Surface passivation with an electron-donating sulfonate group for high-performance and stable perovskite solar cells†
Qingquan
He
,
Zenan
Zhang
,
An
Chen
,
Tao
Zhang
,
Xiuyuan
Chen
,
Xiaolong
Bian
,
Gaopeng
Xu
,
Ting
Chen
,
Shicheng
Pan
,
Jiewen
Yu
,
Guochao
Lu
,
Jing
Li
and
Jun
Pan
 *
*
Zhejiang University of Technology, China. E-mail: panjun0123@zjut.edu.cn
First published on 25th April 2024
Abstract
Perovskite solar cells (PSCs) have gained significant attention due to their potential for high power conversion efficiency (PCE). However, the presence of surface traps has hindered further improvements in their performance and stability. To address this issue, a post-annealing treatment with functional molecules has emerged as an effective method for mitigating trap-mediated non-radiative recombination. Herein, 4-aminophenyl sulfone (APS), containing a Lewis base group (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), could interact with uncoordinated Pb2+ on the perovskite surface, which not only reduces trap state density but also induces a more p-type surface of the perovskite film. As a result of the APS modification, the device achieved a higher PCE of 23.03%, compared to a pristine device of 21.26%, and the values of open-current voltage (VOC), short-circuit current density (JSC), and fill factor (FF) were also improved obviously. Moreover, the APS-modified device demonstrated excellent environmental and thermal stability, maintaining 93% and 90% of its initial PCE after 1000 h at a relative humidity of 30% at room temperature and 200 h in an inert environment at 70 °C, respectively.
O), could interact with uncoordinated Pb2+ on the perovskite surface, which not only reduces trap state density but also induces a more p-type surface of the perovskite film. As a result of the APS modification, the device achieved a higher PCE of 23.03%, compared to a pristine device of 21.26%, and the values of open-current voltage (VOC), short-circuit current density (JSC), and fill factor (FF) were also improved obviously. Moreover, the APS-modified device demonstrated excellent environmental and thermal stability, maintaining 93% and 90% of its initial PCE after 1000 h at a relative humidity of 30% at room temperature and 200 h in an inert environment at 70 °C, respectively.
Introduction
Perovskite solar cells (PSCs) have received tremendous attention over the past decade due to their exceptional light absorption coefficient, low exciton binding energy, high carrier mobility, and low-cost fabrication processes.1–8 These characteristics make them suitable for potential commercialization, especially given that their power conversion efficiency (PCE) has reached 26.1%.9,10 Formamidinium (FA)-based lead triiodide perovskites, with a narrow bandgap (Eg ≈ 1.5 eV) and promising thermal stability, are commonly employed as light absorbers.11–13 However, excess lead iodide (PbI2) is inevitably retained during the preparation of FA-based perovskite films.14,15 As a result, the device experiences rapid degradation and severe nonradiative recombination due to the presence of uncoordinated Pb2+, significantly impacting both device performance and long-term stability.16,17Surface passivation has emerged as a key strategy to reduce extrinsic defects in perovskites and consequently enhance PSC efficiency.18 Various methods, including 2D/3D heterojunction layer formation,19–21 organic polymer modification,22–24 and wide bandgap materials passivation,25 have been explored to improve surface morphology, regulate energy level alignment, and reduce non-radiative recombination. Additionally, small organic molecules containing Lewis-based functional groups such as O-donor, S-donor and N-donor Lewis bases have been investigated for their ability to effectively passivate the perovskite surface.26–30 The use of S-donor Lewis bases has proven to be more effective than that of O-donor bases in PSCs with structures incorporating thiophene, thiourea, and their derivatives.31 Functional groups within these small molecules form coordinate bonds with lone electron pairs to minimize non-radiative recombination and modify the Pb–I surface termination.32–34 While most reports have focused on utilizing sulfonate-based salts to enhance perovskite crystallization and reduce trap density during perovskite film processing, there are few reports about covalent compounds containing the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group.35–37
O group.35–37
In this study, a covalent compound molecule named 4-aminophenyl sulfone (APS) with symmetric space charge distribution was introduced to passivate the perovskite surface. Theoretical calculations and experimental studies demonstrated that APS with the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group strongly coordinates with Pb2+ on the perovskite surface, reducing surface defects. Additionally, the thin film formed between the perovskite layer and the hole transport layer proved beneficial for minimizing charge carrier recombination. The device with APS modification exhibited improved efficiency, achieving over 23% PCE with a high fill factor (FF) over 80% as well as excellent environmental and thermal stability, maintaining 93% and 90% of its initial PCE after 1000 h at a relative humidity of 30% at room temperature and 200 h in an inert environment at 70 °C, respectively.
O group strongly coordinates with Pb2+ on the perovskite surface, reducing surface defects. Additionally, the thin film formed between the perovskite layer and the hole transport layer proved beneficial for minimizing charge carrier recombination. The device with APS modification exhibited improved efficiency, achieving over 23% PCE with a high fill factor (FF) over 80% as well as excellent environmental and thermal stability, maintaining 93% and 90% of its initial PCE after 1000 h at a relative humidity of 30% at room temperature and 200 h in an inert environment at 70 °C, respectively.
Results and discussion
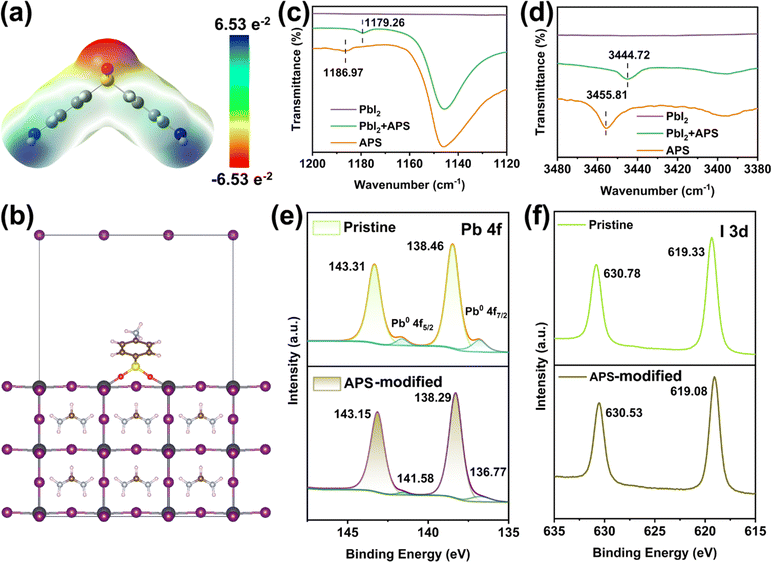
The passivation strategy employed in this study involved the use of an APS molecule with a symmetrical spatial distribution, as depicted in its chemical structure in Fig. S1.† This molecule containing a negatively charged S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional group, a benzene ring, and a positively charged –NH2 functional group was applied to modify the top surface of the perovskite film. The electron distribution, as revealed using the electrostatic potential (ESP) (Fig. 1a), showed that the positive region is primarily distributed in benzene rings and the –NH2 group, while the S
O functional group, a benzene ring, and a positively charged –NH2 functional group was applied to modify the top surface of the perovskite film. The electron distribution, as revealed using the electrostatic potential (ESP) (Fig. 1a), showed that the positive region is primarily distributed in benzene rings and the –NH2 group, while the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in APS exhibited a high-density electron cloud, providing suitable conditions to form stronger bonds with uncoordinated Pb2+ ions.38 Density functional theory calculations indicated that the high electron density of oxygen atoms on sulfonate anions effectively bonded with Pb2+ due to their Lewis base properties (Fig. 1b), leading to fewer Pb2+ defects on the surface after APS modification.
O group in APS exhibited a high-density electron cloud, providing suitable conditions to form stronger bonds with uncoordinated Pb2+ ions.38 Density functional theory calculations indicated that the high electron density of oxygen atoms on sulfonate anions effectively bonded with Pb2+ due to their Lewis base properties (Fig. 1b), leading to fewer Pb2+ defects on the surface after APS modification.
Fourier transform infrared spectroscopy (FTIR) was carried out to analyse the chemical mechanism. The pure APS power exhibited slight peaks at 1186.97 cm−1 and 3455.81 cm−1 (Fig. 1c and d), caused by the symmetric stretching vibrations of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and –NH2 bond, respectively.39 After mixing APS with PbI2 powder, the peaks of the S
O bond and –NH2 bond, respectively.39 After mixing APS with PbI2 powder, the peaks of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and –NH2 bond shifted to lower wavenumbers (1179.26 and 3444.72 cm−1, respectively), suggesting strong coordination between APS and PbI2. This shift demonstrated that the S
O bond and –NH2 bond shifted to lower wavenumbers (1179.26 and 3444.72 cm−1, respectively), suggesting strong coordination between APS and PbI2. This shift demonstrated that the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in APS affected the surface chemical environment.
O group in APS affected the surface chemical environment.
We also performed X-ray photoemission spectroscopy (XPS) tests to investigate the interaction between APS and perovskite films. As shown in Fig. 1e, the main peaks for the pristine perovskite film, located at 143.31 and 138.46 eV, were attributed to Pb 4f5/2 and Pb 4f7/2, respectively.40 After APS modification, these peaks shifted to lower binding energies at 143.15 and 138.29 eV, respectively, suggesting that the high electron density of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups could form a strong band with Pb. The presence of Pb0, a by-product resulting from the degradation of PbI2 under light or X-ray conditions, has the potential to induce deep-level defects and cause serious degradation of the PSCs.41–45 Its noticeable reduction after APS modification suggests that APS can efficiently suppress perovskite defects with electron-donating groups.46 Additionally, the I 3d3/2 and I 3d5/2 peaks of the APS-modified perovskite film at 630.53 and 619.08 eV slightly shifted to the lower binding energy region compared to the pristine film (630.78 and 619.33 eV, respectively), as shown in Fig. 1f, confirming the chemical environment changed by APS modification. The shift observed in the Pb 4f and I 3d peaks indicated effective passivation of both cationic and anionic defects on the perovskite surface.47 Thereby, we speculate that the electro-donor of the S
O groups could form a strong band with Pb. The presence of Pb0, a by-product resulting from the degradation of PbI2 under light or X-ray conditions, has the potential to induce deep-level defects and cause serious degradation of the PSCs.41–45 Its noticeable reduction after APS modification suggests that APS can efficiently suppress perovskite defects with electron-donating groups.46 Additionally, the I 3d3/2 and I 3d5/2 peaks of the APS-modified perovskite film at 630.53 and 619.08 eV slightly shifted to the lower binding energy region compared to the pristine film (630.78 and 619.33 eV, respectively), as shown in Fig. 1f, confirming the chemical environment changed by APS modification. The shift observed in the Pb 4f and I 3d peaks indicated effective passivation of both cationic and anionic defects on the perovskite surface.47 Thereby, we speculate that the electro-donor of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group interacted with the Pb2+ defects and the –NH2 provides a hydrogen bond to interact with I 3d. With the synergistic effect of the two groups, the Pb–I framework could coordinate strongly. Furthermore, a strong peak at 168.1 eV was observed after the APS modification (Fig. S3†), confirming the existence of the S element at the perovskite surface. Importantly, the S 2p signal remained even after washing the APS-modified film with pure chlorobenzene (Fig. S4†), further suggesting the strong interaction between APS and the perovskite film.
O group interacted with the Pb2+ defects and the –NH2 provides a hydrogen bond to interact with I 3d. With the synergistic effect of the two groups, the Pb–I framework could coordinate strongly. Furthermore, a strong peak at 168.1 eV was observed after the APS modification (Fig. S3†), confirming the existence of the S element at the perovskite surface. Importantly, the S 2p signal remained even after washing the APS-modified film with pure chlorobenzene (Fig. S4†), further suggesting the strong interaction between APS and the perovskite film.
To further understand the impact of APS modification on the morphology of perovskite films, scanning electron microscopy (SEM) and atomic force microscopy (AFM) measurements were conducted. As shown in Fig. 2a and b, there was no significant change in grain size between pristine and APS-modified films. Interestingly, a passivation layer was observed on the surface of the film, and we speculated that APS may have formed a buffer layer on the surface. Additionally, AFM analysis revealed that the APS-modified perovskite film (17.4 nm) became smoother compared to the pristine film (20.9 nm) (Fig. 2c and d). Furthermore, wettability assessment through contact angle measurements showed an increase in the contact angle for the APS-modified perovskite film (75.6°) compared to the pristine film (57.9°) (Fig. 2e and f), indicating improved hydrophilicity and long-term stability.
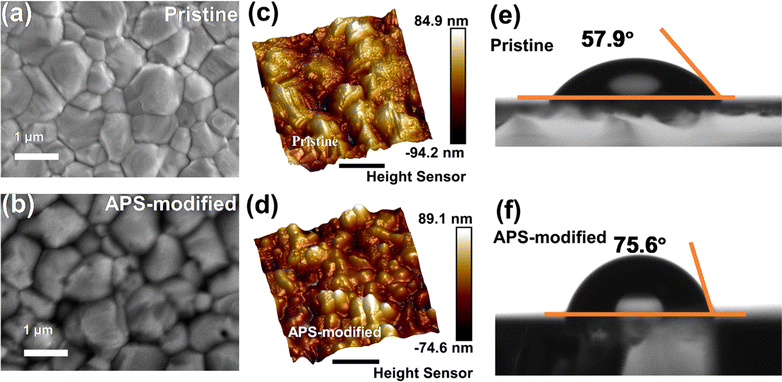 | ||
| Fig. 2 Top-view SEM and AFM images of (a and c) pristine and (b and d) APS-modified perovskite films. The contact angle measurements of water on (e) pristine and (f) APS-modified perovskite films. | ||
To investigate the effect of APS on the photoelectric properties of perovskite films, we conducted UV-vis absorption, excitation–emission matrix (EEM), time-resolved PL (TRPL), and space charge-limited current (SCLC) measurements on pristine and APS-modified perovskite films. The APS-modified perovskite film showed a characteristic absorption spectrum similar to that of the pristine one (Fig. S5†), with an even higher absorption intensity in the lower wavelength region, suggesting that the passivation of the APS molecule does not alter the light absorption capacity of the perovskite layer and may potentially enhance photon absorption.48 Furthermore, both films exhibited an identical band gap (1.56 eV), as determined using the Tauc plot method.
The EEM spectra of the APS-modified films demonstrated a higher emission peak intensity at around 800 nm compared to the pristine film (Fig. 3a and b), with the structure of an ITO/perovskite/APS layer. This phenomenon was attributed to the decrease in the nonradiative recombination after APS modification. TRPL decay spectra (Fig. 3c) and the fitting curve data (Table S1†) indicate a slower charge carrier recombination process in the APS-modified film, suggesting a reduction in trap defects and successful passivation of APS at the perovskite-hole transport layer interface. The SCLC was employed to explore the passivation effect of APS using an electron-only device structure of ITO/SnO2/perovskite/with or without APS/PCBM/Ag under dark conditions. The APS-modified devices presented a lower trap-filled limit voltage (0.38 V) and reduced trap-state densities (1.95 × 1016 cm−3) compared to the pristine device, 0.61 V (Fig. 3d), suggesting improved performance and reduced interface defects after APS modification.
To verify the advantage of APS modification, a device with the structure of ITO/SnO2/perovskite/spiro-OMeTAD/Ag was constructed (Fig. 4a). After optimizing the concentration of APS modification on the perovskite surface, a concentration of 3 mg mL−1 was selected for demonstrating the best PCE of 23.03% (details in Fig. S6 and Table S2†). Subsequently, we conducted measurements of the photocurrent density–voltage (J–V) characteristics of the best device, as illustrated in Fig. 4b, with the corresponding photovoltaic data listed in Table S3.† Comparative analysis revealed that the APS-modified device exhibited enhanced performance compared to the pristine device, primarily attributed to higher open-current voltage (VOC) and FF. Moreover, the J–V hysteresis of the APS-modified device was 7.79% compared to that of pristine device (11.42%). The lower hysteresis confirms that APS could effectively passivate surface defects and decrease carrier recombination. The boxplot representations of PCE (Fig. 4c), short-circuit current density (JSC, Fig. 4d), VOC (Fig. 4e), and FF (Fig. 4f) for several pristine and APS-modified PSCs indicated good reproducibility, displaying narrow distributions for both sets of devices. These observations underscore the consistent and reliable enhancement achieved through APS modification.
Furthermore, Fig. S7† displays the external quantum efficiency (EQE) spectrum of the champion device in the 300–900 nm range. Interestingly, the APS-modified device outperformed the pristine device with a higher integrated photocurrent density. Notably, the data reveal a stronger absorbance in the lower wavelength region for the APS-modified perovskite film, suggesting that APS-modified films generate more charge carriers and mitigate defects. This finding aligns with the UV measurement presented in Fig. S5,† which exhibits a higher absorption in the lower wavelength region for the APS-modified perovskite film. The enhancement mechanism associated with APS modification was further explored through ultraviolet photoelectron spectroscopy (UPS) and electrochemical impedance spectra (EIS) measurements. The UPS spectra and energy level diagrams of pristine and APS-modified perovskite films are depicted in Fig. S8.† The conduction band and valence band maximum (VBM) of the pristine film changed from −4.28 and −5.84 eV to −4.18 and −5.74 eV, respectively. Furthermore, the energy offset between the VBM of the pristine film and the highest occupied molecular orbital of spiro-OMeTAD was 0.62 eV, which reduced to 0.52 eV after APS-modification, demonstrating an improved band alignment with the hole transport layer after APS modification. Moreover, EIS measurements revealed that the APS-modified PSCs could augment charge mobility and impede charge recombination (Fig. S9†). These findings collectively validate the positive impact of APS modification on charge transport and recombination processes, ultimately leading to improved PSC performance.
The environmental stability of PSCs is an important factor in assessing their quality. Long-term stability measurements of PSCs with and without APS modification were performed at a relative humidity (RH) of 30% at room temperature without encapsulation, as depicted in Fig. 5a. Both the APS-modified and pristine devices exhibited an increase in PCE over 200 h. This phenomenon was attributed to the oxidation of the spiro-OMeTAD layer.49 However, after 200 h, the efficiency gradually decreased due to perovskite layer degradation. Notably, the device with APS modification retained 90% of its initial efficiency after 1000 h under ambient conditions, demonstrating superior stability compared to the pristine device. Additionally, thermal stability testing at 70 °C in a N2 atmosphere was conducted, as shown in Fig. 5b. The APS-modified PSC exhibited improved thermal stability compared to the pristine device. The efficiency of the pristine device rapidly decreased to less than 60% of its initial PCE after 80 h, while the APS-modified PSC retained more than 90% of its initial PCE. The X-ray diffraction (XRD) results in Fig. S10† reveal that the perovskite film with APS modification exhibits a lower PbI2 peak and (111) peak compared to the pristine film, which may be attributed to the strong Lewis acid–base interactions between APS and free PbI2, indicating the passivation effect of APS on the perovskite film.50–53 Furthermore, the absence of a new peak after APS modification suggested that APS does not combine with perovskites to form two-dimensional phases. Long-term stability of the APS-modified perovskite films probed by phase evolution after storage was measured by XRD, as shown in Fig. 5c and d. These XRD patterns illustrate that the perovskite (100) orientation decreased after 1000 h due to the degradation of the perovskite layer. Moreover, the perovskite with APS modification exhibited a lower decrease under the same conditions. Additionally, a new peak at around 12° in the pristine film was identified as the δ-phase perovskite resulting from the degradation of the FA-based perovskite film.54–57 These results highlight excellent performance with APS modification, illustrating its ability to passivate perovskite films and suppress perovskite degradation.
Conclusions
In conclusion, our study showcased a multifunctional optimization strategy through the utilization of APS molecules for surface modification of the perovskite layer. The electron-donating sulfonate group within APS effectively passivated the surface defects, interacted with Pb2+, and ultimately enhanced device performance. Notably, several film characteristics were improved, leading to an increase in VOC from 1.11 V to 1.14 V and an enhancement in PCE from 21.26% to 23.03%, respectively. Moreover, the optimized PSCs exhibited robust stability, retaining 93% and 90% of their initial PCE after 1000 h at a relative humidity of 30% at room temperature and 200 h in an inert environment at 70 °C, respectively. This work introduces a new small molecule for modifying the perovskite film surface in PSCs, thereby advancing the development of high-performance and long-term stability PSCs.Author contributions
J. P. supervised the project. Q. H., J. L. and J. P. conceived the ideas for the project and designed the experiments. Z. Z., A. C., T. Z., X. Y., X. B., G. P., T. C., S. P., J. Y. and G. L. characterized the films and devices. Q. H., Z. Z. and J. P. co-wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Project of China (2022YFE0113800), the National Natural Science Foundation of China (grant no. 52172160 and 21805181), the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (2020R01002), and the Fundamental Research Funds for the Provincial Universities of Zhejiang (RF-C2022005 and RF-A2022010).Notes and references
- B. Kim, B. Gil, S. Ryu, J. Kim and B. Park, Adv. Funct. Mater., 2023, 33, 2307640 CrossRef CAS.
- H. Cui, Y. Ning, Y. Yang, D. He, W. Chen, Y. Huang, P. Zhao, Y. Feng and B. Zhang, Sol. RRL, 2023, 7, 2300080 CrossRef CAS.
- Y. Rong, Y. Hu, A. Mei, H. Tan, M. I. Saidaminov, S. I. Seok, M. D. McGehee, E. H. Sargent and H. Han, Science, 2018, 361, 6408 CrossRef PubMed.
- S. Hou, Z. Ma, Y. Li, Z. Du, Y. Chen, J. Yang, W. You, Q. Yang, T. Yu, Z. Huang, G. Li, H. Wang, Q. Liu, G. Yan, H. Li, Y. Huang, W. Zhang, M. Abdi-Jalebi, Z. Ou, K. Sun, R. Su and W. Long, Adv. Funct. Mater., 2023, 34, 2310133 CrossRef.
- L. Liang, Z. Zhang, Y. Li, X. Yu, F. Lin, Y. Xu, Z. Lan, M. Cavazzini, G. Pozzi, S. Orlandi and P. Gao, Sol. RRL, 2023, 7, 2300415 CrossRef CAS.
- J. Barbe, A. Pockett, V. Stoichkov, D. Hughes, H. K. H. Lee, M. Carnie, T. Watson and W. C. Tsoi, J. Mater. Chem. C, 2020, 8, 1715–1721 RSC.
- G. Divitini, S. Cacovich, F. Matteocci, L. Cina, A. Di Carlo and C. Ducati, Nat. Energy, 2016, 1, 15012 CrossRef CAS.
- C. Fei and H. Wang, Org. Electron., 2019, 68, 143–150 CrossRef CAS.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- NREL, Best Research-Cell Efficiency Chart, 2023, https://www.nrel.gov/pv/cell-efficiency.html, accessed, February 2024 Search PubMed.
- S. Yuan, T. Zhang, H. Chen, Y. Ji, Y. Hao, H. Zheng, Y. Wang, Z. D. Chen, L. Chen and S. Li, Chem. Eng. J., 2022, 433, 133227 CrossRef CAS.
- Q. Sun, B. Tuo, Z. Ren, T. Xue, Y. Zhang, J. Ma, P. Li and Y. Song, Adv. Funct. Mater., 2022, 32, 2208885 CrossRef CAS.
- D.-H. Choi, H.-J. Seok, S.-K. Kim, D.-H. Kim, B. Hou and H.-K. Kim, Sol. RRL, 2021, 5, 2100660 CrossRef CAS.
- Y. Gao, F. Ren, D. Sun, S. Li, G. Zheng, J. Wang, H. Raza, R. Chen, H. Wang, S. Liu, P. Yu, X. Meng, J. He, J. Zhou, X. Hu, Z. Zhang, L. Qiu, W. Chen and Z. Liu, Energy Environ. Sci., 2023, 16, 2295–2303 RSC.
- G. Tumen-Ulzii, C. Qin, D. Klotz, M. R. Leyden, P. Wang, M. Auffray, T. Fujihara, T. Matsushima, J.-W. Lee, S.-J. Lee, Y. Yang and C. Adachi, Adv. Mater., 2020, 32, 1905035 CrossRef CAS PubMed.
- F. Gao, Y. Zhao, X. Zhang and J. You, Adv. Energy Mater., 2020, 10, 1902650 CrossRef CAS.
- J. Ye, M. M. Byranvand, C. O. Martinez, R. L. Z. Hoye, M. Saliba and L. Polavarapu, Angew. Chem., Int. Ed., 2021, 60, 21636–21660 CrossRef CAS PubMed.
- Y. Du, J. Wu, X. Zhang, Q. Zhu, M. Zhang, X. Liu, Y. Zou, S. Wang and W. Sun, J. Energy Chem., 2021, 52, 84–91 CrossRef CAS.
- Y. Jiang, J. Yuan, Y. Ni, J. Yang, Y. Wang, T. Jiu, M. Yuan and J. Chen, Joule, 2018, 2, 1356–1368 CrossRef.
- Q. Jiang, Y. Zhao, X. Zhang, X. Yang, Y. Chen, Z. Chu, Q. Ye, X. Li, Z. Yin and J. You, Nat. Photonics, 2019, 13, 460–466 CrossRef CAS.
- J. Park, J. Kim, H.-S. Yun, M. J. Paik, E. Noh, H. J. Mun, M. G. Kim, T. J. Shin and S. I. Seok, Nature, 2023, 616, 724–730 CrossRef CAS PubMed.
- Q. Fu, X. Tang, Y. Gao, H. Liu, M. Chen, R. Wang, Z. Song, Y. Yang, J. Wang and Y. Liu, Small, 2023, 19, 2301175 CrossRef CAS PubMed.
- H. Na, M. Q. Li, J. Cha, S. Kim, H. Jin, D. Baek, M. K. Kim, S. Sim, M. Lee, M. Kim, J. Lim, J. Lee and M. Kim, Appl. Surf. Sci., 2023, 626, 157209 CrossRef CAS.
- B. Zhang, C. Chen, X. Wang, X. Du, D. Liu, X. Sun, Z. Li, L. Hao, C. Gao, Y. Li, Z. Shao, X. Wang, G. Cui and S. Pang, Angew. Chem., Int. Ed., 2023, 62, e202213478 CrossRef CAS PubMed.
- D. Koushik, W. J. H. Verhees, Y. Kuang, S. Veenstra, D. Zhang, M. A. Verheijen, M. Creatore and R. E. I. Schropp, Energy Environ. Sci., 2017, 10, 91–100 RSC.
- J. Peng, J. I. Khan, W. Liu, E. Ugur, T. Duong, Y. Wu, H. Shen, K. Wang, H. Dang, E. Aydin, X. Yang, Y. Wan, K. J. Weber, K. R. Catchpole, F. Laquai, S. De Wolf and T. P. White, Adv. Energy Mater., 2018, 8, 1801208 CrossRef.
- T. Niu, J. Lu, R. Munir, J. Li, D. Barrit, X. Zhang, H. Hu, Z. Yang, A. Amassian, K. Zhao and S. Liu, Adv. Mater., 2018, 30, 1706576 CrossRef PubMed.
- X. Liu, Y. Cheng, C. Liu, T. Zhang, N. Zhang, S. Zhang, J. Chen, Q. Xu, J. Ouyang and H. Gong, Energy Environ. Sci., 2019, 12, 1622–1633 RSC.
- M. Wang, Y. Zhao, X. Jiang, Y. Yin, I. Yavuz, P. Zhu, A. Zhang, G. S. Han, H. S. Jung, Y. Zhou, W. Yang, J. Bian, S. Jin, J.-W. Lee and Y. Yang, Joule, 2022, 6, 1032–1048 CrossRef CAS.
- G. Yang, P. Qin, G. Fang and G. Li, Sol. RRL, 2018, 2, 1800055 CrossRef.
- F. Zhang and K. Zhu, Adv. Energy Mater., 2020, 10, 1902579 CrossRef CAS.
- W. Hou, G. Han, T. Ou, Y. Xiao and Q. Chen, Angew. Chem., Int. Ed., 2020, 59, 21409–21413 CrossRef CAS PubMed.
- J. He, J. Liu, Y. Hou, Y. Wang, S. Yang and H. G. Yang, Nat. Commun., 2020, 11, 4237 CrossRef CAS PubMed.
- B. Yu, Y. Sun, J. Zhang, K. Wang and H. Yu, Small, 2024, 20, 2307025 CrossRef CAS PubMed.
- X. Zheng, Y. Deng, B. Chen, H. Wei, X. Xiao, Y. Fang, Y. Lin, Z. Yu, Y. Liu, Q. Wang and J. Huang, Adv. Mater., 2018, 30, 1803428 CrossRef PubMed.
- Q. Tai, X. Guo, G. Tang, P. You, T.-W. Ng, D. Shen, J. Cao, C.-K. Liu, N. Wang, Y. Zhu, C.-S. Lee and F. Yan, Angew. Chem., Int. Ed., 2019, 58, 806–810 CrossRef CAS PubMed.
- S. Zhou, S. Fu, C. Wang, W. Meng, J. Zhou, Y. Zou, Q. Lin, L. Huang, W. Zhang, G. Zeng, D. Pu, H. Guan, C. Wang, K. Dong, H. Cui, S. Wang, T. Wang, G. Fang and W. Ke, Nature, 2023, 624, 69–73 CrossRef CAS PubMed.
- Y. Cai, J. Cui, M. Chen, M. Zhang, Y. Han, F. Qian, H. Zhao, S. Yang, Z. Yang, H. Bian, T. Wang, K. Guo, M. Cai, S. Dai, Z. Liu and S. Liu, Adv. Funct. Mater., 2021, 31, 2005776 CrossRef CAS.
- C. Xu, S. Zhang, W. Fan, F. Cheng, H. Sun, Z. Kang and Y. Zhang, Adv. Mater., 2023, 35, 2207172 CrossRef CAS PubMed.
- R. Sun, Q. Tian, M. Li, H. Wang, J. Chang, W. Xu, Z. Li, Y. Pan, F. Wang and T. Qin, Adv. Funct. Mater., 2023, 33, 2210071 CrossRef CAS.
- Y. Wang, M. I. Dar, L. K. Ono, T. Zhang, M. Kan, Y. Li, L. Zhang, X. Wang, Y. Yang, X. Gao, Y. Qi, M. Gratzel and Y. Zhao, Science, 2019, 365, 591–595 CrossRef CAS PubMed.
- J. Liang, X. Hu, C. Wang, C. Liang, C. Chen, M. Xiao, J. Li, C. Tao, G. Xing, R. Yu, W. Ke and G. Fang, Joule, 2022, 6, 816–833 CrossRef CAS.
- L. Wang, H. Zhou, J. Hu, B. Huang, M. Sun, B. Dong, G. Zheng, Y. Huang, Y. Chen, L. Li, Z. Xu, N. Li, Z. Liu, Q. Chen, L.-D. Sun and C.-H. Yan, Science, 2019, 363, 265–270 CrossRef CAS PubMed.
- B. Roose, K. Dey, Y. H. Chiang, R. H. Friend and S. D. Stranks, J. Phys. Chem. Lett., 2020, 11, 6505–6512 CrossRef CAS PubMed.
- Z. Wu, M. Jiang, Z. Liu, A. Jamshaid, L. K. Ono and Y. Qi, Adv. Energy Mater., 2020, 10, 1903696 CrossRef CAS.
- Y. Sun, J. Zhang, B. Yu, S. Shi and H. Yu, Nano Energy, 2024, 121, 109245 CrossRef CAS.
- D. Du, G. Liu, L. Zhang, Y. Tao, H. Zhang, T. Guo, H. Xu, S. Xu, J. Ye, H. Zheng and X. Pan, Chem. Eng. J., 2023, 467, 143392 CrossRef CAS.
- G. Yang, D. Zhou, J. Li and J. Yu, Photonics, 2022, 9, 3 CrossRef CAS.
- F. M. Rombach, S. A. Haque and T. J. Macdonald, Energy Environ. Sci., 2021, 14, 5161–5190 RSC.
- Y. Lin, L. Shen, J. Dai, Y. Deng, Y. Wu, Y. Bai, X. Zheng, J. Wang, Y. Fang, H. Wei, W. Ma, X. C. Zeng, X. Zhan and J. Huang, Adv. Mater., 2017, 29, 1604545 CrossRef PubMed.
- K. Wang, J. Liu, J. Yin, E. Aydin, G. T. Harrison, W. Liu, S. Chen, O. F. Mohammed and S. De Wolf, Adv. Funct. Mater., 2020, 30, 2002861 CrossRef CAS.
- M. Hou, X. Liu, Y. Fu, H. Wang, W. Zhao, H. Li, Y. Ni, Y. Lei, W. Zou, Y. Peng, H. Luo, Q. Feng, P. Ye, S. Liu and Y. Tang, Opt. Mater., 2023, 145, 114426 CrossRef CAS.
- X. Jiang, J. Zhang, X. Liu, Z. Wang, X. Guo and C. Li, Angew. Chem., Int. Ed., 2022, 61, e202115663 CrossRef CAS PubMed.
- T. Chen, J. Xie, B. Wen, Q. Yin, R. Lin, S. Zhu and P. Gao, Nat. Commun., 2023, 14, 6125 CrossRef CAS PubMed.
- H. Min, M. Kim, S. U. Lee, H. Kim, G. Kim, K. Choi, J. H. Lee and S. I. Seok, Science, 2019, 366, 749–753 CrossRef CAS PubMed.
- Y. H. Lee, J. Luo, M.-K. Son, P. Gao, K. T. Cho, J. Seo, S. M. Zakeeruddin, M. Grätzel and M. K. Nazeeruddin, Adv. Mater., 2016, 28, 3966–3972 CrossRef CAS PubMed.
- M. Liu, M. Li, Y. Li, Y. An, Z. Yao, B. Fan, F. Qi, K. Liu, H.-L. Yip, F. R. Lin and A. K.-Y. Jen, Adv. Energy Mater., 2024, 14, 2303742 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta01039f |
| This journal is © The Royal Society of Chemistry 2024 |