Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Atomistic picture of electronic metal support interaction and the role of water†
Lukáš
Fusek
 ab,
Matteo
Farnesi Camellone‡
ab,
Matteo
Farnesi Camellone‡
 c,
Michal
Ronovský§
c,
Michal
Ronovský§
 b,
Maximilian
Kastenmeier
b,
Maximilian
Kastenmeier
 a,
Tomáš
Skála
a,
Tomáš
Skála
 b,
Pankaj
Kumar Samal
b,
Pankaj
Kumar Samal
 b,
Nataliya
Tsud
b,
Nataliya
Tsud
 b,
Sascha
Mehl
b,
Sascha
Mehl
 d,
Jan
Škvára
b,
Tomáš
Dolák
b,
Vitalii
Uvarov
d,
Jan
Škvára
b,
Tomáš
Dolák
b,
Vitalii
Uvarov
 b,
Martin
Setvín
b,
Martin
Setvín
 b,
Viktor
Johánek
b,
Viktor
Johánek
 b,
Stefano
Fabris
b,
Stefano
Fabris
 c,
Olaf
Brummel
c,
Olaf
Brummel
 a,
Jörg
Libuda
a,
Jörg
Libuda
 a,
Josef
Mysliveček
a,
Josef
Mysliveček
 *b,
Simone
Piccinin
*b,
Simone
Piccinin
 *c and
Yaroslava
Lykhach
*c and
Yaroslava
Lykhach
 *a
*a
aInterface Research and Catalysis, ECRC, Friedrich-Alexander-Universität Erlangen-Nürnberg, Egerlandstrasse 3, Erlangen 91058, Germany. E-mail: yaroslava.lykhach@fau.de
bCharles University, Faculty of Mathematics and Physics, Department of Surface and Plasma Science, V Holešovičkách 2, Prague 18000, Czech Republic. E-mail: josef.myslivecek@mff.cuni.cz
cIstituto Officina dei Materiali, Consiglio Nazionale delle Ricerche (CNR-IOM), Via Bonomea 265, Trieste 34136, Italy. E-mail: piccinin@iom.cnr.it
dElettra-Sincrotrone Trieste SCpA, Strada Statale 14, km 163.5, Basovizza-Trieste 34149, Italy
First published on 12th January 2024
Abstract
Single atom catalysis (SAC) represents an emerging area of heterogeneous catalysis but faces challenges related to the low density of active sites and poor thermal stability. In this work, we present new fundamental insights into the nature of electronic metal support interactions (EMSI) coupled with cation exchange, which yield high density of atomically dispersed noble metals on defect-free terraces of cation-terminated reducible oxides. On well-ordered Co3O4(111) films, the mechanism involves temperature-controlled substitution of surface Co2+ and sub-surface Co3+ cations by Pt2+ and Pt4+ species, respectively. The cation exchange with Co2+ is coupled with the partial reduction of Co3O4(111), while the cation exchange with Co3+ involves the charge disproportionation within the Pt species. In the presence of co-adsorbed water, the Pt4+ species are stabilized at the surface in the form of triaqua complexes.
Electronic metal support interactions (EMSIs) are ubiquitous in heterogeneous catalysis.1–5 The phenomenon is associated with the charge transfer at the metal/oxide interface resulting in a partial oxidation of the noble metal deposit and a partial reduction of the support.5–7 The magnitude of the charge transfer strongly depends on the size of the noble metal nanoparticles and the structure of the support.7 In the regime of low metal loadings, noble metals can be stabilized in the form of cations which serve as active catalytic sites in single-atom catalysts (SAC).8–19 The concept of SAC gained significant attention due to ultimate noble metal utilization which allowed for remarkable reduction of costs.8–12 However, SAC faces significant challenges associated with low-dispersion of noble metal and poor stability.19–22 Both factors are controlled by the availability of the specific anchoring sites and the coordination environment of the noble metal cations. In particular, the atomic dispersion of noble metals on the energetically most favorable, oxygen-terminated oxide surfaces is controlled by defects.23,24 For instance, on the most studied cerium oxide supports, the stabilization of the noble metals in the form of cations requires the presence of specific sites formed by nanostructuring.25–27 However, nanostructuring of the reducible oxides does not provide sufficient densities of defects to achieve a high coverage of atomically dispersed noble metals in SAC. Therefore, current research focuses on new strategies to fabricate SACs with a high density of single-atom sites, ideally on defect-free supports.28–36 The most successful approaches employ the EMSI associated with cation substitution on the cation-terminated surfaces of reducible oxides.37–40 In this respect, significant insights have been obtained on model systems involving e.g. TiO2, Fe2O3, Fe3O4, Co3O4 substrates.13,37 On these surfaces, noble metal atoms exchange places with the surface cations assuming their coordination environment.13 In some cases, the presence of water or hydroxyl groups has been found to promote the dispersion.41,42 However, the complete mechanism of the EMSI, specifically the aspect of the charge transfer between the noble metal atoms and the reducible oxide during the cationic exchange and the role of water, has never been revealed. This is mostly because the evaluation of the charge transfer requires the quantification of the oxidation states of the reducible oxides (e.g. Ce3+/Ce4+ ratio in CeO2).7 This task, however, is often challenging due to the complex shape of the transition metals core levels.43–45
Here, we combined scanning tunnelling spectroscopy (STM), synchrotron radiation photoelectron spectroscopy (SRPES), and density functional theory (DFT) to draw a comprehensive picture of the EMSI in the Pt/Co3O4(111)/Ir(100) model system. For the first time, the EMSI associated with the cation exchange is characterized with respect to both the structure of the Pt sites and the charge transfer between the Pt atoms and the Co3O4(111) substrate. Our findings provide unprecedented atomistic insights into the details of the charge transfer during the cation exchange at the surface and sub-surface regions which can be employed for the rational design of SAC with high density of atomically dispersed noble metal species.
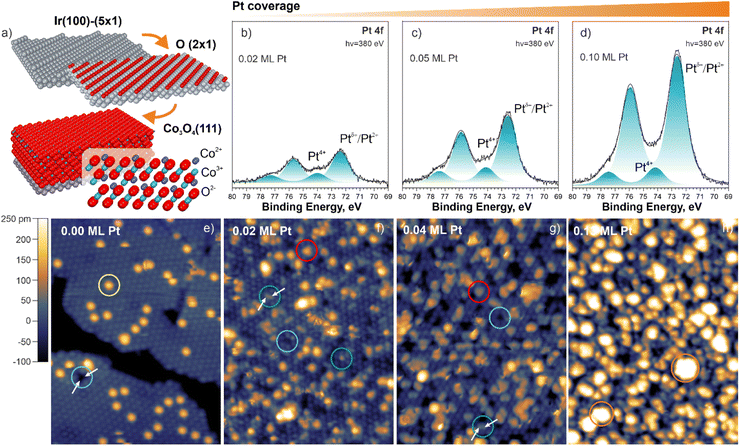
Our study is based on the use of well-ordered Co3O4(111) films prepared by means of physical vapor deposition onto the Ir(100) in a multistep procedure46 schematically shown in Fig. 1a (see ESI for details, Section S1†). The resulting structure is a normal spinel with Co3+ and Co2+ cations in octahedral and tetrahedral coordination, respectively. In the near-surface region, Co2+ and Co3+ cations occupy the first and the second cationic layers, respectively (Fig. 1a). Deposition of Pt onto the Co3O4(111) support in ultrahigh vacuum yields Pt species which give rise to two Pt 4f photoemission doublets at 72.3 eV (Pt 4f7/2) and 74.0 eV (Pt 4f7/2) (Fig. 1b–d). Based on the binding energy, the peak at 72.3 eV (Pt 4f7/2) can be attributed to both atomically dispersed Pt2+ species and ultra-small Ptδ+ aggregates.47,48 Note that the binding energy of supported metallic clusters is a function of their size.49,50 As a result, the spectral contribution from small Ptδ+ aggregates is shifted by as much as 1.0 eV reaching a binding energy of 72.0–72.3 eV.47 In contrast, the peak at 74.0 eV (Pt 4f7/2) can be exclusively assigned to Pt4+ species.48
Scanning tunneling microscopy (STM) revealed very characteristic structural changes when Pt was deposited on the Co3O4(111) surface (Fig. 1e–h). Prior to Pt deposition, we observe well-ordered Co3O4(111) terraces terminated by Co2+ cations in agreement with literature.46 On the clean surface, we observed two types of specific structural features which appear as bright protrusions (circled yellow, species I) and dark depressions (circled cyan, species II) in Fig. 1e. After Pt deposition at 300 K in UHV, the species I disappears and the density of species II increases. In addition, we observe several new features. Among these, we identify the bright protrusions in registry with Co3O4(111) (circled green, species III) and larger bright protrusions of a triangular shape located at threefold hollow sites (FCC sites) (circled red, species IV). At Pt coverages of 0.04 and 0.13 monolayers (ML), we found greater bright features that represent ultra-small Ptδ+ aggregates (circled orange) consisting of 2–3 and 5–6 atoms on average, respectively, in agreement with our earlier study.47 The complete assignment of all surface species observed in Fig. 1f and g is provided in ESI (Fig. S1, Tables S1 and S2).† A more detailed analysis of the structural parameters of the species II–IV with respect to the surface Co2+ cations is given in ESI (Section S2).† Most importantly, the corresponding analysis verified atomic dispersion of the species III and IV (Section S2 and Table S2†) in Fig. 1f and g.
In order to identify the nature of the species I–IV, we simulated the STM patterns of a large number of structural motifs by means of density functional theory (DFT) using the Tersoff–Hamann approach (ESI, Section S1†). The simulated and experimental STM patterns were compared with respect to the position, symmetry, and apparent height of the species I–IV (see ESI, Section S3†). The results are summarized in Fig. 2. For the species I, the best agreement was obtained by locating a single OH− group on top of a surface Co2+ cation and a corresponding H+ on a nearby oxygen anion. The less favorable configurations are listed in ESI (Section S3).† The coverage of OH− groups on as-prepared Co3O4(111) determined by temperature programmed desorption (TPD) is 9% of a surface Co2+ density (see ESI, Section S4†).
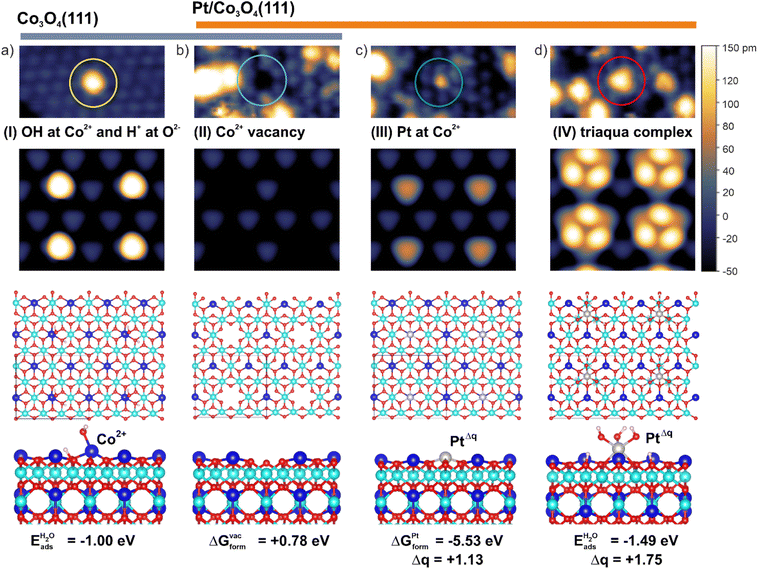 | ||
| Fig. 2 Assignment of species I–IV: the features in the STM images (top) of Co3O4(111) (a) and model Pt/Co3O4(111) systems (b–d) are assigned based on simulated STM patterns (middle). The sizes of the STM images are 5 × 2.5 nm2 (top) and 2.3 × 2.0 nm2 (middle). The STM images in the top and middle panels are plotted on the same apparent height scale. The ball models (bottom) represent the structure and coordination environment of the species (I–IV). The blue and cyan balls represent Co2+ and Co3+ cations while grey, red, and small white balls represent Pt, oxygen, and hydrogen, respectively. Eads indicates adsorption energies while ΔGform indicate formation free energies. Both quantities are defined in the ESI, Section S1.3.† | ||
With respect to the nature of species II, the most satisfactory interpretation of the dark depression is a void associated with a missing Co2+ cation (Fig. 2b). The arguments supporting this assignment are given in the ESI (Section S4).† Most interestingly, the assignment of species III is consistent with a substitution of Co2+ cations by Pt atoms (Fig. 2c). We assume that the substituted Co2+ cation will be incorporated into Co3O4, for example via diffusion to a step edge, and its chemical potential is therefore equal to μCo (see ESI, Section S1.3†). Noteworthy, this structure represents the energetically most favourable configuration with respect to adsorption of single Pt atoms in top, bridge, and FCC sites on Co3O4(111) (ESI, Section S5†).
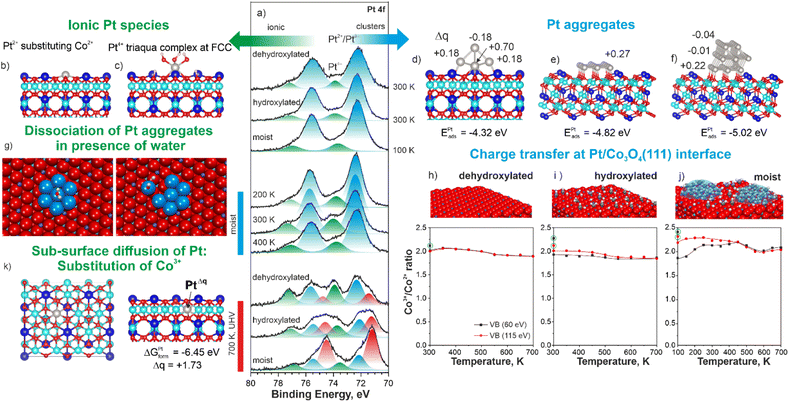
The Bader charge on Pt atom substituting Co2+ is +1.13e which corresponds to the oxidation state Pt2+ (ESI, Section S5†). Formally, the cation exchange is accompanied by the charge transfer from the Pt atom to Co3+ yielding Co2+ cations in the sub-surface region. Finally, the appearance of species IV (triangular protrusions at FCC sites) in STM are best represented by simulated patterns of triaqua complexes (Fig. 2d). These complexes result from the dissociation of three water molecules at a Pt atom in the FCC site followed by its decoration with three OH− groups interacting with three H+ adsorbed on oxygen anions. In this configuration, Pt becomes octahedrally coordinated and gains a Bader charge +1.75e which corresponds to the oxidation state Pt4+ (ESI, Section S5†). For comparison, the dissociation of a single water molecule at the Pt atom in the FCC site yields an adsorption energy similar to that calculated for the triaqua complex (ESI, Section S6†). However, the Bader charge on the Pt atom decorated by a single OH− group at the FCC site merely increases from +0.79e to +0.99e corresponding to a formal oxidation state of approximately Pt2+. If such species exist, they would contribute to the intensity of the Pt 4f contribution at 72.3 eV (Pt 4f7/2). The formation of a triaqua complex at Pt2+ cations substituting Co2+ is less favorable (see ESI, Section S6†). However, we believe that the formation of the triaqua complexes at Pt atoms substituting surface Co2+ would lead to their relocation to the more favorable FCC site. This hypothesis is consistent with the increasing number of cationic vacancies (species II).
It is noteworthy, that the species I associated with hydroxyl groups at the surface Co2+ cations vanish upon deposition of Pt (see ESI, Table S1†). We found that the adsorption energy of a single dissociated water molecule on the Co2+ cation is −1.00 eV, which is lower (i.e. less strongly bound) than that calculated for configurations with OH− groups adsorbed on Pt atoms at the FCC site (−1.51 eV) and Pt2+ substituting the surface Co2+ cations (−1.24 eV). This observation suggests a strong driving force for a migration of water molecules from Co2+ sites in the presence of the Pt atoms.
In order to elucidate the role of water and hydroxyl groups in the atomic dispersion of Pt on Co3O4(111), we investigated the interaction of Pt with three different types of Co3O4(111) substrates denoted as (i) dehydroxylated, (ii) hydroxylated, and (iii) moist. The dehydroxylated substrate was prepared by a brief annealing of the Co3O4(111) film at 650 K in UHV. The hydroxylated and moist substrates were prepared first by a brief annealing of the Co3O4(111) film at 650 K in UHV followed by its exposure to 20 L (1 Langmuir (L) = 1.33 × 10−6 mbar × s) of water at 300 K (hydroxylated) and at 100 K followed by brief annealing to 170 K in UHV (moist). The O 1 s spectra obtained from the dehydroxylated, hydroxylated, and moist Co3O4(111) surfaces are discussed in ESI (Section S7).† Respectively, we obtained a dehydroxylated substrate, which is virtually free of hydroxyl groups and molecular water, and, hydroxylated and moist substrates containing predominantly hydroxyls and molecular water in the form of mixed OH−/H2O clusters.51 The Pt 4f spectra obtained after the deposition of 0.1 ML Pt onto the dehydroxylated, hydroxylated, and moist Co3O4(111) surfaces and subsequent annealing in UHV are shown in Fig. 3a.
Note, that in the case of the moist surface, Pt was deposited at 100 K in order to prevent desorption of molecularly adsorbed water which typically occurs around 200 K.51 In the Pt 4f spectra obtained from the three samples, we resolved two Pt 4f contributions discussed above. Thus, the main ionic species are Pt2+ substituting Co2+ cations (Fig. 3b) and triaqua complexes (Fig. 3c). In addition, all samples contain ultra-small Ptδ+ aggregates. We analyzed the structure and the distribution of Bader charge in ultra-small Ptδ+ aggregates as a function of size (Fig. 3d–f and ESI, Section S8†). We found that only those Pt atoms that are in direct contact with the oxide substrate exhibit significant positive charge. The adsorption energy per Pt atom increases with size, suggesting a tendency for Pt atoms to form clusters (Fig. 3d–f).
Surprisingly, the amounts of Pt4+ species formed upon Pt deposition on all three surfaces are similar (Fig. 3a) despite pronounced differences in the oxidation state of the Co3O4(111) supports. The corresponding Co3+/Co2+ concentration ratios are plotted in Fig. 3h–j. Typically, we quantify the oxidation state of Co3O4(111) based on the changes in the Co3+/Co2+ concentration ratios obtained by the analysis of the valence band spectra.52 The Co3+/Co2+ concentration ratio was monitored using two different photon energies yielding surface (60 eV) and sub-surface (115 eV) information. Prior to Pt deposition (see circled dots in Fig. 3h–j), the Co3+/Co2+ ratios deviate from the stoichiometric ratio; the deviation is the highest on moist substrate suggesting partial oxidation of the surface layer triggered by the reaction with water. The evolution of the Co3+/Co2+ ratio upon annealing of Pt-free Co3O4(111) exposed to 20 L of water is shown in ESI (Section S10).†
Deposition of Pt results in partial reduction of Co3O4(111) substrates due to the EMSI, i.e. charge transfer from Pt to Co3O4. A more pronounced charge transfer upon Pt deposition on the moist substrate results from the migration of water/hydroxyls from Co2+ cations to Pt sites. As predicted by DFT, this process results in an increase of the Bader charges on the Pt sites as a function of the degree of hydroxylation. In the next step, annealing at 350 K leads to an increase of the amount of Pt4+ at the expense of the Pt2+/Ptδ+ species on all three samples: the amount of the Pt4+ species is significantly higher on the moist surface. This observation suggests a clear preference for the formation of triaqua complexes. We modelled the energetics of the formation of the triaqua complexes also by dissociation of the Ptδ+ aggregates (Fig. 3g). Our study suggests that such scenario is thermodynamically favorable based on the residual partial pressure of water in the analysis chamber (see ESI, Section S9†). Upon further annealing, the Pt4+ species are converted to Pt2+/Ptδ+ below 450 K and metallic Pt0 above 450 K (see ESI, Section S7†). This process is accompanied by re-oxidation of the Co3O4(111) substrate mostly due to H2 desorption, which has been reported previously for different systems under similar conditions.53 We experimentally verified H2 desorption from Pt-free Co3O4(111) substrate and Pt/Co3O4(111) system under relevant conditions (ESI, Section S10†). Surprisingly, annealing above 600 K once again triggers the formation of Pt4+ species accompanied by Pt0 at the expense of the Pt2+/Ptδ+ species. The depth profiling of the Pt4+ distribution within the Pt/Co3O4(111) system, suggests its sub-surface location (ESI, Section S11†). Our DFT calculations indicate that the most favorable configuration for the Pt4+ species corresponds to the substitution of Co3+ cations by Pt atoms. This process has a formation free energy of −6.45 eV and leads to an increase of the Bader charge on Pt to +1.73e (Fig. 3k). Noteworthy, this process is not accompanied by the reduction of the Co3O4(111) support (see Fig. 3h–j). We excluded the formation of metallic Pt–Co alloys (see ESI, Section S12†). Accordingly, the charge transfer must occur exclusively between Pt species (e.g. charge disproportionation between two Pt2+ species yielding Pt4+ and Pt0).
The role of water is to form triaqua complexes at FCC sites either by stabilizing the deposited Pt atoms at FCC sites or by conversion of Pt2+ at substitutional Co2+ sites. The latter channel involves relocation of a triaqua complexes to the FCC sites leaving a cationic vacancy behind. This pathway is irreversible, i.e. after the loss of hydroxyl groups, Pt species in FCC sites are prone to sintering. As a result, after annealing at 700 K, the amount of Pt0 is the highest and the amount of Pt4+ species is the lowest on the moist substrate and vice versa on the dehydroxylated substrate (Fig. 3a).
Conclusions
We have demonstrated that atomically dispersed Pt species can be formed at a high density on a flat surface of a reducible oxide. A key element is the termination of the surface by surface cations, which can be substituted by the noble metal species. Specifically, on the well-ordered Co3O4(111) surface, atomically dispersed Pt species are formed in the oxidation states of Pt2+ and Pt4+. The mechanism associated with the EMSI involves temperature-controlled substitution of surface Co2+ and sub-surface Co3+ cations by Pt2+ and Pt4+ cations, respectively. In the presence of co-adsorbed water, Pt4+ species can be stabilized at the surface in the form of triaqua complexes at FCC sites. This pathway potentially lowers the number of surface Pt2+ species yielding cationic vacancies. Under these conditions, substitution of sub-surface Co3+ by Pt4+ cations is suppressed which leads to the formation of metallic Pt0. This complex mechanism highlights the dynamic nature of the catalyst surface. In this respect, we provide the atomic-level understanding of the EMSI and the specifics of the charge transfer upon the cation exchange which can guide the design of the high-density SAC.Data availability
Source data are provided at Zenodo54: https://doi.org/10.5281/zenodo.10229984.Author contributions
Conceptualization: M. F. C., J. M., S. P., and Y. L.; funding acquisition: J. M. O. B. J. L. P. K. S. investigation: L. F., M. F. C., M. R., M. K., T. S., P. K. S., N. T., S. M., J. Š., T. D., V. U., V. J., Y. L; supervision: M. S., V. J., S. F., O. B., J. L., J. M., S. P., Y. L.; validation: L. F., M. F. C., P. K. S., M. S., V. J., J. M., YL.; writing – original draft: Y. L.; writing – review and editing: L. F., M. F. C., M. R., M. K., T. S., N. T., S. M., M. S., V. J., O. B., J. L., J. M., S. P., Y. L.; all authors have given approval for the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support by the Deutsche Forschungsgemeinschaft (DFG) (project 431733372) and the Czech Science Foundation (project GAČR 20-11688J). This project has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement no. 101007417 having benefitted from the access provided by CNR-IOM in Trieste within the framework of the NFFA-Europe Pilot Transnational Access Activity, proposals ID865 and ID186. Additional support is acknowledged by the DFG Collaborative Research Centre SFB 1452 – Catalysis at Liquid Interfaces (project 431791331) and project 453560721. The authors acknowledge financial support from ICSC – Centro Nazionale di Ricerca in High Performance Computing, Big Data and Quantum Computing, funded by European Union – NextGenerationEU. P. K. S. and J. Š. thank for the support of the Grant Agency of Charles University (project GAUK 326122). The authors acknowledge the CERIC-ERIC Consortium for the access to experimental facilities and financial support. The authors also acknowledge the support by Czech Ministry of Education, Youth and Sports (project LM2018116 and project QM4ST, reg. no. CZ.02.01.01/00/22_008/0004572). The authors thank Dr Kevin Charles Prince for the support and organizing the beamtime. The authors thank Prof. Dr M. Alexander Schneider for help with interpretation of STM images and IV-LEED.References
- M. Li, A. Groß and R. J. Behm, ACS Catal., 2022, 12, 10065–10079 CrossRef CAS.
- T. W. van Deelen, C. Hernández Mejía and K. P. de Jong, Nat. Catal., 2019, 2, 955–970 CrossRef CAS.
- J. Chen, Y. Zhang, Z. Zhang, D. Hou, F. Bai, Y. Han, C. Zhang, Y. Zhang and J. Hu, J. Mater. Chem. A, 2023, 11, 8540–8572 RSC.
- C. T. Campbell, Nat. Chem., 2012, 4, 597–598 CrossRef CAS.
- A. Bruix, J. A. Rodriguez, P. J. Ramírez, S. D. Senanayake, J. Evans, J. B. Park, D. Stacchiola, P. Liu, J. Hrbek and F. Illas, J. Am. Chem. Soc., 2012, 134, 8968–8974 CrossRef CAS PubMed.
- G. Pacchioni and H.-J. Freund, Chem. Soc. Rev., 2018, 47, 8474–8502 RSC.
- Y. Lykhach, S. M. Kozlov, T. Skála, A. Tovt, V. Stetsovych, N. Tsud, F. Dvořák, V. Johánek, A. Neitzel, J. Mysliveček, S. Fabris, V. Matolín, K. M. Neyman and J. Libuda, Nat. Mater., 2016, 15, 284–288 CrossRef CAS.
- A. K. Datye and H. Guo, Nat. Commun., 2021, 12, 895 CrossRef CAS.
- S. Mitchell and J. Pérez-Ramírez, Nat. Commun., 2020, 11, 4302 CrossRef CAS PubMed.
- Q. Zhang and J. Guan, Adv. Funct. Mater., 2020, 30, 2000768 CrossRef CAS.
- W. Li, Z. Guo, J. Yang, Y. Li, X. Sun, H. He, S. Li and J. Zhang, Electrochem. Energy Rev., 2022, 5, 9 CrossRef CAS.
- X. Liang, N. Fu, S. Yao, Z. Li and Y. Li, J. Am. Chem. Soc., 2022, 144, 18155–18174 CrossRef CAS PubMed.
- F. Kraushofer and G. S. Parkinson, Chem. Rev., 2022, 122, 14911–14939 CrossRef CAS.
- A. Bruix, Y. Lykhach, I. Matolínová, A. Neitzel, T. Skála, N. Tsud, M. Vorokhta, V. Stetsovych, K. Ševčiková, J. Mysliveček, R. Fiala, M. Václavů, K. C. Prince, S. Bruyere, V. Potin, F. Illas, V. Matolín, J. Libuda and K. M. Neyman, Angew. Chem., Int. Ed., 2014, 53, 10525–10530 CrossRef CAS PubMed.
- J. Jones, H. Xiong, A. T. DeLaRiva, E. J. Peterson, H. Pham, S. R. Challa, G. Qi, S. Oh, M. H. Wiebenga, X. I. Pereira Hernández, Y. Wang and A. K. Datye, Science, 2016, 353, 150–154 CrossRef CAS PubMed.
- R. Lang, X. Du, Y. Huang, X. Jiang, Q. Zhang, Y. Guo, K. Liu, B. Qiao, A. Wang and T. Zhang, Chem. Rev., 2020, 120, 11986–12043 CrossRef CAS PubMed.
- N. J. O'Connor, A. S. M. Jonayat, M. J. Janik and T. P. Senftle, Nat. Catal., 2018, 1, 531–539 CrossRef.
- B. T. Qiao, A. Q. Wang, X. F. Yang, L. F. Allard, Z. Jiang, Y. T. Cui, J. Y. Liu, J. Li and T. Zhang, Nat. Chem., 2011, 3, 634–641 CrossRef CAS PubMed.
- J. Geiger and N. López, J. Phys. Chem. C, 2022, 126, 13698–13704 CrossRef CAS.
- N. Cheng, L. Zhang, K. Doyle-Davis and X. Sun, Electrochem. Energy Rev., 2019, 2, 539–573 CrossRef.
- T. Cui, L. Li, C. Ye, X. Li, C. Liu, S. Zhu, W. Chen and D. Wang, Adv. Funct. Mater., 2022, 32, 2108381 CrossRef CAS.
- S. Duan, R. Wang and J. Liu, Nanotechnology, 2018, 29, 204002 CrossRef PubMed.
- G. Di Liberto, S. Tosoni, L. A. Cipriano and G. Pacchioni, Acc. Mater. Res., 2022, 3, 986–995 CrossRef CAS.
- Y. Ren, Y. Tang, L. Zhang, X. Liu, L. Li, S. Miao, D. Sheng Su, A. Wang, J. Li and T. Zhang, Nat. Commun., 2019, 10, 4500 CrossRef PubMed.
- M. Farnesi Camellone, F. Dvořák, M. Vorokhta, A. Tovt, I. Khalakhan, V. Johánek, T. Skála, I. Matolínová, S. Fabris and J. Mysliveček, ACS Catal., 2022, 12, 4859–4871 CrossRef CAS.
- A. Tovt, L. Bagolini, F. Dvořák, N.-D. Tran, M. Vorokhta, K. Beranová, V. Johánek, M. Farnesi Camellone, T. Skála, I. Matolínová, J. Mysliveček, S. Fabris and V. Matolín, J. Mater. Chem. A, 2019, 7, 13019–13028 RSC.
- H. N. Pham, A. DeLaRiva, E. J. Peterson, R. Alcala, K. Khivantsev, J. Szanyi, X. S. Li, D. Jiang, W. Huang, Y. Sun, P. Tran, Q. Do, C. L. DiMaggio, Y. Wang and A. K. Datye, ACS Sustainable Chem. Eng., 2022, 10, 7603–7612 CrossRef CAS.
- J. R. Regalbuto and A. K. Datye, Nat. Nanotechnol., 2022, 17, 110–111 CrossRef CAS PubMed.
- J. Shan, J. Liao, C. Ye, J. Dong, Y. Zheng and S.-Z. Qiao, Angew. Chem., Int. Ed., 2022, 61, e202213412 CrossRef CAS PubMed.
- W. Yang, X. Zhao, Y. Wang, R. Wang, W. Yang, Y. Peng and J. Li, Nano Res., 2023, 16, 219–227 CrossRef CAS.
- J. Wu, L. Xiong, B. Zhao, M. Liu and L. Huang, Small Methods, 2020, 4, 1900540 CrossRef CAS.
- Y. Wang, S. Lee, J. Zhou, J. Fu, A. Foucher, E. Stach, L. Ma, N. Marinkovic, S. Ehrlich, W. Zheng and D. G. Vlachos, Catal. Sci. Technol., 2022, 12, 2920–2928 RSC.
- H. Shin, J. Ko, C. Park, D.-H. Kim, J. Ahn, J.-S. Jang, Y. H. Kim, S.-H. Cho, H. Baik and I.-D. Kim, Adv. Funct. Mater., 2022, 32, 2110485 CrossRef CAS.
- X. Yang and G. Wu, Angew. Chem., Int. Ed., 2023, 62, e202216490 CrossRef CAS PubMed.
- R. Lang, W. Xi, J.-C. Liu, Y.-T. Cui, T. Li, A. F. Lee, F. Chen, Y. Chen, L. Li, L. Li, J. Lin, S. Miao, X. Liu, A.-Q. Wang, X. Wang, J. Luo, B. Qiao, J. Li and T. Zhang, Nat. Commun., 2019, 10, 234 CrossRef PubMed.
- X. Hai, S. Xi, S. Mitchell, K. Harrath, H. Xu, D. F. Akl, D. Kong, J. Li, Z. Li, T. Sun, H. Yang, Y. Cui, C. Su, X. Zhao, J. Li, J. Pérez-Ramírez and J. Lu, Nat. Nanotechnol., 2022, 17, 174–181 CrossRef CAS PubMed.
- J. Hulva, M. Meier, R. Bliem, Z. Jakub, F. Kraushofer, M. Schmid, U. Diebold, C. Franchini and G. S. Parkinson, Science, 2021, 371, 375–379 CrossRef CAS PubMed.
- Z. Jiang, X. Feng, J. Deng, C. He, M. Douthwaite, Y. Yu, J. Liu, Z. Hao and Z. Zhao, Adv. Funct. Mater., 2019, 29, 1902041 CrossRef.
- Z. Jakub, J. Hulva, M. Meier, R. Bliem, F. Kraushofer, M. Setvin, M. Schmid, U. Diebold, C. Franchini and G. S. Parkinson, Angew. Chem., Int. Ed., 2019, 58, 13961–13968 CrossRef CAS PubMed.
- E. Bianchetti, D. Perilli and C. Di Valentin, ACS Catal., 2023, 13, 4811–4823 CrossRef CAS PubMed.
- F. Kraushofer, L. Haager, M. Eder, A. Rafsanjani-Abbasi, Z. Jakub, G. Franceschi, M. Riva, M. Meier, M. Schmid, U. Diebold and G. S. Parkinson, ACS Energy Lett., 2022, 7, 375–380 CrossRef CAS PubMed.
- L. Puntscher, K. Daninger, M. Schmid, U. Diebold and G. S. Parkinson, Electrochim. Acta, 2023, 449, 142190 CrossRef CAS.
- M. C. Biesinger, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2010, 257, 887–898 CrossRef CAS.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS.
- Y. Lykhach, S. Piccinin, T. Skála, M. Bertram, N. Tsud, O. Brummel, M. Farnesi Camellone, K. Beranová, A. Neitzel, S. Fabris, K. C. Prince, V. Matolín and J. Libuda, J. Phys. Chem. Lett., 2019, 10, 6129–6136 CrossRef CAS.
- W. Meyer, K. Biedermann, M. Gubo, L. Hammer and K. Heinz, J. Phys.: Condens. Matter, 2008, 20, 265011 CrossRef CAS PubMed.
- M. Bertram, C. Prössl, M. Ronovský, J. Knöppel, P. Matvija, L. Fusek, T. Skála, N. Tsud, M. Kastenmeier, V. Matolín, K. J. J. Mayrhofer, V. Johánek, J. Mysliveček, S. Cherevko, Y. Lykhach, O. Brummel and J. Libuda, J. Phys. Chem. Lett., 2020, 11, 8365–8371 CrossRef CAS PubMed.
- NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20, National Institute of Standards and Technology, Gaithersburg MD, 2000, p. 20899, DOI:10.18434/T4T88K.
- M. G. Mason, Phys. Rev. B: Condens. Matter Mater. Phys., 1983, 27, 748–762 CrossRef CAS.
- G. K. Wertheim and S. B. DiCenzo, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 844–847 CrossRef CAS PubMed.
- M. Schwarz, F. Faisal, S. Mohr, C. Hohner, K. Werner, T. Xu, T. Skála, N. Tsud, K. C. Prince, V. Matolín, Y. Lykhach and J. Libuda, J. Phys. Chem. Lett., 2018, 9, 2763–2769 CrossRef CAS PubMed.
- M. Kastenmeier, L. Fusek, F. Mohamed, C. Schuschke, M. Ronovský, T. Skála, M. Farnesi Camellone, N. Tsud, V. Johánek, S. Fabris, J. Libuda, S. Piccinin, Y. Lykhach, J. Mysliveček and O. Brummel, J. Phys. Chem. C, 2023, 127, 6034–6044 CrossRef CAS.
- Y. Fujimori, W. E. Kaden, M. A. Brown, B. Roldan Cuenya, M. Sterrer and H.-J. Freund, J. Phys. Chem. C, 2014, 118, 17717–17723 CrossRef CAS.
- Y. Lykhach, J. Mysliveček, J. Libuda, S. Piccinin and M. Farnesi Camellone, Atomistic Picture of Electronic Metal Support Interaction and the Role of Water, Zenodo, 2023, DOI:10.5281/zenodo.10229984.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, STM evaluation, DFT computation, IV-LEED analysis, full set of SRPES data, TPD. See DOI: https://doi.org/10.1039/d3ta06595b |
| ‡ This author contributed equally and should be also considered as first author. |
| § Present address: European Synchrotron Radiation Facility, 38043 Grenoble, France. |
| This journal is © The Royal Society of Chemistry 2024 |