Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Direct observation of phase transitions between delta- and alpha-phase FAPbI3via defocused Raman spectroscopy†
Bernd K.
Sturdza
 ,
Benjamin M.
Gallant
,
Benjamin M.
Gallant
 ,
Philippe
Holzhey
,
Elisabeth A.
Duijnstee
,
Marko W.
von der Leyen
,
Harry C.
Sansom
,
Henry J.
Snaith
,
Philippe
Holzhey
,
Elisabeth A.
Duijnstee
,
Marko W.
von der Leyen
,
Harry C.
Sansom
,
Henry J.
Snaith
 ,
Moritz K.
Riede
and
Robin J.
Nicholas
*
,
Moritz K.
Riede
and
Robin J.
Nicholas
*
Department of Physics, Clarendon Laboratory, University of Oxford, Parks Road, Oxford OX1 3PU, UK. E-mail: robin.nicholas@physics.ox.ac.uk
First published on 5th February 2024
Abstract
The ability to characterise perovskite phases non-destructively is key on the route to ensuring their long-term stability in operando. Raman spectroscopy holds the promise to play an important role in this task. Among all perovskites, formamidinium lead iodide (FAPbI3) has emerged as one of the most promising candidates for single-junction photovoltaic cells. However, Raman spectroscopy of FAPbI3 remains challenging as is evidenced by conflicting reports in the literature. Here, we demonstrate that due to the vulnerability of FAPbI3 to laser-induced degradation, the detected Raman spectrum depends strongly on the experimental conditions. This can lead to conflicting results and is revealed as the origin of discrepancies in the literature. We overcome this issue by deploying defocused Raman spectroscopy, preventing laser-induced damage to the sample and simultaneously improving the signal-to-noise ratio, allowing us to furthermore resolve much weaker Raman modes than was previously possible. We offer step-by-step instructions on how to apply this technique to a given spectrometer. Non-destructive characterisation of the FAPbI3 phases further enables us to quantify the phase stability of pristine FAPbI3 crystals and FAPbI3 grown with the high-performance additive methylenediammonium chloride (MDACl2). This shows that the neat crystals fully degrade within two weeks, whereas in samples grown with the additive only about 2% of the crystal bulk is in the δ-phase after 400 days. This establishes defocused Raman spectroscopy as a powerful tool for the characterisation of FAPbI3 and other perovskite materials.
1 Introduction
Hybrid organic–inorganic metal–halide perovskites have emerged as a promising class of semiconductor materials. Their tuneable bandgaps,1 high absorption coefficients2 and long charge-carrier diffusion lengths3 make them attractive for a range of optoelectronic applications. For single-junction photovoltaic cells, FAPbI3 (FA+ is formamidinium, HC(NH2)2+) is the most promising candidate out of all the ABX3 lead-halide perovskites reported to date4 since FAPbI3 has the narrowest achievable bandgap (Eg ≈ 1.45 eV) for a Pb-based halide perovskite.5 This allows for the highest theoretical photovoltaic power conversion efficiency (PCE) for a lead halide perovskite in a single-junction architecture6 and is reflected in the majority of recent world record PCE perovskite PV devices employing FAPbI3-based materials as their photo-absorbing layer.7–10The main challenge with the use of FAPbI3 is the thermodynamic instability of the cubic α-phase in ambient conditions which tends to transform into a photo-inactive hexagonal 2H δ-phase. This hexagonal phase has a wider band gap, larger defect density and poor quantum efficiency and is hence unsuitable for photovoltaic applications.11–13
Several approaches aiming to stabilise the α-phase have been investigated, including the synthesis of low dimensional perovskite materials, compositional engineering, and the addition of external additives.7,14,15
To study FAPbI3's phase instability it is necessary to isolate the effect of the intended adaptation from other processing variables. For this purpose, single crystalline materials are most suitable, as the properties of thin film and powder samples are highly dependent on their fabrication conditions. Moreover, FAPbI3 single crystals have several advantages over polycrystalline films, such as lower defect densities, longer carrier diffusion lengths, and better-defined structure since the influence of interfaces and grain boundaries can be eliminated.16–19 Therefore, single crystals provide a more reliable platform to study phase transitions as well as the optoelectronic properties of FAPbI3.20,21
The ability to quantify the phase stability of single crystalline FAPbI3 under operational ageing is a critical tool for these investigations. Characterisation of single crystals by X-ray techniques either requires single crystal X-ray diffraction (SCXRD) or powder X-ray diffraction (PXRD). SCXRD of mixed-phase materials is often highly complex, and moreover cannot be used to quantify sample phase composition. On the other hand, PXRD requires single crystals to be powdered, thus destroying the sample and potentially artificially inducing changes in phase composition. Moreover, both techniques are sensitive only to bulk crystalline phases, and are insensitive to either amorphous phases or crystalline nanodomains. Thus, although instructive, neither is suitable for operational phase stability studies, which requires a technique that can be applied many times to the same FAPbI3 single crystal without affecting its properties.
The different symmetries of the δ- and α-phases of FAPbI3 suggest Raman spectroscopy as a powerful characterisation tool. The Raman activity of lead halide perovskites can be divided into two regions. The low wavenumber region (<500 cm−1) contains modes of the inorganic framework and coupled modes, whereas the individual molecular modes of the organic part are represented in the higher wavenumber region (500–3000 cm−1).22 Consequently, only wavenumbers below 500 cm−1 have to be considered to distinguish the δ and α-phase of FAPbI3.
The hexagonal space group of the δ-phase gives rise to four expected Raman modes in the low-frequency region: octahedral distortion (35 cm−1), molecular in-plane rotation around a corner H (63 cm−1), molecular out-of-plane rotation around the N–N axis (90 cm−1) and molecular translation (108 cm−1).23 These four distinct modes make the δ-phase straightforward to identify.
However, the literature is inconsistent in the assignment of Raman modes to the α-phase. Han et al. were the first to report Raman signatures for both phases, assigning peaks at 111 cm−1 and 135 cm−1 to the δ- and α-phase, respectively.13 This assignment has been used in numerous studies24–26 since, even though the cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry of the α-phase is predicted to be Raman inactive by group theory. Two recent reports by Driscoll et al.27 and Ibaceta-Jana et al.28 dissent from these previous assignments and provide experimental data supporting the assignment of the α-phase as Raman inactive.
m symmetry of the α-phase is predicted to be Raman inactive by group theory. Two recent reports by Driscoll et al.27 and Ibaceta-Jana et al.28 dissent from these previous assignments and provide experimental data supporting the assignment of the α-phase as Raman inactive.
This discrepancy in the literature is surprising, as FAPbI3 crystals can, once degraded into the δ-phase, be transformed back into the α-phase via heating to temperatures above the phase transition (observed at 145 °C under 3 K min−1 heating, see Fig. S1†) and α-phase Raman peaks should therefore be straightforward to identify. The origin of this controversy can be found in the experimental details of the Raman measurements.
2 Experimental
2.1 Materials
γ-Butyrolactone (GBL, Alfa Aesar), formamidinium iodide (HC(NH2)2I, Greatcell Solar), methylenediammonium dichloride (H2C(NH3)2Cl2, Sigma Aldrich), lead iodide (PbI2, TCI). All chemicals were used as received and without any further purification.2.2 Synthesis of single crystals
All single crystals were fabricated in N2 from a seed crystal following a previously published experimental protocol.29,30 FAPbI3 single crystals are prepared by dissolving equimolar FAI and PbI2 in GBL. The solution is then stirred at 60 °C for 4 h. Then the solution is filtered with a 25 mm diameter 0.45 μm GMF filter. 4 mL of the filtrate is then placed in a vial that contains a seed FAPbI3 crystal, and the vial is kept in an oil bath undisturbed at 95 °C for 12 h. The crystals were then dried in a vacuum oven at 180 °C for 45 min. For the FAPbI3-M single crystals, alongside equimolar FAI and PbI2 3.8 mol% MDACl2 is added. Then the same procedure as for the FAPbI3 crystals was followed.2.3 Raman spectroscopy
Raman spectra were acquired with a Jobin Yvon T64000 triple spectrometer and an Andor DU420A-OE CCD. The spectrometer was calibrated with the 520.7 cm−1 line of a silicon wafer. Samples were excited with a Ventus solo Nd:YAG laser (λ = 532 nm) and the incident laser irradiation of the samples was strictly monitored.3 Results and discussion
3.1 Raman spectroscopy of FAPbI3
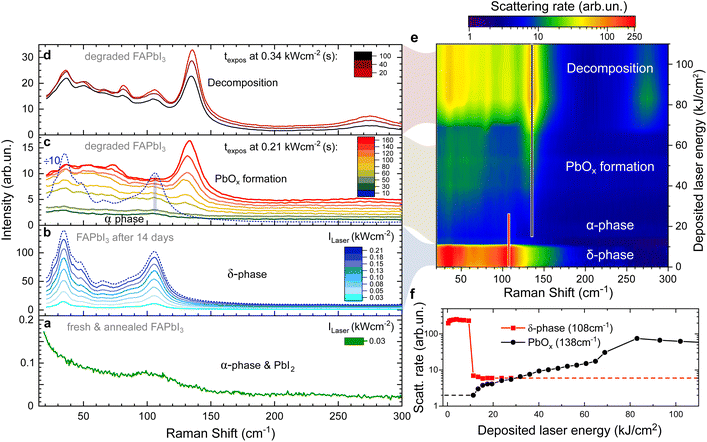
The high absorption coefficient (1 × 105 cm−1 at Elaser = 2.33 eV)31 and low thermal conductivity32 of α-FAPbI3 make it sensitive to laser-induced heating. In the presence of oxygen, this has been shown to cause the formation of Raman active PbOx compounds with a peak at 138 cm−1 and a wider two-phonon peak at 276 cm−1.33,34Chemical decomposition of FAPbI3 single crystals is thought to occur first through abstraction of H+ from FA+ to produce formamidine (FA), which is volatile, leading to the formation of lead iodide (PbI2), polyiodides (Ix−) and molecular iodine (I2) which have low wavenumber peaks in the range of 40–110 cm−1.34,35
Here, we demonstrate all of these degradation stages on a single FAPbI3 crystal in Fig. 1 as a function of laser intensity and deposited laser energy. Initially, the freshly grown and vacuum annealed sample is in the α-phase (Fig. 1a) as is confirmed by X-ray scattering data (Fig. S2†). Apart from the laser line background, there is only a small shoulder present at 110 cm−1 which we attribute to the presence of traces of PbI2 which can be formed as a side effect of annealing.34 No peak is observed at 138 cm−1 and we conclude that the α-phase of FAPbI3 is Raman inactive as predicted by theory.
Raman spectra of the same crystal after ambient exposure for 14 days are given in Fig. 1b. The surface of the FAPbI3 crystal has now degraded into the δ-phase and shows the characteristic four peaks. Upon increasing the laser intensity, the Raman intensity of the δ-phase peaks grows linearly. However, after 10 s at a threshold laser intensity of 0.21 kW cm−2, we observe a 50-fold intensity drop of the Raman modes (Fig. 1b and c) that happens faster than the 5 s acquisition time used. We assign this to laser-induced heating causing a δ-to α-phase transition.
The rapid nature of the switch (see also Fig. 1f) can be explained by the much higher absorption coefficient of the α-phase, resulting in the laser energy being deposited in a smaller volume of the crystal. About 90% of the laser energy is deposited in the first 200 nm of an α-phase crystal (using α = 105 cm−1), resulting in a rapidly increasing temperature in this surface layer and formation of PbOx begins. Note that due to the low thermal conductivity of FAPbI3 (ref. 32) this is a local effect and limited to a small area around the laser spot on the surface (see next section).
The weak remaining peaks at 35 cm−1 and 108 cm−1 (bottom of Fig. 1c) originate from the remaining δ-phase in the periphery of the laser spot on the crystal surface that has not reached the phase transition temperature. Besides these, there are no modes visible, as expected for the α-phase.
Under continuing exposure at the same laser intensity, the PbOx mode at 138 cm−1 becomes visible within 60 seconds of the phase transition (Fig. 1c).
At even higher laser intensities (0.34 kW cm−2), additional modes arise at 36, 50, 67, 82 and 106 cm−1 (Fig. 1d) caused by the formation of PbI2, Ix− and I2 upon decomposition.34,36 The overall signal intensity decreases over time, a clear indication of decomposition which results in the crystal surface moving out of focus.
Fig. 1e provides a visualisation of the laser-induced phase transition and consecutive degradation of the Raman data in Fig. 1b–d. In the false-colour map, the Raman shift is plotted against the cumulative laser energy deposited into the single crystal while the colour indicates the Raman scattering rate. This demonstrates that the Raman scattering cross-section of the α-phase is at least two orders of magnitude weaker than the δ-phase.24
The peak scattering rates of the δ-phase peak at 108 cm−1 and the PbOx peak at 138 cm−1 are plotted against the cumulative deposited laser energy in Fig. 1f. The peak at 108 cm−1 was picked over the stronger peak at 35 cm−1 as it is less affected by the laserline background. Tracking the peak intensity of these two peaks highlights the drop in Raman intensity when the surface of the crystal is transformed from the δ-into the α-phase, even on a logarithmic scale.
3.2 Defocused Raman spectroscopy
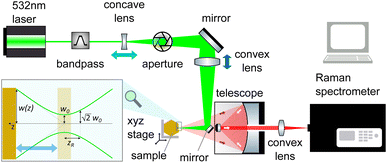
The sensitivity of FAPbI3 to laser-induced degradation demonstrated above requires the laser intensity to be reduced as far as possible. This can be realised by reducing the laser power or, alternatively, increasing the laser spot size on the sample. Lowering the laser power is accompanied by a proportional decrease in Raman signal intensity, such that longer acquisition times are required to maintain sufficient signal-to-noise ratios (SNRs). This is not an ideal solution, as the longer laser exposure at a lower intensity might still degrade the crystal surface. Therefore, increasing the laser spot size is the better approach. This can be achieved in two ways, either by increasing the beam radius at the focal point or by moving the sample out of the focal plane.The spot size at the focal point can be calculated for a Gaussian beam in the paraxial approximation as 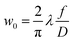 with the focal length of the focusing lens f and the diameter D of the beam at the focusing lens, which is controlled by the aperture. Adjusting the optical system so the sample is not in the focal plane requires a more complex approach. Raman spectrometers typically consist of two separate optical systems, one to focus the laser onto the sample (green rays in Fig. 2) and the second to collect and focus the scattered light onto the detector (red rays in Fig. 2). This means that simply moving the sample out of focus is not a good option, as the detection system would have to be adjusted simultaneously. Instead, de-focusing the excitation system by repositioning either of the two lenses (see Fig. 2) in the direction of the beam is the better option. This moves the focal point of the excitation beam away from the sample surface while keeping the alignment of the detection system intact.
with the focal length of the focusing lens f and the diameter D of the beam at the focusing lens, which is controlled by the aperture. Adjusting the optical system so the sample is not in the focal plane requires a more complex approach. Raman spectrometers typically consist of two separate optical systems, one to focus the laser onto the sample (green rays in Fig. 2) and the second to collect and focus the scattered light onto the detector (red rays in Fig. 2). This means that simply moving the sample out of focus is not a good option, as the detection system would have to be adjusted simultaneously. Instead, de-focusing the excitation system by repositioning either of the two lenses (see Fig. 2) in the direction of the beam is the better option. This moves the focal point of the excitation beam away from the sample surface while keeping the alignment of the detection system intact.
The beam radius at a given distance to the focal point w(z) can be calculated via the Raleigh length zR = πw02/λ as 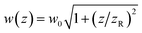 (see Fig. 2). In combination with the equation for the focal length, this allows us to calculate how far a given lens has to be moved to achieve a given spot size at the sample position.
(see Fig. 2). In combination with the equation for the focal length, this allows us to calculate how far a given lens has to be moved to achieve a given spot size at the sample position.
Since the FAPbI3 samples investigated are all single crystals, the outcome of the Raman measurement is not affected by the spot size. We have increased the spot size from a diameter of d = 10 ± 3 μm to d = 65 ± 5 μm by repositioning the concave lens (light blue arrow in Fig. 2). This simple adjustment allows us to retain 50% of the original SNR. Since the area of the defocused spot is about 40 times larger than before, the defocused Raman setup produces a 20-fold increase in the SNR at the same laser intensity. The spot sizes were measured by moving the spot across the edge of a Silicon wafer with a micrometre stage, see Fig. S3.†
The defocused Raman setup, therefore, allows us to resolve much weaker Raman modes than was previously possible. To demonstrate this, we reported the molecular modes of the organic cation FA+ in a recent study37 (see also Fig. S4†) which have previously only been detected in FAPbI3 with laser energies below the band gap.24,25
3.3 Spatially resolved laser-induced phase changes and degradation
In addition to reducing the laser intensity required for a given Raman measurement, the defocused Raman setup also gives us access to new experimental techniques.At very high laser powers, the defocused spot can be used to intentionally change the sample surface. Subsequently, the laser power is strongly reduced and the laser spot focused, which means it can be used as a probe to resolve the spatial variation of the laser-induced changes which now happen on a larger scale.
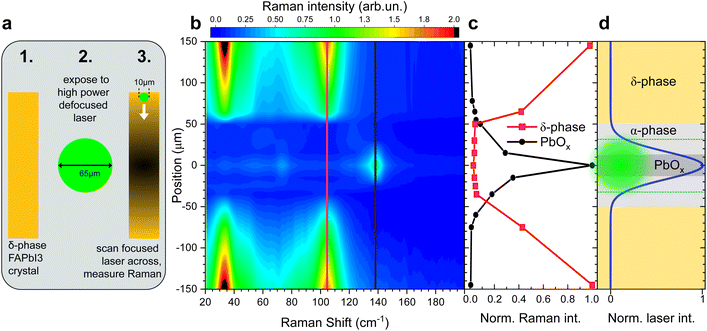
To demonstrate this, we performed Raman measurements on a FAPbI3 crystal, in the δ-phase, in the defocused setting (65 μm spot size) and gradually increased the laser power, until the Raman response indicated a phase transition into the α-phase, similar to the experiment described in Fig. 1b and c.
As soon as the phase change was observed, the laser power was strongly reduced and the setup returned to the focused setting (10 μm spot size). The smaller laser spot can now act as a probe and be scanned across the crystal surface that was previously irradiated with the larger high-power spot, see illustration in Fig. 3a.
This allows us to sample the Raman response across the large spot on the crystal surface. Since the intensity distribution across a focal spot follows a Gaussian distribution, we can observe the effect of a continuum of laser intensities on the δ-phase FAPbI3 crystal. The false-colour map in Fig. 3b reflects the Raman scattering intensity across the defocused laser spot. Far away from the laser spot, the crystal is still entirely in the δ-phase. Starting at about 150 μm from the spot centre, the δ-phase Raman intensity decreases until it vanishes entirely at 50 μm from the centre.
Towards the centre of the large spot, from about 20 μm out, a strong PbOx peak emerges at 138 cm−1 and the iodine peaks indicative of crystal decomposition are detected.
The position-dependent Raman peak intensities of the best resolved δ-phase and PbOx peaks are displayed in Fig. 3c.
The intensity profile of a Gaussian beam with d = 65 μm and the predominant crystal phases are illustrated in Fig. 3d. Since the α-phase is Raman inactive, the absence of Raman scattering in the range of 20 up to 50 cm−1 from the centre suggests that the crystal surface is converted into the α-phase there. The size of the defocused spot is highlighted in green in Fig. 3d. The surface area partially transformed into the α-phase is much larger than the irradiated area, suggesting laser-induced heating rather than photodegradation as the driving mechanism behind the phase transition, in accordance with previous observations on MAPbI3.32
The presence of the PbOx peak (138 cm−1) on a δ-phase crystal that was just briefly exposed to a higher intensity laser shows that the presence of the PbOx peak is uncorrelated to the phase of the FAPbI3 sample prior to the Raman measurement. At sufficient laser intensity, it can be observed in both α-phase and δ-phase crystals, either, via degrading the α-phase sample directly, or, by first transforming the δ-into the α-phase and then degrading it. The PbOx peak is thus not suited as an indicator for the presence of the α-phase.
Even so, the fraction of the crystal that is in the δ-phase can be estimated from the intensity of the δ-phase peaks compared to a purely δ-phase crystal. In the absence of degradation-related peaks like PbOx, the missing fraction of the δ-phase peak intensity can be assumed to be in the α-phase. This insight will be used for tracking the phase stability of FAPbI3 single crystals in the following.
3.4 Quantifying the phase stability of FAPbI3
To quantitatively track the phase stability of FAPbI3 crystals, we compare pristine FAPbI3 crystals and crystals grown with the methylenediammonium (MDA) additive which has been shown to improve stability.7,9,10 We have reported the mechanism of the stability improvement and early Raman results elsewhere,37 here we provide an instructive description of the evolved approach.In Fig. 1 we have shown that the Raman scattering cross-section of α-phase FAPbI3 is at least two orders of magnitude weaker than for the δ-phase (Fig. 1). The overall Raman response of any given FAPbI3 sample will consequently be dominated by δ-phase peaks as soon as a small fraction of the probed layer is transformed into the δ-phase.
The enhanced SNR of the defocused Raman setup thus allows us to track the degraded fraction of the probed surface layer by monitoring the δ-phase peak intensities, even if the crystal is predominantly in the α-phase. We note that peak area is always proportional to the amount of δ-phase present in the crystal and, as is the case here, if peak width is constant then peak height correlates directly with peak area. We monitor the absolute Raman intensity of the best resolved δ-phase peak at a Raman shift of 108 cm−1 under ambient conditions, see Fig. 4.
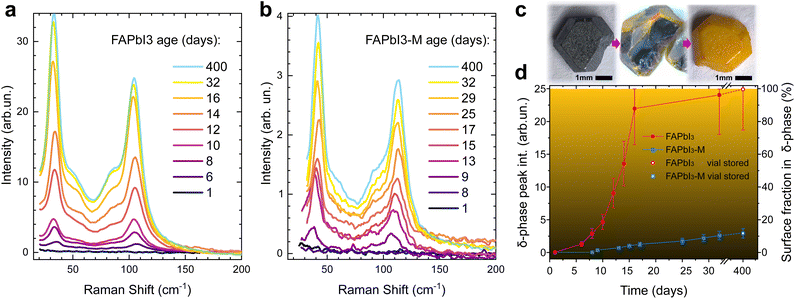
Crystals were used directly after growth and consecutive vacuum annealing. All spectra were taken on the same crystal and are representative of multiple spots on the crystal surface. Crystals were kept in the sample holder of the Raman spectrometer under exposure to ambient air for the duration of the experiment to ensure consistent experimental conditions. We observed the formation of the PbOx Raman peak at laser intensities as low as 25 W cm−2. The defocused Raman setup allows us to keep the laser intensity in our Raman measurements strictly below 5 W cm−2 while maintaining a good SNR.
Neat FAPbI3 (Fig. 4a) undergoes detectable δ-phase formation after 6 days of open ambient exposure. This onset occurs after 8 days for FAPbI3-M (Fig. 4b). After 32 days, the δ-phase peak intensity of neat FAPbI3 stabilises at 24 ± 6, which means the probed crystal volume is fully degraded into the δ-phase. The δ-phase peak intensity is about 10 times smaller for FAPbI3-M after 32 days, suggesting that only about 10% of the probed surface layer has degraded into the δ-phase (Fig. 4d).
Raman spectra of fully degraded FAPbI3 crystals from the same batch that were stored in air-filled vials for 400 days show an intensity of 25 ± 5 which we thus use as reference for a 100% δ-phase crystal. FAPbI3-M shows an intensity of 3.0 ± 0.7 in the same experiments, demonstrating that only about 12% of the probed surface layer is in the δ-phase.
Fig. 4c shows a FAPbI3 crystal cleaved after 8 hours of ambient exposure, demonstrating that our FAPbI3 crystals degrade from the surface inwards. We verify that this surface degradation does not correspond to regions probed during defocused Raman analysis, and thus confirm that surface-initiated phase transformation is the general mechanism for degradation of α-FAPbI3. The thickness of the probed layer for α-phase FAPbI3 is estimated to be 100 nm via the absorption coefficient (α = 105 cm−1 at the Raman laser wavelength of 532 nm).31
Since the absorption coefficient is much lower in the δ-phase, this does not affect the accuracy of the measurement. The estimated δ-phase fraction of the crystal is limited to the probed surface layer. However, the technique can be expanded to the crystal bulk by probing freshly cleaved crystals of a given age. Performing this on our vial stored crystals after 400 days yields an intensity ratio of Ibulk/Isurface = 0.15 ± 0.04 for FAPbI3-M. Combined with our measurements on the surface, this suggests that merely a thin surface layer is degraded while the bulk remains largely in the α-phase (compare centre of Fig. 4c) with only about 0.15 × 0.12 = 2% δ-phase detected. By contrast, the neat FAPbI3 is fully degraded into the δ-phase and the intensity ratio of bulk and surface is close to unity.
4 Conclusions
Non-destructive phase determination of perovskites is a crucial step in assessing and improving their stability. Raman spectroscopy holds the promise to play a key role in this task. Here, we demonstrate the challenges involved in Raman experiments on perovskites with the example of FAPbI3. The vulnerability of metal halide perovskites to laser-induced degradation means that depending on prior history and the experimental conditions used virtually any result can be produced for the same sample. Defocused Raman spectroscopy offers a solution which eliminates this problem while simultaneously improving the SNR. We offer step-by-step instructions on how to apply this technique to a given spectrometer.Applying this allows us to resolve the weak molecular FA+ modes of FAPbI3 for the first time with standard visible laser energies above the band gap. Non-destructive characterisation of the FAPbI3 phase further enables us to quantify the phase stability of neat FAPbI3 crystals and FAPbI3 grown with the high-performance additive MDACl2. This shows that the neat crystals fully degrade within two weeks, whereas in samples grown with the additive only about 12% of the crystal surface and 2% of the crystal bulk are in the δ-phase after 400 days. Overall, this work establishes defocused Raman spectroscopy as a powerful tool for the characterisation of FAPbI3 and opens the door for a better characterisation of other perovskite materials.
Author contributions
B. K. S. performed Raman experiments and wrote the manuscript. B. K. S., M. W. vdL, and R. J. N. conceptualised the defocused Raman setup. B. K. S., P. H., and R. J. N. curated and analysed the Raman data. B. M. G. and E. A. D. synthesised the samples. H. C. S. performed and analysed single crystal XRD measurements. H. J. S., M. K. R., and R. J. N. supervised the work. All authors reviewed and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
B. K. S. acknowledges University College, Oxford, for the Oxford-Radcliffe scholarship and thanks EPSRC for the funding through the Centre for Doctoral Training in New and Sustainable PV (EP/L01551X/1). This work was supported through UKRI-STFC Grants (ST/P002048/1 and No. ST/V001655/1). H. C. S. thanks EPSRC for support under the EPSRC Prosperity Partnership EP/S004947/1. This project was supported by the Processable Electronics Centre for Doctoral Training.Notes and references
- M. Saliba, J. P. Correa-Baena, M. Grätzel, A. Hagfeldt and A. Abate, Angew. Chem., Int. Ed., 2018, 57, 2554–2569 CrossRef CAS PubMed
.
- S. De Wolf, J. Holovsky, S. J. Moon, P. Löper, B. Niesen, M. Ledinsky, F. J. Haug, J. H. Yum and C. Ballif, J. Phys. Chem. Lett., 2014, 5, 1035–1039 CrossRef CAS PubMed
.
- J. Lim, M. T. Hörantner, N. Sakai, J. M. Ball, S. Mahesh, N. K. Noel, Y. H. Lin, J. B. Patel, D. P. McMeekin, M. B. Johnston, B. Wenger and H. J. Snaith, Energy Environ. Sci., 2019, 12, 169–176 RSC
.
- G. E. Eperon, S. D. Stranks, C. Menelaou, M. B. Johnston, L. M. Herz and H. J. Snaith, Energy Environ. Sci., 2014, 7, 982–988 RSC
.
- L. Chen, J. W. Yoo, M. Hu, S. U. Lee and S. I. Seok, Angew. Chem., Int. Ed., 2022, 61, 1–7 Search PubMed
.
- W. Shockley and H. Queisser, J. Appl. Phys., 1961, 32(3), 510–519 CrossRef CAS
.
- H. Min, M. Kim, S. U. Lee, H. Kim, G. Kim, K. Choi, J. H. Lee and S. I. Seok, Science, 2019, 366, 749–753 CrossRef CAS PubMed
.
- G. Kim, H. Min, K. S. Lee, D. Y. Lee, S. M. Yoon and S. I. Seok, Science, 2020, 370, 108–112 CrossRef CAS PubMed
.
- H. Min, D. Y. Lee, J. Kim, G. Kim, K. S. Lee, J. Kim, M. J. Paik, Y. K. Kim, K. S. Kim, M. G. Kim, T. J. Shin and S. I. Seok, Nature, 2021, 598, 444–450 CrossRef CAS PubMed
.
- J. Park, J. Kim, H.-S. Yun, M. J. Paik, E. Noh, H. J. Mun, M. G. Kim, T. J. Shin and S. I. Seok, Nature, 2023, 616, 724–730 CrossRef CAS PubMed
.
- C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis, Inorg. Chem., 2013, 52, 9019–9038 CrossRef CAS PubMed
.
- F. Ma, J. Li, W. Li, N. Lin, L. Wang and J. Qiao, Chem. Sci., 2016, 8, 800–805 RSC
.
- Q. Han, S. H. Bae, P. Sun, Y. T. Hsieh, Y. Yang, Y. S. Rim, H. Zhao, Q. Chen, W. Shi, G. Li and Y. Yeng, Adv. Mater., 2016, 28, 2253–2258 CrossRef CAS PubMed
.
- S. Masi, A. F. Gualdrón-Reyes and I. Mora-Seró, ACS Energy Lett., 2020, 5, 1974–1985 CrossRef CAS
.
- S. P. Dunfield, L. Bliss, F. Zhang, J. M. Luther, K. Zhu, M. F. van Hest, M. O. Reese and J. J. Berry, Adv. Energy Mater., 2020, 10, 1–35 Search PubMed
.
- D. Shi, V. Adinolfi, R. Comin, M. Yuan, E. Alarousu, A. Buin, Y. Chen, S. Hoogland, A. Rothenberger, K. Katsiev, Y. Losovyj, X. Zhang, P. A. Dowben, O. F. Mohammed, E. H. Sargent and O. M. Bakr, Science, 2015, 347, 519–522 CrossRef CAS PubMed
.
- Q. Dong, Y. Fang, Y. Shao, P. Mulligan, J. Qiu, L. Cao and J. Huang, Science, 2015, 347, 967–970 CrossRef CAS PubMed
.
- Z. Chen, Q. Dong, Y. Liu, C. Bao, Y. Fang, Y. Lin, S. Tang, Q. Wang, X. Xiao, Y. Bai, Y. Deng and J. Huang, Nat. Commun., 2017, 8, 1–7 CrossRef PubMed
.
- M. T. Weller, O. J. Weber, J. M. Frost and A. Walsh, J. Phys. Chem. Lett., 2015, 6, 3209–3212 CrossRef CAS
.
- T. Chen, B. J. Foley, C. Park, C. M. Brown, L. W. Harriger, J. Lee, J. Ruff, M. Yoon, J. J. Choi and S. H. Lee, Sci. Adv., 2016, 2, 1–7 Search PubMed
.
- Z. A. Nan, L. Chen, Q. Liu, S. H. Wang, Z. X. Chen, S. Y. Kang, J. B. Ji, Y. Y. Tan, Y. Hui, J. W. Yan, Z. X. Xie, W. Z. Liang, B. W. Mao and Z. Q. Tian, Chem, 2021, 7, 2513–2526 CAS
.
- A. M. Leguy, A. R. Goñi, J. M. Frost, J. Skelton, F. Brivio, X. Rodríguez-Martínez, O. J. Weber, A. Pallipurath, M. I. Alonso, M. Campoy-Quiles, M. T. Weller, J. Nelson, A. Walsh and P. R. Barnes, Phys. Chem. Chem. Phys., 2016, 18, 27051–27066 RSC
.
- J. Ibaceta-Jaña, R. Muydinov, P. Rosado, H. Mirhosseini, M. Chugh, O. Nazarenko, D. N. Dirin, D. Heinrich, M. R. Wagner, T. D. Kühne, B. Szyszka, M. V. Kovalenko and A. Hoffmann, Phys. Chem. Chem. Phys., 2020, 22, 5604–5614 RSC
.
- J. A. Steele, H. Yuan, C. Y. Tan, M. Keshavarz, C. Steuwe, M. B. Roeffaers and J. Hofkens, ACS Nano, 2017, 11, 8072–8083 CrossRef CAS PubMed
.
- A. G. Kontos, G. K. Manolis, A. Kaltzoglou, D. Palles, E. I. Kamitsos, M. G. Kanatzidis and P. Falaras, J. Phys. Chem. C, 2020, 124, 8479–8487 CrossRef CAS
.
- U. Erkılıç, H. G. Ji, E. Nishibori and H. Ago, Phys. Chem. Chem. Phys., 2020, 22, 21512–21519 RSC
.
- E. H. Driscoll, A. Orera, P. A. Anderson, M. L. Sanjuán and P. Slater, Dalton Trans., 2021, 3315–3323 RSC
.
- J. Ibaceta-Jaña, R. Muydinov, P. Rosado, S. H. B. Vinoth Kumar, R. Gunder, A. Hoffmann, B. Szyszka and M. R. Wagner, Phys. Chem. Chem. Phys., 2021, 3, 9476–9482 RSC
.
- P. K. Nayak, D. T. Moore, B. Wenger, S. Nayak, A. A. Haghighirad, A. Fineberg, N. K. Noel, O. G. Reid, G. Rumbles, P. Kukura, K. A. Vincent and H. J. Snaith, Nat. Commun., 2016, 7, 1–8 Search PubMed
.
- B. Wenger, P. K. Nayak, X. Wen, S. V. Kesava, N. K. Noel and H. J. Snaith, Nat. Commun., 2017, 8, 1–10 CrossRef CAS PubMed
.
- J. Chang, L. Jiang, G. Wang, Y. Huang and H. Chen, New J. Chem., 2021, 45, 4393–4400 RSC
.
- Y. Zhou, H. F. Garces and N. P. Padture, Front. Optoelectron., 2016, 9, 81–86 CrossRef
.
- E. Climent-Pascual, B. C. Hames, J. S. Moreno-Ramírez, A. L. Álvarez, E. J. Juarez-Perez, E. Mas-Marza, I. Mora-Seró, A. De Andrés and C. Coya, J. Mater. Chem. A, 2016, 4, 18153–18163 RSC
.
- N. N. Udalova, A. S. Tutantsev, Q. Chen, A. Kraskov, E. A. Goodilin and A. B. Tarasov, ACS Appl. Mater. Interfaces, 2020, 12, 12755–12762 CrossRef CAS PubMed
.
- S. B. Sharp and G. I. Gellene, J. Phys. Chem., 1997, 101(11), 2192–2197 CrossRef CAS
.
- S. Wang, Y. Jiang, E. J. Juarez-Perez, L. K. Ono and Y. Qi, Nat. Energy, 2017, 2, 1–8 Search PubMed
.
- E. A. Duijnstee, B. M. Gallant, P. Holzhey, D. J. Kubicki, S. Collavini, B. K. Sturdza, H. C. Sansom, J. Smith, M. J. Gutmann, S. Saha, M. Gedda, M. I. Nugraha, M. Kober-Czerny, C. Xia, A. D. Wright, Y. H. Lin, A. J. Ramadan, A. Matzen, E. Y. Hung, S. Seo, S. Zhou, J. Lim, T. D. Anthopoulos, M. R. Filip, M. B. Johnston, R. J. Nicholas, J. L. Delgado and H. J. Snaith, J. Am. Chem. Soc., 2023, 145, 10275–10284 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2299142 and 2299143. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ta06411e |
| This journal is © The Royal Society of Chemistry 2024 |