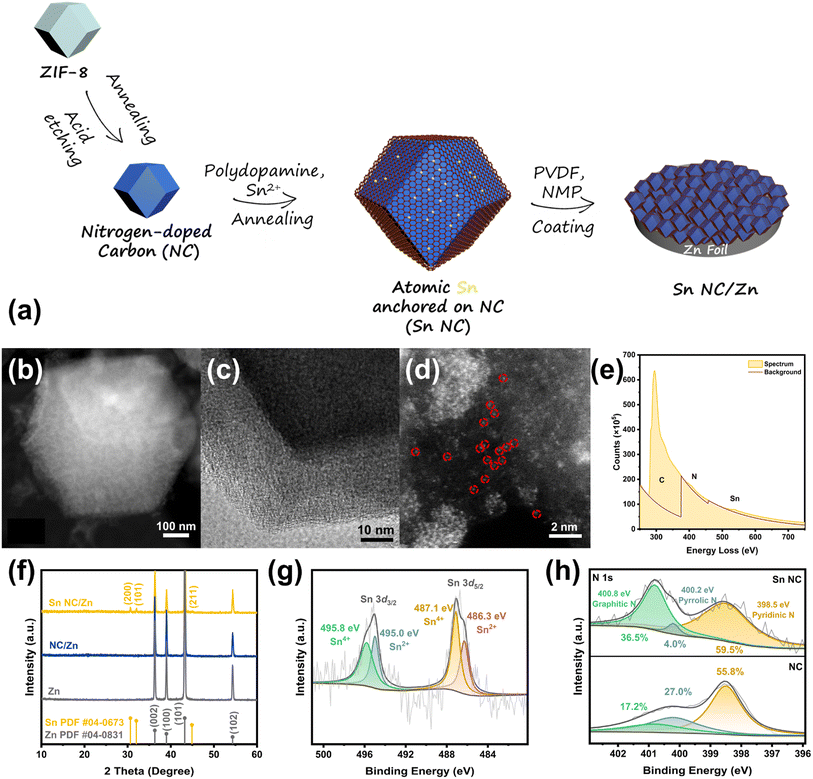
Atomic Sn sites on nitrogen-doped carbon as a zincophilic and hydrophobic protection layer for stable Zn anodes†
Yijie
Wang
,
Yan
Tan
and
Chuanwei
Cheng
 *
*
Shanghai Key Laboratory of Special Artificial Microstructure Materials and Technology, School of Physics Science and Engineering, Tongji University, Shanghai 200092, P. R. China. E-mail: cwcheng@tongji.edu.cn
First published on 25th November 2023
Abstract
Anodic dendrites and side reaction inhibition are crucial for high-performance, long-lifespan aqueous Zn-ion batteries. Herein, a multifunctional protection layer for stable zinc anodes, i.e., atomic Sn sites anchored on nitrogen-doped carbon supports (Sn NC), is reported. The Sn NC layer can guide Zn nucleation at a nano-level owing to rich atomic zincophilic sites and deliver an even electron distribution with facilitated charge transfer by conductive nitrogen-doped carbon (NC). A fast Zn2+ diffusion pathway is built owing to a gradient concentration field created by the hydrophobic/hydrophilic bi-layer configuration of porous Sn NC, leading to an oriented deposition within and at the surface of Sn NC. Accordingly, a surface-inside-interspace sequential deposition is achieved with a three-dimensional (3D) structured coating to ensure a smooth electrode surface in deep cycling. The hydrophobic surficial Sn–N–C layer further blocks water permeation to prevent hydrogen evolution. Therefore, an ultralow nucleation overpotential of 7.5 mV and a stable cycling performance (over 280 h@10 mA h cm−2) are achieved in symmetric cells. Both coin-type and pouch-type Zn//MnO2 full cells exhibit a high rate capability and superior long-term cycling performance. Our work presents a new insight into the design of interface engineering for robust metal anodes in advanced energy storage systems.
Introduction
The continuous expansion of application prospects of electrical energy has led to a high market demand for efficient and environment-friendly energy storage systems.1 Aqueous Zn-ion batteries (ZIBs) are currently a hotspot in academia and industry owing to their low redox potential (−0.762 V vs. standard hydrogen electrode), high theoretical capacity (820 mA h g−1), high safety, and metal resource abundance.2 However, ZIBs still suffer from performance decay in discharge capacity and coulombic efficiency (CE) caused by dendrite formation, electrode corrosion, and unfavorable side reactions at Zn anodes.3 The accumulation of Zn dendrites appears as surface protrusions and aggravates the roughness of the anode surface, further leading to the formation of electrochemically inactive “dead zinc” and even the puncturing of separators.4 Moreover, the disordered morphology of Zn anodes brings about an irregular distribution of electrons and a high local current density, as well as the subsequent hydrogen evolution reaction (HER) by interfacial water molecules.5 To tackle the above issues and create a smoother and purer zinc anode surface during cycling, numerous means have been devoted to optimizing the solid/liquid interface, including electrolyte additives,6–11 interface decoration layers,12–17 zincophilic hosts,18–21 and electrode structure design.22–24One major goal of dendrite and side reaction inhibition is to achieve uniform Zn nucleation at the anode surface while blocking the permeation of water molecules. To achieve this goal, creating rich zincophilic sites and building a large hydrophobic interface area are two key points to regulate solid/liquid reactions.25–28 For the former requirement, the conventional means is to design a zincophilic host for Zn deposition.29,30 However, it would increase the complexity of electrodes and cause severe side reactions and sometimes require the preloading of Zn.31,32 For the latter, the traditional artificial solid electrolyte interphase (SEI) layer cannot provide a structured interface towards the regulation of electric field distribution.33,34 Moreover, inorganic two-dimensional SEI layers cannot prevent Zn anodes from volume expansion and struggle with deep cycling owing to poor Zn2+ diffusion.35 Accordingly, designing an interface modification layer that can simultaneously achieve dendrite and side reaction inhibition under high-capacity cycling is essential and ideal for high-performance zinc electrodes.
The construction of zincophilic metal sites is a conventional approach to improving the nucleation and deposition behaviors in interface engineering.36 Previous studies have demonstrated that Sn nanoparticles or Sn–Zn alloys can be effective in reducing energy barriers in nucleation and the subsequent plating of Zn and inhibiting hydrogen evolution.29,37 However, larger Sn sites inevitably face the risk of dissolution and passivation in mild acidic aqueous electrolytes. Under such consideration, creating atomic zincophilic Sn metal sites in nitrogen-doped carbon (Sn–N–C) can perfectly meet the requirement to modulate Zn nucleation, with high metal utilization efficiency and electrochemical stability.38 Although single-atom materials have received extensive attention in electrocatalysis, their application in metal anode optimization is still at an early stage.
Herein, we design and achieve atomic Sn sites anchored on nitrogen-doped carbon supports (Sn NC) as a surface modification coating for Zn anodes with the merits of both a hydrophobic 3D structured artificial SEI layer and a zincophilic conductive support. The delicate composition and structure endow the as-fabricated Zn anode with the following features: (a) the atomic zincophilic Sn sites at the interface can function as the nucleation seeds to regulate Zn nucleation, while the conductive carbon support contributes to a uniform electric field distribution, resulting in the achievement of a nano-level control for stable and uniform Zn deposition; (b) the hydrophobic/hydrophilic bi-layer configuration of porous Sn NC creates a gradient distribution of electrolyte concentration and endows a fast Zn2+ diffusion path to achieve an oriented deposition within and at the surface of Sn NC; (c) the surface-inside-interspace sequential deposition can be achieved by 3D structured coating of Sn NC to ensure a smooth electrode surface in deep cycling; and (d) the hydrophobic surface created by polydopamine-derived Sn–N–C layer can reduce the permeation of water molecules, ease the decomposition of water at the interphase, and minimize the formation of hydrogen and byproducts. Consequently, Sn NC/Zn-based symmetric cells achieved a significantly lowered nucleation overpotential of 7.5 mV, a steady low overpotential of ∼23.8 mV at 1 mA cm−2, and stable cycling performance (1 mA h cm−2 for 1000 h and 10 mA h cm−2 over 280 h). The Sn NC/Zn//MnO2 coin cells and pouch cells also exhibited excellent electrochemical performance in terms of a high-rate capability and superior long-term cycling performance (76.2 mA h g−1 at 2 A g−1 after 2800 cycles for coin cells and 164.2 mA h g−1 at 0.5 A g−1after 1500 cycles for pouch cells).
Results and discussion
Atomic Sn sites anchored on nitrogen-doped carbon supports (Sn NC) are prepared based on a metal–organic framework (MOF)-derived nitrogen-doped carbon (NC) as a conductive skeleton and the surface decoration of the Sn–N–C layer, as schematically illustrated in Fig. 1a. The synthesis procedure mainly comprises two steps. First, the Zeolitic Imidazolate Framework-8 (ZIF-8) is converted to NC by annealing and acid etching. Second, polydopamine is selected as the precursor and carrier for the synthesis of single-atom materials owing to its easy preparation and fine control.39,40 A polydopamine layer with rich Sn sites is self-polymerized on the NC and further annealed to a Sn–N–C layer coated outside the NC to obtain the desired Sn NC. To facilitate large-scale preparation, Sn NC powders are coated with Zn foil, and the ideal Zn anode with modification, Sn NC/Zn, is acquired. The morphology of a single Sn NC was observed in transmission electron microscopy (TEM), as depicted in Fig. 1b. A dodecahedron structure from ZIF-8 is maintained in the final product. From the element mappings in Fig. S1,† Sn is uniformly distributed from a macro perspective. Fig. 1c and d illustrates the detailed distribution of Sn sites. No obvious metal lattices were observed on the surface of Sn NC under a high-resolution TEM image, as depicted in Fig. 1c. With the aid of high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM), atomic Sn highlighted by red circles as well as a few Sn clusters can be observed. According to the Inductively Coupled Plasma (ICP) results, the content of Sn in Sn NC is 1.12 wt%. Further, the spectrum based on electron energy loss spectroscopy (EELS) in Fig. 1e proves the interaction between Sn and N, evidencing the coordination of Sn and N atoms to form Sn–Nx configurations.41 According to the X-ray diffraction (XRD) patterns in Fig. 1f, tiny peaks in Sn NC/Zn distinguished from NC/Zn can be attributed to the (200), (101), and (211) planes of tetragonal Sn (PDF #04-0673), which correspond to the HAADF-STEM image in Fig. 1d, indicating that small amounts of Sn clusters exist in Sn NC. Fig. 1g and h shows the X-ray photoelectron spectroscopy (XPS) spectra of Sn 3d orbit of Sn NC and N 1s orbit of Sn NC and NC, respectively. The overall spectrum of Sn NC is depicted in Fig. S2,† from which the elements Sn, N, C, and O can be observed. In the Sn 3d spectra (Fig. 1g), owing to surface oxidation, Sn4+ (495.8 eV and 487.1 eV) and Sn2+ (495.0 eV and 486.3 eV) are deconvoluted.42 No Sn0 appears in Sn NC according to XPS analysis, verifying that Sn clusters are in low content. For the N 1s spectra in Fig. 1h, the nitrogen composition of polydopamine-derived Sn–N–C differs from that of the inner NC in a larger proportion of graphitic-N (from 17.2% to 36.5%).38 The proportion of pyridinic-N in Sn–N–C, which is normally considered the main zincophilic species in nitrogen-doped carbon, shows slight advantages over that in NC.3,43 The XPS analysis of nitrogen composition indicates that pyridinic-N and graphitic-N are the major species of N in Sn–N–C that participate in reactions at the solid/liquid interface. Fig. S3† shows the C 1s spectra, from which C–C (286.3 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (285.3 eV), and C–N (286.3 eV) can be deconvoluted.43 The Brunauer–Emmett–Teller (BET) surface area of Sn NC and NC is analyzed, as shown in Fig. S4.† Sn NC (916.45 m2 g−1) shows a similar BET surface area to NC (946.96 m2 g−1), with an extended average diameter from 1.88 nm to 4.72 nm based on the Barrett–Joyner–Halenda (BJH) Model. It is expected that the appropriate nano-level pore diameter is suitable for Zn accommodation.21
N (285.3 eV), and C–N (286.3 eV) can be deconvoluted.43 The Brunauer–Emmett–Teller (BET) surface area of Sn NC and NC is analyzed, as shown in Fig. S4.† Sn NC (916.45 m2 g−1) shows a similar BET surface area to NC (946.96 m2 g−1), with an extended average diameter from 1.88 nm to 4.72 nm based on the Barrett–Joyner–Halenda (BJH) Model. It is expected that the appropriate nano-level pore diameter is suitable for Zn accommodation.21
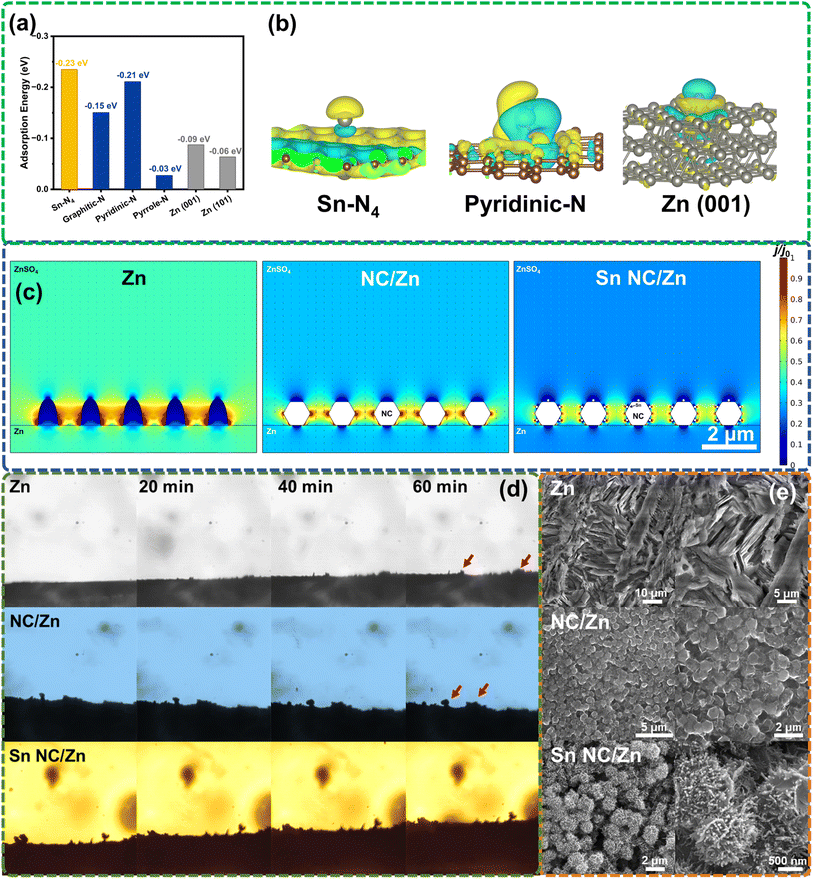
To verify the functions in the Zn deposition of different configurations in Sn NC and Zn, density functional theory (DFT) calculations are performed (Fig. 2a, b and S5–S7†). The models of Sn–N4, pyridinic-N, graphitic-N, pyrrole-N, Zn (101), and Zn (001) are presented in Fig. S5,† wherein the crystal plane orientation of Zn is selected in accordance with the XRD results. The calculated adsorption energy (Eads) between the Zn atoms and various configurations of Sn NC and Zn are listed in Fig. 2a. Sn–N4 configuration is calculated to have the most negative Eads (−0.23 eV), indicating an optimal zincophilic feature. In N–C configurations, pyridinic-N (−0.21 eV) and graphitic-N (−0.15 eV) are more zincophilic than pyrrole-N. As illustrated by the XPS results in Fig. 1h, the major N species are pyridinic-N and graphitic-N. Therefore, as a mutual confirmation, the N species in Sn NC are also conducive to uniform Zn nucleation.3 Based on the charge density difference results depicted in Fig. 2b and S6† (yellow for positive and blue for negative charge differences, respectively), the Sn–N4 configuration shows a high affinity to Zn and verifies a strong interaction with Zn, which is favorable for oriented Zn deposition.38 To exhaustively evaluate the roles of Sn NC in Zn deposition, we further present the DFT calculations on Sn (101) and Sn (200) as depicted in Fig. S7,† based on the XRD results and HAADF-STEM images, wherein small amounts of Sn clusters exhibit a zincophilic nature. Meanwhile, we simulate the electric field distribution, as illustrated in Fig. 2c. The three-dimensional (3D) dodecahedron structure of Sn NC and NC can increase the electrochemically active surface area and lower the current densities at the interface. Furthermore, with the introduction of Sn sites, the local current densities near the solid/liquid interface can be further reduced, and the electric field distribution can be in a more uniform status.23,44 More importantly, in the illustration relating to Sn NC/Zn, the accumulated electrons, especially on the Sn sites, can guide the initial Zn deposition on the surface Sn NC. The relative current density at the Zn surface can be lowered to avoid or relieve the direct deposition on Zn foil in low-depth cycling compared with NC/Zn. Therefore, the redirection of Zn deposition sites by Sn NC can lead to an optimal dendrite inhibition effect.
Based on the high zincophilicity and the electric field regulation of Sn NC/Zn, to intuitively understand the deposition behavior of Zn on our samples, we first observe the cross-sectional morphology of Zn, NC/Zn, and Sn NC/Zn in situ using an optical microscope at 100× magnification (Fig. 2d) in a deposition process for 60 min at 1 mA cm−2. Both Zn and NC/Zn show evident changes at the interface of the electrodes, while Zn remains a flat and even growth tendency on Sn NC. Moreover, we compare the morphology of three samples before and after 100 cycles at 1 mA cm−2 and 0.5 mA h cm−2, respectively. Fig. S8† shows the surface morphology of Sn NC/Zn NC/Zn, and Zn, and Fig. S9† depicts the cross-sectional image of Sn NC/Zn. The thickness of the Sn NC layer on Zn foil is measured to be ∼15 μm. As can be seen from the scanning electron microscopy (SEM) images in Fig. 2e, Zn shows the most significant deformation with protrusions. On the surface of NC/Zn, the deposited Zn in sheets of a larger size can be noticed, with no regular patterns in distribution, which may lead to further irregular growth of Zn dendrites and protrusions. Meanwhile, the Zn at the surface of NC is at a very small amount; therefore, it can be deduced that in low-depth cycling, Zn shows an initially confined deposition inside the NC. Our champion sample Sn NC/Zn exhibits an exciting morphology, wherein Zn is deposited evenly on the surface of Sn NC in nanosheets of a smaller size, which is favorable for uniform Zn deposition, and surface deformation can be avoided. The improvement in morphology can be attributed to the rich Sn sites on the surface of Sn NC and the conductive micro-structure, which perfectly correspond to the theoretical calculation of zincophilicity and the numerical simulation of lowered and guided local current densities.
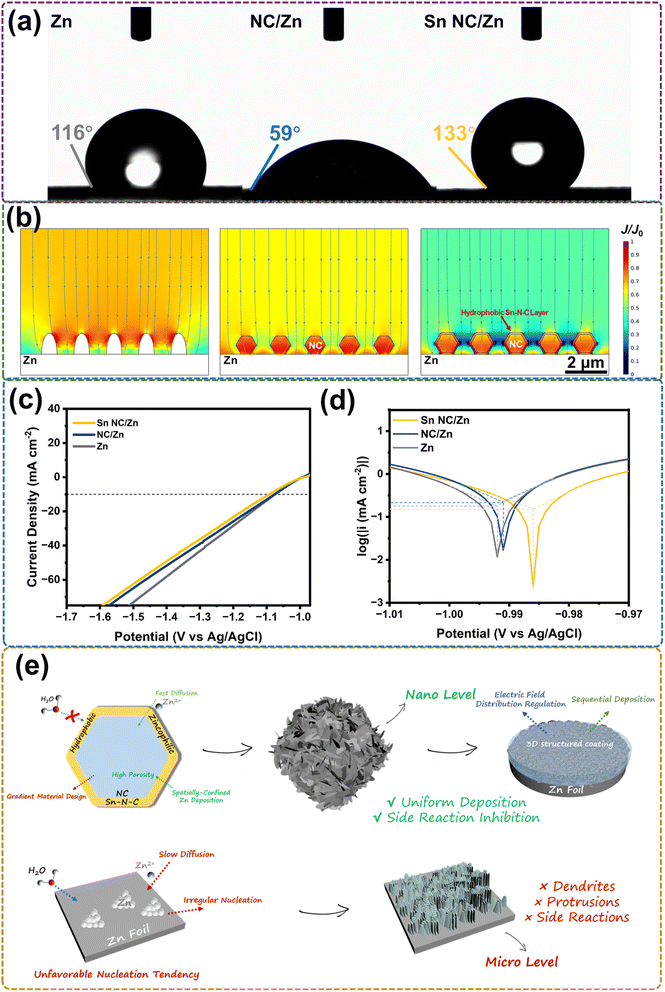
The hydrophobic/hydrophilic feature of the samples directly relates to the nucleation property of electrodes and the anti-corrosion feature.45,46 In comparison, a hydrophilic surface is beneficial to Zn deposition as better access to electrolytes can be granted, yet the side reactions and corrosion cannot be inhibited. The unfavorable decomposition of H2O and hydrogen evolution causes electrode corrosion and side product formation.6,47 However, a hydrophobic surface inevitably increases surface polarization, making it harder for Zn nucleation, while blocking water molecules to the electrode surface can significantly prevent the decomposition of water and hydrogen evolution. Accordingly, the construction of a zincophilic-hydrophobic layer is ideal for both Zn deposition and side reaction inhibition.48,49 Contact angle measurement is performed to test the hydrophobicity/hydrophilicity of the samples. The initial contact angle between Sn NC/Zn and 2 M ZnSO4 electrolyte under ambient environment is 133°, while NC/Zn and Zn exhibit smaller angles of 59° and 113°, respectively (Fig. 3a). The highest contact angle leads to the highest hydrophobicity of Sn NC, which is caused by the hydrophobic polydopamine-derived nitrogen-doped carbon outside the NC support, and is expected to have the optimal anti-corrosion feature.48 The Zn2+ flux and concentration distribution are also simulated via COMSOL, as shown in Fig. 3b and S10,† respectively, wherein the normalized values of ion flux and concentration reflect the accumulation of Zn2+. From the qualitative results of Sn NC/Zn, the hydrophobic Sn–N–C layer contributes to a faster Zn2+ diffusion by repelling the permeation of water. The highest Zn ion flux at the hydrophobic layer is also observed, indicating a nucleation priority at the surface of Sn NC.6 Based on Fig. S10,† the Sn NC coating can further lower the Zn2+ concentration near the surface of Zn foil so that a reduced nucleation tendency directly on Zn foil can be achieved. Therefore, in conjunction with the electric field distribution simulation results, in the low-depth cycling, we can conclude that Zn deposition on Sn NC/Zn mainly follows a sequence at the surface and inside Sn NC. We further test the corrosion resistance ability of the electrodes from an electrochemical perspective. Linear polarization measurements are performed to test the hydrogen evolution performance in 2 M ZnSO4, as shown in Fig. 3c and d. The introduction of Sn sites in Sn NC/Zn enlarges the overpotential by 16 mV, compared with NC/Zn, at a current density of 10 mA cm−2. More importantly, the corrosion current density of Sn NC/Zn is only 151.7 μA cm−2 (215 μA cm−2 for NC/Zn and 178 μA cm−2 for Zn), and the corrosion potential of Sn NC/Zn is the most positive at 986 mV (991 mV for NC/Zn and 992 mV for Zn). The results verify that the hydrophobic surface of Sn NC/Zn in ZnSO4 electrolyte contributes to the excellent anti-corrosion performance of electrodes.18,44,45 It is also worth mentioning that the anti-corrosion parameters of NC/Zn are better than those of Zn although NC/Zn has a more hydrophilic surface, which may be attributed to a larger HER inactive surface area by the NC coating.10Fig. 3e depicts a summary of the modification mechanism of the Sn NC/Zn electrode in an aqueous Zn electrolyte. The atomic-rich zincophilic Sn sites on the nitrogen-doped carbon support ensure a fast Zn2+ diffusion, guide the initial deposition of Zn at a nano level on the surface of Sn NC, and facilitate uniform nucleation. Conductive Sn NC with high porosity can also achieve a confined deposition in Sn NC. The 3D-structured coating layer on Zn foil can lower the local current density at the Zn surface to avoid or relieve the direct deposition on Zn foil in low-depth cycling.18,27,50 In further plating, Zn can fill in the interspace of the 3D-structured coating to achieve a uniform anode surface, which is extremely important for long-time deep cycling. The surface-inside-interspace sequential deposition is expected to handle all depths of stable stripping/plating processes. Moreover, the hydrophobic Sn–N–C layer at the surface of Sn NC derived from polydopamine endows the electrode with an excellent anti-corrosion effect. By contrast, commercial Zn foil endures severe side reactions and irregular nucleation and deposition of Zn, which is easy to incur dendrite growth and hydrogen evolution.
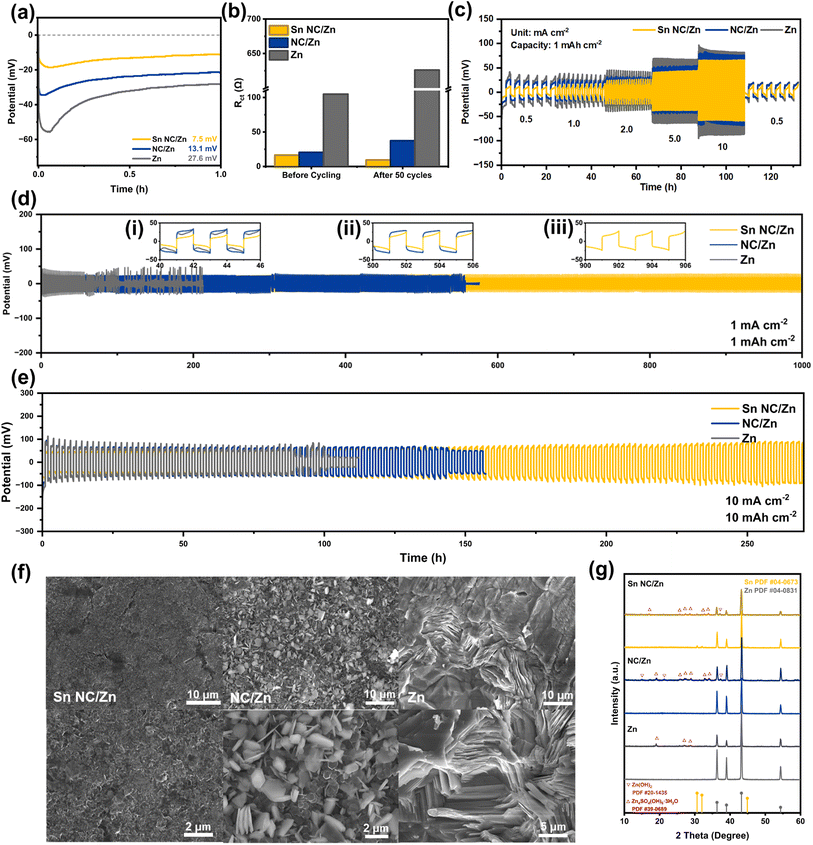
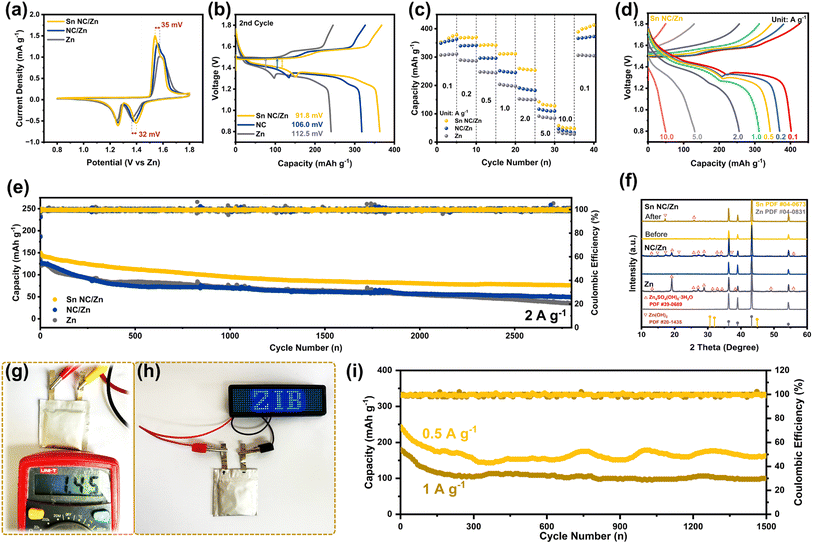
The boost in reversibility of the Sn NC layer is evaluated in Ti//Zn asymmetric cells first, wherein Sn NC/Ti foil performs as the cathode and Zn foil functions as the anode. As shown in Fig. S11a,† Sn NC/Ti//Zn cell achieves better durability in the plating/stripping process for 450 h at 1 mA cm−2 and 1 mA h cm−2 than both control samples, with a low voltage hysteresis of 30.9 mV (Fig. S11b†). By contrast, as illustrated in Fig. S11c and d,† NC/Zn and Zn show obvious failure after 60 and 50 cycles, respectively, which is caused by severe side reactions and dendrite growth. Galvanostatic cycling of symmetric cells is also performed to analyze long-term reversibility and durability. The initial nucleation stage at the surface of zinc electrodes is essential for investigating the deposition process and dendrite growth. Thus, the nucleation overpotential is a vital parameter that reflects the nucleation behavior, which is defined as the potential gap in the first deposition process between the lowest potential and the equilibrium potential in cathodic polarization. Sn NC/Zn possesses the lowest nucleation overpotential of 7.5 mV, as shown in Fig. 4a, compared with NC/Zn (13.1 mV) and Zn (27.6 mV) at 1 mA cm−2 and 1 mA h cm−2, respectively, which proves that the introduction of zincophilic Sn sites leads to a reduced energy barrier for the nucleation of Zn0 and is expected to elevate the reversibility of Zn electrodes. As depicted in Fig. S12,† chronoamperometry tests on Sn NC/Zn, NC/Zn, and Zn are performed. The current on Sn NC/Zn almost stabilizes after 60 s of 2D diffusion for nucleation and turns into the longest 3D diffusion. The Zn2+ is reduced to Zn0 at the anode/electrolyte surface, and Zn0 is subsequently absorbed on the zincophilic Sn sites so that the 2D diffusion process is inhibited, and 3D diffusion continues to promote uniform Zn growth. Fig. 4b shows a comparison of the fitted quantitative results regarding electrochemical impedance spectroscopy (EIS) profiles before cycling and after 50 cycles (at 1 mA cm−2 and 1 mA h cm−2, respectively). The original Nyquist plots at high-frequency regions and the corresponding fitted curves are presented in Fig. S13.† As depicted in Fig. 4b and S13a,† Sn NC shows the lowest initial charge transfer resistance (Rct) of 16.36 Ω. After 50 cycles (Fig. S13b†), owing to poor reversibility and the growth of dead zinc, the value of Rct of Zn increases significantly from 104.8 Ω to 626 Ω. NC also shows slight performance decay, with an increased Rct of 37.3 Ω. Our Sn NC/Zn achieves a remarkably low Rct of 9.39 Ω after 50 cycles because rich zincophilic Sn sites contribute to more nucleation sites, and the NC support yields more efficient electron transfer and ion diffusion, leading to lowered polarization. With respect to the rate performance, Sn NC/Zn symmetric cells demonstrate the lowest and the steadiest overpotential from 0.5 mA cm−2 to 10 mA cm−2 at a fixed capacity of 1 mA h cm−2. The ultralow overpotential of Sn NC/Zn demonstrates the profound role of zincophilic Sn sites and fast electron transfer by the nitrogen-doped carbon layer in the plating/stripping processes of Zn. In the 1000 h stability tests, Sn NC/Zn-based symmetric cells show a steady low overpotential of ∼23.8 mV at 1 mA cm−2 and 1 mA h cm−2, as illustrated in Fig. 4d. Inset (i)–(iii) depicts the detail potential profiles at 40–46 h, 500–506 h, and 900–906 h, and Sn NC/Zn can still maintain a low overpotential of 24.1 mV at 900 h. By contrast, symmetric cells based on NC/Zn and Zn fail at around 550 h and 50 h, respectively. The Sn NC/Zn-based symmetric cell also shows profound edges over the control samples at a current density of 0.1 mA cm−2, as shown in Fig. S14.† Even at a large cycling current density of 10 mA cm−2 and a deep cycling capacity of 10 mA h cm−2, our Sn NC/Zn-based symmetric cell can still maintain stable operation for 280 h, corresponding to a remarkable cumulative capacity of 2800 mA h cm−2. The impressive deep cycling performance can be attributed to the sequential deposition of Sn NC towards uniform plating/stripping, including the exquisite nano-level deposition at the surface of Sn NC, the confined deposition within porous Sn NC, and the accommodation among the interspace of the 3D structured coating. To further support the profound role of zincophilic Sn sites in Sn NC/Zn, symmetric cells based on a set of examples synthesized by applying the same procedure as Sn NC without the addition of Sn precursors (denoted as PDA NC) are also tested. As depicted in Fig. S15,† PDA NC/Zn-based symmetric cells cannot be parallel to Sn NC/Zn-based cells owing to both zincophilicity and stability. We further compare the cycling performance of Sn NC/Zn-based symmetric cells with other recent studies, as presented in Table S1.† Our Sn NC/Zn still possesses evident advantages in voltage hysteresis at high cycling areal capacity, proving the successful design and achievement of a protective layer with multiple advantages. Given the ultralow loading of Sn (1.12 wt%) in Sn NC, the Sn NC/Zn anode shows evident superiority with high zincophilicity, high utilization efficiency, and thus high anode performance over other particle-/layer-based anode materials. The influence of Sn content in Sn NC is also studied, and the product with a larger SnCl2 addition (100 mg, 5 times the amount in the synthesis of Sn NC) is denoted as Sn NC-100. The SEM images of Sn NC-100 in Fig. S16† show that large particles of Sn are presented, and the Sn content is measured to be 15.2 wt% based on the ICP test. The symmetric cells based on Sn NC-100 show a poor cycling performance (∼80 h) at 10 mA cm−2 and 10 mA h cm−2 (Fig. S17†), which can be attributed to the negative regulation of Zn deposition by randomly distributed Sn in large particles.
To explore the mechanism of the long lifespan of the Sn NC/Zn electrode, we disassemble the symmetric cells after long-term tests and observe the surface morphology changes before and after the durability tests, as shown in Fig. 4f. An almost smooth surface belonging to Sn/NC Zn is observed, proving that the scale modulation at the nano level in Zn deposition can stimulate the uniform distribution of Zn cations and cause no protrusion or dendrite growth in long-time plating/stripping. In comparison, NC/Zn and Zn exhibit messy stacks of Zn at varying degrees, which may easily lead to a disordered current distribution at the surface of the electrodes and upcoming battery failures.44 The ex situ XRD spectra in Fig. 4g indicate that, after 1000 h, a small amount of byproducts, comprising β-Zn(OH)2 (PDF #20-1435) and Zn4SO4(OH)6·4H2O (PDF #39-0689), are generated at the surface of Sn NC/Zn, resulting in the slight performance deterioration in the 1000 h cycling. However, the small flaws do not detract from the overall excellence of Sn NC/Zn. The above byproducts also appear in the failed cells of NC/Zn and Zn with larger intensities, indicating a severe byproduct formation that causes the direct failure of the symmetric cells together with protruded morphology and “dead zinc”. It is worth mentioning that in the post-cycling Sn NC/Zn electrode, peaks belonging to Sn are presented at a smaller intensity, proving the slow dissolution of a small amount of Sn clusters in mild acidic electrolytes. This phenomenon also indicates that the zincophilic feature of the Sn NC/Zn anode is mainly caused by the Sn–N4 configuration. We can further calculate the peak intensity ratio of I(002)/I(101) after cycling, as depicted in Fig. 4g.51 The value of I(002)/I(101) for Sn NC/Zn is 1.35, while that for NC/Zn is just 0.96. The overall growth tendency corresponds with the SEM images depicted in Fig. 4f, wherein NC/Zn shows a dominant (101) plane preference in Zn deposition, leading to a rough electrode surface.
Based on the excellent stability and reversibility of Sn NC/Zn anodes, the anodes are further tested in Zn//MnO2 full batteries in the types of both coin cells and pouch cells, wherein MnO2 nanorods are synthesized using a hydrothermal method and function as cathodic active materials. Fig. S18† illustrates the SEM images of the synthesized MnO2 nanorods, and the phase information is evidenced by XRD spectra depicted in Fig. S19,† in which the MnO2 is in an α-MnO2 phase (PDF #72-1982). A smaller polarization of 67 mV and higher current density in cyclic voltammetry sweeping are observed, as illustrated in Fig. 5a, compared with the coin cells based on Sn NC/Zn and Zn, signifying that the optimization of anodes can endow the battery with higher performance in electrochemical storage. Fig. 5b depicts the detailed profiles of charging and discharging in the 2nd cycle at 100 mA g−1. Sn NC/Zn//MnO2 coin cells deliver the lowest voltage gap of 91.8 mV, further proving the low polarization of the champion sample in the electrochemical processes. We further test the rate capability of the batteries, which is a vital parameter in evaluating the practical application of batteries. As demonstrated in Fig. 5c, the Sn NC/Zn-based coin cell shows a clear dominance in current densities ranging from 0.1 A g−1 to 10 A g−1. As shown in Fig. 5d and S20,† a high capacity of 49.7 mA h g−1 can still be maintained by Sn NC/Zn-based full batteries at 10 A g−1, which is significantly higher than that of NC/Zn (38.8 mA h g−1) and Zn (30.5 mA h g−1). Based on the Ragone plot in Fig. S21,† Sn NC/Zn achieves a high maximum energy density of 558.4 W h kg−1 (0.1 A g−1) and a peak power density of 2615.1 W kg−1 (2 A g−1). With respect to the long-term cycling illustrated in Fig. 5d, the Sn NC/Zn//MnO2 coin cell can maintain a high capacity of 76.2 mA h g−1 at 2 A g−1 after 2800 cycles. The initial capacity decay of Zn//MnO2 batteries is attributed to the H+ intercalation/extraction, and with the increased Zn2+ intercalation/extraction, the performance decay is retarded.52 The CE of Sn NC/Zn-based coin cells maintains a stable value of ∼100%. In contrast, huge variations are observed for those of NC/Zn and Zn-based batteries, reflecting unstable and poor reversibility. Table S2† lists the comparison between Sn NC/Zn-based coin cells and the results from other recent studies, and the Sn NC/Zn//MnO2 coin cells are still comparable with other high-quality works in terms of capacity retention given a long cycling number. To probe into the mechanism of performance decay caused by anodes, SEM images of post-cycling anodes are illustrated in Fig. S22.† Sn NC/Zn possesses a smooth surface after long-term cycling, which is favorable for uniform stripping/plating processes. In combination with the post-cycling XRD spectra depicted in Fig. 5f, the Sn NC/Zn anode shows the least byproduct generation that causes direct performance decay, which validates the stable and robust cycling performance of the Sn NC/Zn anode. We further assemble Zn//MnO2 pouch cells to test the application prospects of batteries in larger areas, wherein the anode preparation with an area of 9 cm−2 can be easily achieved using a coating method. Fig. 5g presents a digital graph of the measurement of the open-circuit voltage (OCV) of the assembled pouch cell, and our Sn NC/Zn-based pouch cell delivers a high OCV of 1.45 V. From Fig. 5h, one single pack of battery based on Sn NC/Zn can power the operation of a blue LED display. We further test the cycling performance of Sn NC/Zn-based pouch cells at 0.5 and 1 A g−1, as depicted in Fig. 5i. High final capacities of 164.2 and 99.8 mA h g−1 are achieved after 1500 cycles at 0.5 and 1 A g−1, respectively, and the pouch cells show a stable capacity platform after the initial decay in capacities. The results of the pouch cells confirm the satisfactory application prospects of Sn NC coating in large-scale battery devices. Overall, the hydrophobic nitrogen-doped carbon-based functional layer with atomic zincophilic Sn sites can inhibit dendrite growth and relieve side reactions simultaneously, thereby expanding the lifespan of Zn-based aqueous batteries and possessing bright prospects in large-scale applications.
Conclusions
In this study, a multifunctional interface modification Sn NC layer was achieved to simultaneously relieve dendrite growth, byproduct formation, side reactions, and surface protrusions of Zn anodes. Atomically dispersed, rich zincophilic Sn sites, as demonstrated by advanced characterizations and theoretical calculations, ensured the uniform nucleation of Zn with a nano-level modification. The numerical simulation further revealed that the porous dodecahedron NC support led to a reduced current density distribution at the solid/liquid interface, causing fewer protrusions in plating/stripping. The hydrophobic/hydrophilic bi-layer structure allowed for a gradient distribution of Zn2+ for the guidance of oriented Zn deposition. In conjunction with the 3D-structured coating of porous Sn NC, a surface-inside-interspace sequential deposition can be guaranteed towards a smooth electrode surface during deep cycling. The hydrophobic surface of the Sn–N–C layer further reduced the decomposition of water molecules and minimized the formation of hydrogen and byproducts. Based on the features above, Sn NC/Zn-based symmetric cells delivered extremely low nucleation overpotential and stable cycling performance. The coin-type and pouch-type Zn//MnO2 full cells show excellent electrochemical performance with high anodic reversibility. This study offers insights into the rational design of multifunctional interface modification layers in high-performance aqueous metal-ion batteries.Author contributions
Yijie Wang: conceptualization, data curation, formal analysis, investigation, theoretical computation and simulation, visualization, writing – original draft, writing – review & editing. Yan Tan: investigation, theoretical computation and simulation. Chuanwei Cheng: supervision, writing – review & editing, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support by National Natural Science Foundation of China (Grant no. 51772213), and Fundamental Research Funds for the Central Universities.References
- Y. Tian, Y. An, C. Wei, B. Xi, S. Xiong, J. Feng and Y. Qian, Recent Advances and Perspectives of Zn-Metal Free “Rocking-Chair”-Type Zn-Ion Batteries, Adv. Energy Mater., 2020, 11, 2002529, DOI:10.1002/aenm.202002529.
- Q. Cao, Y. Gao, J. Pu, A. M. Elshahawy and C. Guan, Materials and structural design for preferable Zn deposition behavior toward stable Zn anodes, SmartMat, 2023, 1194, DOI:10.1002/smm2.1194.
- F. Xie, H. Li, X. Wang, X. Zhi, D. Chao, K. Davey and S. Z. Qiao, Mechanism for Zincophilic Sites on Zinc-Metal Anode Hosts in Aqueous Batteries, Adv. Energy Mater., 2021, 11, 2003419, DOI:10.1002/aenm.202003419.
- Q. Zhang, J. Luan, Y. Tang, X. Ji and H. Wang, Interfacial Design of Dendrite-Free Zinc Anodes for Aqueous Zinc-Ion Batteries, Angew. Chem., Int. Ed., 2020, 59, 13180–13191, DOI:10.1002/anie.202000162.
- Z. Yi, G. Chen, F. Hou, L. Wang and J. Liang, Strategies for the Stabilization of Zn Metal Anodes for Zn-ion Batteries, Adv. Energy Mater., 2020, 11, 2003065, DOI:10.1002/aenm.202003065.
- P. Zou, R. Lin, T. P. Pollard, L. Yao, E. Hu, R. Zhang, Y. He, C. Wang, W. C. West, L. Ma, O. Borodin, K. Xu, X. Q. Yang and H. L. Xin, Localized hydrophobicity in aqueous zinc electrolytes improves zinc metal reversibility, Nano Lett., 2022, 22, 7535–7544, DOI:10.1021/acs.nanolett.2c02514.
- Q. Zhang, Y. Ma, Y. Lu, Y. Ni, L. Lin, Z. Hao, Z. Yan, Q. Zhao and J. Chen, Halogenated Zn2+ Solvation Structure for Reversible Zn Metal Batteries, J. Am. Chem. Soc., 2022, 144, 18435–18443, DOI:10.1021/jacs.2c06927.
- L. Zhang, L. Miao, W. Xin, H. Peng, Z. Yan and Z. Zhu, Engineering zincophilic sites on Zn surface via plant extract additives for dendrite-free Zn anode, Energy Storage Mater., 2022, 44, 408–415, DOI:10.1016/j.ensm.2021.10.033.
- W. Wu, X. Yang, K. Wang, Z. Lin, H. Y. Shi and X. Sun, Inducing the Solid–Liquid Conversion of Zinc Metal Anode in Alkaline Electrolytes by a Complexing Agent, Adv. Funct. Mater., 2022, 32, 2207397, DOI:10.1002/adfm.202207397.
- H. Qin, W. Kuang, N. Hu, X. Zhong, D. Huang, F. Shen, Z. Wei, Y. Huang, J. Xu and H. He, Building Metal-Molecule Interface towards Stable and Reversible Zn Metal Anodes for Aqueous Rechargeable Zinc Batteries, Adv. Funct. Mater., 2022, 32, 2206695, DOI:10.1002/adfm.202206695.
- C. Meng, W. He, L. Jiang, Y. Huang, J. Zhang, H. Liu and J. J. Wang, Ultra-Stable Aqueous Zinc Batteries Enabled by β-Cyclodextrin: Preferred Zinc Deposition and Suppressed Parasitic Reactions, Adv. Funct. Mater., 2022, 32, 2207732, DOI:10.1002/adfm.202207732.
- J. Zhu, Z. Bie, X. Cai, Z. Jiao, Z. Wang, J. Tao, W. Song and H. J. Fan, A Molecular-Sieve Electrolyte Membrane enables Separator-Free Zinc Batteries with Ultralong Cycle Life, Adv. Mater., 2022, 34, 2207209, DOI:10.1002/adma.202207209.
- C. Zhao, Y. Du, Z. Guo, A. Chen, N. Liu, X. Lu, L. Fan, Y. Zhang and N. Zhang, Missing-linker bifunctional MIL-125(Ti)-Zn interface modulation layer to simultaneously suppress hydrogen evolution reaction and dendrites for Zn metal anodes, Energy Storage Mater., 2022, 53, 322–330, DOI:10.1016/j.ensm.2022.09.014.
- T. Wang, P. Wang, L. Pan, Z. He, L. Dai, L. Wang, S. Liu, S. C. Jun, B. Lu, S. Liang and J. Zhou, Stabling Zinc Metal Anode with Polydopamine Regulation through Dual Effects of Fast Desolvation and Ion Confinement, Adv. Energy Mater., 2022, 13, 2203523, DOI:10.1002/aenm.202203523.
- H. Wang, Y. Chen, H. Yu, W. Liu, G. Kuang, L. Mei, Z. Wu, W. Wei, X. Ji, B. Qu and L. Chen, A Multifunctional Artificial Interphase with Fluorine-Doped Amorphous Carbon layer for Ultra-Stable Zn Anode, Adv. Funct. Mater., 2022, 32, 2205600, DOI:10.1002/adfm.202205600.
- B. Luo, Y. Wang, S. Zheng, L. Sun, G. Duan, J. Lu, J. Huang and Z. Ye, Ion pumping synergy with atomic anchoring for dendrite-free Zn anodes, Energy Storage Mater., 2022, 51, 610–619, DOI:10.1016/j.ensm.2022.07.010.
- H. Fan, M. Li and E. Wang, Anion-functionalized interfacial layer for stable Zn metal anodes, Nano Energy, 2022, 103, 107751, DOI:10.1016/j.nanoen.2022.107751.
- Q. Cao, Y. Gao, J. Pu, X. Zhao, Y. Wang, J. Chen and C. Guan, Gradient design of imprinted anode for stable Zn-ion batteries, Nat. Commun., 2023, 14, 641, DOI:10.1038/s41467-023-36386-3.
- Y. Zeng, P. X. Sun, Z. Pei, Q. Jin, X. Zhang, L. Yu and X. W. D. Lou, Nitrogen-Doped Carbon Fibers Embedded with Zincophilic Cu Nanoboxes for Stable Zn-Metal Anodes, Adv. Mater., 2022, 34, 2200342, DOI:10.1002/adma.202200342.
- J.-L. Yang, P. Yang, W. Yan, J.-W. Zhao and H. J. Fan, 3D zincophilic micro-scaffold enables stable Zn deposition, Energy Storage Mater., 2022, 51, 259–265, DOI:10.1016/j.ensm.2022.06.050.
- H. Li, C. Guo, T. Zhang, P. Xue, R. Zhao, W. Zhou, W. Li, A. Elzatahry, D. Zhao and D. Chao, Hierarchical Confinement Effect with Zincophilic and Spatial Traps Stabilized Zn-Based Aqueous Battery, Nano Lett., 2022, 22, 4223–4231, DOI:10.1021/acs.nanolett.2c01235.
- Z. Bie, Q. Yang, X. Cai, Z. Chen, Z. Jiao, J. Zhu, Z. Li, J. Liu, W. Song and C. Zhi, One-Step Construction of a Polyporous and Zincophilic Interface for Stable Zinc Metal Anodes, Adv. Energy Mater., 2022, 12, 2202683, DOI:10.1002/aenm.202202683.
- G. Zhang, X. Zhang, H. Liu, J. Li, Y. Chen and H. Duan, 3D-Printed Multi-Channel Metal Lattices Enabling Localized Electric-Field Redistribution for Dendrite-Free Aqueous Zn Ion Batteries, Adv. Energy Mater., 2021, 11, 2003927, DOI:10.1002/aenm.202003927.
- Y. Wang, A. Li and C. Cheng, Three-dimensional ordered macroporous patterned structure for dendrite-free and stable zinc anodes, Mater. Today Chem., 2022, 26, 101057, DOI:10.1016/j.mtchem.2022.101057.
- L. Zeng, J. He, C. Yang, D. Luo, H. Yu, H. He and C. Zhang, Direct 3D printing of stress-released Zn powder anodes toward flexible dendrite-free Zn batteries, Energy Storage Mater., 2023, 54, 469–477, DOI:10.1016/j.ensm.2022.10.061.
- H. Lu, J. Hu, Y. Zhang, K. Zhang, X. Yan, H. Li, J. Li, Y. Li, J. Zhao and B. Xu, 3D Cold-Trap Environment Printing for Long-Cycle Aqueous Zn-Ion Batteries, Adv. Mater., 2023, 35, 2209886, DOI:10.1002/adma.202209886.
- Y. Gao, Q. Cao, J. Pu, X. Zhao, G. Fu, J. Chen, Y. Wang and C. Guan, Stable Zn Anodes with Triple Gradients, Adv. Mater., 2023, 35, 2207573, DOI:10.1002/adma.202207573.
- M. Zhang, P. Yu, K. Xiong, Y. Wang, Y. Liu and Y. Liang, Construction of Mixed Ionic-Electronic Conducting Scaffolds in Zn Powder: A Scalable Route to Dendrite-Free and Flexible Zn Anodes, Adv. Mater., 2022, 34, 2200860, DOI:10.1002/adma.202200860.
- H. Yu, Y. Zeng, N. W. Li, D. Luan, L. Yu and X. W. D. Lou, Confining Sn nanoparticles in interconnected N-doped hollow carbon spheres as hierarchical zincophilic fibers for dendrite-free Zn metal anodes, Sci. Adv., 2022, 8, eabm5766, DOI:10.1126/sciadv.abm5766.
- Q. Cao, H. Gao, Y. Gao, J. Yang, C. Li, J. Pu, J. Du, J. Yang, D. Cai, Z. Pan, C. Guan and W. Huang, Regulating Dendrite-Free Zinc Deposition by 3D Zincopilic Nitrogen-Doped Vertical Graphene for High-Performance Flexible Zn-Ion Batteries, Adv. Funct. Mater., 2021, 31, 2103922, DOI:10.1002/adfm.202103922.
- Y. Zeng, X. Zhang, R. Qin, X. Liu, P. Fang, D. Zheng, Y. Tong and X. Lu, Dendrite-Free Zinc Deposition Induced by Multifunctional CNT Frameworks for Stable Flexible Zn-Ion Batteries, Adv. Mater., 2019, 31, 1903675, DOI:10.1002/adma.201903675.
- Q. Zhang, J. Luan, X. Huang, L. Zhu, Y. Tang, X. Ji and H. Wang, Simultaneously Regulating the Ion Distribution and Electric Field to Achieve Dendrite-Free Zn Anode, Small, 2020, 16, 2000929, DOI:10.1002/smll.202000929.
- L. Zhang, B. Zhang, T. Zhang, T. Li, T. Shi, W. Li, T. Shen, X. Huang, J. Xu, X. Zhang, Z. Wang and Y. Hou, Eliminating Dendrites and Side Reactions via a Multifunctional ZnSe Protective Layer toward Advanced Aqueous Zn Metal Batteries, Adv. Funct. Mater., 2021, 31, 2100186, DOI:10.1002/adfm.202100186.
- J. Hao, B. Li, X. Li, X. Zeng, S. Zhang, F. Yang, S. Liu, D. Li, C. Wu and Z. Guo, An In-Depth Study of Zn Metal Surface Chemistry for Advanced Aqueous Zn-Ion Batteries, Adv. Mater., 2020, 32, 2003021, DOI:10.1002/adma.202003021.
- A. Chen, C. Zhao, J. Gao, Z. Guo, X. Lu, J. Zhang, Z. Liu, M. Wang, N. Liu, L. Fan, Y. Zhang and N. Zhang, Multifunctional SEI-like structure coating stabilizing Zn anodes at a large current and capacity, Energy Environ. Sci., 2023, 16, 275–284, 10.1039/d2ee02931f.
- R. Zhao, X. Dong, P. Liang, H. Li, T. Zhang, W. Zhou, B. Wang, Z. Yang, X. Wang, L. Wang, Z. Sun, F. Bu, Z. Zhao, W. Li, D. Zhao and D. Chao, Prioritizing Hetero-Metallic Interfaces via Thermodynamics Inertia and Kinetics Zincophilia Metrics for Tough Zn-Based Aqueous Batteries, Adv. Mater., 2023, 2209288, DOI:10.1002/adma.202209288.
- L. Wang, W. Huang, W. Guo, Z. H. Guo, C. Chang, L. Gao and X. Pu, Sn Alloying to Inhibit Hydrogen Evolution of Zn Metal Anode in Rechargeable Aqueous Batteries, Adv. Funct. Mater., 2021, 32, 2108533, DOI:10.1002/adfm.202108533.
- S. Chen, J. Chen, X. Liao, Y. Li, W. Wang, R. Huang, T. Zhao, S. Yan, Z. Yan, F. Cheng and H. Wang, Enabling Low-Temperature and High-Rate Zn Metal Batteries by Activating Zn Nucleation with Single-Atomic Sites, ACS Energy Lett., 2022, 7, 4028–4035, DOI:10.1021/acsenergylett.2c02042.
- H. Tian, X. Cui, H. Dong, G. Meng, F. Kong, Y. Chen, L. Peng, C. Chen, Z. Chang and J. Shi, Engineering single MnN4 atomic active sites on polydopamine-modified helical carbon tubes towards efficient oxygen reduction, Energy Storage Mater., 2021, 37, 274–282, DOI:10.1016/j.ensm.2021.02.017.
- N. Guo, H. Xue, R. Ren, J. Sun, T. Song, H. Dong, Z. Zhao, J. Zhang, Q. Wang and L. Wu, S-block potassium single-atom electrocatalyst with K-N4 configuration derived from K+/polydopamine for efficient oxygen reduction, Angew. Chem., Int. Ed., 2023, 202312409, DOI:10.1002/anie.202312409.
- F. Luo, A. Roy, L. Silvioli, D. A. Cullen, A. Zitolo, M. T. Sougrati, I. C. Oguz, T. Mineva, D. Teschner, S. Wagner, J. Wen, F. Dionigi, U. I. Kramm, J. Rossmeisl, F. Jaouen and P. Strasser, P-block single-metal-site tin/nitrogen-doped carbon fuel cell cathode catalyst for oxygen reduction reaction, Nat. Mater., 2020, 19, 1215–1223, DOI:10.1038/s41563-020-0717-5.
- F. Li, H. J. Noh, W. Che, J. P. Jeon, G. F. Han, T. J. Shin, M. G. Kim, Y. Wang, Y. Bu, Z. Fu, Y. Lu and J. B. Baek, Tin Nanoclusters Confined in Nitrogenated Carbon for the Oxygen Reduction Reaction, ACS Nano, 2022, 16, 18830–18837, DOI:10.1021/acsnano.2c07589.
- Y. Li, D. Zhao, J. Cheng, Y. Lei, Z. Zhang, W. Zhang and Q. Zhu, A bifunctional nitrogen doped carbon network as the interlayer for dendrite-free Zn anode, Chem. Eng. J., 2023, 452, 139264, DOI:10.1016/j.cej.2022.139264.
- H. Liu, J. Li, D. Wei, X. Liu, Z. Cai, H. Zhang, Z. Lv, L. Chen, H. Li, H. Luo, Y. Zhao, H. Yu, X. Wang, F. Chen, G. Zhang and H. Duan, Biomimetic honeycomb Zn anode enabled multi-field regulation toward highly stable flexible Zn-ion batteries, Adv. Funct. Mater., 2023, 2300419, DOI:10.1002/adfm.202300419.
- K. Guan, L. Tao, R. Yang, H. Zhang, N. Wang, H. Wan, J. Cui, J. Zhang, H. Wang and H. Wang, Anti-corrosion for reversible zinc anode via a hydrophobic interface in aqueous zinc batteries, Adv. Energy Mater., 2022, 12, 2103557, DOI:10.1002/aenm.202103557.
- L. Miao, R. Wang, S. Di, Z. Qian, L. Zhang, W. Xin, M. Liu, Z. Zhu, S. Chu, Y. Du and N. Zhang, Aqueous electrolytes with hydrophobic organic cosolvents for stabilizing zinc metal anodes, ACS Nano, 2022, 16, 9667–9678, DOI:10.1021/acsnano.2c02996.
- M.-C. Liu, C.-Y. Tian, D.-T. Zhang, Y.-S. Zhang, B.-M. Zhang, Y.-Y. Wang, C.-Y. Li, M.-J. Liu, B. Gu, K. Zhao, L.-B. Kong and Y.-L. Chueh, Design on modified-zinc anode with dendrite- and side reactions-free by hydrophobic organic-inorganic hybrids for ultra-stable zinc ion batteries, Nano Energy, 2022, 103, 107805, DOI:10.1016/j.nanoen.2022.107805.
- S. Tao, C. Zhang, J. Zhang, Y. Jiao, M. Li, W. Lin, L. Ran, B. Clement, M. Lyu, I. Gentle, L. Wang and R. Knibbe, A hydrophobic and fluorophilic coating layer for stable and reversible aqueous zinc metal anodes, Chem. Eng. J., 2022, 446, 136607, DOI:10.1016/j.cej.2022.136607.
- X. Zhou, R. Chen, E. Cui, Q. Liu, H. Zhang, J. Deng, N. Zhang, C. Xie, L. Xu and L. Mai, A novel hydrophobic-zincophilic bifunctional layer for stable Zn metal anodes, Energy Storage Mater., 2023, 55, 538–545, DOI:10.1016/j.ensm.2022.12.019.
- H. Liu, J. Li, X. Zhang, X. Liu, Y. Yan, F. Chen, G. Zhang and H. Duan, Ultrathin and Ultralight Zn Micromesh-Induced Spatial-Selection Deposition for Flexible High-Specific-Energy Zn-Ion Batteries, Adv. Funct. Mater., 2021, 31, 2106550, DOI:10.1002/adfm.202106550.
- J. Hao, L. Yuan, Y. Zhu, X. Bai, C. Ye, Y. Jiao and S. Z. Qiao, Low-cost and Non-flammable Eutectic Electrolytes for Advanced Zn-I2 Batteries, Angew. Chem., Int. Ed., 2023, 62, 202310284, DOI:10.1002/anie.202310284.
- Y. Liao, H.-C. Chen, C. Yang, R. Liu, Z. Peng, H. Cao and K. Wang, Unveiling performance evolution mechanisms of MnO2 polymorphs for durable aqueous zinc-ion batteries, Energy Storage Mater., 2022, 44, 508–516, DOI:10.1016/j.ensm.2021.10.039.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ta06372k |
| This journal is © The Royal Society of Chemistry 2024 |