Aromatic polyaroxydiazole pseudocapacitive anode materials with tunable electrochemical performance through side group engineering†
Yan
Jiang
ab,
Chen
Yang
ab,
Yuanyuan
Yu
ab,
Yulin
Zhou
ab,
Zhoutai
Shang
ab,
Shengchang
Zhang
ab,
Pengqing
Liu
 ab,
Jiadeng
Zhu
ab,
Jiadeng
Zhu
 cd and
Mengjin
Jiang
cd and
Mengjin
Jiang
 *ab
*ab
aCollege of Polymer Science and Engineering, Sichuan University, Chengdu 610065, China. E-mail: memoggy@126.com
bState Key Laboratory of Polymer Materials Engineering, Chengdu 610065, China
cChemical Sciences Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
dSmart Devices and Printed Electronics Foundry, Brewer Science Inc., Springfield, MO 65806, USA
First published on 27th November 2023
Abstract
Conductive polymers (CPs) are promising electrode materials for pseudocapacitors due to their high electronic conductivity and outstanding pseudocapacitive charge storage capability. Herein, aromatic polyaroxydiazole (POD) with high electron deficient oxadiazole rings were synthesized to develop n-type CPs by side group engineering. Electron-withdrawing side groups (i.e., –F, –Br, –NO2) were imported to phenylenes to tune the main chain's electronic structure, making the oxadiazole rings more electron-deficient. The lowest unoccupied molecular orbital (LUMO) and energy gap of the resulting POD can be achieved using the side group of –NO2, which has the LUMO energy level of −4.16 eV with the onset doping potential of −0.46 V (vs. Ag/AgCl). Meanwhile, its energy gap and electronic conductivity are 2.98 eV and 4.07 × 10−4 S cm−1, respectively, indicating good electronic conductivity. A binder-free anode composed of poly(1,4-phenylene-1,3,4-oxadiazole) (p-POD) and commercial activated carbon (YP80F) was prepared and used to construct an asymmetric supercapacitor, which could deliver a high operating voltage of 2.35 V, a maximum energy density of 20 W h kg−1 with a power density of 6832.2 W kg−1. Therefore, POD is a promising pseudocapacitive anode material with a tunable chemical structure, high capacitance, and high conductivity for pseudocapacitors.
1. Introduction
With the flourishing development of portable and wearable electronic devices, efficient energy storage systems have received tremendous attention.1–4 Supercapacitors (SCs) stand out for their high-power output and long-cycle lifespan.5,6 Nevertheless, the energy density of commercial SCs is about 10 W h kg−1 due to the low electrical double-layer capacitance of the used activated carbon (AC) electrodes. In contrast, pseudocapacitive electrode materials store charges via reversible surface/near-surface faradaic redox reactions and depict remarkable specific capacitances (>200 F g−1).7 Adopting a pseudocapacitive electrode in SCs is a practical approach for constructing SCs with considerable energy densities (10–50 W h kg−1). Even though several conductive polymers (CPs) (i.e., polyaniline (PANI), polypyrrole (PPy), and poly(3,4-ethylenedioxythiophene) (PEDOT)) have been proven to be promising pseudocapacitive materials with high capacitance and excellent conductivity,8 they are p-type doping CPs and usually performed as positive electrode materials in aqueous electrolyte devices. The development of n-type CPs utilized as negative electrodes lags far behind p-type CPs regarding material diversity and device performance.9–11 Therefore, developing new n-type CPs with large pseudocapacitance in nonaqueous electrolytes is essential for constructing high-energy SCs by integrating large capacitances and high-voltage windows. Benzothiadiazole (BT),11 naphthalene diimides (NDIs),12,13 diketopyrrolopyrrole14 (DPP), and perylene diimides (PDIs)15 units are common electron-accepting moieties used in electrochemical systems to construct n-type CPs. Among them, poly((N,N′-bis(2-octyl dodecyl)-naphthalene-1,4,5,8-bis(dicarboximide)-2,6-diyl)-alt-5,5′-(2,20-bithiophene)) (P(NDI2OD-T2)) was reported by Facchetti, which has been considered one of the most studied n-type polymers.16 However, the synthesis process of these n-type CPs is usually complicated, and the yields are relatively low.In contrast, polyaroxydiazole (POD), whose main chain comprises alternative aromatic and oxadiazole rings, endows a long conducting skeleton.17 Moreover, POD can be easily synthesized, and the most widely used method to obtain POD is the one-step polycondensation of dicarboxylic acids with hydrazine sulfate in fuming sulfuric acid. Oxadiazole ring is a typical electron-deficient heteroaromatic ring, making POD an attractive n-type CP. Most importantly, the structure of POD is tunable, and diverse POD can be prepared through synthesis engineering.18 For example, solution-processible POD was achieved by introducing ether bonds and sulfonic acid side groups into POD, and the obtained product was used as a binder to prepare the Si microparticle anode in lithium-ion batteries.19,20 S. JANIETZ and coworkers reported that p-POD film could be reversibly n-doped in 0.1 M Bu4NClO4 in the acetonitrile (AN) electrolyte.21 Using the method described by De Pra et al., when p-POD was reversibly n-doped, the number of electrons transferred in the process was about 0.5 per p-POD repeat unit.17,22 That result stated the great potential of p-POD for high-capacitance pseudocapacitive materials. However, the electrochemical doping potential of p-POD is too negative because AN begins to decompose violently at −2 V (vs. Ag/Ag+). Therefore, it is crucial to modify p-POD to improve its electrochemical doping potential.
The onset doping potential is determined by the LUMO energy level of the CPs and shifts to high potential with a decreased LUMO energy level.23 Bin Meng and coworkers utilized p–π conjugation with embedded heteroatoms to tune the electronic structure of CPs.24 N-type CPs with low-lying LUMO and highest occupied molecular orbital (HOMO) energy levels were synthesized and demonstrated the photovoltaic applications. Dong et al. proposed an effective method to develop a high-performance n-type conducting polymer through acceptor–acceptor (A–A) copolymerization.25 The resulting polymers showed an ultralow LUMO energy level of −4.4 eV and an electric conductivity of 7.8 × 10−4 S cm−1, suggesting that increasing the electron affinity of the CP backbone is an effective strategy to lower the LUMO energy level of the material.11
In this work, we propose an approach to increase the electronic affinity of the POD backbone by importing withdrawing substituents (–F, –Br, –NO2) to phenylenes. Herein, n-type CPs composed of electron-deficient oxadiazole rings and withdrawing substituents were successfully synthesized. The energy levels and the energy gap are decreased because of the improved electron affinity of the main chain.26 As a result, the LUMO energy level of NO2-POD is −4.16 eV with the onset doping potential of −0.46 V (vs. Ag/AgCl). The energy gap and electronic conductivity of NO2-POD are 2.98 eV and 4.07 × 10−4 S cm−1, respectively. The steric hindrance of side groups hinders the intercalation of counterions in the doping process, resulting in a decreased doping degree. Herein, the specific capacitance of Br-POD decreases to 300 F g−1 compared with the p-POD's 500 F g−1. A binder-free anode composed of POD and commercial AC (YP80F) was prepared and used to construct an asymmetric supercapacitor (ASC). Such prepared cell exhibits excellent specific discharge capacitance of 136 F g−1 at a current density of 1 A g−1 even after 500 cycles. The capacitance retention is 81.6% after 200 cycles, which is improved due to the π–π interaction between p-POD and active carbon. The ASCs deliver a high operating voltage of 2.35 V, a maximum energy density of 20 W h kg−1 with a power density of 6832.2 W kg−1. Therefore, POD shows great application potential in the preparation of pseudocapacitor anodes.
2. Experimental section
2.1 Synthesis and characterization
All raw materials for the synthesis of POD materials were provided by Chengdu Kelong Chemical Co., Ltd. Oleum (20 wt% and 50 wt%) and concentrated sulfuric acid were purchased from Yangzhou Lubao Chemical Reagent Co., Ltd. The electrolyte (1.0 M Et4NBF4 in AN) used in the supercapacitor was purchased from Guangdong Candlelight New Energy Technology Co., LTD. All the chemicals were used as received.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05 were mixed in a three-necked flask. Then, concentrated sulfuric acid was introduced to dissolve hydrazine sulfate and aromatic diacid at 75 °C for 1 h. After that, Oleum (50 wt%) was added as a dehydration and sulfonation agent, and the molar mass ratio of aromatic diacid and SO3 was 1
1.05 were mixed in a three-necked flask. Then, concentrated sulfuric acid was introduced to dissolve hydrazine sulfate and aromatic diacid at 75 °C for 1 h. After that, Oleum (50 wt%) was added as a dehydration and sulfonation agent, and the molar mass ratio of aromatic diacid and SO3 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. The oligomers were prepared by pre-polymerization at 85 °C for 2 h. Then, the resultant's temperature was raised to 120 °C and maintained for 4 h. At last, benzoic acid (1% of aromatic diacid) was added to the three-necked flask as an end-capping reagent. Finally, the sulfuric acid solution of POD was obtained and diluted to the desired concentration.
8. The oligomers were prepared by pre-polymerization at 85 °C for 2 h. Then, the resultant's temperature was raised to 120 °C and maintained for 4 h. At last, benzoic acid (1% of aromatic diacid) was added to the three-necked flask as an end-capping reagent. Finally, the sulfuric acid solution of POD was obtained and diluted to the desired concentration.
2.2 Structural characterization of polymers
2.3 Fabrication of composite anodes
Commercial AC (YP80F) and Super C65 were added to the sulfuric acid solutions of p-POD with a mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, and the mass concentration of the slurry was 10%. The specific preparation process of composite electrodes is identical to that of POD electrodes.
6, and the mass concentration of the slurry was 10%. The specific preparation process of composite electrodes is identical to that of POD electrodes.
2.4 Fabrication of organic ASCs
Commercial AC (YP80F), Super C65, and PVDF were added into NMP with a mass ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the mass concentration of the slurry was 10%. The slurry was coated on the Cu foil with a 50 μm applicator and dried in a vacuum oven at 80 °C overnight. The prepared AC electrode was cut into 12 mm diameter slices with a 1 mg cm−2 mass density and used as the cathode in ASCs.
1, and the mass concentration of the slurry was 10%. The slurry was coated on the Cu foil with a 50 μm applicator and dried in a vacuum oven at 80 °C overnight. The prepared AC electrode was cut into 12 mm diameter slices with a 1 mg cm−2 mass density and used as the cathode in ASCs.
The ASC device was sandwich-like with the p-POD/YP80F anode and the AC cathode separated by a glass fiber membrane and 1 M TEABF4/AN electrolyte. The weight balance of the two electrodes was carried out based on the equation (C+ × ΔE+ × m+ = C– × ΔE– × m–), where C is the specific capacitance, ΔE is the voltage range, and m is the mass of the electrode. According to the specific capacitances of the p-POD/YP80F anode and AC cathode, the mass ratio of the two electrodes (m+/m−) is determined to be 2. The devices were assembled in an Ar-filled glovebox under dry and oxygen-free conditions. The energy density and power density (normalized on the total mass of active materials inboth cathode and anode) were calculated based on eqn (1) and (2), as shown below:
| E = 0.5 × C × (ΔV)2 | (1) |
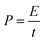 | (2) |
In which E refers to the energy density, C is the specific capacitance, ΔV is the operation voltage window, P is the power density and t is the discharge time.
3. Results and discussion
3.1 Preparation and structure characterization of PODs
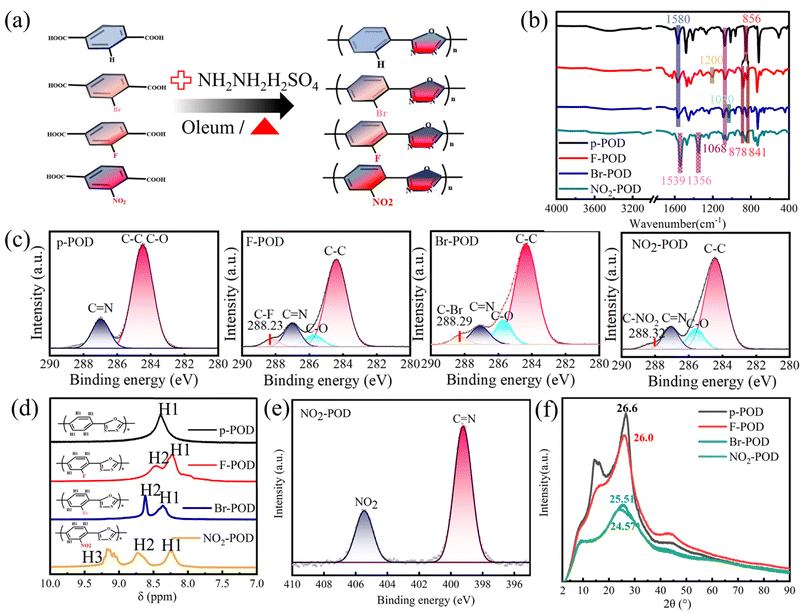
Hydrazine sulfate and terephthalic acids with different side groups (–H, –F, –Br, –NO2) at 2-position were condensed in Oleum, and POD (p-POD, F-POD, Br-POD, NO2-POD) samples with different electron-withdrawing substituents were obtained (Fig. 1a). The molecular weight of POD samples was characterized by intrinsic viscosity, and the fitting processes are shown in Fig. S1.† The intrinsic viscosity values of p-POD, F-POD, and NO2-POD are 5.78, 3.45, and 2.03, respectively. While the molecular weight of Br-POD is too low to be characterized by intrinsic viscosity. The reactivity of terephthalic acids decreased with the increase of steric hindrance effect of side groups, resulting in decreased molecular weight.Attenuated Total Reflection Infrared spectra (ATR-FTIR) were conducted to confirm the chemical structure and successful preparation of PODs (Fig. 1b). The peaks at around 1580 cm−1 and 1068 cm−1 are ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration and C–O–C stretching vibration of oxadiazole rings.17,27 The benzene rings of p-POD are disubstituted, and the C–H stretching vibration peak is at 856 cm−1. In F-POD, Br-POD, and NO2-POD, the C–H stretching vibration of benzene ring peaks are at 841 cm−1 and 878 cm−1, demonstrating that the benzene rings are trisubstituted. The peaks at 1200 cm−1 and 1030 cm−1 are characteristic peaks of benzene ring-F and benzene ring-Br. The peaks at 1356 cm−1 and 1539 cm−1 correspond to the N
N stretching vibration and C–O–C stretching vibration of oxadiazole rings.17,27 The benzene rings of p-POD are disubstituted, and the C–H stretching vibration peak is at 856 cm−1. In F-POD, Br-POD, and NO2-POD, the C–H stretching vibration of benzene ring peaks are at 841 cm−1 and 878 cm−1, demonstrating that the benzene rings are trisubstituted. The peaks at 1200 cm−1 and 1030 cm−1 are characteristic peaks of benzene ring-F and benzene ring-Br. The peaks at 1356 cm−1 and 1539 cm−1 correspond to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of NO2. The C1s spectra of different POD samples are represented in Fig. 1c. The peaks at 284.5 eV, 285.7 eV, and 287 eV correspond to C–C in benzene, C–O–C, and C
O stretching vibration of NO2. The C1s spectra of different POD samples are represented in Fig. 1c. The peaks at 284.5 eV, 285.7 eV, and 287 eV correspond to C–C in benzene, C–O–C, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the oxadiazole ring. The new peaks at 288.23, 288.29, and 288.32 eV can be ascribed to C–F, C–Br, and C–NO2, respectively. The above results show the chemical structure of POD, demonstrating the successful importing of F, –Br, and –NO2 side groups. 1H NMR (Fig. 1d) and XPS were utilized to investigate the chemical structure of POD further. The peaks between 8–9.5 ppm are related to H of benzene. There is only one peak at 8.44 ppm in the spectra of p-POD since the chemical environment of H atoms is identical. The chemical shift of H atoms at different positions is unequal after importing electron-withdrawing side groups. The peak of the H atom adjacent to the F, Br, and NO2 side group shifts to 8.47, 8.62, and 9.12 ppm. The N1s XPS spectra of p-POD and NO2-POD are represented in Fig. S2† and 1e, respectively. The peak at 399.2 eV is ascribed to the C
N in the oxadiazole ring. The new peaks at 288.23, 288.29, and 288.32 eV can be ascribed to C–F, C–Br, and C–NO2, respectively. The above results show the chemical structure of POD, demonstrating the successful importing of F, –Br, and –NO2 side groups. 1H NMR (Fig. 1d) and XPS were utilized to investigate the chemical structure of POD further. The peaks between 8–9.5 ppm are related to H of benzene. There is only one peak at 8.44 ppm in the spectra of p-POD since the chemical environment of H atoms is identical. The chemical shift of H atoms at different positions is unequal after importing electron-withdrawing side groups. The peak of the H atom adjacent to the F, Br, and NO2 side group shifts to 8.47, 8.62, and 9.12 ppm. The N1s XPS spectra of p-POD and NO2-POD are represented in Fig. S2† and 1e, respectively. The peak at 399.2 eV is ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in oxadiazole rings, and a new peak emerges at 405.5 eV, corresponding to the NO2 in NO2-POD.
N in oxadiazole rings, and a new peak emerges at 405.5 eV, corresponding to the NO2 in NO2-POD.
X-ray diffraction (XRD) was conducted (Fig. 1f) to investigate POD's structural arrangement and microstructure. The peak with 2θ value of ≈25° is the crystalline peak of POD, and the peaks with 2θ values of 14° and 44° originate from the π–π stacking of polymer backbones. The crystalline peak intensity of Br-POD and NO2-POD decreases and shifts to the left, owing to the steric hindrance effect of Br and NO2, indicating their less crystalline nature and larger interchain distance. The crystalline peak intensity of F-POD is close to that of p-POD due to the small volumetric change of the H atom to the F atom. Binding energy was obtained from molecular simulation and is summarized in Fig. S3.† The binding energy increases with the side group's electron-withdrawing ability. The TG and DTG curves of different POD samples are shown in Fig. S4.† The maximum thermal decomposition temperature exceeds 300 °C, demonstrating their excellent thermal stability.
3.2 Molecular theoretical calculation
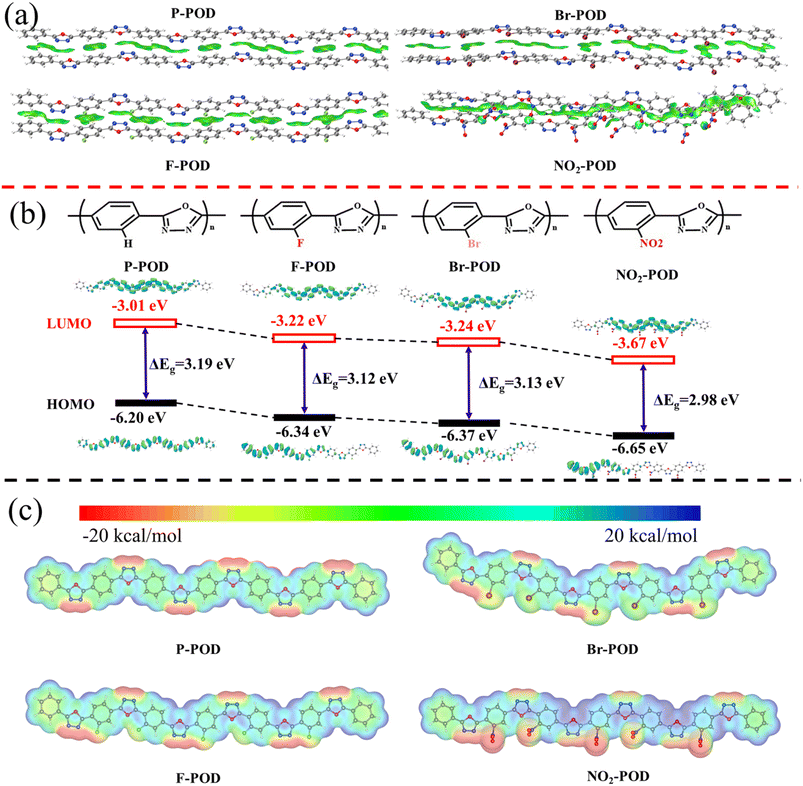
Density-functional theory (DFT) calculations at the BLYP-D3BJ/def2-SV(P) level were performed for each of the POD chains with six repeating units to elucidate the effect of electron-withdrawing side groups on the electronic structure of the main chain backbone.24 The non-covalent interactions (NCI) between two hexamer layers are shown in Fig. 2a, where the green reduced density gradient (RDG) iso-surface painted between the two layers shows extended π–π stacking effects ascribed to the electrostatic interactions between the long-range conducting structures of POD chains.28 As a result of strong intermolecular binding forces provided by substitutions, the electrostatic interactions increase with the electron-withdrawing ability of substitutions, especially for NO2-POD. The NIC result is consistent with that of binding energy. The planarity of the polymeric backbone and the inter-layer distance were investigated by analyzing the dihedral angles and the C–C distance between two stacked phenylene rings of the optimized hexamer layers (Fig. S5†). The incorporation of side groups with significant steric hindrance increases the dihedral angle and inter-layer distance, resulting in decreased planarity of the backbone and loss stack of polymer chains. The dihedral angle and inter-layer distance results are consistent with the XRD results.Fig. 2b exhibits the molecular orbital energy levels and band gaps. The calculated ELUMO/EHOMO of PODs with electron-withdrawing side groups are decreased, and the reduced degree of ELUMO is greater than that of EHOMO, resulting in a low band gap. Moreover, the reduction degree of the band gap is proportional to the electron-withdrawing ability of side groups. The orbital energy levels and band gap significantly affect the electrochemical performance of CPs because the onset doping potential is determined by the LUMO energy level, and a narrow band gap enhances the electronic conductivity of the polymer.
In addition, the electrostatic potential (ESP) maps were calculated from the optimized geometries (Fig. 2c). ESP maps describe the electronic distribution on the polymer backbone, which can be used to predict the active sites. The scale from blue to red indicates a positive to negative electrostatic potential. N atoms with lone pair electrons in PODs are in red, suggesting a negative electrostatic potential, and the lone pair electrons are the least constrained. O atoms are in blue, suggesting a positive electrostatic potential, and electrons are the most constrained. However, the number of electrons in the outer orbital of the O atom is full, and no more electrons can be accommodated. Meanwhile, the color of POD's backbone turns bluer when electron-withdrawing substituents are introduced into phenylene, suggesting increased electron deficiency of POD's backbone. Moreover, the increase of the backbone's electron affinity is proportional to the electron absorption capacity of the substituent (NO2 > F > Br > H). Due to the unsaturated characteristic of delocalized π electrons, the backbone turns to get additional electrons easily. As a result, we suppose the redox activity and the motion of π electrons will be improved by improving the electron-deficient backbone. The results are consistent with the decreased theoretical LUMO energy levels and smaller band gaps. The electronic structure of the POD-backbone has been successfully tuned through side group engineering.
3.3 Electrochemical performance and energy storage mechanism of PODs
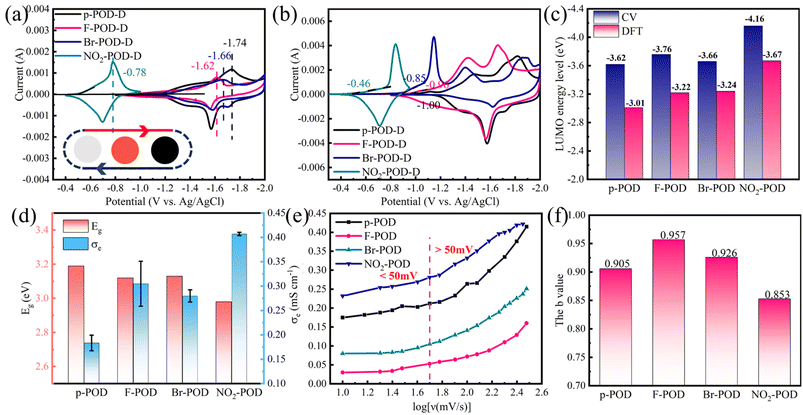
POD solutions were spin-coated onto stainless steel washers as working electrodes and tested in a three-electrode system comprising Ag/AgCl as a reference electrode and Pt as a counter electrode to evaluate the electrochemical performance of PODs. The cyclic voltammetry (CV) analysis was tested at a scan rate of 10 mV s−1 in 1.0 M Et4NBF4 in the AN electrolyte. The CV curves of POD electrodes are demonstrated in Fig. 3a, and POD could be n-doped reversibly and show electrochromic properties. The color changed from transparent to red, followed by dark black in the doping process, and back to transparent in the de-doping process (see Videos 1–4 in the ESI†). CV tests of p-POD, Br-POD, and F-POD were conducted in a potential range of −0.8 V to −2.0 V, and significant redox peaks were observed with cathodic peak current potentials at −1.76 V, −1.70 V, −1.65 V. The CV test of NO2-POD was operated at a potential range of −0.4 V to −1.0 V, and the cathodic peak current potential is at −0.8 V. Reversible redox peaks observed in CV tests indicate a pseudocapacitive characteristic of POD material.According to the formula ELUMO = −eE(red, onset) − 4.4 eV (vs. SCE), LUMO energy level can be obtained if the onset doping potential of the material is known.29 As we can see from Fig. 3b, the onset doping potential can be extracted from the first CV curves of PODs, and the onset doping potential of p-POD, F-POD, Br-POD, NO2-POD are −0.78 V, −0.74 V, −0.64 V, and −0.24 V (vs. SCE) respectively. The calculated LUMO energy levels are −3.62 eV, −3.76 eV, −3.66 eV, and −4.16 eV, respectively. Imparting different electron-withdrawing substituents increases the backbone's delocalized degree and electron affinity. As a result, the HOMO and LUMO energy levels are decreased.30,31 Moreover, the reduction degree is proportional to the electron absorption ability of the substituent. The LUMO energy levels obtained from DFT and CV tests show identical changing trends (Fig. 3c). The onset doping potential of NO2-POD increases by 0.54 V for the strong electron-withdrawing ability of –NO2.
The schematic diagram of the structure and conductance of p-POD is shown in Fig. S6a.† When POD is doped, a quinone structure with a more planar conformation is formed, and charges exist as polarons and bipolarons are generated, accompanied by the intercalation of counterions. As a result, the conductivity of undoped polymer can be increased by ten or more orders of magnitude through doping.30 In order to investigate the influence of band gap on the conductivity of POD, the electronic conductivity of the doped POD electrode was tested, and the specific operation is shown in Fig. S6b.† PODs were coated on the Cu foil with a thickness of around 20 μm. The doping process was performed in an argon glovebox at room temperature. A three-electrode system was assembled, and the POD electrode served as the working electrode to be doped electrochemically. A constant potential was applied to the working electrode and was held for 300 s to achieve a fully doped state. The potential applied to the p-POD electrode was −1.8 V (vs. Ag/AgCl) and −1.6 V (vs. Ag/AgCl) for F-POD, Br-POD, and NO2-POD. Then, a two-electrode cell was assembled using a doped POD electrode as the negative electrode and a stainless-steel washer as the counter electrode. The electronic conductivity (σe) can be calculated according to eqn (3), where L is the thickness of the POD film, U is the applied voltage, S is the area, and Ie is the current obtained from the i–t test. The i–e test curves of POD electrodes are shown in Fig. S6c–f.†
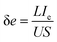 | (3) |
The electronic conductivity and energy gap of POD electrodes are shown in Fig. 3d. The electronic conductivity of doped POD is in order of 10−4 and increases with the increasing electron-withdrawing ability of side groups (–NO2 > –F > –Br > –H). The electronic conductivity of NO2-POD is up to 4.07 × 10−4 S cm−1. The energy gap decreases with the increasing electron-withdrawing ability of side groups, which plays a significant role in the conductivity of the conducting polymer. In general, we can conclude that the electronic conductivity is inversely proportional to the energy gap of POD.
CV tests were performed at a scan rate of 10–300 mV s−1 (Fig. S7a–d†) to investigate POD's charge storage kinetics and capacitive performance. The response current varies with scan rates, and the shift of differential potential between cathodic and anodic current peak (peak shift) increases with the scan rates.32 Pseudocapacitive electrodes store charges through diffusion-controlled faradaic and surface-controlled capacitive processes. We use peak shift and the slope of plot log(i)–log(v) to evaluate the charge storage kinetics of PODs,33,34 where i is the second peak current of anodic current and v is the scan rate. Fig. 3e demonstrates the peak shift of POD samples as a function of the sweep rate. At low rates (<50 mV s−1), the peak shift increases slowly with scan rates as surface-controlled redox reactions limit the process. At high rates (>50 mV s−1), the peak shift increases dramatically, and the redox reaction begins to be controlled by a diffusion-controlled process. Generally, the peak shift of F-POD and Br-POD is under 0.25 V, even at a high scan rate of 300 mV s−1, indicating a fast kinetics performance. The peak shift of p-POD and NO2-POD is from 0.15 V to 0.45 V, demonstrating a relatively slow kinetics performance. In order to investigate the contribution of surface-controlled capacitive (electric double layer and pseudocapacitance) to the total capacity, the relationship between peak current (i) and sweep rate (v) was analyzed. At a certain potential, the peak current (i) and the scan rate obey the power law in eqn (4):
| i ∝ vb | (4) |
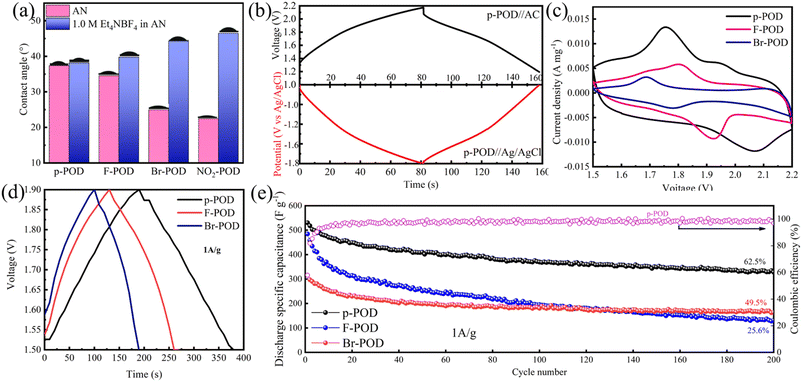
A contact angle test was performed to explore the electrolyte wettability of POD films. The contact angle between AN, 1 M Et4NBF4 in AN electrolyte, and POD films was measured, and the results are exhibited in Fig. 4a. With the importing of polar side groups, the contact angles of acetonitrile with POD films are decreased for the improved polarity of POD films. However, the contact angles between electrolyte and POD films are increased. It shows that the additional electrolyte salt affects the degree of freedom and polarity of AN, and thus, the solvent AN does not easily interact with POD, resulting in relatively poor electrolyte wettability.
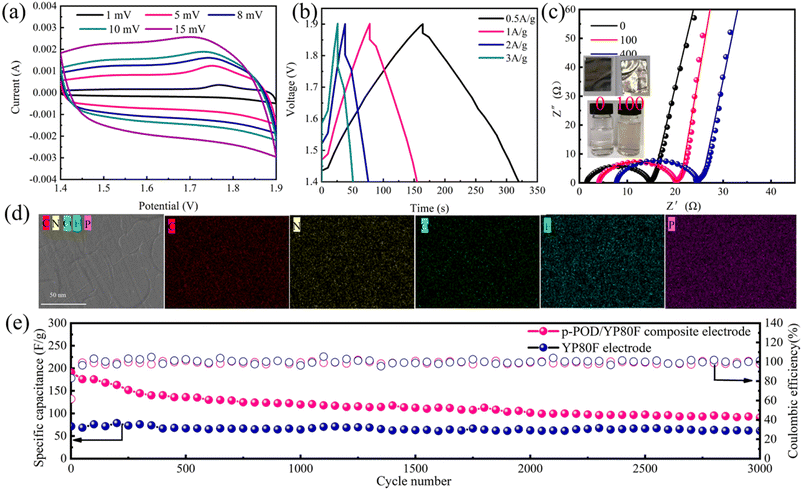
The specific capacity of pure POD was measured in a coin-typed half-cell with the POD film coated on the Cu foil as a working electrode and a thick AC sheet as the counter electrode. The actual mass loading of the POD electrode is about 0.3 mg cm−2. Due to the poor electrolyte wettability of NO2-POD, the NO2-POD pure electrode could not be doped and tested. To test the low potential tolerance of the electrolyte, a three-electrode system comprising Pt as the working electrode and counter electrode, Ag/AgCl as the reference electrode, was prepared. CV tests were conducted at different potential ranges (Fig. S8†), and the electrolyte decomposes violently beyond −1.5 V. In order to confirm the voltage window of the half-cell, a three-electrode system comprising a p-POD electrode as the working electrode, Ag/AgCl as the reference electrode, and a thick AC sheet as the counter electrode was designed to monitor the potential of p-POD (vs. Ag/AgCl) and the voltage between p-POD and AC electrode. In the voltage range, the pseudocapacitive POD should exert the highest specific capacitance; meanwhile, the electrolyte maintains stability. Finally, the specific capacity of POD samples was tested in a voltage range of 1.4–1.9 V (the potential of p-POD is −1 to −1.43 V vs. Ag/AgCl, Fig. 4b). The CV curves (Fig. 4c) at a scan rate of 10 mV s−1 exhibit reversible redox peaks, revealing that the main capacitance is from pseudocapacitance. The constant current charging-discharging (GCD) profiles (Fig. 4d) at 1 A g−1 are symmetric triangular, indicating that the energy storage process is surface-controlled. The discharge specific capacitance and CE at a 1 A g−1 are shown in Fig. 4e. The initial specific discharge capacity of p-POD and F-POD is around 500 F g−1 and decreases as the test progresses. The steric hindrance effect of Br suppresses the pairing of electrons and counterions, and the initial discharge specific capacity down to about 300 F g−1, ascribed to the decreased doping degree. The CE of p-POD is higher than 98% after the tenth cycle. However, the specific capacitance retentions of p-POD, F-POD, and Br-POD are 62.5%, 25.6%, and 49.5%, respectively, indicating poor cycling performance.
3.4 Electrochemical performance of p-POD/YP80F composite anode and ASC
A binder-free composite electrode composed of p-POD and YP80F was prepared to investigate the application of p-POD in pseudocapacitors. The mass loading of the composite electrode is about 0.8 mg cm−1. The electrochemical tests were measured in a coin-typed half-cell with a thick AC sheet as the counter electrode. CV curves (Fig. 5a) and GCD profiles (Fig. 5b) are similar to p-POD electrodes, suggesting that the surface-controlled pseudocapacitance is still the main composition of the total capacitance.Fig. 5c displays the EIS curves of the p-POD/YP80F composite anode after 0, 100, and 400 cycles, and the corresponding equivalent circuit is shown in Fig. S9.† At high frequencies, a semi-cycle loop appears and shifts from 15 Ω to 25 Ω after 400 cycles, which is undesirable as it decreases the performances of the cell in terms of resistance and power. The imaginary part sharply increases at low frequency, and a line near vertical is observed, traducing a capacitive behaviour.35 As an n-type CP, p-POD gradually exfoliates from the electrode and dissolves in the electrolyte after repeated volumetric change. In a three-electrode system using the pure p-POD electrode as the working electrode and Pt as the reference electrode, the color of the electrolyte changed from transparent to yellow after 100 cycles, and the dissolved p-POD gradually deposited on Pt. The EDS results are shown in Fig. 5d. The C and N elements from p-POD, O, F, and P from the electrolyte are detected. The gradually increased exfoliation of p-POD results in increased resistance. The electrochemical cycling performance of the p-POD/YP80F composite electrode is shown in Fig. 5e. The composite electrode exhabits excellent specific discharge capacitance of 136 F g−1 even after 500 cycles at a current density of 1 A g−1, in stark contrast with that of the YP80F electrode (67 F g−1). The composite anode's first cycle CE is 61.7%, ascribed to the unreversible doping of p-POD, and the CE from the 2nd to 3000th cycle is over 95%. The capacitance retention is 85.4% after 200 cycles, much higher than that of pure POD (62.5%), resulting from the π–π interaction between p-POD and active carbon.36
3.5 Electrochemical performance of p-POD/YP80F//AC
ASCs can maximize the full device's operating voltage and provide a solution to achieve both high energy and power density. The p-POD/YP80F was used as the anode, and the AC electrode was chosen as the cathode to fabricate a full cell. In order to balance the capacity of cathode and anode, the mass ratio of cathode and anode materials is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
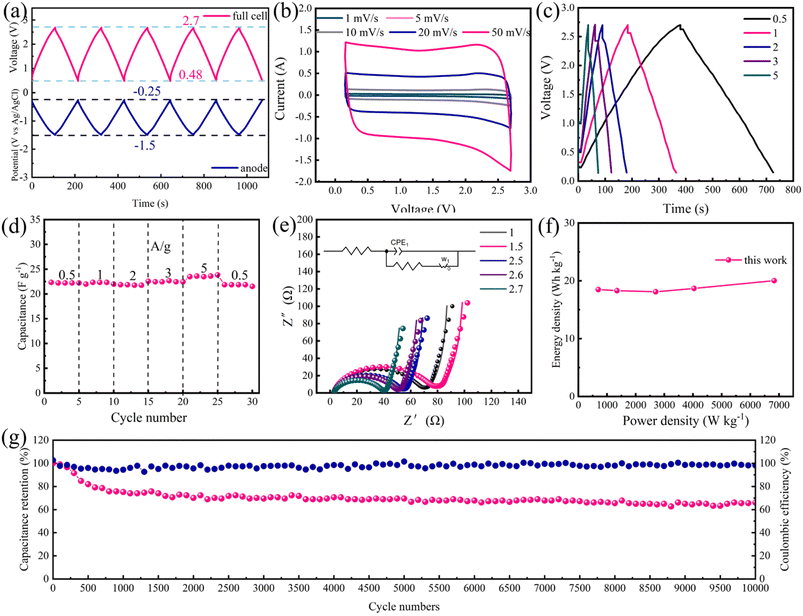
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Voltage variations of the cell and anode were tested in a three-electrode system to determine the voltage window of the device, and results are shown in Fig. 6a. The cutoff doping potential was chosen as −1.5 V (vs. Ag/AgCl), and the corresponding voltage between p-POD/YP80F and AC was 2.7 V to ensure the cycling stability of p-POD. The open circuit voltage of the device is around 0.1 V; thus, the final operating voltage range was confirmed as 0.15–2.7 V.
1. Voltage variations of the cell and anode were tested in a three-electrode system to determine the voltage window of the device, and results are shown in Fig. 6a. The cutoff doping potential was chosen as −1.5 V (vs. Ag/AgCl), and the corresponding voltage between p-POD/YP80F and AC was 2.7 V to ensure the cycling stability of p-POD. The open circuit voltage of the device is around 0.1 V; thus, the final operating voltage range was confirmed as 0.15–2.7 V.
From Fig. 6b, the CV curves display redox peaks at different scan rates, which indicates an excellent combination of pseudocapacitance and electrical double-layer capacitance. Fig. 6c further shows GCD curves at different current densities, and all curves have quasi-symmetrical shapes, manifesting the excellent reversibility of the device.37 The specific capacitance of POD/YP80F//AC was calculated to be 22.22 F g−1 at 0.5 A g−1 and 23.49 F g−1 at 5 A g−1 (Fig. 6d), suggesting its remarkable rate capability. EIS curves at different voltages are displayed in Fig. 6e. The semi-cycle loop represents the device's charge-transfer resistance (Rct). According to CV curves, the onset doping voltage of p-POD is about 2 V, and the Rct decreases with the doping of p-POD. As a result, the Rct of the device at 2.7 V is 40 Ω. The Ragone plot (Fig. 6f) further explains the energy and power density attributes of organic ASCs. Owing to the large electrochemical potential window of 1 M TEABF4 in the AN organic electrolyte, the ASC could deliver an energy density of 20 W h kg−1 with a power density of 6832.2 W kg−1, which is superior to the previously reported capacitors using AC as electrodes by Simon and Burke.38 Finally, the cycling stability was evaluated by repeating the charge/discharge test at 2 A g−1(Fig. 6g). Thanks to the superior adhesive property of p-POD, the device maintains 69.5% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, suggesting its good cycling stability.
000 cycles, suggesting its good cycling stability.
4. Conclusion
We have successfully synthesized four n-type CPs with tunable electronic structures through side group engineering, which exhibit promising performance for pseudocapacitors. Moreover, the backbone's electronic affinity is proportional to the electron absorption ability of the substituent. The increase in electronic affinity and electron delocalization results in decreased energy levels and band gaps. The onset doping potential of POD is advanced due to the decreased LUMO energy level. The decreased band gap leads to improved electronic conductivity. Reversible redox peaks are observed in the CV curve of POD, indicating a pseudocapacitive characteristic. The energy storage mechanism of POD is surface controlled with a b value of higher than 0.85. Extended π–π stacking effects ascribed to the electrostatic interactions occurred due to substitution's strong intermolecular binding forces. The initial specific discharge capacity of p-POD is above 500 F g−1 at 1 A g−1. The steric hindrance of side groups inhabits the pairing of electrons and counterions, causing the decreased doping degree and capacitance. Besides, the repeated volumetric change during reversible doping and de-doping will lead to the exfoliation of POD and poor cycling performance. A binder-free composite electrode comprising p-POD and YP80F AC was prepared, exhibiting excellent specific discharge capacitance of 136 F g−1 even after 500 cycles. When p-POD/YP80F was used as the anode in the p-POD/YP80F//AC device, the ASC could deliver an energy density of 20 W h kg−1 with a power density of 6832.2 W kg−1, which is superior to the previously reported capacitors using AC as electrodes. Our work reveals the structure–performance relationships of POD anodes for pseudocapacitors and provides new insight into the design of n-type CPs.Author contributions
Yan Jiang: conceptualization, methodology, investigation, data curation, writing – original draft. Chen Yang: assay, data curation. Yuanyuan Yu: assay, data curation. Yulin Zhou: methodology, software. Zhoutai Shang: methodology, data curation. Shengchang Zhang: methodology. Pengqing Liu: supervision, data analysis. Jiadeng Zhu: methodology, writing – review & editing. Mengjin Jiang: conceptualization, funding acquisition, writing – review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the State Key Laboratory of Polymer Materials Engineering (Grant No. sklpme2022-2-04). The authors gratefully acknowledge the State Key Laboratory of Polymer Materials Engineering, College of Polymer Science and Engineering, Sichuan University, and the Analytical &Testing Centre of Sichuan University.References
- C. Lethien, J. Le Bideau and T. Brousse, Energy Environ. Sci., 2019, 12, 96–115 RSC.
- D. P. Dubal, N. R. Chodankar, D.-H. Kim and P. Gomez-Romero, Chem. Soc. Rev., 2018, 47, 2065–2129 RSC.
- F. Wang, X. Wu, X. Yuan, Z. Liu, Y. Zhang, L. Fu, Y. Zhu, Q. Zhou, Y. Wu and W. Huang, Chem. Soc. Rev., 2017, 46, 6816–6854 RSC.
- B.-W. Zhang, L. Ren, Y.-X. Wang, X. Xu, Y. Du and S.-X. Dou, Interdiscip. Mater., 2022, 1, 354–372 CrossRef.
- X. Zhang, Z. Xiao, X. Liu, P. Mei and Y. Yang, Renew. Sust. Energ. Rev., 2021, 147, 111247 CrossRef CAS.
- A. Burke, J. Power Source, 2000, 91, 37–50 CrossRef CAS.
- R. Dhilip Kumar, S. Nagarani, V. Sethuraman, S. Andra and V. Dhinakaran, Chem. Pap., 2022, 76, 3371–3385 CrossRef CAS.
- N. Kurra, R. Wang and H. N. Alshareef, J. Mater. Chem. A, 2015, 3, 7368–7374 RSC.
- Y. Deng, B. Sun, Y. He, J. Quinn, C. Guo and Y. Li, Angew. Chem., Int. Ed., 2016, 55, 3459–3462 CrossRef CAS PubMed.
- Z. Yuan, B. Fu, S. Thomas, S. Zhang, G. DeLuca, R. Chang, L. Lopez, C. Fares, G. Zhang, J.-L. Bredas and E. Reichmanis, Chem. Mater., 2016, 28, 6045–6049 CrossRef CAS.
- Z. Zhao, Z. Yin, H. Chen, L. Zheng, C. Zhu, L. Zhang, S. Tan, H. Wang, Y. Guo, Q. Tang and Y. Liu, Adv. Mater., 2017, 29, 1602410 CrossRef PubMed.
- Y. Guo, Y. Li, O. Awartani, H. Han, J. Zhao, H. Ade, H. Yan and D. Zhao, Nat. Commun., 2017, 29, 1700309 CrossRef PubMed.
- J. Yang, B. Xiao, A. Tang, J. Li, X. Wang and E. Zhou, Adv. Mater., 2019, 31, 1804699 CrossRef CAS PubMed.
- W. Li, W. S. C. Roelofs, M. Turbiez, M. M. Wienk and R. A. J. Janssen, Adv. Mater., 2014, 26, 3304–3309 CrossRef CAS PubMed.
- M. B. Miltenburg, S. Y. An, N. K. Obhi, E. Grignon, B. T. McAllister and D. S. Seferos, ACS Appl. Polym. Mater., 2020, 2, 5574–5580 CrossRef CAS.
- R. A. Schlitz, F. G. Brunetti, A. M. Glaudell, P. L. Miller, M. A. Brady, C. J. Takacs, C. J. Hawker and M. L. Chabinyc, Adv. Sci., 2014, 26, 2825–2830 CrossRef CAS PubMed.
- B. Schulz, M. Bruma and L. Brehmer, Adv. Mater., 1997, 9, 601–613 CrossRef CAS.
- D. Gomes and S. P. Nunes, J. Membr. Sci., 2008, 321, 114–122 CrossRef CAS.
- Y. Yu, J. Zhu, K. Zeng and M. Jiang, J. Mater. Chem. A, 2021, 9, 3472–3481 RSC.
- F. Wang, X. Liao, H. Wang, Y. Zhao, J. Mao and D. G. Truhlar, Interdiscip. Mater., 2022, 1, 517–525 CrossRef.
- S. Janietz, B. Schulz, M. Törrönen and G. Sundholm, Eur. Polym. J., 1993, 29, 545–549 CrossRef CAS.
- B. Schulz, G. Knochenhauer, L. Brehmer and S. Janietz, Synth. Met., 1995, 69, 603–604 CrossRef CAS.
- Q. Tang, G. Hu, V. Fung and D.-e. Jiang, Acc. Chem. Res., 2018, 51, 2793–2802 CrossRef CAS PubMed.
- B. Meng, Y. Ren, J. Liu, F. Jäkle and L. Wang, Angew. Chem., Int. Ed., 2018, 57, 2183–2187 CrossRef CAS PubMed.
- C. Dong, S. Deng, B. Meng, J. Liu and L. Wang, Angew. Chem., Int. Ed., 2021, 60, 16184–16190 CrossRef CAS PubMed.
- F. Borrmann, T. Tsuda, O. Guskova, N. Kiriy, C. Hoffmann, D. Neusser, S. Ludwigs, U. Lappan, F. Simon, M. Geisler, B. Debnath, Y. Krupskaya, M. Al-Hussein and A. Kiriy, Adv. Sci., 2022, 9, 2203530 CrossRef CAS PubMed.
- D. Li, H. Wang, L. Luo, J. Zhu, J. Li, P. Liu, Y. Yu and M. Jiang, ACS Appl. Energy Mater., 2021, 4, 879–887 CrossRef CAS.
- S. K. Pati, D. Patra, S. Muduli, S. Mishra and S. Park, Small, 2023, 19, 2300689 CrossRef CAS PubMed.
- Y. Li, Y. Cao, J. Gao, D. Wang, G. Yu and A. J. Heeger, Synth. Met., 1999, 99, 243–248 CrossRef CAS.
- T.-H. Le, Y. Kim and H. Yoon, Polymers, 2017, 9, 150 CrossRef PubMed.
- D. Mori, H. Benten, H. Ohkita, S. Ito and K. Miyake, ACS Appl. Mater. Interfaces, 2012, 4, 3325–3329 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2020, 19, 1151–1163 CrossRef CAS PubMed.
- J. C. Russell, V. A. Posey, J. Gray, R. May, D. A. Reed, H. Zhang, L. E. Marbella, M. L. Steigerwald, Y. Yang, X. Roy, C. Nuckolls and S. R. Peurifoy, Nat. Mater., 2021, 20, 1136–1141 CrossRef CAS PubMed.
- N. R. Chodankar, H. D. Pham, A. K. Nanjundan, J. F. S. Fernando, K. Jayaramulu, D. Golberg, Y.-K. Han and D. P. Dubal, Small, 2020, 16, 2002806 CrossRef CAS PubMed.
- C. Portet, P. L. Taberna, P. Simon and C. Laberty-Robert, Electrochim. Acta, 2004, 49, 905–912 CrossRef CAS.
- S. V. Bhosale, C. H. Jani and S. J. Langford, Chem. Soc. Rev., 2008, 37, 331–342 RSC.
- P. Zhang, M. Wang, Y. Liu, Y. Fu, M. Gao, G. Wang, F. Wang, Z. Wang, G. Chen, S. Yang, Y. Liu, R. Dong, M. Yu, X. Lu and X. Feng, J. Am. Chem. Soc., 2023, 145, 6247–6256 CrossRef CAS PubMed.
- P. Simon and A. Burke, Electrochem. Soc. Interface, 2008, 17, 38 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ta06374g |
| This journal is © The Royal Society of Chemistry 2024 |