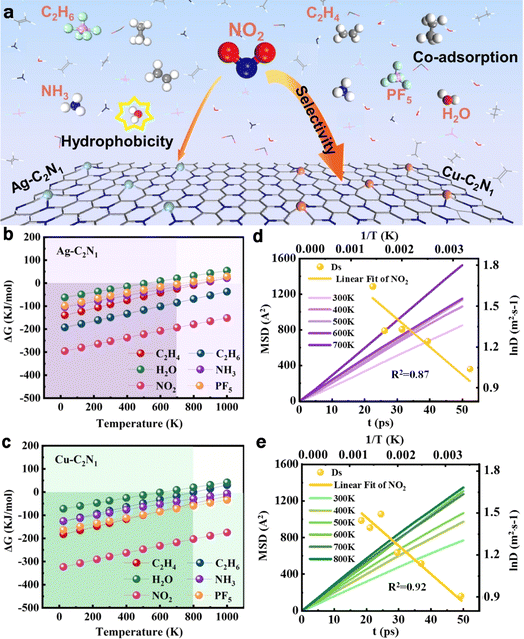
Metal-modified C3N1 monolayer sensors for battery instability monitoring†
Mingyang
Gu
a,
Lin
Tao
 *a,
Davoud
Dastan
b,
Jie
Dang
*a,
Davoud
Dastan
b,
Jie
Dang
 c,
Timing
Fang
c,
Timing
Fang
 d and
Baigang
An
*a
d and
Baigang
An
*a
aSchool of Chemical Engineering, University of Science and Technology Liaoning, Anshan 114051, China. E-mail: taolin@ustl.edu.cn; bgan@ustl.edu.cn
bDepartment of Materials Science and Engineering, Cornell University, Ithaca, New York 14850, USA
cCollege of Materials Science and Engineering, Chongqing University, Chongqing 400044, China
dSchool of Chemistry and Chemical Engineering, Qingdao University, Qingdao 266071, China
First published on 18th March 2024
Abstract
The pressing need for affordable gas sensors with enhanced sensitivity and selectivity in identifying hazardous gases released after the battery becomes unstable cannot be overstated. In this study, a C3N1 monolayer modified with Cu and Ag atoms (Cu/Ag–C2N1) was selected to achieve selective adsorption of NO2 under the coexistence of multiple gases (PF5, NH3, H2O, C2H4, and C2H6) based on density functional theory. The results demonstrate that securely anchoring metal atoms to the monolayer, as indicated by cohesion energy and ab initio molecular dynamics simulations, concurrently enhances the material's conductivity. Analyses of electrostatic potential and work function identified high activity sites and electron-releasing capabilities. Furthermore, the gas–solid interface structures of multiple gases on the Cu/Ag–C2N1 monolayers are revealed by the adsorption energy and distance. Importantly, NO2 exhibits stronger adsorption energy on Cu/Ag–C2N1, reaching −3.54 and −3.27 eV, respectively. Crystal Orbital Hamilton Population and d-band center theory unveiled differences in adsorption energy resulting from the modification involving the two metals. Fascinatingly, density of states calculation demonstrates, for the first time, that the two doped metal monolayers generate a distinct response solely to NO2 in a multi-gas coexistence setting, effectively excluding interference from water. In practice, based on Gibbs free energy and Einstein diffusion law calculations, Cu–C2N1 exhibits superior hydrophobicity, a broader temperature range and a lower diffusion activation energy barrier (2.5 kJ mol−1). Our theoretical calculations demonstrate Cu's efficacy in substituting expensive Ag, yielding cost-effectiveness without compromising selectivity, response, stability, and versatility.
1 Introduction
Widespread in portable electronics and integral to the surge in electric vehicles, batteries play a pivotal role, significantly impacting the tapestry of modern society.1–3 However, the pursuit of high-energy-density batteries raises safety concerns due to increased gas evolution, particularly with lithium metal.4,5 Safety monitoring is vital as battery defects can trigger severe risks like electrolyte-induced heat leading to fire and explosion. This thermal runaway results in the release of harmful gases.6 Lithium metal poses challenges due to its reactivity, potentially causing dendritic formation and a hot mixture release, a precursor to fire or explosion. Early detection of gases like NO2, PF5, NH3, C2H4, H2O, and C2H6 before thermal runaway is imperative to prevent safety hazards in practical applications.7,8In recent years, an abundance of theoretical studies has delved into the design and fabrication of monolayers for detecting toxic gases. Theoretical calculations, employing density functional theory (DFT), have played a pivotal role in accurately predicting the sensing efficiency of two-dimensional (2D) monolayers and elucidating their micro-sensing mechanisms.9–17 For instance, Sun et al.18 utilized DFT calculations to analyze the adsorption behavior of six gases on an indium nitride monolayer, demonstrating its potential in detecting SO2 and NO2 molecules. Additionally, first-principles calculations were employed to explore the structural and electronic properties of group III nitrides and phosphides in gas adsorption. It was concluded that significant potential exists for the detection of CO2 by these sensors. Furthermore, the introduction of transition metal doping can enhance the sensing capabilities of 2D monolayers.19–21
In gas-sensitive applications, 2D monolayers demonstrate exceptional performance owing to their high specific monolayer area, thermal conductivity, unique optoelectronic properties, and catalytic activity.22–25 As chemical gas sensors, these monolayer offer abundance, cost-effectiveness, and easy manufacturability, and play a pivotal role in sensor technology. Among numerous 2D material-based gas sensors, their sensitivity to various toxic and explosive gases stems from gas-induced resistance changes,26 finding wide application across diverse fields. Graphene and C3N monolayer films, among others, have theoretically shown promising gas-sensing characteristics.27–29 Despite this, inherent defects limit the full application potential of 2D monolayers in sensing technologies. Hence, exploring and discovering novel types of two-dimensional monolayers for gas detection become imperative to address these limitations and expand the sensor monolayer repertoire.
Creating new gas sensors to detect typical electrolyte decomposition products under harsh conditions is crucial. 2D monolayers stand out as prime candidates for such sensing applications, operating within a high temperature range and exhibiting the highest response levels.30 Enhancing gas sensor characteristics involves monolayer material modification. Semiconductor-based metal oxide gas sensors also require temperature resistance during thermal runaway, making safety-based detectors particularly promising. These devices offer stability under high humidity conditions but demand high-temperature resilience during thermal runaway.31,32 Metal-doped 2D material gas sensors exhibit notably high responses, showcasing how metals create a platform to merge diverse physical and chemical properties within a system. Past research has amalgamated experiments and intricate computational analyses to validate the applicability of metal-doped structures in sensor applications.33–36 These studies have visualized the gas sensing traits, portraying the adsorption performance of gas sensors for practical implementation.
However, the electrical response of the sensing monolayer originates from the cumulative effect of each gas. The significant scientific challenge lies in the absence of an effective method to systematically allocate the contribution of each gas. Consequently, in the case of the simultaneous adsorption of multiple gases, the strategy to enhance selectivity involves eliminating the electrical response to other interfering gases, focusing solely on one specific gas.
In this study, DFT was employed to calculate the response of Cu/Ag–C2N1 in the presence of multiple gases (PF5, NO2, NH3, H2O, C2H4, and C2H6). Initially, 2D monolayers doped with these two metals were constructed, and their stability and plausibility were examined from various perspectives. Subsequently, the adsorption behavior of multiple gases on the doped monolayer was comprehensively calculated, elucidating differences in adsorption performance based on methods such as d-band center theory and Crystal Orbital Hamiltonian Population (COHP). Utilizing the density of states (DOS) method, the electrical signal response behavior of the monolayer during the adsorption of multiple gases for selective adsorption was evaluated. Finally, the practical applicability of the sensors was explored by computing the Gibbs free energy of gas adsorption and the recovery time of the electrical signal. Our theoretical exploration offers novel insights for designing highly selective gas sensors in battery instability monitoring.
2 Results and discussion
2.1 Structural characterization
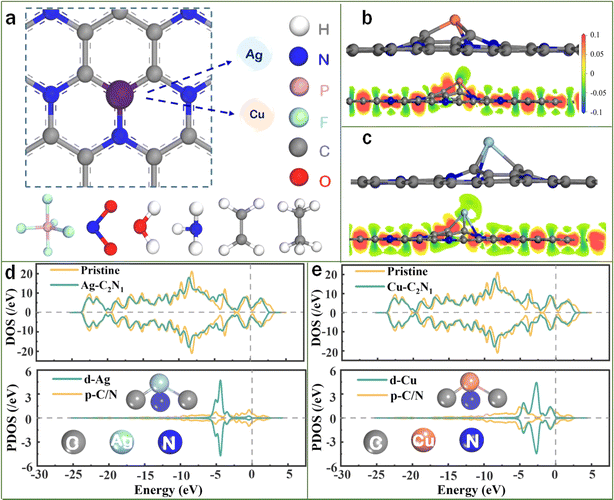
The optimized structures of the Cu/Ag–C2N1 monolayer and six gas molecules (PF5, NO2, NH3, H2O, C2H4, and C2H6) are shown in Fig. 1a. It exhibits a top-down view of the metal-modified C2N1 monolayer, comprising a pattern of corner rings formed by C–N–C–C sequences, while also illustrating the interaction sites of Ag and Cu. In detail, the P–F bond length in PF5 is 1.58 Å, the N–O bond length in NO2 is 1.27 Å, the N–H bond length in NH3 is 1.03 Å, and the H–O bond length in H2O is 0.98 Å. For C2H4, the C–H bond length is 1.09 Å, and the C–C bond length is 1.33 Å. For C2H6, the C–H bond length is 1.06 Å, and the C–C bond length is 1.53 Å. These data calculated in this work are consistent with those previously reported,37–44 demonstrating that the selected calculation parameters are reasonable and accurate. Fig. 1b and c depict the main view of the monolayer of Ag–C2N1 and Cu–C2N1, respectively. Clearly, both doped metals are situated atop the 2D material, creating the most stable structure, in accordance with earlier research observations.28 So as to understand the monolayer stability of doped metal atoms from the point of view of charge density, Fig. 1b and c also illustrate distinct charge density distribution. The noticeable increase in red indicates a heightened charge density of electronic wavefunction, predominantly around the C and N atoms, confirming its high charge acceptance. Both Ag and Cu act as electron donors, transferring electrons to C2N1. Therefore, to maintain an optimal stable structure, the doped metal atoms ultimately position themselves above the monolayer, resulting in substrate deformation.Additionally, cohesion energy (Ecoh) analyses were conducted to quantitatively explore the anchoring of metal atoms onto the monolayer. First, the Ecoh was analyzed as:45
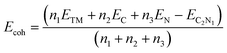 | (1) |
Based on previous research,12,19,46–49 the height of the DOS curve at the Fermi level can reflect the quality of material conductivity. In other words, higher peaks indicate better conductivity. Conductivity stands as a crucial criterion for high-performance gas sensors. Therefore, this study employs this approach to assess the impact of introducing doping metals on the material's conductivity. In Fig. 1d and e, the overall DOS demonstrates a slight increase in the curve around the Fermi level after the introduction of doping metals, indicating an enhancement in the material's conductivity. Additionally, partial density of states (PDOS) calculations were conducted to delve deeper into the behavior of the doped metal atoms. Fig. 1d and e display the d orbitals of the metal atoms and the p-orbitals of nonmetallic C/N in the monolayer. Typically, transition metal d-orbitals play a significant role in bonding. The outcomes reveal a noticeable alignment between the d orbital of Ag and the p orbital of C/N within the material. Furthermore, the hybridization of Cu's d orbital with C/N's p orbital is more prominent and situated at a higher energy level. The findings indicate that Cu and Ag can be securely affixed to the C2N1 monolayer. This indicates a robust interaction between the doped metal atoms and the monolayers, ensuring structural stability.
2.2 Gas sensing performance
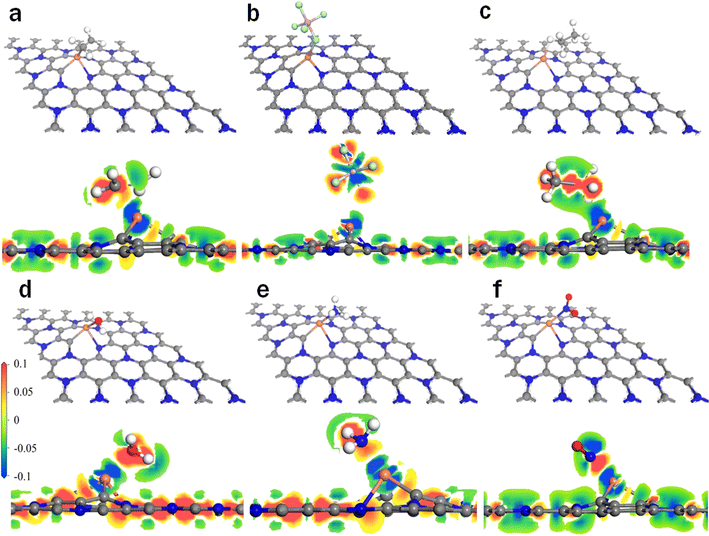 | ||
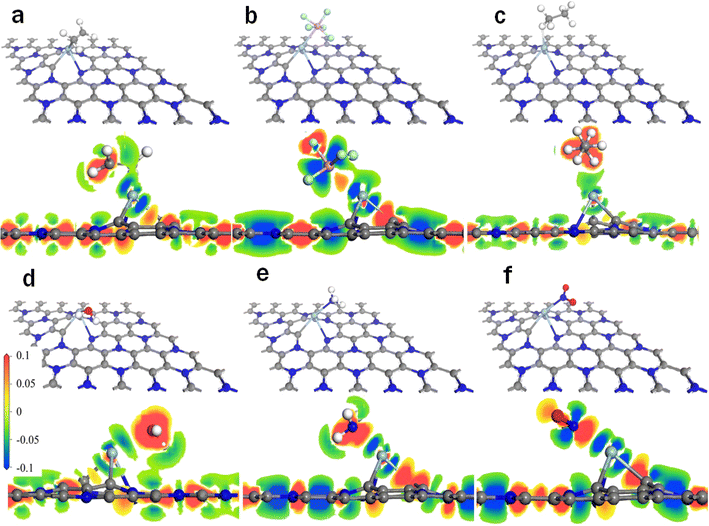
| Fig. 3 Stable configurations and charge density of gas adsorbed on the Cu–C2N1 monolayer: (a) Cu–C2N1–C2H4, (b) Cu–C2N1–C2H6, (c) Cu–C2N1–PF5, (d) Cu–C2N1–H2O, (e) Cu–C2N1–NH3, and (f) Cu–C2N1–NO2. | ||
In Fig. 2, the shortest atomic distances observed are 2.3 Å for Ag–C2N1–C2H4, 2.5 Å for Ag–C2N1–PF5, 2.2 Å for Ag–C2N1–C2H6, 2.5 Å for Ag–C2N1–H2O, 2.3 Å for Ag–C2N1–NH3, and 2.2 Å for Ag–C2N1–NO2. In Fig. 3, the shortest atomic distances are 2.1 Å for Cu–C2N1–C2H4, 2.5 Å for Cu–C2N1–PF5, 2.0 Å for Cu–C2N1–C2H6, 2.1 Å for Cu–C2N1–H2O, 2.1 Å for Cu–C2N1–NH3, and 1.9 Å for Cu–C2N1–NO2. After optimization, the positions of all gas molecules have shifted. The shortest distance of each gas molecule in the Ag–C2N1 system is slightly longer than that of the Cu–C2N1 system. Additionally, for a more visual examination of the charge accumulation and depletion during gas interaction with Cu/Ag–C2N1, Fig. 2 and 3 present the results of charge density analysis. The charge densities of electronic wavefunction illustrate that both Ag–C2N1 and Cu–C2N1 transfer a significant amount of charge during gas adsorption. The distinct dark red color observed around the gas molecules (C2H4, C2H6, PF5, H2O, NH3, and NO2) clearly indicates the acquisition of electrons from Ag–C2N1 and Cu–C2N1 by the gas molecules, demonstrating a strong interaction between the gas and Cu/Ag–C2N1. For specific charge transfer values, refer to Table S1.† The results indicate that among the six gases considered, NO2 exhibited the most significant charge transfer to Cu/Ag–C2N1, suggesting its superior adsorption effect on it, particularly on the Cu–C2N1 monolayer.
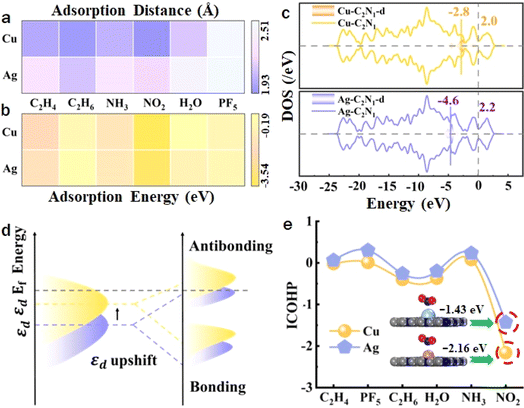
For a more comprehensive understanding of gas adsorption behavior and to quantitatively assess the interaction forces between gases and Cu/Ag–C2N1, it becomes imperative to compare adsorption distances and energies. Fig. 4a and b show respectively the adsorption distance and adsorption energy. The adsorption energy is defined as:52–54
| Eads = Etotal − (Emonolayer + Egas) | (2) |
Combining the different gas adsorption properties caused by the above two doped metal atoms, it can be found that the performance of Cu is better than that of Ag. For example, the adsorption energy of Cu–C2N1–NO2 is −3.53 eV, which is stronger than that of Ag–C2N1–NO2 (−3.26 eV). Given that d-band center theory is a useful tool for elucidating the interaction between transition metals and gas molecules, the calculation of the d-band center (εd) becomes imperative to unveil the underlying mechanism. The εd can be calculated as:55–57
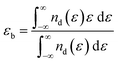 | (3) |
To expose disparities in gas adsorption energy on Cu–C2N1 and Ag–C2N1 from a chemical bonding perspective, the chemical bond is categorized into bonding and anti-bonding, with bonding playing the decisive role. COHP analysis is employed for a more accurate examination of interatomic forces during gas adsorption.58–61 The detailed calculation results are displayed in Fig. S17 and S18.† COHP represents the respective contributions of bonding and antibonding and shows the strength of the chemical bond between atoms, and the larger the bonding state below the Fermi level, the smaller the antibonding state, the stronger the bonding ability between the two atoms. In the example Fig. S17† of Ag–C2N1–PF5, there are a large number of anti-bonds below the Fermi level, resulting in a huge internal stress between Ag and F atoms, and the anti-bonds in the system cause repulsion between the atoms, and the interaction cannot be stable. In contrast, there is a large amount of bonding below the Fermi level in Cu–C2N1–NO2 in Fig. S18,† indicating that there is a strong force between Cu and N, which confirms the strong interaction between Cu–C2N1 and NO2. A more quantitative explanation can be provided by calculating the integral COHP (ICOHP) value obtained by calculating the energy integral from negative infinity to the Fermi level. The more negative the ICOHP value, the stronger the interaction between metal atoms and gas atoms, as shown in Fig. 4e. Compared to the other five gases, NO2 exhibits the most prominent ICOHP values on Cu–C2N1 and Ag–C2N1. Therefore, this result substantiates the clear advantage of competitive adsorption for NO2 on Cu–C2N1.
In Fig. 5, the conductivity of NO2 adsorption was significantly higher than that of the background, leading to a higher electron count near this level, ultimately enhancing conductivity. In the context of NO2 adsorption, the redistribution of charges is the primary driver behind this shift, causing a significant alteration in conductivity. The C2N1 monolayer modified with metal atoms (Ag and Cu) displays a selective response to NO2 gas amidst various other gases (C2H4, C2H6, PF5, H2O, and NH3). Notably, the DOS for all orbitals on the right side of the Cu–C2N1–NO2 Fermi level surpasses that of Ag–C2N1–NO2, indicating a more robust response of Cu–C2N1 to NO2 compared to its Ag–C2N1 counterpart.
The discernible charge transfer path is directly reflected in the DOS alteration seen in Fig. S19 and S20.† The conductivity of C2H4, C2H6, H2O, and NH3 on the monolayer of Ag–C2N1 changed little after adsorption, and the conductivity decreased slightly after PF5 adsorption. The conductivity of C2H6, PF5, H2O and NH3 on the monolayer of Cu–C2N1 did not change significantly after adsorption, but the conductivity of C2H4 decreased slightly. There was no significant change in the conductivity of the gas after adsorption, which was consistent with the observed trend. Observing the changes of the DOS before and after adsorption of H2O in Fig. S19 and S20d,† it can be found that the DOS curves before and after adsorption change very small, and there is no change at the Fermi level, which indicates that Ag–C2N1 and Cu–C2N1 have no electrical signal response to H2O and the monolayer is hydrophobic. Interestingly, when NO2 is adsorbed on the monolayer of Ag–C2N1 and Cu–C2N1, the Cu/Ag–C2N1 monolayer conductivity increases significantly, which is reflected in the obvious rise and left shift of the DOS curve at the Fermi level after NO2 adsorption in the DOS diagram. This is in contrast to the DOS changes after adsorption of other gases. It is proved that the monolayers of Ag–C2N1 and Cu–C2N1 can generate a selective electrical signal response to NO2 gas. It is worth noting that the DOS change of NO2 on the monolayer of Cu–C2N1 is more obvious than that of Ag–C2N1, which proves that Cu–C2N1 has a stronger effect on NO2 and has a better effect.
In order to explore the reason why Cu/Ag–C2N1 can respond selectively to NO2 but not to the other five gases, the adsorption mechanism was explored by PDOS. The PDOS distribution analysis showed that the more overlapping peaks in different orbitals, the stronger the hybridization between the orbitals, as shown in Fig. S19 and S20.† The conspicuous overlap observed between the metal (Ag and Cu) d-orbitals and N p-orbitals substantiates strong chemical interactions between NO2 and Ag–C2N1, as well as Cu–C2N1. Conversely, for the remaining five gases (C2H4, C2H6, PF5, H2O, and NH3), which exhibit weak adsorption on Ag–C2N1 and Cu–C2N1, their PDOS plots indicate limited interactions. In addition, the interaction between NO2 and Ag–C2N1 is weak, evident from the small overlapping region between Ag-d and N-p orbitals. However, during the adsorption of NO2, the hybridization between the p orbital of the N atom and the d orbital of the Cu atom is significantly enhanced, resulting in a significant increase of the PDOS of the d orbital of the Cu atom at the Fermi level, which ultimately affects the overall electrical response of the material. In summary, compared with Ag–C2N1, Cu–C2N1 has the most obvious response to NO2 selective electrical signals and has the best effect.
Notably, the conductivity of H2O adsorbed on the Cu/Ag–C2N1 monolayer changes very little, as depicted in Fig. 5, and no discernible changes were observed. In Fig. S19 and S20,† the overlap between the metal (Ag and Cu) d orbital and the O p orbital is still small. This observation supports the conclusion that NO2 can be selectively detected by Ag–C2N1 and Cu–C2N1, affirming that humidity does not impede the selective electrical signal response of the doped monolayer to NO2 in multifarious gas environments.
2.3 Applications of gas sensors
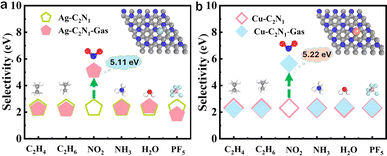
In this section, firstly, based on the detailed discussion of high sensitivity and selectivity to NO2, the gas sensor is shown in actual usage scenarios, while also showcasing the high humidity resistance of the Cu–C2N1 monolayer. Subsequently, as a response to the various hazardous gases generated after the battery becomes thermally unstable, it is imperative to conduct a thorough assessment of the operational environment to determine the temperature tolerance of high-performance sensors. Furthermore, the gas diffusion performance of gas-sensitive materials, which determines their responsiveness, is evaluated comprehensively through molecular dynamics simulations to assess the diffusion behavior of the six gases. Finally, the desorption time of the detected gases from the gas-sensitive material is analyzed.In Fig. 6a, the practicality of Cu–C2N1 and Ag–C2N1 monolayer is illustrated when exposed to a range of gases emitted due to battery instability. This exposure highlights their distinctive capacity for selectively adsorbing NO2, notably emphasized within Cu–C2N1. Both monolayers exhibit commendable hydrophobic properties. Additionally, considering the impact of temperature on the material's performance, it is essential to analyze the Gibbs free energy of the material to assess the practical temperature range for its use. The detailed calculation process can be referred to the ESI.†
The more negative the free energy, the more likely a spontaneous adsorption occurs. This study calculates Gibbs free energy at various temperatures to identify intervals where the free energy is below zero. Therefore, the actual applicable temperature range of Ag–C2N1 and Cu–C2N1 is determined. Fig. 6b and c show the linear relationship between the Gibbs free energy and temperature of the six gases adsorbed on Cu/Ag–C2N1 at different temperatures. In Fig. 6b, ΔG < 0 is observed at a minimum temperature of 500 K, indicating that Ag–C2N1 cannot adsorb certain gases at temperatures higher than 500 K. Meanwhile, Fig. 6c displays ΔG < 0 at a minimum temperature of 600 K. Consequently, the high-temperature resistance of Cu–C2N1 to the adsorption of the six gases surpasses that of Ag–C2N1. Therefore, the application scope of Cu–C2N1 is broader. Selective detection of NO2 on Cu–C2N1 in high-temperature environments is superior and applicable over a wider temperature range compared to Ag–C2N1. The noteworthy aspect is the maximum applicable temperature of 500 K on Ag–C2N1 and 600 K on Cu–C2N1, which are the upper limits for H2O adsorption. The results of DOS analysis confirmed that humidity has no impact on the selective adsorption performance. Consequently, the actual response temperature upper limit of Ag–C2N1 is 700 K (determined by Ag–C2N1–PF5), while for Cu–C2N1, it's 800 K (determined by Cu–C2N1–C2H6). The Cu–C2N1 monolayer's applicable temperature surpasses the hot mixture's maximum temperature released after battery damage (470 to 720 K) and remains minimally affected by humidity, making it suitable for a wide range of environments.
For the gas diffusion performance of Cu/Ag–C2N1, molecular dynamics simulations were used to calculate the diffusion coefficient. The detailed calculation can be found in the ESI.† The diffusion coefficient plays a critical role as it quantifies the gas's diffusion capacity. A larger diffusion coefficient corresponds to a faster diffusion rate, indicating a lower energy barrier required for diffusion.62,63 In order to explore the diffusion behavior of gases on the monolayer, the diffusion coefficients of the six gas molecules on the monolayer were calculated. The initial and the final stable configurations are shown in Fig. S21 and S22.† Cu–C2N1–NO2 has the smallest diffusion coefficient as shown in Table S2,† which further proved that the monolayer of Cu–C2N1 can achieve selective adsorption of NO2 gas. Interestingly, the diffusion coefficient of water is the largest, indicating that the monolayer does not attract much water.
NO2 exhibits the lowest diffusion rate on the monolayer. To comprehend its underlying mechanism from a physicochemical perspective, NO2 diffusion activation energy was obtained by fitting the Arrhenius equation according to the diffusion coefficient. The detailed calculation can be referred to the ESI.† The stable structures of Cu–C2N1–NO2 at different temperatures are shown in Fig. S23 and S24.† The level of activation energy directly affects both the difficulty and the rate of adsorption in the process.64,65 The smaller the activation energy, the less energy is required, making the adsorption more likely to occur. As shown in Fig. 6d and e, the mean-squared displacement of NO2 on Ag/Cu–C2N1 in the effective applicable temperature range shows that the diffusion activation energy of Cu–C2N1 (2.5 kJ mol−1) is less than that of Ag–C2N1 (2.7 kJ mol−1). Thus, it is confirmed that Cu–C2N1 has more advantages than Ag–C2N1 in the field of NO2 detection.
In the practical sensor usage, assessing gas molecule recovery time performance is crucial. The recovery time (τ) is defined as the time taken by a sensor to return to its original state, when the gas is removed. According to transition state theory, τ is calculated as:16
τ = ν−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−Eads/kT e−Eads/kT | (4) |
3 Conclusions
In summary, the gas-sensitive behavior on Cu/Ag–C2N1 was clarified by dispersion-corrected DFT calculations. The results show that Cu and Ag can be anchored to the monolayer of the C2N1 material. After doping metal atoms, the cohesion energy was compared to illustrate the monolayer stability. By DOS analysis, Cu decorated C2N1 improved conductivity better than the Ag atom. Moreover, NO2 prefers to adsorb on Cu–C2N1 due to its obvious electronic overlap. The adsorption energy of NO2 for Cu–C2N1 is −3.54 eV, and that of NO2 for Ag–C2N1 is −3.27 eV. The adsorption of NO2 by Cu–C2N1 is significant as also demonstrated by charge transfer, bond length and COHP. Thus, Cu–C2N1 has a stronger affinity for NO2. Notably, we clearly show for the first time in the DOS analysis that Cu–C2N1 achieves a single electrical response to NO2 while eliminating other gaseous disturbances. Furthermore, we also use d-band-center theory to understand the underlying mechanism of gas adsorption on the Cu/Ag–C2N1 monolayer. In terms of practicality, the temperature application range of the modified Cu–C2N1 (<800 K) is higher than that of Ag–C2N1 (<700 K). Interestingly, the adsorption and response of H2O were extremely poor, demonstrating the minimal effect of humidity on the monolayer. Molecular dynamics simulations revealed that the fundamental reason for the difference in the diffusion performance of NO2 on the monolayers is that Cu–C2N1 has a lower diffusion activation energy barrier (2.5 kJ mol−1). When considering cost, Cu proves to be considerably more economical than Ag. Our research confirms that Cu–C2N1 surpasses the Ag–C2N1 in both adsorption and response to NO2 gas. Thus, the Cu–C2N1 monolayer can be used as a gas sensor for gas generated by degassed products, greatly increasing the chances of successfully developing a new prototype of safety monitoring.Data availability
Data will be made available on request.Author contributions
Mingyang Gu: data curation, formal analysis, investigation, methodology, writing – original draft, and writing – review & editing. Lin Tao: validation, data curation, methodology, supervision, funding acquisition, conceptualization, validation, and writing – review & editing. Davoud Dastan: methodology, supervision, and resources. Jie Dang: methodology, formal analysis, and software. Timing Fang: methodology, formal analysis, and software. Baigang An: supervision, formal analysis, and writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The funding from the National Natural Science Foundation of China (Grant No. 52304330), the University of Science and Technology Liaoning Talent Project Grants (6003000317), the Outstanding Youth Fund of University of Science and Technology Liaoning (2023YQ11), and the Youth Fund of the Education Department of Liaoning Province (LJKQZ20222324) are gratefully acknowledged.References
- J. B. Goodenough and K. S. Park, The Li-ion rechargeable battery: a perspective, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- A. Manthiram, An Outlook on Lithium Ion Battery Technology, ACS Cent. Sci., 2017, 3, 1063–1069 CrossRef CAS PubMed.
- M. Armand and J. M. Tarascon, Building better batteries, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- A. M. Bates, Y. Preger, L. Torres-Castro, K. L. Harrison, S. J. Harris and J. Hewson, Are solid-state batteries safer than lithium-ion batteries?, Joule, 2022, 6, 742–755 CrossRef CAS.
- Y. Chen, Y. Kang, Y. Zhao, L. Wang, J. Liu, Y. Li, Z. Liang, X. He, X. Li, N. Tavajohi and B. Li, A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards, J. Energy Chem., 2021, 59, 83–99 CrossRef CAS.
- D. Santos-Carballal, O. Lupan, N. Magariu, N. Ababii, H. Krüger, M. T. Bodduluri, N. H. de Leeuw, S. Hansen and R. Adelung, Al2O3/ZnO composite-based sensors for battery safety applications: An experimental and theoretical investigation, Nano Energy, 2023, 109, 108301 CrossRef CAS.
- O. Lupan, N. Magariu, D. Santos-Carballal, N. Ababii, J. Offermann, P. Pooker, S. Hansen, L. Siebert, N. H. de Leeuw and R. Adelung, Development of 2-in-1 Sensors for the Safety Assessment of Lithium-Ion Batteries via Early Detection of Vapors Produced by Electrolyte Solvents, ACS Appl. Mater. Interfaces, 2023, 15, 27340–27356 CrossRef CAS PubMed.
- P. Liu, L. Yang, B. Xiao, H. Wang, L. Li, S. Ye, Y. Li, X. Ren, X. Ouyang, J. Hu, F. Pan, Q. Zhang and J. Liu, Revealing Lithium Battery Gas Generation for Safer Practical Applications, Adv. Funct. Mater., 2022, 32, 2208586 CrossRef CAS.
- H. Cui, C. Yan, P. Jia and W. Cao, Adsorption and sensing behaviors of SF6 decomposed species on Ni-doped C3N monolayer: A first-principles study, Appl. Surf. Sci., 2020, 512, 145759 CrossRef CAS.
- D. Chen, X. Zhang, H. Xiong, Y. Li, J. Tang, S. Xiao and D. Zhang, A First-Principles Study of the SF6 Decomposed Products Adsorbed Over Defective WS2 Monolayer as Promising Gas Sensing Device, IEEE Trans. Device Mater. Reliab., 2019, 19, 473–483 CAS.
- S. Zhai, X. Jiang, D. Wu, L. Chen, Y. Su, H. Cui and F. Wu, Single Rh atom decorated pristine and S-defected PdS2 monolayer for sensing thermal runaway gases in a lithium-ion battery: A first-principles study, Surf. Interfaces, 2023, 37, 102735 CrossRef CAS.
- K. Boonpalit, J. Kinchagawat, C. Prommin, S. Nutanong and S. Namuangruk, Efficient exploration of transition-metal decorated MXene for carbon monoxide sensing using integrated active learning and density functional theory, Phys. Chem. Chem. Phys., 2023, 25, 28657–28668 RSC.
- B. Mondal, X. Zhang, S. Kumar, F. Long, N. K. Katiyar, M. Kumar, S. Goel and K. Biswas, A resistance-driven H(2) gas sensor: high-entropy alloy nanoparticles decorated 2D MoS(2), Nanoscale, 2023, 15, 17097–17104 RSC.
- P. Li, R. Zhou, B. Liu, Y. Yuan, H. Cui, Z.-H. Pu and T. Wu, Adsorption of a MoSe2-Based Sensor for Fluorocarbon Gas Decomposition Products in Gas-Insulated Switchgear: A First Principles Analysis, J. Phys. Chem. C, 2023, 127, 11176–11185 CrossRef CAS.
- H. Cui, T. Yang, X. Peng and G. Zhang, First-principles screening upon Janus PtXY (X, Y = S, Se and Te) monolayer under applied biaxial strains and electric fields, J. Mater. Res. Technol., 2022, 18, 1218–1229 CrossRef CAS.
- D. Chen, X. Zhang, J. Tang, Z. Cui and H. Cui, Pristine and Cu decorated hexagonal InN monolayer, a promising candidate to detect and scavenge SF6 decompositions based on first-principle study, J. Hazard. Mater., 2019, 363, 346–357 CrossRef CAS PubMed.
- C. Anichini, W. Czepa, D. Pakulski, A. Aliprandi, A. Ciesielski and P. Samori, Chemical sensing with 2D materials, Chem. Soc. Rev., 2018, 47, 4860–4908 RSC.
- X. Sun, Q. Yang, R. Meng, C. Tan, Q. Liang, J. Jiang, H. Ye and X. Chen, Adsorption of gas molecules on graphene-like InN monolayer: A first-principle study, Appl. Surf. Sci., 2017, 404, 291–299 CrossRef CAS.
- L. Tao, D. Dastan, W. Wang, P. Poldorn, X. Meng, M. Wu, H. Zhao, H. Zhang, L. Li and B. An, Metal-Decorated InN Monolayer Senses N2 against CO2, ACS Appl. Mater. Interfaces, 2023, 15, 12534–12544 CrossRef CAS PubMed.
- H. Cui, X. Zhang, Y. Li, D. Chen and Y. Zhang, First-principles insight into Ni-doped InN monolayer as a noxious gases scavenger, Appl. Surf. Sci., 2019, 494, 859–866 CrossRef CAS.
- H. Ye, S. Liu, K. Peng, Q. Zheng, J. Hu and H. Cui, Exploration of Pt-doped Janus WSSe monolayer as a typical gas sensor for condition assessment in XLPE cables, Chem. Phys. Lett., 2023, 832, 140880 CrossRef CAS.
- B. Pecz, G. Nicotra, F. Giannazzo, R. Yakimova, A. Koos and A. Kakanakova-Georgieva, Indium Nitride at the 2D Limit, Adv. Mater., 2021, 33, 2006660 CrossRef CAS PubMed.
- P. Hess, Bonding, structure, and mechanical stability of 2D materials: the predictive power of the periodic table, Nanoscale Horiz., 2021, 6, 856–892 RSC.
- T. Zhang, L. Li, T. Huang, H. Wan, W.-Y. Chen, Z.-X. Yang, G.-F. Huang, W. Hu and W.-Q. Huang, Correlation between spin state and activity for hydrogen evolution of PtN2 monolayer, Appl. Phys. Lett., 2024, 124, 063903 CrossRef CAS.
- W.-Y. Chen, L. Li, T. Huang, Z.-X. Yang, T. Zhang, G.-F. Huang, W. Hu and W.-Q. Huang, Extending Schottky–Mott rule to van der Waals heterostructures of 2D Janus materials: Influence of intrinsic dipoles, Appl. Phys. Lett., 2023, 123, 171601 CrossRef CAS.
- Y. Liu, L. Gao, S. Fu, S. Cheng, N. Gao and H. Li, Highly efficient VOC gas sensors based on Li-doped diamane, Appl. Surf. Sci., 2023, 611, 155694 CrossRef CAS.
- X. Tang, G. Guo, Y. Peng, M. You, S. Luo, C. He, C. Tang, Z. Huang and J. Zhong, Tunable properties of two-dimensional bilayer C3N as anode material: Bandgap, binding energy, and diffusion barrier, J. Energy Storage, 2024, 77, 109906 CrossRef.
- X. Wang, H. Niu, X. Wan, A. Wang, F. R. Wang and Y. Guo, Impact of Coordination Environment on Single-Atom-Embedded C3N for Oxygen Electrocatalysis, ACS Sustain. Chem. Eng., 2022, 10, 7692–7701 CrossRef CAS.
- Z. Chen, J. Zhao, C. R. Cabrera and Z. Chen, Computational Screening of Efficient Single-Atom Catalysts Based on Graphitic Carbon Nitride (g-C3N4) for Nitrogen Electroreduction, Small Methods, 2018, 3, 1800368 CrossRef.
- P. Kaur, S. Bagchi, D. Gribble, V. G. Pol and A. P. Bhondekar, Impedimetric Chemosensing of Volatile Organic Compounds Released from Li-Ion Batteries, ACS Sens., 2022, 7, 674–683 CrossRef CAS PubMed.
- P. Kaur, S. Bagchi, V. G. Pol and A. P. Bhondekar, Early Detection of Mixed Volatile Organic Compounds to Circumvent Calamitous Li-Ion Battery Thermal Runaway, J. Phys. Chem. C, 2023, 127, 8373–8382 CrossRef CAS.
- M. Wu, S. Han, S. Liu, J. Zhao and W. Xie, Fire-safe polymer electrolyte strategies for lithium batteries, Energy Storage Mater., 2024, 66, 103174 CrossRef.
- H. Xiong, H. Zhang and L. Gan, A new bifunctional C3N nanosheet of NO2, SO2 gas sensor and CO2 separation: A first-principles study, Phys. E, 2021, 126, 114463 CrossRef CAS.
- H. Cui, G. Zhang, X. Zhang and J. Tang, Rh-doped MoSe2 as a toxic gas scavenger: a first-principles study, Nanoscale Adv., 2019, 1, 772–780 RSC.
- L. Li, H. Cao, Z. Liang, Y. Cheng, T. Yin, Z. Liu, S. Yan, S. Jia, L. Li, J. Wang and Y. Gao, First-Principles Study of Ti-Deficient Ti3C2 MXene Nanosheets as NH3 Gas Sensors, ACS Appl. Nano Mater., 2022, 5, 2470–2475 CrossRef CAS.
- D. Dastan, K. shan, A. Jafari, T. Marszalek, M. K. A. Mohammed, L. Tao, Z. Shi, Y. Chen, X.-T. Yin, N. D. Alharbi, F. Gity, S. Asgary, M. Hatamvand and L. Ansari, Influence of heat treatment on H2S gas sensing features of NiO thin films deposited via thermal evaporation technique, Mater. Sci. Semicond. Process., 2023, 154, 107232 CrossRef CAS.
- P. Lv, D. Wu, B. He, X. Li, R. Zhu, G. Tang, Z. Lu, D. Ma and Y. Jia, An efficient screening strategy towards multifunctional catalysts for the simultaneous electroreduction of NO3−, NO2− and NO to NH3, J. Mater. Chem. A, 2022, 10, 9707–9716 RSC.
- M. Tang, D. Zhang, D. Wang, J. Deng, D. Kong and H. Zhang, Performance prediction of 2D vertically stacked MoS2-WS2 heterostructures base on first-principles theory and Pearson correlation coefficient, Appl. Surf. Sci., 2022, 596, 153498 CrossRef CAS.
- D. Chen, Y. Li, S. Xiao, C. Yang, J. Zhou and B. Xiao, Single Ni atom doped WS2 monolayer as sensing substrate for dissolved gases in transformer oil: A first-principles study, Appl. Surf. Sci., 2022, 579, 152141 CrossRef CAS.
- L. T. Ta, I. Hamada, Y. Morikawa and V. A. Dinh, Adsorption of toxic gases on borophene: surface deformation links to chemisorptions, RSC Adv., 2021, 11, 18279–18287 RSC.
- W. Nong, H. Liang, S. Qin, Y. Li and C. Wang, Computational Design of Two-Dimensional Boron-Containing Compounds as Efficient Metal-free Electrocatalysts toward Nitrogen Reduction Independent of Heteroatom Doping, ACS Appl. Mater. Interfaces, 2020, 12, 50505–50515 CrossRef CAS PubMed.
- M. I. Ahmed, L. J. Arachchige, Z. Su, D. B. Hibbert, C. Sun and C. Zhao, Nitrogenase-Inspired Atomically Dispersed Fe–S–C Linkages for Improved Electrochemical Reduction of Dinitrogen to Ammonia, ACS Catal., 2022, 12, 1443–1451 CrossRef CAS.
- Q. Yue, Z. Shao, S. Chang and J. Li, Adsorption of gas molecules on monolayer MoS2 and effect of applied electric field, Nanoscale Res. Lett., 2013, 8, 425 CrossRef PubMed.
- H. Wang, X. Li, J. Wu and D. Zhang, An Experimental and Density Functional Theory Simulation Study of NO Reduction Mechanisms over Fe(0) Supported on Graphene with and without CO, Langmuir, 2023, 39, 15369–15379 CrossRef CAS PubMed.
- L. Tao, J. Huang, D. Dastan, T. Wang, J. Li, X. Yin and Q. Wang, CO2 capture and separation on charge-modulated calcite, Appl. Surf. Sci., 2020, 530, 147265 CrossRef CAS.
- I. S. Amiinu, X. Liu, Z. Pu, W. Li, Q. Li, J. Zhang, H. Tang, H. Zhang and S. Mu, From 3D ZIF Nanocrystals to Co-Nx/C Nanorod Array Electrocatalysts for ORR, OER, and Zn-Air Batteries, Adv. Funct. Mater., 2018, 28, 1704638 CrossRef.
- M. Wang, W. Ma, C. Tan, Z. Qiu, L. Hu, X. Lv, Q. Li and J. Dang, Designing Efficient Non-Precious Metal Electrocatalysts for High-Performance Hydrogen Production: A Comprehensive Evaluation Strategy, Small, 2023, e2306631 CrossRef PubMed.
- Z. Lv, W. Ma, M. Wang, J. Dang, K. Jian, D. Liu and D. Huang, Co-Constructing Interfaces of Multiheterostructure on MXene (Ti3C2Tx)-Modified 3D Self-Supporting Electrode for Ultraefficient Electrocatalytic HER in Alkaline Media, Adv. Funct. Mater., 2021, 31, 2102576 CrossRef CAS.
- K. He, W. Li, L. Tang, L. Chen, G. Wang, Q. Liu, X. Xin, C. Yang, Z. Wang, S. Lv and D. Xing, Insight into the design of a Ti3C2 MXene/Ti4O7 composite ceramic membrane boosts the electrocatalytic activity for 1,4-dioxane electro-oxidation, Appl. Catal., B, 2023, 338, 123077 CrossRef CAS.
- S. Nie, L. Tao, J. Li, W. Wang, P. Poldorn, Y. He, X. Yin and M. Wu, A single response to reducing gases by NiO-TiO2 heterojunction nanocrystals, Appl. Surf. Sci., 2024, 644, 158821 CrossRef CAS.
- S. Nie, J. Li, L. Tao, Y. He, D. Dastan, X. Meng, P. Poldorn and X. Yin, Insights into Selective Mechanism of NiO-TiO(2) Heterojunction to H(2) and CO, ACS Sens., 2023, 8, 4121–4131 CrossRef CAS PubMed.
- L. Tao, J. Huang, D. Dastan, T. Wang, J. Li, X. Yin and Q. Wang, New insight into absorption characteristics of CO2 on the surface of calcite, dolomite, and magnesite, Appl. Surf. Sci., 2021, 540, 148320 CrossRef CAS.
- Y. He, L. Tao, J. Li, M. Wu, P. Poldorn, D. Dastan, S. Abbasi, S. Nie, X. Yin and Q. Wang, Atomic-level insights into selective adsorption of H2 and CO on SnO2/CoO heterojunctions, Mater. Today Nano, 2023, 22, 100334 CrossRef CAS.
- Y. He, J. Li, L. Tao, S. Nie, T. Fang, X. Yin and Q. Wang, First-principles calculations on the resistance and electronic properties of H2 adsorption on a CoO-SnO2 heterojunction surface, Phys. Chem. Chem. Phys., 2021, 24, 392–402 RSC.
- M. Wang, L. Kong, X. Lu and C.-M. Lawrence Wu, First-row transition metal embedded pyrazine-based graphynes as high-performance single atom catalysts for the CO2 reduction reaction, J. Mater. Chem. A, 2022, 10, 9048–9058 RSC.
- J. Li, C. Hou, C. Chen, W. Ma, Q. Li, L. Hu, X. Lv and J. Dang, Collaborative Interface Optimization Strategy Guided Ultrafine RuCo and MXene Heterostructure Electrocatalysts for Efficient Overall Water Splitting, ACS Nano, 2023, 17, 10947–10957 CrossRef CAS PubMed.
- L. Tao, Z. Li, G.-C. Wang, B.-Y. Cui, X.-T. Yin and Q. Wang, Evolution of calcite surface reconstruction and interface adsorption of calcite-CO2 with temperature, Mater. Res. Express, 2018, 6, 025035 CrossRef.
- H. Zhang, R. Zhang, Y. Ni, M. Chen, C. Sun and F. Dong, SO2 adsorption and conversion on pristine and defected calcite {1 0 4} surface: A density functional theory study, Appl. Surf. Sci., 2022, 596, 153575 CrossRef CAS.
- L. Lin, S. Yao, R. Gao, X. Liang, Q. Yu, Y. Deng, J. Liu, M. Peng, Z. Jiang, S. Li, Y. W. Li, X. D. Wen, W. Zhou and D. Ma, A highly CO-tolerant atomically dispersed Pt catalyst for chemoselective hydrogenation, Nat. Nanotechnol., 2019, 14, 354–361 CrossRef CAS PubMed.
- X. Liu, Y. Jiao, Y. Zheng, M. Jaroniec and S.-Z. Qiao, Building Up a Picture of the Electrocatalytic Nitrogen Reduction Activity of Transition Metal Single-Atom Catalysts, J. Am. Chem. Soc., 2019, 141, 9664–9672 CrossRef CAS PubMed.
- H. Hu, P. Zhang, B.-B. Xiao and J.-L. Mi, Theoretical study of p-block metal–nitrogen–carbon single-atom catalysts for the oxygen reduction reaction, Catal. Sci. Technol., 2022, 12, 6751–6760 RSC.
- L. Tao, J. Huang, X. Yin, Q. Wang, Z. Li, G. Wang and B. Cui, Adsorption Kinetics of CO2 on a Reconstructed Calcite Surface: An Experiment-Simulation Collaborative Method, Energy Fuels, 2019, 33, 8946–8953 CrossRef CAS.
- L. Tao, J. Huang, D. Dastan, J. Li, X. Yin and Q. Wang, Flue gas separation at organic-inorganic interface under geological conditions, Surf. Interfaces, 2021, 27, 101462 CrossRef CAS.
- T. Fang, C. Wei, X. Meng, G. Zhou and X. Liu, How homogeneous and biphasic membranes contribute to the gas transfer: A molecular dynamics simulation study, Int. J. Heat Mass Transfer, 2023, 201, 123644 CrossRef CAS.
- X. Meng, T. Fang, G. Zhou, S. Liu and X. Liu, Selectivity and permeability of gas separation in SILMs: Effect of collapsed structure, J. Mol. Liq., 2023, 388, 122834 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta00645c |
| This journal is © The Royal Society of Chemistry 2024 |