Open Access Article
Open Access ArticleUnveiling the ligand-mediated phase engineering mechanism in two-dimensional transition metal chalcogenides through coordination geometry control†
Sungju
Jun‡
 ac,
Joo-Won
Lee‡
ac,
Joo-Won
Lee‡
 a,
Sung-Chul
Kim
a,
Sung-Chul
Kim
 b,
Soong Ju
Oh
b,
Soong Ju
Oh
 c and
Sohee
Jeong
c and
Sohee
Jeong
 *a
*a
aMaterials Architecturing Research Center, Korea Institute of Science and Technology, 5, Hwarang-ro 14 gil, Seongbuk-gu, Seoul, 02792, South Korea. E-mail: soheejeong@kist.re.kr
bAdvanced Analysis and Data Center, Korea Institute of Science and Technology, 5, Hwarang-ro 14 gil, Seongbuk-gu, Seoul, 02792, South Korea
cDepartment of Materials Science and Engineering, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul, 02841, South Korea
First published on 5th March 2024
Abstract
While metastable metallic phases of group-VI transition metal dichalcogenide (TMDC) nanosheets exhibit intriguing and unprecedented characteristics, the development of reliable synthetic methodologies, particularly in direct solution-based synthesis, remains a challenge due to the lack of understanding of molecular leverage of the metal–ligand coordination geometry for obtaining metastable phases in two-dimensional (2D) TMDCs. Here, we describe an effective solution-based approach for directly synthesizing metastable metallic phases by unveiling the criterion for phase-selective formation, using appropriate ligands. Specifically, metallic 1T′-WSe2 and 1T-WS2 nanosheets were obtained with trioctylphosphine oxide, whereas 2H-WSe2 and 2H-WS2 nanosheets were formed using oleylamine. Spectroscopic analysis, including X-ray pre-edge absorption, revealed that phosphine oxide ligands (–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P) induce the distorted octahedral metal–ligand geometry, followed by the phase-selective formation of metallic 1T′ and 1T phases. Meanwhile, amine ligands (–NH2), accompanied by the trigonal prismatic metal–ligand geometry, exclusively lead to the production of 2H phases. This strategy was applied using hexadecylamine and triphenylphosphine oxide to produce 2H and 1T′ phases, respectively. As a proof-of-concept study, metallic 1T′-WSe2 shows enhanced hydrogen evolution activity with long-term durability. This strategy, controlling the metal–ligand coordination geometry by the choice of suitable ligands, offers a new guideline for securing metastable phases in 2D TMDCs.
P) induce the distorted octahedral metal–ligand geometry, followed by the phase-selective formation of metallic 1T′ and 1T phases. Meanwhile, amine ligands (–NH2), accompanied by the trigonal prismatic metal–ligand geometry, exclusively lead to the production of 2H phases. This strategy was applied using hexadecylamine and triphenylphosphine oxide to produce 2H and 1T′ phases, respectively. As a proof-of-concept study, metallic 1T′-WSe2 shows enhanced hydrogen evolution activity with long-term durability. This strategy, controlling the metal–ligand coordination geometry by the choice of suitable ligands, offers a new guideline for securing metastable phases in 2D TMDCs.
Interest has surged in 2D layered nanomaterials such as TMDCs because these TMDCs exhibit remarkable catalytic, optical, and electronic properties due to the presence of d-electrons in transition metals distinct from graphene.1–5 Also, with an emerging direct band gap in the monolayer and diverse electrical properties from semiconducting to metallic,6,7 TMDCs have found applications in energy harvesting and storage. Notably, semiconducting TMDCs like 2H-MoS2 and 2H-WS2 served as photocatalysts,8,9 while metallic 1T-MoS2 and 1T-MoSe2 were utilized as electrochemical catalysts.10,11 Existing synthetic protocols for group-VI TMDCs (MCh2, M = Mo, W; Ch = S, Se) primarily focused on producing thermodynamically stable 2H-MCh2 with the trigonal prismatic (TPR-6) coordination geometry of M using conventional methods like chemical vapor deposition and solution-based synthesis.12–14 Conversely, metastable 1T′-MCh2 and 1T-MCh2 with distorted octahedral and octahedral (dOC-6 and OC-6) coordination geometries of M have been obtained through post-treatments on 2H-MCh2, such as intercalation/exfoliation and chemical treatment.15–17 These procedures, however, introduce chemical impurities like cations inside of TMDCs, compelling a need for the direct solution-based synthesis of metallic MCh2 to avoid interference from chemical impurities. While some reports provided colloidal strategies for the fabrication of phase-controlled MCh2,18–23 the crucial chemical factor for precise phase engineering, especially into metallic phases with dOC-6 and OC-6 geometries, remains underexplored.
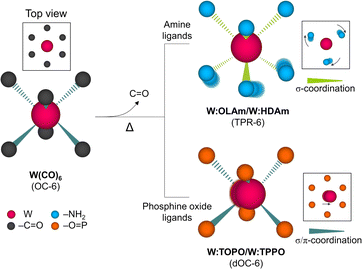
In this study, we present a direct solution-based synthetic approach for obtaining metastable metallic WCh2 by meticulously manipulating the metal–ligand coordination geometry in W complex molecules with the choice of suitable ligands as a pivotal factor guiding the synthesis towards the selective production of 2H-WCh2 and 1T′-/1T-WCh2. According to the theoretical study,24 metastable phases of WCh2 are more stable and feasible due to their lower unit lattice energies compared to MoCh2. Therefore, this study focuses on the phase control in WCh2 as a representative case. X-ray spectroscopic investigation along with X-ray pre-edge absorption reveals that specific functional groups (–NH2 and –O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P) in the amine ligands (OLAm and HDAm, standing for oleylamine and hexadecylamine) and phosphine oxide ligands (TOPO and TPPO, abbreviations for trioctylphosphine oxide and triphenylphosphine oxide) drive into W complex molecules with the TPR-6 geometry (W
P) in the amine ligands (OLAm and HDAm, standing for oleylamine and hexadecylamine) and phosphine oxide ligands (TOPO and TPPO, abbreviations for trioctylphosphine oxide and triphenylphosphine oxide) drive into W complex molecules with the TPR-6 geometry (W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and W
OLAm and W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDAm) and the dOC-6 geometry (W
HDAm) and the dOC-6 geometry (W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO and W
TOPO and W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPPO), respectively, as shown in Scheme 1. In addition, Raman analysis bridges the gap between the control of metal–ligand coordination geometry and the phase-selective formation of 2H-WCh2 and 1T′-/1T-WCh2. As a test for electrocatalytic applications, 1T′-WSe2 proves the improved hydrogen evolution reaction (HER) performance regarding overpotential, Tafel slope, and durability. While ligands have been extensively studied in 2D layered nanomaterial synthesis, the role of metal–ligand coordination geometry in phase control has been overlooked. To the best of our knowledge, this is the first attempt to demonstrate that the metal–ligand coordination geometry at the molecular level significantly influences the phase engineering of 2D TMDCs, which even facilitates the formation of metastable phases.
TPPO), respectively, as shown in Scheme 1. In addition, Raman analysis bridges the gap between the control of metal–ligand coordination geometry and the phase-selective formation of 2H-WCh2 and 1T′-/1T-WCh2. As a test for electrocatalytic applications, 1T′-WSe2 proves the improved hydrogen evolution reaction (HER) performance regarding overpotential, Tafel slope, and durability. While ligands have been extensively studied in 2D layered nanomaterial synthesis, the role of metal–ligand coordination geometry in phase control has been overlooked. To the best of our knowledge, this is the first attempt to demonstrate that the metal–ligand coordination geometry at the molecular level significantly influences the phase engineering of 2D TMDCs, which even facilitates the formation of metastable phases.
 | ||
| Scheme 1 Illustration showing the control of metal–ligand coordination geometry in W complex molecules by using the amine ligands and phosphine oxide ligands. | ||
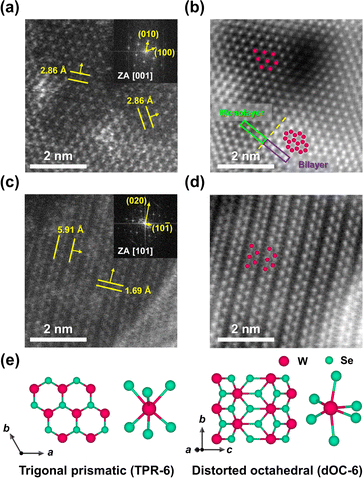
We first conducted the synthesis of WSe2 nanosheets by reacting W(CO)6 and Se in the presence of OLAm and TOPO as a representative of amine ligands and phosphine oxide ligands, affording 2H-WSe2 and 1T′-WSe2, respectively. For WSe2 nanosheets synthesized with OLAm (2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm), W hexagonal arrays which correspond to the atomic configuration of the 2H phase with the TPR-6 coordination were observed (Fig. 1a, b and e) by high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) imaging. In contrast, when the TOPO ligand was utilized instead of OLAm, WSe2 nanosheets exhibited a clear characteristic atomic arrangement indicative of the metallic 1T′ phase with the dOC-6 coordination (1T′-WSe2
OLAm), W hexagonal arrays which correspond to the atomic configuration of the 2H phase with the TPR-6 coordination were observed (Fig. 1a, b and e) by high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) imaging. In contrast, when the TOPO ligand was utilized instead of OLAm, WSe2 nanosheets exhibited a clear characteristic atomic arrangement indicative of the metallic 1T′ phase with the dOC-6 coordination (1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO), displaying aligned W zig–zag chains (Fig. 1c–e). Both 2H-WSe2
TOPO), displaying aligned W zig–zag chains (Fig. 1c–e). Both 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and 1T′-WSe2
OLAm and 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO were found to show less than 4 layers in thickness and 10–15 nm in diameter and have an interlayer distance of 0.64 nm based on high-resolution TEM images (Fig. S1 and S2†). The 1T′ phase uniquely features zig–zag chains on the basal plane even when it has multilayers, unlike other polytypes such as Td and 2M phases.25–30 Both monolayer and bilayer regions were detected in 2H-WSe2
TOPO were found to show less than 4 layers in thickness and 10–15 nm in diameter and have an interlayer distance of 0.64 nm based on high-resolution TEM images (Fig. S1 and S2†). The 1T′ phase uniquely features zig–zag chains on the basal plane even when it has multilayers, unlike other polytypes such as Td and 2M phases.25–30 Both monolayer and bilayer regions were detected in 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm from the intensity profile (Fig. S3†). The fast Fourier transform (FFT) analysis of WSe2 nanosheets confirmed d-spacings along the basal planes of 2H-WSe2
OLAm from the intensity profile (Fig. S3†). The fast Fourier transform (FFT) analysis of WSe2 nanosheets confirmed d-spacings along the basal planes of 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm (d100 = d010 = 2.86 Å) and 1T′-WSe2
OLAm (d100 = d010 = 2.86 Å) and 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO (d10−1 = 5.91 Å and d020 = 1.69 Å).31,32
TOPO (d10−1 = 5.91 Å and d020 = 1.69 Å).31,32
To further investigate the distinguishing features of both WSe2 nanosheets, powder X-ray diffraction (PXRD), Raman spectroscopy, and X-ray photoelectron spectroscopy (XPS) were employed. In Fig. 2a, the PXRD patterns of 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and 1T′-WSe2
OLAm and 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO closely match with the reported crystallographic structures.31,32 Specifically, the diffraction peaks corresponding to (100) and (103) planes in 2H-WSe2
TOPO closely match with the reported crystallographic structures.31,32 Specifically, the diffraction peaks corresponding to (100) and (103) planes in 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm indicate the regular interval of W atoms, while the (40−2) diffraction peak in 1T′-WSe2
OLAm indicate the regular interval of W atoms, while the (40−2) diffraction peak in 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO reflects the Peierls distortion (Fig. S4†).33 From the Raman measurements which specialize in distinguishing the phase of TMDCs, 2H-WSe2
TOPO reflects the Peierls distortion (Fig. S4†).33 From the Raman measurements which specialize in distinguishing the phase of TMDCs, 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm showed typical vibration modes of E12g and A1g, while 1T′-WSe2
OLAm showed typical vibration modes of E12g and A1g, while 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO exhibited three peaks with low intensities at 217, 236, and 257 cm−1 (Fig. 2b). This tripartite splitting aligns with recent studies on the phonon dispersion relationships of 1T′-WSe2.23,32,34 XPS analysis ascertained differences in binding energy between 2H-WSe2
TOPO exhibited three peaks with low intensities at 217, 236, and 257 cm−1 (Fig. 2b). This tripartite splitting aligns with recent studies on the phonon dispersion relationships of 1T′-WSe2.23,32,34 XPS analysis ascertained differences in binding energy between 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and 1T′-WSe2
OLAm and 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO. As shown in Fig. S5,† 1T′-WSe2
TOPO. As shown in Fig. S5,† 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO exhibits lower binding energies in W 4f and Se 3d levels compared to those of 2H-WSe2
TOPO exhibits lower binding energies in W 4f and Se 3d levels compared to those of 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm, indicating the redistribution of d-electrons due to the dOC-6 coordination in 1T′-WSe2
OLAm, indicating the redistribution of d-electrons due to the dOC-6 coordination in 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO.35 Additionally, both 2H-WSe2
TOPO.35 Additionally, both 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and 1T′-WSe2
OLAm and 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO presented minor W6+ signals attributed to oxidized W edge sites in nanosheets.23 Nevertheless, 1T′-WSe2
TOPO presented minor W6+ signals attributed to oxidized W edge sites in nanosheets.23 Nevertheless, 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO nanosheets with few layers exhibited the structural persistence of the metastable phase (Fig. S6†) even when stored under ambient conditions for up to 60 days at least.
TOPO nanosheets with few layers exhibited the structural persistence of the metastable phase (Fig. S6†) even when stored under ambient conditions for up to 60 days at least.
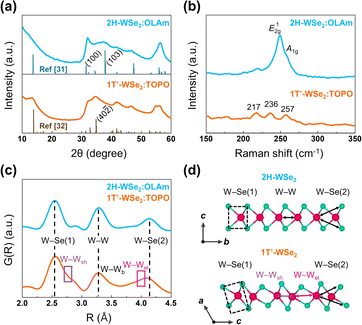 | ||
Fig. 2 (a) PXRD results with standard patterns, (b) Raman spectra, and (c) XPDF curves of 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and 1T′-WSe2 OLAm and 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO. (d) Side-view representations of 2H-WSe2 and 1T′-WSe2. TOPO. (d) Side-view representations of 2H-WSe2 and 1T′-WSe2. | ||
We exploited X-ray atomic pair distribution function (XPDF) analysis to discover structural differences at the atomic scale. Theoretical atomic-pair distances of 2H-WSe2 and 1T′-WSe2 are listed in Table S1.†. To start with the nearest W–Se pair, denoted as W–Se(1) in Fig. 2d, the average distances in 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and 1T′-WSe2
OLAm and 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO (DW–Se(1)/2H and DW–Se(1)/1T′) are marked as 2.54 and 2.58 Å in Fig. 2c, respectively. The dOC-6 coordination of W in 1T′-WSe2
TOPO (DW–Se(1)/2H and DW–Se(1)/1T′) are marked as 2.54 and 2.58 Å in Fig. 2c, respectively. The dOC-6 coordination of W in 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO (see Fig. 1e and Table S1†) is expected to correlate with the elongation of W–Se(1) average distance (2.58 Å). Also, arising from the Peierls distortion in the dOC-6 structure, 1T′-WSe2
TOPO (see Fig. 1e and Table S1†) is expected to correlate with the elongation of W–Se(1) average distance (2.58 Å). Also, arising from the Peierls distortion in the dOC-6 structure, 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO revealed three distinct types of W–W pairs in the measured range:22,32,36 one is W–W in the b-direction (W–Wb, see Fig. 1e) and the others are referred to as shortened (W–Wsh) and elongated (W–Wel) pairs (Fig. 2d). These structural features were clearly confirmed, as observed in the purple box (DW–Wsh/1T′ = 2.79 Å) and pink box (DW–Wel/1T′ = 4.03 Å) in Fig. 2c. In contrast, 2H-WSe2
TOPO revealed three distinct types of W–W pairs in the measured range:22,32,36 one is W–W in the b-direction (W–Wb, see Fig. 1e) and the others are referred to as shortened (W–Wsh) and elongated (W–Wel) pairs (Fig. 2d). These structural features were clearly confirmed, as observed in the purple box (DW–Wsh/1T′ = 2.79 Å) and pink box (DW–Wel/1T′ = 4.03 Å) in Fig. 2c. In contrast, 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm exhibits only one type of W–W pair (DW–W/2H = 3.28 Å). The peak of W–Se(2) in 1T′-WSe2
OLAm exhibits only one type of W–W pair (DW–W/2H = 3.28 Å). The peak of W–Se(2) in 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO is relatively broadened compared to that in 2H-WSe2
TOPO is relatively broadened compared to that in 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm, attributed to the dOC-6 coordination of W again.
OLAm, attributed to the dOC-6 coordination of W again.
The ligand-mediated phase engineering syntheses of 2H-WSe2 and 1T′-WSe2 were further validated by using alternative amine (HDAm) and phosphine oxide (TPPO). PXRD and Raman results clearly verified the identical phase-selective formation into 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDAm and 1T′-WSe2
HDAm and 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPPO (Fig. S7–S10†). The ligands are pivotal in influencing the phase of WSe2 nanosheets, as observed in the experiments. This phenomenon can be examined by the difference in the ligand-mediated coordination geometry of W complex molecules, which determines the resultant crystal structure grown in solution.37 Thus, we systematically investigated the W precursor complexes (W(CO)6
TPPO (Fig. S7–S10†). The ligands are pivotal in influencing the phase of WSe2 nanosheets, as observed in the experiments. This phenomenon can be examined by the difference in the ligand-mediated coordination geometry of W complex molecules, which determines the resultant crystal structure grown in solution.37 Thus, we systematically investigated the W precursor complexes (W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and W(CO)6
OLAm and W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
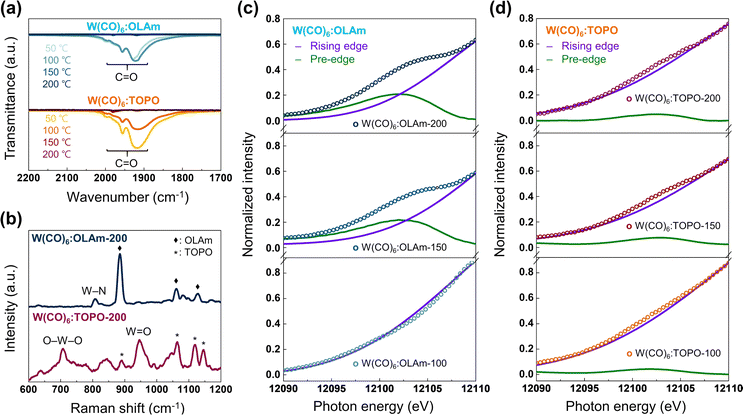
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO, obtained from the reaction of W(CO)6 and each ligand only) to identify the underlying phase engineering mechanism of WSe2 nanosheets by Fourier-transform infrared (FTIR), Raman, and XPS spectroscopic analyses. A decrease in IR active C
TOPO, obtained from the reaction of W(CO)6 and each ligand only) to identify the underlying phase engineering mechanism of WSe2 nanosheets by Fourier-transform infrared (FTIR), Raman, and XPS spectroscopic analyses. A decrease in IR active C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching signals with increasing temperature was observed in both W(CO)6
O stretching signals with increasing temperature was observed in both W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and W(CO)6
OLAm and W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO, ultimately leading to the absence of C
TOPO, ultimately leading to the absence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching signals (Fig. 3a). The C
O stretching signals (Fig. 3a). The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching signals of W(CO)6
O stretching signals of W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and W(CO)6
OLAm and W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO at 50 °C are comparable to those of pristine W(CO)6 (Fig. S11†). However, the C
TOPO at 50 °C are comparable to those of pristine W(CO)6 (Fig. S11†). However, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signals nearly disappear above 150 °C, implying the formation of W
O signals nearly disappear above 150 °C, implying the formation of W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and W
OLAm and W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO (see next), as depicted in Scheme 1.
TOPO (see next), as depicted in Scheme 1.
In Fig. 3b, Raman spectra of W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm-200 and W(CO)6
OLAm-200 and W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO-200, obtained at 200 °C, exhibit distinct vibrational modes corresponding to the coordination environments of W. Notably, Raman peaks of W–N (808.1 cm−1) and W–O (707.7 and 946.5 cm−1), analogous to six-coordinate W compounds such as WN38,39 and WO3,40 were measured in W(CO)6
TOPO-200, obtained at 200 °C, exhibit distinct vibrational modes corresponding to the coordination environments of W. Notably, Raman peaks of W–N (808.1 cm−1) and W–O (707.7 and 946.5 cm−1), analogous to six-coordinate W compounds such as WN38,39 and WO3,40 were measured in W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm-200 and W(CO)6
OLAm-200 and W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO-200, respectively. Unlike WO3, the vibration mode implying a bulk network structure (805 cm−1) was not detected in the spectrum of W(CO)6
TOPO-200, respectively. Unlike WO3, the vibration mode implying a bulk network structure (805 cm−1) was not detected in the spectrum of W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO-200, but the terminal W
TOPO-200, but the terminal W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal was noticeable, indicating the molecular structure of W(CO)6
O signal was noticeable, indicating the molecular structure of W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO-200.40 The W 4f XPS spectrum of W(CO)6
TOPO-200.40 The W 4f XPS spectrum of W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm-200 (Fig. S12†) in connection with the Raman result implies W6− species (37.1 and 35.0 eV)41 by forming W–NH2 bonds. Similarly, the spectrum of W(CO)6
OLAm-200 (Fig. S12†) in connection with the Raman result implies W6− species (37.1 and 35.0 eV)41 by forming W–NH2 bonds. Similarly, the spectrum of W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO-200 exhibits W6+ doublet peaks (37.5 and 35.6 eV) contributed by W–O
TOPO-200 exhibits W6+ doublet peaks (37.5 and 35.6 eV) contributed by W–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P bonds. The broadened spectral feature in W(CO)6
P bonds. The broadened spectral feature in W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO-200 attributed to the peak indicating a lower valence (34.0 eV) can be assigned to a slightly lower coordination number by TOPO (<6) compared to the W(CO)6
TOPO-200 attributed to the peak indicating a lower valence (34.0 eV) can be assigned to a slightly lower coordination number by TOPO (<6) compared to the W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm-200 due to its relatively weak affinity with W and bulkiness.42 In summary, W(CO)6 undergoes the removal of C
OLAm-200 due to its relatively weak affinity with W and bulkiness.42 In summary, W(CO)6 undergoes the removal of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and the formation of W–NH2 and W–O
O and the formation of W–NH2 and W–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P bonds in the presence of OLAm and TOPO, respectively.
P bonds in the presence of OLAm and TOPO, respectively.
To directly understand the change in metal–ligand coordination geometry of W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and W(CO)6
OLAm and W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO, we performed pre-edge analysis at the W L1-edge region using X-ray absorption near edge structure (XANES) measurements. According to a selection rule and p–d hybridization (Fig. S13†),43,44 pristine W(CO)6 with the OC-6 metal–ligand geometry showed no pre-edge features (Fig. S14†). In contrast, both W(CO)6
TOPO, we performed pre-edge analysis at the W L1-edge region using X-ray absorption near edge structure (XANES) measurements. According to a selection rule and p–d hybridization (Fig. S13†),43,44 pristine W(CO)6 with the OC-6 metal–ligand geometry showed no pre-edge features (Fig. S14†). In contrast, both W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm-150 (reacted at 150 °C) and W(CO)6
OLAm-150 (reacted at 150 °C) and W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm-200 exhibited prominent pre-edge peaks at ∼12
OLAm-200 exhibited prominent pre-edge peaks at ∼12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 102 eV (Fig. 3c), strongly indicating the existence of TPR-6 metal–ligand geometry (Fig. S13†). For W(CO)6
102 eV (Fig. 3c), strongly indicating the existence of TPR-6 metal–ligand geometry (Fig. S13†). For W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm-100 (reacting at 100 °C), only a rising-edge curve was observed, suggesting the retention of the OC-6 metal–ligand geometry and W–C
OLAm-100 (reacting at 100 °C), only a rising-edge curve was observed, suggesting the retention of the OC-6 metal–ligand geometry and W–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds from W(CO)6,18 in agreement with FTIR results. Interestingly, minimal pre-edge absorption was observed in all W(CO)6
O bonds from W(CO)6,18 in agreement with FTIR results. Interestingly, minimal pre-edge absorption was observed in all W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO samples (Fig. 3d), verifying the dOC-6 metal–ligand geometry.44 A similar pattern of pre-edge absorption was detected in XANES spectra of W(CO)6
TOPO samples (Fig. 3d), verifying the dOC-6 metal–ligand geometry.44 A similar pattern of pre-edge absorption was detected in XANES spectra of W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDAm-200 (Fig. S15†) and W(CO)6
HDAm-200 (Fig. S15†) and W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPPO-200 (Fig. S16†), which shows that an identical W-ligand coordination geometry was produced using the same type of ligands. These XANES results confirmed that amine ligands (OLAm and HDAm) and phosphine oxide ligands (TOPO and TPPO) are capable of manipulating the metal–ligand geometry in W complex molecules into the TPR-6 and the dOC-6 geometry, respectively.
TPPO-200 (Fig. S16†), which shows that an identical W-ligand coordination geometry was produced using the same type of ligands. These XANES results confirmed that amine ligands (OLAm and HDAm) and phosphine oxide ligands (TOPO and TPPO) are capable of manipulating the metal–ligand geometry in W complex molecules into the TPR-6 and the dOC-6 geometry, respectively.
According to the criterion for the metal–ligand coordination geometry of six-coordinate W complexes, the OC-6 coordination is dominant except for the condition of a high-valence W and exclusive σ-coordination between W and ligands.45 W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm-200 with the oxidation state of +6 is expected to form σ-coordination bonds between W and –NH2 functional groups, referring to previous studies on W–NH2 complexes about bonding parameters,46 molecular geometries (Fig. S17†),47 and coordination reaction.48 Thus, it was revealed that W(CO)6
OLAm-200 with the oxidation state of +6 is expected to form σ-coordination bonds between W and –NH2 functional groups, referring to previous studies on W–NH2 complexes about bonding parameters,46 molecular geometries (Fig. S17†),47 and coordination reaction.48 Thus, it was revealed that W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm-200 and W(CO)6
OLAm-200 and W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDAm-200 show the TPR-6 metal–ligand geometry. In contrast, W(CO)6
HDAm-200 show the TPR-6 metal–ligand geometry. In contrast, W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO samples maintain the W(CO)6 geometry in the form of dOC-6 (Fig. 3d) due to σ/π-coordination bonds between W and –O
TOPO samples maintain the W(CO)6 geometry in the form of dOC-6 (Fig. 3d) due to σ/π-coordination bonds between W and –O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P. The π-coordination between W and –O
P. The π-coordination between W and –O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P is highly attributed to molecular π bonds between O and P in phosphine oxide ligands.46,49 In this context, ex situ Raman measurements in Fig. 4 completely concluded the effect of controlling the metal–ligand coordination geometry on the phase engineering of WSe2 nanosheets. The additional selenization process for 60 min converted W(CO)6
P is highly attributed to molecular π bonds between O and P in phosphine oxide ligands.46,49 In this context, ex situ Raman measurements in Fig. 4 completely concluded the effect of controlling the metal–ligand coordination geometry on the phase engineering of WSe2 nanosheets. The additional selenization process for 60 min converted W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm-200 and W(CO)6
OLAm-200 and W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO-200 into 2H-WSe2 and 1T′-WSe2 nanosheets, respectively, conserving each coordination geometry of W.
TOPO-200 into 2H-WSe2 and 1T′-WSe2 nanosheets, respectively, conserving each coordination geometry of W.
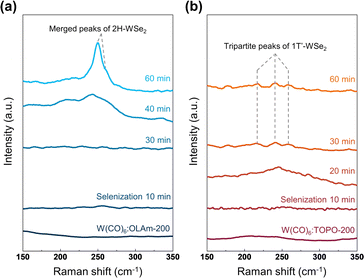 | ||
Fig. 4 Raman spectra of (a) W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm-200 and (b) W(CO)6 OLAm-200 and (b) W(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO-200, treated by the selenization process from 10 min to 60 min. TOPO-200, treated by the selenization process from 10 min to 60 min. | ||
Our phase engineering mechanism was also applicable for synthesizing WS2 nanosheets. The resulting products grown with each OLAm and TOPO (2H-WS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and 1T-WS2
OLAm and 1T-WS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO) were verified to show the atomic configurations of 2H and 1T phases (Fig. S18a–d†), which agrees with the previously reported structures (Fig. S18e†).50,51 PXRD patterns confirmed a slight shift of the (100) diffraction peak in 1T-WS2
TOPO) were verified to show the atomic configurations of 2H and 1T phases (Fig. S18a–d†), which agrees with the previously reported structures (Fig. S18e†).50,51 PXRD patterns confirmed a slight shift of the (100) diffraction peak in 1T-WS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO compared to the 2H-WS2
TOPO compared to the 2H-WS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm (Fig. S19†), reflecting a slight expansion of the (100) d-spacing in 1T-WS2.52
OLAm (Fig. S19†), reflecting a slight expansion of the (100) d-spacing in 1T-WS2.52
As a proof-of-concept application study, semiconducting and metallic WCh2 nanosheets were utilized as HER electrocatalysts. In Fig. 5a–c, 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO exhibited lower overpotential at −10 mA cm−2 (0.21 V), lower Tafel slope (115 mV dec−1), and higher double-layer capacitance (18.21 mF cm−2) compared to those of 2H-WSe2
TOPO exhibited lower overpotential at −10 mA cm−2 (0.21 V), lower Tafel slope (115 mV dec−1), and higher double-layer capacitance (18.21 mF cm−2) compared to those of 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm. Double-layer capacitances were obtained by sequential cyclic voltammetry measurements with varying scan rates (Fig. S20†). Also, in Fig. S21,† 1T-WS2
OLAm. Double-layer capacitances were obtained by sequential cyclic voltammetry measurements with varying scan rates (Fig. S20†). Also, in Fig. S21,† 1T-WS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO showed lower overpotential (0.37 V) compared to the 2H-WS2
TOPO showed lower overpotential (0.37 V) compared to the 2H-WS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm (0.68 V). Notably, 1T′-WSe2
OLAm (0.68 V). Notably, 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO proved long-term durability for 180 h (Fig. 5d) and still showed an identical polarization curve even after the stability test (Fig. 5a). This can be attributed to the conserved metallic phase and nanosheet morphology of the 1T′-WSe2
TOPO proved long-term durability for 180 h (Fig. 5d) and still showed an identical polarization curve even after the stability test (Fig. 5a). This can be attributed to the conserved metallic phase and nanosheet morphology of the 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO catalyst (Fig. S22 and S23†). Also, the structural persistence of 1T′-WSe2
TOPO catalyst (Fig. S22 and S23†). Also, the structural persistence of 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO was further confirmed by measuring polarization curves of samples stored under ambient conditions for seven days (1T-WSe2-7 days) and 60 days (1T′-WSe2-60 days), as shown in Fig. S24.† Accordingly, the HER performance of 1T′-WSe2
TOPO was further confirmed by measuring polarization curves of samples stored under ambient conditions for seven days (1T-WSe2-7 days) and 60 days (1T′-WSe2-60 days), as shown in Fig. S24.† Accordingly, the HER performance of 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO is comparable to that of other WCh2 catalysts (Table S2†). Thus, we found the possibility of phase-engineered WSe2 nanosheets for practical electrocatalytic applications.
TOPO is comparable to that of other WCh2 catalysts (Table S2†). Thus, we found the possibility of phase-engineered WSe2 nanosheets for practical electrocatalytic applications.
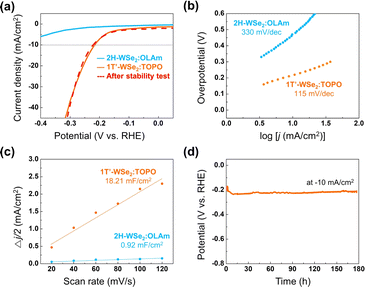 | ||
Fig. 5 (a) Polarization curves, (b) Tafel slopes, and (c) double-layer capacitances of 2H-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OLAm and 1T′-WSe2 OLAm and 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO. (d) Long-term durability of 1T′-WSe2 TOPO. (d) Long-term durability of 1T′-WSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO. TOPO. | ||
In conclusion, our investigation has revealed that achieving the phase-controlled WCh2 by a direct solution-phase synthesis necessitates the modulation of metal–ligand coordination geometry in W complex molecules through the use of appropriate ligands. Various spectroscopic analyses confirmed that phosphine oxide ligands, which form σ/π-coordination with W through an –O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P functional group, can exclusively induce the formation of metastable metallic WCh2. This process proceeds from W
P functional group, can exclusively induce the formation of metastable metallic WCh2. This process proceeds from W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO and W
TOPO and W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPPO with the dOC-6 metal–ligand coordination geometry. Otherwise, amine ligands lead to the formation of 2H phases of WCh2. The phase-engineered 1T′-WSe2 nanosheets show promising HER performance in activity and durability. Therefore, once suitable ligands facilitating selective geometry formation are found, our approach holds significant potential for synthesizing and exploring various metastable phases in 2D TMDCs.
TPPO with the dOC-6 metal–ligand coordination geometry. Otherwise, amine ligands lead to the formation of 2H phases of WCh2. The phase-engineered 1T′-WSe2 nanosheets show promising HER performance in activity and durability. Therefore, once suitable ligands facilitating selective geometry formation are found, our approach holds significant potential for synthesizing and exploring various metastable phases in 2D TMDCs.
Author contributions
S. Jun and J.-W. Lee contributed equally to this work. S. Jun and J.-W. Lee synthesized WSe2 and WS2, conducted XRD and Raman measurements, carried out TEM and XANES, analysed HER measurement results and discussed about the raw data. S.-C. Kim conducted XPDF measurements and helped with the discussion of the results. S. J. Oh contributed suggestions on the synthesis. S. Jeong supervised the work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National R&D Program (NRF-2020M3H4A3106354) and the program of Future Hydrogen Original Technology Development (NRF-2021M3I3A1083946) through the National Research Foundation of Korea (NRF) funded by the Korean government (Ministry of Science and ICT (MSIT)), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1A6A3A01087461), and the Future Key Technology Program (Project No. 2E32511) funded by the Korea Institute of Science and Technology.References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- Y. Zhang, L. Zhang and C. Zhou, Acc. Chem. Res., 2013, 46, 2329–2339 CrossRef CAS PubMed.
- H. Li, C. Tsai, A. L. Koh, L. Cai, A. W. Contryman, A. H. Fragapane, J. Zhao, H. S. Han, H. C. Manoharan, F. Abild-Pedersen, J. K. Norskov and X. Zheng, Nat. Mater., 2016, 15, 48–53 CrossRef CAS PubMed.
- C. Lattyak, M. Vehse, M. A. Gonzalez, D. Pareek, L. Gütay, S. Schäfer and C. Agert, Adv. Opt. Mater., 2022, 10, 2102226 CrossRef CAS.
- R. Kappera, D. Voiry, S. E. Yalcin, B. Branch, G. Gupta, A. D. Mohite and M. Chhowalla, Nat. Mater., 2014, 13, 1128–1134 CrossRef CAS PubMed.
- S. Krishnamurthi and G. Brocks, NPJ 2D Mater. Appl., 2021, 5, 43 CrossRef CAS.
- S. Chowdhury, P. Venkateswaran and D. Somvanshi, Phys. B: Condens., 2023, 653, 414668 CrossRef CAS.
- A. Rahman, J. R. Jennings, A. L. Tan and M. M. Khan, ACS Omega, 2022, 7, 22089–22110 CrossRef CAS PubMed.
- P. Xiao, J. Lou, H. Zhang, W. Song, X.-L. Wu, H. Lin, J. Chen, S. Liu and X. Wang, Catal. Sci. Technol., 2018, 8, 201–209 RSC.
- Z. Zhu, A. Mosallanezhad, D. Sun, X. Lei, X. Liu, Z. Pei, G. Wang and Y. Qian, Energy Fuels, 2021, 35, 5613–5626 CrossRef CAS.
- Q. Xia, L. Zhao, D. Li, J. Wang, L. Liu, C. Hou, X. Liu, H. Xu, F. Dang and J. Zhang, J. Mater. Chem. A, 2021, 9, 19922–19931 RSC.
- B. Kalanyan, W. A. Kimes, R. Beams, S. J. Stranick, E. Garratt, I. Kalish, A. V. Davydov, R. K. Kanjolia and J. E. Maslar, Chem. Mater., 2017, 29, 6279–6288 CrossRef CAS PubMed.
- R. Konar, Rosy, I. Perelshtein, E. Teblum, M. Telkhozhayeva, M. Tkachev, J. J. Richter, E. Cattaruzza, A. Pietropolli Charmet, P. Stoppa, M. Noked and G. D. Nessim, ACS Omega, 2020, 5, 19409–19421 CrossRef CAS PubMed.
- A. Midya, A. Ghorai, S. Mukherjee, R. Maiti and S. K. Ray, J. Mater. Chem. A, 2016, 4, 4534–4543 RSC.
- K. Leng, Z. Chen, X. Zhao, W. Tang, B. Tian, C. T. Nai, W. Zhou and K. P. Loh, ACS Nano, 2016, 10, 9208–9215 CrossRef CAS PubMed.
- E. Er, H.-L. Hou, A. Criado, J. Langer, M. Möller, N. Erk, L. M. Liz-Marzán and M. Prato, Chem. Mater., 2019, 31, 5725–5734 CrossRef CAS.
- Y. Ma, B. Liu, A. Zhang, L. Chen, M. Fathi, C. Shen, A. N. Abbas, M. Ge, M. Mecklenburg and C. Zhou, ACS Nano, 2015, 9, 7383–7391 CrossRef CAS PubMed.
- P. Zhou, P. Schiettecatte, M. Vandichel, A. Rousaki, P. Vandenabeele, Z. Hens and S. Singh, Cryst. Growth Des., 2021, 21, 1451–1460 CrossRef CAS.
- J. Q. Geisenhoff, A. K. Tamura and A. M. Schimpf, Chem. Commun., 2019, 55, 8856–8859 RSC.
- B. Mahler, V. Hoepfner, K. Liao and G. A. Ozin, J. Am. Chem. Soc., 2014, 136, 14121–14127 CrossRef CAS PubMed.
- Z. Liu, K. Nie, X. Qu, X. Li, B. Li, Y. Yuan, S. Chong, P. Liu, Y. Li, Z. Yin and W. Huang, J. Am. Chem. Soc., 2022, 144, 4863–4873 CrossRef CAS PubMed.
- M. S. Sokolikova, P. C. Sherrell, P. Palczynski, V. L. Bemmer and C. Mattevi, Nat. Commun., 2019, 10, 712 CrossRef CAS PubMed.
- A. Niebur, A. Söll, P. Haizmann, O. Strolka, D. Rudolph, K. Tran, F. Renz, A. P. Frauendorf, J. Hübner, H. Peisert, M. Scheele and J. Lauth, Nanoscale, 2023, 15, 5679–5688 RSC.
- K.-A. N. Duerloo, Y. Li and E. J. Reed, Nat. Commun., 2014, 5, 4214 CrossRef CAS PubMed.
- T. Lu, Y. Wang, G. Cai, H. Jia, X. Liu, C. Zhang, S. Meng and M. Liu, Mater. Futures, 2023, 2, 015001 CrossRef.
- Y. M. Itahashi, Y. Nohara, T. Ideue, T. Akiba, H. Takahashi, S. Ishiwata and Y. Iwasa, Phys. Rev. Res., 2023, 5, L022022 CrossRef CAS.
- R. Konar, B. Rajeswaran, A. Paul, E. Teblum, H. Aviv, I. Perelshtein, I. Grinberg, Y. R. Tischler and G. D. Nessim, ACS Omega, 2022, 7, 4121–4134 CrossRef CAS PubMed.
- J. Y. Kim, I. H. Kwak, I. S. Kwon, Q. A. Sial, J. Ihsan, G. M. Zewdie, J. Park and H. S. Kang, J. Mater. Chem. A, 2023, 11, 19619–19628 RSC.
- X. Guo, T. Wu, S. Zhao, Y. Fang, S. Xu, M. Xie and F. Huang, ACS Appl. Energy Mater., 2022, 5, 7674–7680 CrossRef CAS.
- H. Paudyal and E. R. Margine, J. Mater. Chem. C, 2022, 10, 7917–7924 RSC.
- W. Schutte, J. De Boer and F. Jellinek, J. Solid State Chem., 1987, 70, 207–209 CrossRef CAS.
- Z. Lai, Q. He, T. H. Tran, D. V. M. Repaka, D. D. Zhou, Y. Sun, S. Xi, Y. Li, A. Chaturvedi, C. Tan, B. Chen, G. H. Nam, B. Li, C. Ling, W. Zhai, Z. Shi, D. Hu, V. Sharma, Z. Hu, Y. Chen, Z. Zhang, Y. Yu, X. Renshaw Wang, R. V. Ramanujan, Y. Ma, K. Hippalgaonkar and H. Zhang, Nat. Mater., 2021, 20, 1113–1120 CrossRef CAS PubMed.
- D. H. Keum, S. Cho, J. H. Kim, D.-H. Choe, H.-J. Sung, M. Kan, H. Kang, J.-Y. Hwang, S. W. Kim, H. Yang, K. J. Chang and Y. H. Lee, Nat. Phys., 2015, 11, 482–486 Search PubMed.
- W. Chen, X. Xie, J. Zong, T. Chen, D. Lin, F. Yu, S. Jin, L. Zhou, J. Zou, J. Sun, X. Xi and Y. Zhang, Sci. Rep., 2019, 9, 2685 Search PubMed.
- W. Zhao, J. Pan, Y. Fang, X. Che, D. Wang, K. Bu and F. Huang, Chem.–Eur. J., 2018, 24, 15942–15954 CrossRef CAS PubMed.
- J. W. González, E. Flórez and J. D. Correa, J. Mol. Liq., 2024, 396, 123904 CrossRef.
- D. P. Dinega and M. G. Bawendi, Angew. Chem., Int. Ed., 1999, 38, 1788–1791 CrossRef CAS PubMed.
- C. Pardanaud, D. Dellasega, M. Passoni, C. Martin, P. Roubin, Y. Addab, C. Arnas, L. Couëdel, M. Minissale, E. Salomon, G. Giacometti, A. Merlen, E. Bernard, R. Mateus, E. Alves, Z. Siketic, I. B. Radovic and A. Hakola, Nucl. Fusion, 2020, 60, 086004 CrossRef CAS.
- P. Shen, X. Li, Y. Luo, Y. Guo, X. Zhao and K. Chu, ACS Nano, 2022, 16, 7915–7925 CrossRef CAS PubMed.
- B. Pecquenard, H. Lecacheux, J. Livage and C. Julien, J. Solid State Chem., 1998, 135, 159–168 CrossRef CAS.
- S. Nomoto, H. Kitamura, S. Takase and Y. Shimizu, Electrochemistry, 2022, 90, 087005 CrossRef CAS.
- J. Q. Geisenhoff, H. Yin, N. Oget, H. Chang, L. Chen and A. M. Schimpf, Front. Nanotechnol., 2022, 4, 1026635 CrossRef.
- U. Jayarathne, P. Chandrasekaran, A. F. Greene, J. T. Mague, S. DeBeer, K. M. Lancaster, S. Sproules and J. P. Donahue, Inorg. Chem., 2014, 53, 8230–8241 CrossRef CAS PubMed.
- S. Yamazoe, Y. Hitomi, T. Shishido and T. Tanaka, J. Phys. Chem. C, 2008, 112, 6869–6879 CrossRef CAS.
- K. Seppelt, Acc. Chem. Res., 2003, 36, 147–153 CrossRef CAS PubMed.
- R. Brown and G. Dobson, Inorg. Chim. Acta, 1972, 6, 65–71 CrossRef CAS.
- M. Kaupp, J. Am. Chem. Soc., 1996, 118, 3018–3024 CrossRef CAS.
- S. Baskaran, P. Balu and C. Sivasankar, J. Chem. Sci., 2015, 127, 83–94 CrossRef CAS.
- D. G. Gilheany, Chem. Rev., 1994, 94, 1339–1374 CrossRef CAS PubMed.
- B. F. Mentzen and M. Sienko, Inorg. Chem., 1976, 15, 2198–2202 CrossRef CAS.
- N. Lee, I. Y. Choi, K.-Y. Doh, J. Kim, H. Sim, D. Lee, S.-Y. Choi and J. K. Kim, J. Mater. Chem. A, 2019, 7, 26378–26384 RSC.
- M. Srinivaas, C.-Y. Wu, J.-G. Duh and J. M. Wu, ACS Sustainable Chem. Eng., 2019, 7, 10363–10370 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta00326h |
| ‡ S. Jun and J.-W. Lee contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |