Open Access Article
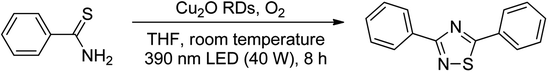
Open Access ArticlePhotocatalytic oxidative cyclization of aromatic thioamides catalyzed by Cu2O rhombic dodecahedra†
Guan-Ru
Wang
and
Michael H.
Huang
 *
*
Department of Chemistry, National Tsing Hua University, Hsinchu 300044, Taiwan. E-mail: hyhuang@mx.nthu.edu.tw
First published on 7th February 2024
Abstract
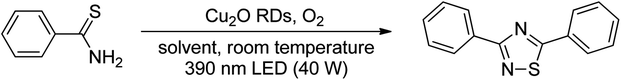
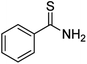
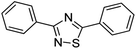
Cu2O rhombic dodecahedra were used to photocatalyze thiobenzamide cyclization in tetrahydrofuran (THF) forming 3,5-diphenyl-1,2,4-thiadiazole under 390 nm light illumination. The rhombic dodecahedra outperform cubic and octahedral Cu2O crystals. They can also photocatalyze thiobenzamides bearing a wide range of substituent groups with generally high product yields in 18 h at room temperature. Electron, hole and various radical scavenging experiments were performed to support the proposed reaction mechanism. This work demonstrates again that the use of surface-controlled metal oxide crystals offers huge cost advantages over the covalent organic framework (COF) photocatalysts for some organic transformations.
Introduction
Cu2O, Ag2O, Ag3PO4, SrTiO3 and other polyhedral crystals have been shown to exhibit facet-dependent photocatalytic properties.1–6 For instance, Cu2O rhombic dodecahedra are notably more photocatalytically active than octahedra, yet cubes are inert even with gold nanoparticle decoration.7 Recent synchrotron X-ray diffraction (XRD) and high-resolution transmission electron microscopy (HR-TEM) characterization has revealed the presence of bulk and surface lattices in all Cu2O crystals, and different surfaces show distinct lattice deviation patterns.8 Bulk cell constants also vary slightly for different particle shapes. Such structural feature should affect the barrier to charge transport across a particular crystal face, yielding the observed photocatalytic facet effect. Moreover, surface deposition of Cu2O polyhedra with various semiconductor nanocrystals including ZnO and ZnS can both enhance and suppress photocatalytic activity of the heterostructures depending on the interfacial planes with either favorable or unfavorable band bending.9,10 Such interfacial contact selection is also useful to achieving current rectification.11 Another way to boost photocatalytic activity is through surface conjugated molecular functionalization.12–14 This is because surface band structure and electron density is greatly tuned with molecular modification.Upon light illumination on semiconductor materials, the photoexcited electrons in the conduction band and holes in the valence band first migrate to the crystal surface. Electrons reduce dissolved oxygen to form superoxide anion radicals (O2˙−), while holes oxidize water or hydroxide ions to give hydroxyl radicals (˙OH).15,16 Other than dye photodegradation and water splitting reactions, Cu2O crystals have been employed in CO2 reduction.17–19 The photogenerated charge carriers and radicals can also be utilized to catalyze some organic transformation reactions.20 Previously, Cu2O rhombic dodecahedra have been demonstrated to efficiently photocatalyze arylboronic acid hydroxylation.21 4-Nitrophenylacetylene (4-NA)-modified Cu2O rhombic dodecahedra also photocatalyze aryl sulfide oxidation.22 Oxidative amine coupling can also be performed using polyhedral SrTiO3 and 4-NA-modified Cu2O crystals.23,24 We further consider the formation of 1,2,4-thiadiazoles through photocatalytic thioamide cyclization reactions. 1,2,4-Thiadiazoles are an important class of heterocyclic compounds with biological and therapeutic applications ranging from anticancer to neuroprotective activity.25,26 In the past, hypervalent-iodine(III)-mediated intramolecular oxidative reaction and CoPcS-catalyzed intramolecular reaction for the synthesis of 1,2,4-thiadiazole derivatives have been reported.27,28 Iodine-mediated synthesis of 1,2,4-thiadiazoles is also known.29 Organic oxidant has also been employed to form thioamides.30 Use of Cu(OTf)2 as a catalyst and K2CO3 as a base in acetonitrile can also produce diverse thiobenzamides at 70 °C.31 These procedures involve the use stoichiometric amounts of halogen as an oxidizing agent and base. More recently, covalent organic frameworks, such as fully conjugated Py-BSZ-COF containing an electron-donating unit and an electron-accepting unit, have been prepared at 120 °C for photocatalytic oxidative cyclization of diverse thioamides to 1,2,4-thiadiazoles or through photo-initiated intramolecular coupling.32–34 Nevertheless, COFs are more complicated to synthesize and characterize. Making Py-BSZ-COF can take nearly 5 days.
Here we demonstrate that simple Cu2O cubes, octahedra, and rhombic dodecahedra can photocatalyze thiobenzamide cyclization to form 3,5-diphenyl-1,2,4-thiadiazole. In particular, Cu2O rhombic dodecahedra show generally high conversion of diverse thioamides to 1,2,4-thiadiazoles in THF. Charge carrier and radical scavenging experiments were also performed to support the proposed reaction mechanism. This work further illustrates the great value of using surface-controlled semiconductor crystals for photocatalytic organic transformations.
Results and discussion
Characterization of Cu2O crystals
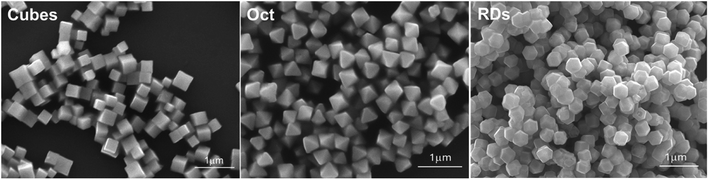
Cu2O cubes, rhombic dodecahedra, and octahedra were synthesized following our reported conditions.35Fig. 1 provides scanning electron microscopy (SEM) images of the synthesized Cu2O crystals, revealing high size and shape uniformity. Fig. S1, ESI† give their size distribution histograms. The average edge length of cubes is 307 nm. The average opposite corner length of octahedra is 292 nm, while the average opposite face length of rhombic dodecahedra is 331 nm. XRD patterns of these samples, available in Fig. S2, ESI,† indicate exclusive formation of Cu2O. While cubes and octahedra have nearly identical peak positions, rhombic dodecahedra have peaks slightly shifted to lower 2θ angles from their larger cell constants. The results are consistent with synchrotron XRD measurements.8 Such lattice constant variations give rise to their various facet-dependent behaviors.For a fair comparison of the photocatalytic activity, the total particle surface area should be the same. With a fixed total surface area of 8.8 × 1015 nm2, the amounts of Cu2O cubes, octahedra, and rhombic dodecahedra needed for the photocatalysis experiments are 2.7, 1.5 and 2.9 mg, respectively (Table S1, ESI†). A degassing and oxygen-filling process was carried out before adding thiobenzamide into a tube containing Cu2O rhombic dodecahedra to optimize the reaction conditions. Initially, solvent selection was examined (see Table 1). With the oxygen-filled glass tube, thiobenzamide conversion to 3,5-diphenyl-1,2,4-thiadiazole did not occur in water under 390 nm light-emitting diode (LED) light irradiation with a power of 40 W at room temperature. In 1,4-dioxane, toluene and acetone, the product yields reached to 23–41% after 21 h. Switching to ethyl acetate and acetonitrile, the yields improved to 52–58%. Use of dichloromethane and dichloroethane delivered yields of 63–69%. Further yield increases to 78–80% were observed in dimethylformamide, ethanol and methanol, so ethanol can be a good and green solvent choice for this reaction. However, the highest product yield of 94% was achieved using tetrahydrofuran as the solvent. Thus, tetrahydrofuran was chosen for subsequent optimization experiments.
| Entry | Solvent | Time (h) | Yield (%) |
|---|---|---|---|
| a Reagents: thiobenzamide (0.4 mmol), Cu2O RDs (2.9 mg) in solvent (3 mL). b Isolated yield. | |||
| 1 | Water | 21 | 0 |
| 2 | 1,4-Dioxane | 21 | 23 |
| 3 | Toluene | 21 | 31 |
| 4 | Acetone | 21 | 41 |
| 5 | Ethyl acetate | 21 | 52 |
| 6 | Acetonitrile | 21 | 58 |
| 7 | Dichloromethane | 21 | 63 |
| 8 | Dichloroethane | 21 | 69 |
| 9 | Dimethylformamide | 21 | 78 |
| 10 | Ethanol | 21 | 78 |
| 11 | Methanol | 21 | 80 |
| 12 | Tetrahydrofuran | 21 | 94 |
The irradiation light wavelength effect was also examined (Table S2, ESI†). Use of 40 W 440 and 370 nm LED lamps gave product yields of 37 and 82%, respectively. With 390 nm light irradiation for 6 and 8 h, the product yields were 51 and 94%, respectively. Therefore, the 390 nm LED lamp remains most effective. Next, a series of control experiments for oxidative cyclization of thiobenzamide was conducted (Table 2). Use of commercial Cu2O powder only delivered 23% thiobenzamide conversion, showing the importance of catalyst surface control to reactivity. Without light irradiation, adding any catalyst, or introducing nitrogen, only a trace of amount of product was obtained. This confirms that proper light illumination, catalyst and oxygen are all indispensable to achieving high product formation. Conducting the reaction in air atmosphere only gave 19% product yield, so filling the tube with oxygen is critically important. Additionally, when the reaction was carried out with the fan turned off, the solution temperature can reach 50 °C. The obtained product yield is 90%, so it is beneficial to use a fan to cool the solution.
Using the optimized reaction conditions, the product yield was 94% for {110}-bound Cu2O rhombic dodecahedra after 8 h of reaction as noted above (Table 3). By contrast, {100}-terminated cubes and {111}-enclosed octahedra showed 52 and 75% yields, respectively. Rhombic dodecahedra are clearly the best catalyst. This is understandable, since they are most efficient in charge transport to the surface for facile radical generation.7,36 The notably high product yield in the case of Cu2O cubes should result from extended light irradiation to cause some degree of surface roughening. SEM images and XRD patterns of the particles taken after the photocatalysis reaction are provided in Fig. S3 and S4, ESI.† While octahedra and rhombic dodecahedra generally preserve their morphologies, some nanostructures appear on the cube surfaces. From X-ray photoelectron spectroscopy analysis, it has been observed before that continued light irradiation on Cu2O nanocubes can produce some CuO.36 This surface change should make Cu2O cubes become photocatalytically active. XRD patterns of the used photocatalysts give only Cu2O diffraction peaks. Again rhombic dodecahedra possess a slightly larger cell constant than those of cubes and octahedra.
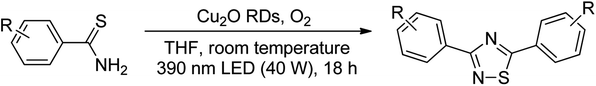
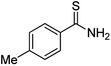
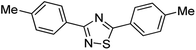
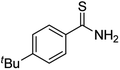
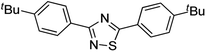
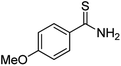
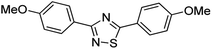
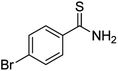
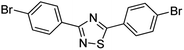
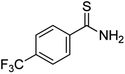
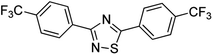
Substrate scope was examined next using the optimized reaction conditions with Cu2O rhombic dodecahedra as the photocatalyst. The reaction time was 18 h. Table 4 shows thiobenzamide bearing electron-donating 4-OCH3, 4-CH3 and tert-butyl substituents can also achieve high product yields in the range of 93–96%. For the halogen-substituted thiobenzamides, generally good product yields of 69% (–F), 73% (–Cl), and 82% (–Br) were obtained. The results suggest that the electron-withdrawing ability of the halogen group is somewhat linked to the reactivity. For thiobenzamide with a highly electronegative trifluoromethyl group, a product yield of 76% was obtained. Furthermore, use of a naphthalene-substituted thioamide for oxidative coupling resulted in 90% product yield. Last, use of a heterocyclic thiophene-substituted thioamide gave a product yield of 80%, but the pyridinyl-substituted thioamide showed no product formation. A lack of reactivity for pyridinylboronic acid hydroxylation has been observed before.21 Despite the pyridinyl inactivity, these experiments demonstrate that Cu2O rhombic dodecahedra are capable of photocatalyzing cyclization reaction of a diverse range of thioamides.
To propose a reaction mechanism, electron, hole, and radical scavenging experiments need to be performed first using the optimized reaction conditions with Cu2O rhombic dodecahedra as the photocatalyst. As seen in Table 5, adding potassium persulfate (K2S2O8) as an electron scavenger led to a significant drop in thiobenzamide conversion to just 21%.37 This infers that the photoexcited electrons are utilized to generate radicals for thioamide cyclization. When KI was introduced as a hole scavenger, 3,5-diphenyl-1,2,4-thiadiazole formation was reduced to 11%. Employing sodium oxalate as another hole scavenger, a trace amount of product formation was recorded. Thus, holes should participate in the photoredox reaction to facilitate thioamide cyclization. Next, addition of 1,4-benzoquinone to the reaction solution to remove superoxide anion radicals led to a significant product decrease to just 27%, indicating the active role of superoxide radicals to product formation. Previous electron paramagnetic resonance (EPR) measurements on photoirradiated Cu2O rhombic dodecahedra dispersed in methanol also revealed the formation of superoxide anion radicals.21,22 Introducing DABCO as singlet oxygen scavenger reduces the product yield to 32%. Singlet oxygen molecules have been suggested to involve in photocatalytic benzylamine coupling reactions.23 Singlet oxygen is produced upon photoexcition of the ground state triplet oxygen. It is subsequently reduced to form a superoxide anion radical. Removal of singlet oxygen molecules lowers the production of superoxide radicals. Last, adding isopropanol as a hydroxyl radical scavenger had a relatively minor effect with a product yield of 82%, so hydroxyl radicals are less important in the photocatalytic process. The above results are consistent with the band positions of these Cu2O crystals. With their conduction band energies at around −1.40 V vs. RHE (reversible hydrogen electrode), oxygen reduction to form superoxide radicals at a standard potential of −0.33 V should happen.38 However, their valence band positions at around 0.70 V is not capable of carrying out direct water oxidation to yield hydroxyl radicals (2.73 V).39 An alternative pathway via water oxidation to molecular oxygen first (1.23 V), followed by the formation of H2O2 at 0.68 V, is more reasonable.40 Subsequent hydrogen peroxide reduction, photolysis, or reaction with superoxide radicals, gives hydroxyl radicals. Water oxidation by Cu2O is possible, considering surface band bending should exist to explain the observed facet-dependent behaviors.
| Entry | Trapping reagent | Yield (%) |
|---|---|---|
| a Reagents: thiobenzamide (0.4 mmol) and Cu2O RDs (2.9 mg) in THF (3 mL). b Isolated yield. c K2S2O8 as electron scavenger. d KI and sodium oxalate as hole scavengers. e 1,4-Benzoquinone as O2˙− radical scavenger. f DABCO as 1O2 scavenger. g Isopropanol as ˙OH scavenger. | ||
| 1c | K2S2O8 | 21 |
| 2d | KI | 11 |
| 3d | Sodium oxalate | Trace |
| 4e | 1,4-Benzoquinone | 27 |
| 5f | DABCO | 32 |
| 6g | Isopropanol | 82 |
From the above results, along with related literature reports, a plausible mechanism of the photocatalytic oxidative cyclization reaction is proposed, as illustrated in Fig. 2.32,34 Under light irradiation, electron–hole pairs are generated within Cu2O rhombic dodecahedra and then migrate to the surface. The photogenerated holes participate in the oxidization of thioamide and convert the molecules into corresponding cationic radicals. The thioamide radical cations first transform into two radical isomers after proton removal, which are linked to give a dimer through radical cross-coupling. The photogenerated electrons would reduce oxygen to form superoxide radicals. Through proton abstraction by superoxide radicals, 1,2,4-thiadiazole is readily obtained via intramolecular cyclization of the dimer and subsequent aromatization with removal of SH− species.
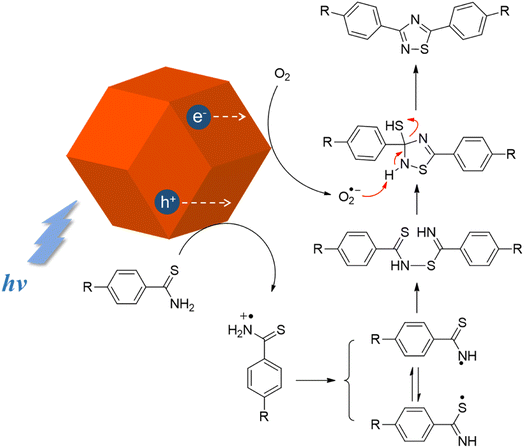 | ||
| Fig. 2 Proposed reaction mechanism of Cu2O-catalyzed photocatalytic oxidative cyclization reaction of thioamides. | ||
Conclusions
Cu2O rhombic dodecahedra were used to optimize the reaction conditions toward photocatalytic oxidative cyclization of thiobenzamide. Under the optimized reaction conditions, rhombic dodecahedra can achieve 94% product yield with 390 nm LED illumination in just 8 h, while cubes and octahedra delivered lower product yields. Cu2O rhombic dodecahedra can also photocatalyze thiobenzamides bearing a broad range of substituent groups with generally high product yields. Charge carrier and radical scavenging experiments were conducted to support a plausible reaction mechanism. The use of polyhedral cuprous oxide crystals for this organic transformation offers tremendous cost advantages over COF photocatalysts.Experimental
Chemicals
Sodium dodecyl sulfate (SDS, 100%, J. T. Baker), anhydrous copper(II) chloride (CuCl2, 98%, Alfa Aesar), sodium hydroxide (NaOH, 98%, SHOWA), hydroxylamine hydrochloride (NH2OH·HCl, 99%, Alfa Aesar), potassium carbonate (K2CO3, 99%, Alfa Aesar), acetone (C3H6O, 97%, Alfa Aesar), acetonitrile (CH3CN, 99%, Alfa Aesar), dichloroethane (C2H4Cl2, 99.8%, Sigma-Aldrich), dichloromethane (CH2Cl2, >95%, Alfa Aesar), N,N-dimethylmethanamide (C3H7NO, 99%, Alfa Aesar), 1,4-dioxane (C4H8O2, 99.8%, Sigma-Aldrich), ethanol (C2H5OH, ≥99.5%, Honeywell), ethyl acetate (C4H8O2, >99.5%, Alfa Aesar), n-hexane (C6H14, ≥95%, Sigma-Aldrich), methanol (CH3OH, ≥99.8%, Honeywell), tetrahydrofuran (C4H8O, THF, ≥99.9%, Sigma-Aldrich), toluene (C6H5CH3, 99.5%, Alfa Aesar), DABCO (C6H12N2, >99%, Sigma-Aldrich), 1,4-benzoquinone (C6H4O2, 98%, Alfa Aesar), potassium persulfate (K2S2O8, ≥99.0%, Sigma-Aldrich), potassium iodide (KI, 99.5%, J. T. Baker), sodium oxalate (Na2C2O4, ≥99.0%, Sigma-Aldrich), isopropanol (C3H8O, 99.5%, Sigma-Aldrich), thiobenzamide (C6H5CSNH2, 98%, Sigma-Aldrich), 4-methylbenzothioamide (C8H9NS, >98.0%, TCI), 4-methoxythiobenzamide (C8H9NOS, 98%, Alfa Aesar), 4-tert-butylthiobenzamide (C11H15NS, 98%, Sigma-Aldrich), 4-fluorothiobenzamide (C7H6FNS, 97%, Alfa Aesar), 4-chlorobenzothioamide (C7H6ClNS, 97%, TCI), 4-bromothiobenzamide (C7H6BrNS, 97%, Thermo), 4-(trifluoromethyl)thiobenzamide (C8H6F3NS, 98%, TCI), naphthalene-2-thiocarboxamide (C11H9NS, 97%, Thermo), thiophene-2-carbothioamide (C5H5NS2, 99%, TCI), and 4-pyridinethioamide (C6H6N2S, 97%, Sigma-Aldrich) were used as received. All the water (18.2 MΩ) used was purified by the Milli-Q technique.Cu2O-photocatalyzed oxidative cyclization of thioamides
Synthesis conditions of Cu2O cubes, octahedra, and rhombic dodecahedra are provided in the ESI.† Also see Fig. S5, ESI† for an illustration of the crystal synthesis procedure. Typically, rhombic dodecahedral Cu2O crystals (2.9 mg) and thioamide (0.4 mmol) were added to a 15 mL quartz test tube with a stir bar and sealed with a rubber septum. The tube was evacuated using a vacuum pump and refilled with oxygen three times. After the evacuation and refill steps, tetrahydrofuran was injected into the tube. The mixture was sonicated for 3 min and placed approximately 2 cm from a blue LED lamp (40 W, λ = 390 nm). See Fig. S6, ESI† for the sample preparation illustration. The photocatalytic reaction was carried out for 8 h with the aid of a cooling fan to maintain the solution temperature near room temperature. Cu2O crystals were separated by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 min. The separated organic layer was concentrated by rotary evaporation to obtain the crude product. The residue was purified by short column chromatography.
000 rpm for 3 min. The separated organic layer was concentrated by rotary evaporation to obtain the crude product. The residue was purified by short column chromatography.
Instrumentation
SEM images of the samples were obtained using a JOEL JSM-7000F electron microscope. XRD patterns were collected using a D2 PHASER desktop diffractometer with Cu Kα radiation (λ = 0.1541 Å). UV-Vis absorption spectra were taken with the use of a JASCO V-770 spectrophotometer. A Kessil KSPR160-L lamp was used in the photocatalytic experiments. 1H and 13C NMR spectra were recorded on a Bruker Avance 400 spectrometer. Chemical shifts (δ) are reported in ppm with the residual solvent signal as internal standard (chloroform at 7.26 and 77.00 ppm for 1H and 13C NMR spectroscopy, respectively). Thin-layer chromatography (TLC) was conducted on silica-gel 60 F254 plates (Merck KGaA).Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support is provided by the National Science and Technology Council, Taiwan (NSTC 112-2113-M-007-016-MY3).References
- M. H. Huang and M. Madasu, Nano Today, 2019, 28, 100768 CrossRef CAS.
- Y.-J. Chen, Y.-W. Chiang and M. H. Huang, ACS Appl. Mater. Interfaces, 2016, 8, 19672–19679 CrossRef CAS PubMed.
- Y.-W. Harn, T.-H. Yang, T.-Y. Tang, M.-C. Chen and J.-M. Wu, ChemCatChem, 2015, 7, 80–86 CrossRef CAS.
- D. Majumder, I. Chakraborty, K. Mandal and S. Roy, ACS Omega, 2019, 4, 4243–4251 CrossRef CAS PubMed.
- M.-S. Hsieh, H.-J. Su, P.-L. Hsieh, Y.-W. Chiang and M. H. Huang, ACS Appl. Mater. Interfaces, 2017, 9, 39086–39093 CrossRef CAS PubMed.
- P.-L. Hsieh, G. Naresh, Y.-S. Huang, C.-W. Tsao, Y.-J. Hsu, L.-J. Chen and M. H. Huang, J. Phys. Chem. C, 2019, 123, 13664–13671 CrossRef CAS.
- C.-Y. Chu and M. H. Huang, J. Mater. Chem. A, 2017, 5, 15116–15123 RSC.
- B.-H. Chen, G. Kumar, Y.-J. Wei, H.-H. Ma, J.-C. Kao, P.-J. Chou, Y.-C. Chuang, I.-C. Chen, J.-P. Chou, Y.-C. Lo and M. H. Huang, Small, 2023, 19, 2303491 CrossRef CAS PubMed.
- S.-C. Wu, C.-S. Tan and M. H. Huang, Adv. Funct. Mater., 2017, 27, 1604635 CrossRef.
- G. Naresh, P.-L. Hsieh, V. Meena, S.-K. Lee, Y.-H. Chiu, M. Madasu, A.-T. Lee, H.-Y. Tsai, T.-H. Lai, Y.-J. Hsu, Y.-C. Lo and M. H. Huang, ACS Appl. Mater. Interfaces, 2019, 11, 3582–3589 CrossRef CAS PubMed.
- A.-T. Lee, C.-S. Tan and M. H. Huang, ACS Cent. Sci., 2021, 7, 1929–1937 CrossRef CAS PubMed.
- S.-J. Chan, J.-C. Kao, P.-J. Chou, Y.-C. Lo, J.-P. Chou and M. H. Huang, J. Mater. Chem. C, 2022, 10, 8422–8431 RSC.
- A. S. Patra, J.-C. Kao, S.-J. Chan, P.-J. Chou, J.-P. Chou, Y.-C. Lo and M. H. Huang, J. Mater. Chem. C, 2022, 10, 3980–3989 RSC.
- P.-J. Chou, W.-Y. Yu, J.-C. Kao, Y.-C. Lo, J.-P. Chou and M. H. Huang, J. Mater. Chem. A, 2023, 11, 19514–19523 RSC.
- D. Nunes, A. Pimental, R. Branquinho, E. Fortunato and R. Martins, Catalysts, 2021, 11, 504 CrossRef CAS.
- Y.-H. Zhang, M.-M. Liu, J.-L. Chen, S.-M. Fang and P.-P. Zhou, Dalton Trans., 2021, 50, 4091–4111 RSC.
- T. Zhu, B. Wu, J. Xie, H. Yang, W. Zhang and Y. Sun, ACS Sustainable Chem. Eng., 2023, 11, 17482–17491 CrossRef CAS.
- J. Lu, L. Yang, Y. Zhang, C. Wang, C. Zhang and X. S. Zhao, ACS Appl. Nano Mater., 2023, 6, 20746–20756 CrossRef CAS.
- C.-f. Li, R.-t. Guo, Z.-r. Zhang, T. Wu and W.-g. Pan, Small, 2023, 19, 2207875 CrossRef CAS PubMed.
- S. Rej, M. Bisetto, A. Naldoni and P. Fornasiero, J. Mater. Chem. A, 2021, 9, 5915–5951 RSC.
- Z.-H. Su, M.-H. Hsieh, Z.-L. Chen, W.-T. Dai, E.-T. Wu and M. H. Huang, Chem. Mater., 2023, 35, 2782–2789 CrossRef CAS.
- M.-H. Hsieh, Z.-H. Su, E.-T. Wu and M. H. Huang, ACS Appl. Mater. Interfaces, 2023, 15, 11662–11669 CrossRef CAS PubMed.
- Z.-L. Chen and M. H. Huang, J. Mater. Chem. A, 2023, 11, 22198–22205 RSC.
- E.-T. Wu and M. H. Huang, ACS Catal., 2023, 13, 14746–14752 CrossRef CAS.
- Y. J. Pragathi, R. Screenivasulu, D. Veronica and R. R. Raju, Arabian J. Sci. Eng., 2021, 46, 225–232 CrossRef CAS PubMed.
- G. L. Perlovich, A. N. Proshin, T. V. Volkova, L. N. Petrova and S. O. Bachurin, Mol. Pharm., 2012, 9, 2156–2167 CrossRef CAS PubMed.
- A. Mariappan, K. Rajaguru, N. M. Chola, S. Muthusubramanian and N. Bhuvanesh, J. Org. Chem., 2016, 81, 6573–6579 CrossRef CAS PubMed.
- L. Yang, L. Song, S. Tang, L. Li, H. Li, B. Yuan and G. Yang, Eur. J. Org Chem., 2019, 1281–1285 CrossRef CAS.
- N. Jatangi, N. Tumula, R. K. Palakodety and M. Nakka, J. Org. Chem., 2018, 83, 5715–5723 CrossRef CAS PubMed.
- D. Cheng, R. Luo, W. Zheng and J. Yan, Synth. Commun., 2012, 42, 2007–2013 CrossRef CAS.
- Y. Sun, W. Wu and H. Jiang, Eur. J. Inorg. Chem., 2014, 4239–4243 CrossRef CAS.
- S. Li, L. Li, Y. Li, L. Dai, C. Liu, Y. Liu, J. Li, J. Lv, P. Li and B. Wang, ACS Catal., 2020, 10, 8717–8726 CrossRef CAS.
- J. Yuan, Q. Xia, W. Zhu, C. Wu, B. Wang, B. Liu, X. Yang, Y. Xu and H. Xu, ChemPhotoChem, 2020, 4, 445–450 CrossRef CAS.
- Y. Liu, X. Jiang, L. Chen, Y. Cui, Q.-Y. Li, X. Zhao, X. Han, Y.-C. Zheng and X.-J. Wang, J. Mater. Chem. A, 2023, 11, 1208–1215 RSC.
- H.-H. Ma and M. H. Huang, J. Mater. Chem. C, 2023, 11, 5857–5866 RSC.
- G.-Z. Yuan, C.-F. Hsia, Z.-W. Lin, C. Chiang, Y.-W. Chiang and M. H. Huang, Chem.–Eur. J., 2016, 22, 12548–12556 CrossRef CAS PubMed.
- M.-Q. Yang, C. Han, N. Zhang and Y.-J. Xu, Nanoscale, 2015, 7, 18062–18070 RSC.
- P. Ren, T. Zhang, N. Jain, H. Y. V. Ching, A. Jaworski, G. Barcaro, S. Monti, J. Silvestre-Albero, V. Celorrio, L. Chouhan, A. Rokicińska, E. Debroye, P. Kuśtrowski, S. Van Doorslaer, S. Van Aert, S. Bals and S. Das, J. Am. Chem. Soc., 2023, 145, 16584–16596 CrossRef CAS PubMed.
- S. Siahrostami, G.-L. Li, V. Viswanathan and J. K. Nørskov, J. Phys. Chem. Lett., 2017, 8, 1157–1160 CrossRef CAS PubMed.
- S. Wu and X. Quan, ACS ES&T Engg, 2022, 2, 1068–1079 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ta08126e |
| This journal is © The Royal Society of Chemistry 2024 |