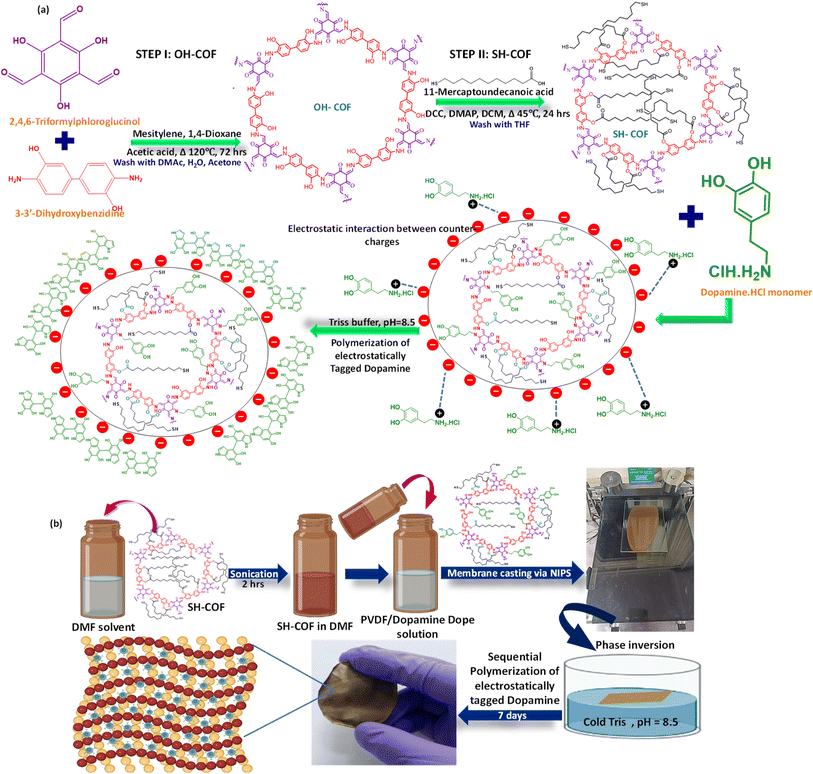
Tuning the surface charge and pore size of IPNs arrests covalent organic nanostructures through in situ exchangeable bonds for the removal of persistent contaminants†
Ria
Sen Gupta
 a,
Sk. Safikul
Islam
a,
Sk. Safikul
Islam
 a,
Amit
Malakar
a,
Tridip
Das
b and
Suryasarathi
Bose
a,
Amit
Malakar
a,
Tridip
Das
b and
Suryasarathi
Bose
 *a
*a
aDepartment of Materials Engineering, Indian Institute of Science, Bengaluru–560012, India. E-mail: sbose@iisc.ac.in; Fax: +91-(0)80-2360 0472
bMaterials and Process Simulation Centre, California Institute of Technology, Pasadena, California – 91125, United States
First published on 19th June 2024
Abstract
Covalent organic frameworks (COFs) have proven to be a wonderful material for water remediation, but their stability has been a long-standing challenge. Herein, to address this problem, a novel ‘giant’ COF containing thiol group (SH-COF) was synthesized and infused into a sequential interpenetrating polymeric matrix through the formation of exchangeable imine bonds in situ. This COF-tagged IPN membrane, besides enhancing its stability, served as a versatile platform for membrane engineering, particularly for efficient interaction with heavy metals such as mercury. Moreover, the in situ formation of dynamic bonds endowed the membrane with recyclability, a focal point of current membrane research, which is not amenable using the conventional routes of introducing COFs on thin-film composite membranes. The structure of the synthesized SH-COF was characterized using DFT simulations to gain insights from a fundamental perspective. The SH-COF inclusion within the membrane structure not only reduced the pore size but also facilitated the incorporation of significant numbers of charge-carrying centers. These engineered membranes exhibited high and sustained water flux for 3 weeks, along with enhanced separation capabilities for dyes (>99%), antibiotics (>97%), and monovalent salts (>98%). Thiol groups facilitated effective mercury removal (up to 97%), while the hydrophilic surface maintained antifouling properties and tolerance to chlorine. Importantly, these membranes are non-cytotoxic and re-processable, making them promising candidates for advancing sustainable water treatment technologies. This research has the potential to address the adverse effects of microplastic pollution resulting from inadequate membrane disposal practices. Furthermore, it presents a timely solution for the development of environmentally friendly and sustainable membranes.
Introduction
Water contamination and water scarcity have been a burning issue that has plagued the global population for many years and would continue to do so for many more centuries to follow.1 Improper and inadequate sanitation has exposed nearly 2.4 billion people to the aftermath of waterborne diseases.2–4 According to official statistical records, nearly two million children die every year from diarrheal diseases alone. With the ever-increasing global population and expanding anthropogenic activities, the universal demand for freshwater has increased manifold, thus exacerbating the scarcity of this fundamental resource. From this perspective, the importance of adopting measures for sustainable water treatment has reached a climax.4,5Membrane separation has emerged as a viable and promising technology to overcome water scarcity issues in the most efficient and affordable manner owing to its scalability and ability to target a wide range of hazardous contaminants.6,7 However, most commercial membranes suffer from fouling and mismanagement towards their end of life.8,9 Both the aforementioned issues hamper membrane performance and sustainability, thus generating huge amounts of membrane wastes with little or no know-how about their effective disposal measures.
To this end, the use of dynamic covalent bonds (or covalent adaptable networks, i.e. CANs) in situ in the membrane matrix can offer an edge in terms of thermal stability and recyclability.10,11 Recycling and reusing the fouled and used polymeric membranes offer opportunities for extending their longevity, thus ensuring sustainability. Additionally, to increase the mechanical robustness of the fabricated membranes, these dynamic bonds or exchangeable bonds should be amalgamated with IPNs or interpenetrating polymeric networks. Thus, within the realm of robust recyclable membranes, we studied the amalgamation of interpenetrating polymeric network-based membranes infused with a novel covalent organic framework (COF).12–14
The synergistic combination of IPNs, CANs and COFs has the potential to yield membranes with highly robust and mechanically stable architecture, exceptional selectivity and adsorption, and ample recycling opportunities. COFs are known to possess highly porous and crystalline structures,15–19 and upon combining them with IPNs in a mixed-matrix pathway, a hybrid material is formed, which enables precise tuning of pore sizes and surface chemistries, thus effectively targeting contaminants.20,21 In this work, the COF precursors and IPN components were chosen selectively, so that they could undergo specific covalent (imine bonds) as well as electrostatic interactions (between the counter charged COF and the IPN component), thus promoting the in situ formation of some exchangeable bonds22,23 that can undergo exchange reactions under the specified reaction parameters without causing any variation in the porous architecture of the membrane.
Moreover, the COF moieties were endowed with thiol functionalities and hence their inclusion into the IPN matrix can facilitate the removal of toxic heavy metals such as mercury from water due to the high affinity of complexation between thiol and Hg(II) ions.24,25 Mercury removal is of utmost importance since it has already been named a “chemical of concern” by WHO right in 2017.26,27 It has the ability to bioaccumulate through food chains and create havoc in the human ecosystem, and is thus regarded as a global threat with the Environmental Protection Agency (EPA) of the United States setting an upper limit of 2 ppb of mercury in potable water.28–31
For the fabrication of these membranes, a novel thiol COF was synthesized in a two-step pathway. In the first step, a hydroxyl-terminated COF was designed and subsequently thiol groups were introduced via simple chemical modification. The SH-COF was carefully synthesized so that it can have selective interaction with the dopamine monomers of the IPN system. This COF had a two-way interaction with the dopamine moieties. On the one hand, some of the SH-COF moieties got directly attached to some of the dopamine molecules via a Schiff-base interaction, leading to the formation of the exchangeable imine bonds. On the other hand, the inclusion of SH-COFs into the matrix was tactfully done, so that the molar ratio of dopamine monomers is always higher than that of the SH-COF. This ensured the presence of unexhausted dopamine monomers, which, being positively charged, can undergo electrostatic tagging with the SH-COF moieties that are already in conjugation with the exhausted dopamine monomers via an imine linkage. The dopamine monomers which experience electrostatic conjugation are left to be polymerized upon availability of suitable polymerization conditions.
Now, during the membrane fabrication step, the in situ sequential polymerization of the unexhausted yet electrostatically tagged dopamine monomers occurs and with it the SH-COF-tagged dopamine molecules get evenly dispersed within the entire membrane matrix. Thus, the matrix gets populated with the imine linkages. Additionally, these SH-COF-dopamine conjugates help in reducing the pore sizes of the membrane due to the large framework architecture and also act as effective charge carrying centres, which endow the membrane with high amounts of negatively charged interfaces, thus facilitating efficient separation performance.
In this study, we put forward the facile fabrication of a novel thiol-functionalised COF and have embedded it into an IPN matrix via the formation of imine bonds, thus generating a recyclable membrane. Such incorporation of exchangeable bonds into the fabrication process itself enhances the membrane's ability to contribute towards a world with lesser carbon footprint generation augmenting closed-loop circular economy. The membranes with thiol functionalization demonstrated high percentages of mercury removal along with other hazardous contaminants including salts, dyes, antibiotics and microplastics. The membranes' compatibility with biological systems was determined by the popular MTT assay. The unique combination of CANs, IPNs and thiol-functionalised COFs represents a promising approach for creating advanced membranes in the near future. These membranes seamlessly integrate strength, resilience, and eco-friendliness, offering a potential blueprint for crafting high-efficiency, environmentally conscious membranes that greatly minimize waste generation.
Results and discussion
‘Jammed network’ in COF-infused IPN membranes: controlling both pores and charges
SC-IPN membranes were synthesized using engineered concepts of IPNs and in situ exchangeable bonds. A novel thiol-functionalized covalent organic framework was fabricated via functionalizing a hydroxyl-terminated COF using esterification chemistry. The final framework could act as a platform to interact swiftly with toxic heavy metals such as mercury. Additionally, exchangeable bonds, generated in the system in situ, provided the membranes with a whip hand over the commercial counterparts. Here the SH-COF molecules were selectively taken in a molar ratio less than that of the dopamine molecules. In doing so, the SH-COF got covalently anchored to some of the dopamine monomers via formation of an imine linkage (exchangeable bonds or covalent adaptable networks) and simultaneously experienced electrostatic interactions with the unexhausted dopamine monomers, which are counter charged to that of the SH-COF moieties. This mixture was introduced into a PVDF dope solution and later cast via the well-known NIPS (Non-Solvent Induced Phase Separation) technique. Upon availability of suitable polymerization conditions, only the electrostatically tagged SH-COF-dopamine molecules polymerized and resulted in a jammed network-like structure. The dopamine molecules which underwent electrostatic interactions with SH-COF-covalently attached dopamine molecules helped in homogeneously dispersing the tagged moieties, and hence distributed the imine bonds throughout the membrane matrix and also inside the pores. Scheme 1 illustrates the synthesis pathway of the hydroxyl COF and its modification yielding the SH-COF. It also involves the membrane fabrication steps and the changes undergone during the entire process. The membranes were endowed with high amounts of negative charges and small pore sizes (enabled by the presence of the large framework structure of the SH-COF moieties), which helped in achieving desirable separation performance. Moreover, the presence of exchangeable covalent adaptable bonds helped in sustaining the mechanical as well as characterizational features over repeated phases of recycling.Fundamental understanding of the designer COF using density functional theory
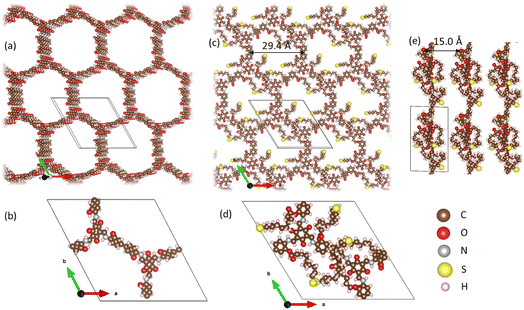
First, the fabricated OH-COF and the novel SH-COF structures were predicted using Density Functional Theory (DFT). DFT calculations were carried out using VASP 5.4.4. The specifics of the computational setup are outlined in the ESI Section (1).† DFT was used to minimize the structure of OH-COFs (Fig. 1a). DFT minimization resulted in a triclinic unit cell with cell dimensions a = 2.86, b = 2.93, and c = 0.46 nm, and α = 79, β = 109, and γ = 120° (Tables 1 and 2 in the ESI Section† provide the unit cell lattice parameters). The OH-COF unit cell was then expanded in the z-direction and thiol ligands (i.e. 11-mercaptoundecanoic acid chains) were attached with full extension. Subsequently, molecular dynamics (MD) minimization was run with Avogadro software32 while modifying the OH-COF moieties to SH-COF. After MD minimization with the universal force field33 thiol ligand chains got shortened and this structure of the SH-COF was found to be energetically favourable over straightened thiol chains after DFT minimization. The final structure of SH-COF was observed to be very close to a hexagonal symmetry with a ≈ b = 2.98, c = 1.5 nm (details of the lattice parameters are provided in the ESI Section†).Characterizing the membranes infused with the designer COF
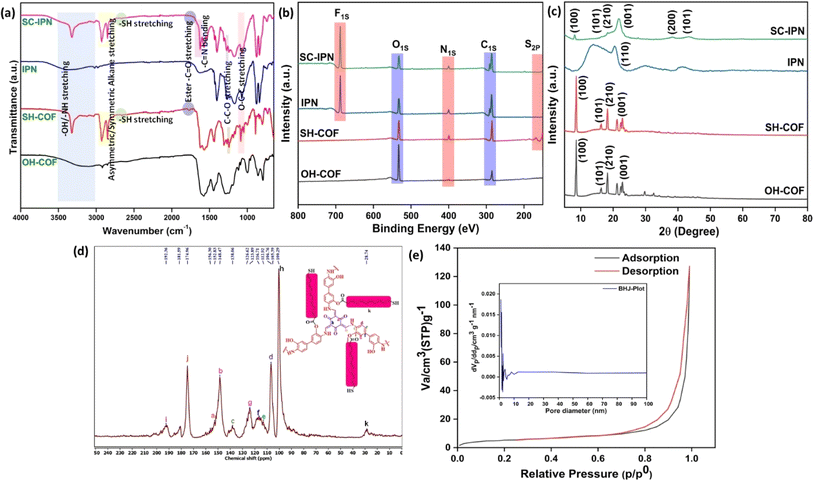
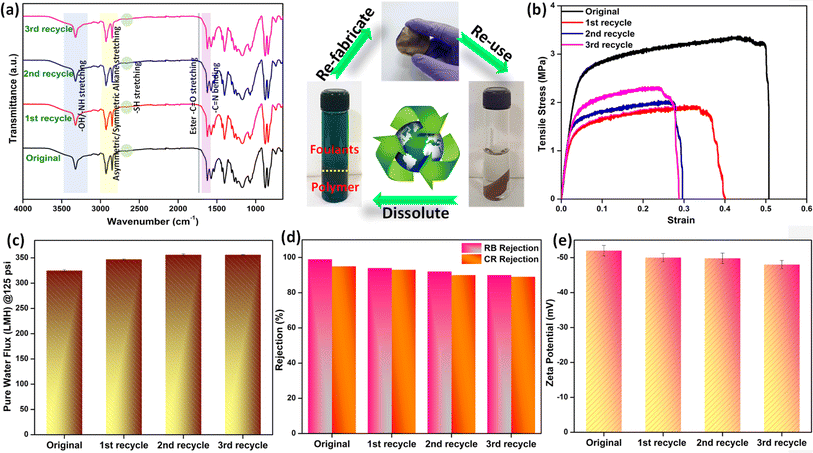
A range of characterization analyses were performed to ascertain the successful synthesis of the OH-COF and the SH-COF particles along with the designed SC-IPN membranes. Intensive characterizations had to be done to elucidate the chemical structure of the novel SH-COF. FTIR studies were the most preliminary technique used to confirm the successful synthesis of the novel covalent organic framework (Fig. 2a). It was observed that the OH-terminated COF particles had characteristic peaks of –OH, –NH, –C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations and C
O stretching vibrations and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending vibrations at around 3200 (broad hump), 3000, 1270, 1650, and 990 cm−1, respectively. Upon modifying the OH-COF with 11-mercaptoundecanoic acid for the formation of the thiol-terminated COF, two new peaks corresponding to the symmetric and asymmetric stretching vibrations of alkanes emerged at around 2920 and 2854 cm−1,34,35 indicating the successful incorporation of the thiol acid into the OH-COF architecture that resulted in the formation of the SH-COF. Additionally, the presence of ester bonds, i.e., C
C bending vibrations at around 3200 (broad hump), 3000, 1270, 1650, and 990 cm−1, respectively. Upon modifying the OH-COF with 11-mercaptoundecanoic acid for the formation of the thiol-terminated COF, two new peaks corresponding to the symmetric and asymmetric stretching vibrations of alkanes emerged at around 2920 and 2854 cm−1,34,35 indicating the successful incorporation of the thiol acid into the OH-COF architecture that resulted in the formation of the SH-COF. Additionally, the presence of ester bonds, i.e., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–C–O, and O–C–C stretching peaks at around 1750, 1236 and 1066 cm−1 respectively indicates bond formation between the OH-terminated COF and the thiol acid. Furthermore, the presence of a thiol –SH stretching peak at around 2658 cm−1 was proof enough for the successful SH-COF formation. Interestingly, the emergence of a sharp peak at ∼3300 cm−1 in the spectra of SH-COF could be ascribed to the secondary amine –NH stretching vibration. In the SC-IPN membranes, apart from the regular peaks for neat IPNs,36 the retention of the SH-COF peaks along with the emergence of the sharp imine C
O, C–C–O, and O–C–C stretching peaks at around 1750, 1236 and 1066 cm−1 respectively indicates bond formation between the OH-terminated COF and the thiol acid. Furthermore, the presence of a thiol –SH stretching peak at around 2658 cm−1 was proof enough for the successful SH-COF formation. Interestingly, the emergence of a sharp peak at ∼3300 cm−1 in the spectra of SH-COF could be ascribed to the secondary amine –NH stretching vibration. In the SC-IPN membranes, apart from the regular peaks for neat IPNs,36 the retention of the SH-COF peaks along with the emergence of the sharp imine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N peak at 1630 cm−1 hinted at the covalent attachment of dopamine and SH-COFs.
N peak at 1630 cm−1 hinted at the covalent attachment of dopamine and SH-COFs.
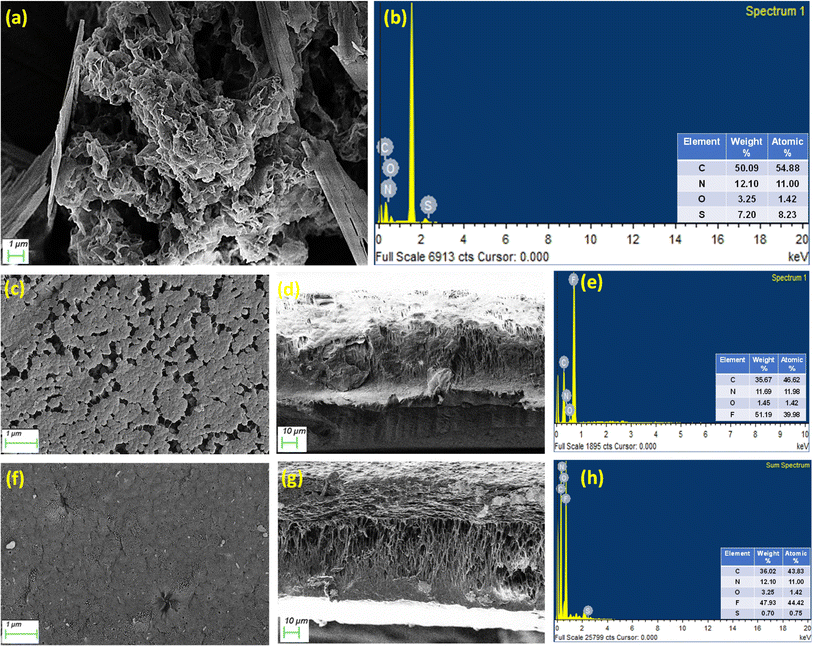
The FTIR findings were further substantiated using XPS. Fig. 2b presents the integrated survey scans of four representative samples, namely OH-COF and SH-COF particles, IPNs, and SC-IPN membranes, to assess their elemental compositions. Notably, the key distinction between OH-COF and SH-COF lies in the appearance of 2p sulfur in the SH-COF moieties. In the integrated survey scans, distinct peaks were observed at 401 eV and 688 eV, indicating the presence of N 1s and F 1s in IPN and SC-IPN samples, which suggests the successful incorporation of polydopamine and the presence of PVDF in both IPN and SC-IPN membranes. After deconvoluting the C 1s core spectra of the SH-COF (Fig. S1†), four distinct peaks were identified at approximately 284.84, 285.84, 286.93, and 288.66 eV. These peaks can be attributed to the presence of sp2/sp3 hybridized carbon, C–S/C–N, carbonyl C–O, and C–N–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively. The core C 1s spectra of the SC-IPN membrane (shown in Fig. S1†), along with the above-mentioned peaks, showed one additional peak at ∼290.68 eV for the –CF2 bond. The deconvoluted O 1s spectra of both SH-COF and SC-IPN exhibited distinct peaks at 532.76 and 532.58 eV, respectively. These peaks provide strong evidence for the presence of the ester bond on the surface of both materials and membranes. The deconvoluted S spectra analysis confirmed the presence of the SH (168.15 eV) bond in the SH-COF, and this SH bond was retained in the final SC-IPN membrane. Interestingly upon deconvolution of the N 1s spectra of IPN and SC-IPN, it was observed that in both cases, the presence of the N–H bond (399 eV) confirmed the polymerization of dopamine molecules, and hence, the hypothesis that the unexhausted dopamine molecules had ample scope for polymerization was proven to be correct.37–40
O bonds, respectively. The core C 1s spectra of the SC-IPN membrane (shown in Fig. S1†), along with the above-mentioned peaks, showed one additional peak at ∼290.68 eV for the –CF2 bond. The deconvoluted O 1s spectra of both SH-COF and SC-IPN exhibited distinct peaks at 532.76 and 532.58 eV, respectively. These peaks provide strong evidence for the presence of the ester bond on the surface of both materials and membranes. The deconvoluted S spectra analysis confirmed the presence of the SH (168.15 eV) bond in the SH-COF, and this SH bond was retained in the final SC-IPN membrane. Interestingly upon deconvolution of the N 1s spectra of IPN and SC-IPN, it was observed that in both cases, the presence of the N–H bond (399 eV) confirmed the polymerization of dopamine molecules, and hence, the hypothesis that the unexhausted dopamine molecules had ample scope for polymerization was proven to be correct.37–40
The crystalline nature of the materials was evaluated by XRD (Fig. 2c). The XRD pattern of OH-COF and SH-COF exhibited a prominent peak at ca. 8.1° (2θ), corresponding to the (100) planes, indicating a highly crystalline and periodic structure. Additional peaks were observed at 16.2°, 18.1°, and at ∼23° (2θ), corresponding to the (101), (210), and (001) planes, respectively.41 The plane (001) indicates the π–π stacking between COF layers. However, the IPN membrane revealed a prominent peak at approximately 20.56°, corresponding to the (110) plane, due to the semi-crystalline nature of PVDF. Subsequently, upon the addition of the SH-COF into the IPN matrix, the XRD pattern of the resulting SC-IPN membranes exhibited new peaks at 8.12°, 16.32°, 18.3°, 21.64°, 38.12°, and 43.26°, corresponding to the (100), (101), (210), (001), (200) and (101) planes for both the SH-COF and IPN components. This observation confirms the successful integration of the SH-COF into the IPN matrix, validating the formation of the desired composite architecture.
Solid-state 13C NMR spectroscopy was employed to investigate the atomic-level orientations in OH-COF and SH-COF frameworks. Fig. 2d displays the obtained spectra, along with the corresponding peak assignments. In the OH-COF (Fig. S2a†), a series of peaks were identified in the 107 to 138 ppm range, as well as at 152 ppm and 154 ppm, providing evidence for the formation of a hierarchical COF structure.42 Especially, post-esterification reactions in SH-COF induced the appearance of three new significant peaks at 28.74 ppm, 181.59 ppm, and 192.36 ppm, corresponding to aliphatic carbons (k), ester carbons (j), and aromatic carbons (i), respectively. These observations offer crucial insights into the structural changes and chemical transformations within the COF frameworks, paving the way for the design and development of advanced functional materials.
Finally, the porous nature of the OH-COF and subsequently SH-COF was analysed using BET. Fig. 2e illustrates the N2 adsorption–desorption curves of SH-COFs at a temperature of 77.35 K. For both the OH-COF (Fig. S2b†) and the SH-COF, adsorption curves exhibited typical IV isotherms, indicating their unique characteristics. In addition, the specific surface areas of OH-COF and SH-COF were calculated by the Brunauer–Emmett–Teller model, which were valued at 84 m2 g−1 and 160 m2 g−1, respectively. Additionally, the average pore diameters of OH-COF and SH-COF were determined to be 21 nm and 16 nm, respectively, using non-local density functional theory (NLDFT).43,44 The observed hysteresis loop shape could be attributed to capillary condensation within the mesoporous structures. The incorporation of the SH-COF in the IPN network is expected to lead to a reduction in pore size.
Improved stability of COF-infused IPN membranes
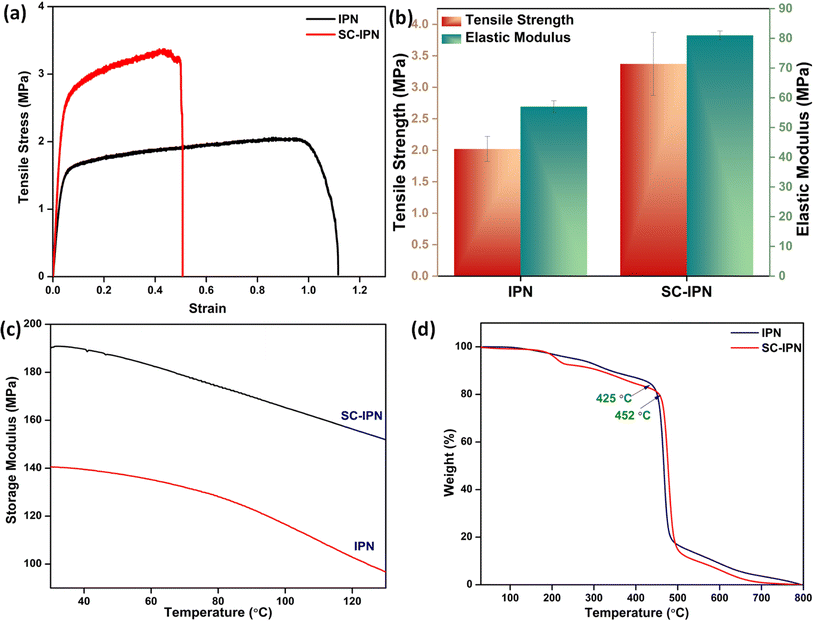
The mechanical properties of the SC-IPN membranes were evaluated by UTM and DMA analysis. For performing the ultimate tensile strength test, a micro UTM machine was employed, and a uniaxial tensile test was undertaken. In accordance with the ASTM D882 standard, the samples of SC-IPN membranes and neat IPN membranes were prepared (Fig. 3a). A preload of 0.1 N was applied and the loading rate was set at 10 mm min−1. Although the neat IPNs demonstrated more toughness, the addition of the SH-COF into the IPN matrix increased the stiffness and led to a considerable reduction in toughness.45,46Additionally, from the slope of the Hookean region of the stress vs. strain graph, elastic modulus was evaluated (Fig. 3b). These results further corroborated the findings, where the presence of SH-COF moieties was found to enhance the modulus as well as the overall tensile strength when compared to the neat IPN membranes. A probable reason for such an observation could be the fact that the SH-COF moieties acted as nanofillers and effectively filled up the available free volume present in the IPN matrix, thus augmenting chain rigidity. Furthermore, this stiffness was further aided by the secondary interactions (polar–polar, π–π, etc.) between the IPN chains and the SH-COF moieties.43
DMA studies were performed to further ascertain the UTM findings (Fig. 3c). The incorporation of the SH-COF into the IPN matrix was found to enhance the storage modulus of the SC-IPN membranes drastically. The SC-IPN membranes exhibited a storage modulus of 190 MPa as compared to the 140 MPa for the neat IPN matrix. Such enhancement can be solely ascribed to the presence of SH-COF moieties because of their high structural integrity.19
Now, in order to gauge the thermal stability of the SC-IPN membranes, TGA analysis was performed (Fig. 3d). It was observed that the neat IPN membranes had a 2-step degradation profile. The first degradation temperature (200–300 °C) could be ascribed to the degradation of the catechol groups present in the IPN architecture and the second degradation step (300–500 °C) was due to the presence of the alkyl spacers in polydopamine.47 On inspecting the SC-IPN membranes, a 2-step degradation profile was achieved. However, the first degradation step is a bit more prominent owing to the combined effect of catechol degradation and framework decomposition.48,49 The second step could again be ascertained by the large alkyl spacers present in the SH-COF moieties (Fig. S3†).50 On closer evaluation, it was observed that the final degradation temperature of the SC-IPN membranes was nearly 30 °C greater than that of the neat IPNs. Thus, it can be rightfully inferred that the covalent inclusion of the SH-COF species significantly helped in enhancing the thermal stability of the membranes.
Surface topology and charge of the designer membranes
The surface charge and nature of the membrane surface are the two key players involved in determining the actual applicability of the membranes in water remediation. In this perspective, the water contact angle is a direct indicator of the nature of the membrane surface since its absolute numerical value decides whether a membrane is hydrophobic or hydrophilic in nature. A membrane, if hydrophilic, is deemed to be non-fouling or resistant to fouling issues. This is due to the fact that hydrophilic surfaces quickly form a thick hydration layer on their surface, and hence, the deposition of unwanted foulants becomes increasingly difficult.51 Water contact angle values lying in the range of 0–90° indicate the presence of a hydrophilic surface, whereas anything beyond 90° indicates a hydrophobic surface. When measured using a contact angle goniometer, the SC-IPN membranes exhibited values in the range of 40 ± 2° as compared to the 68° of the neat IPN membranes (Fig. 4a). This shift towards the more hydrophilic spectrum could be ascribed to the enhancement of hydrophilic functional groups in the system coming from the dopamine-tagged SH-COF and polydopamine molecules. The hydrophilic nature of the membrane can be further corroborated by the fact that the water uptake of the SC-IPN membranes increased to 93% as compared to 85% of neat IPN membranes.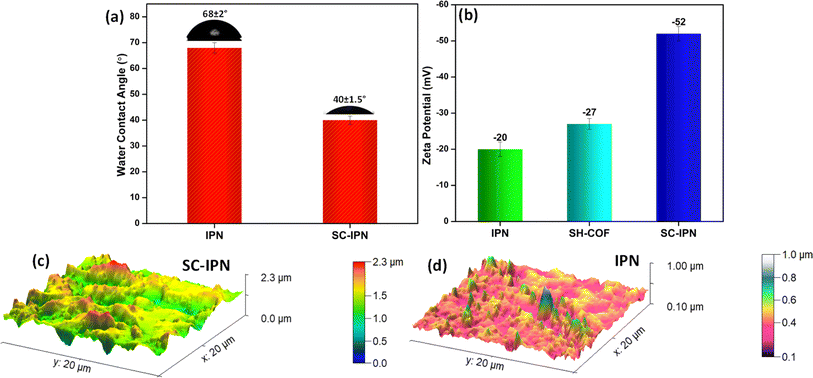 | ||
| Fig. 4 Surface characteristics. (a) Water contact angle of the IPN and SC-IPN membranes. (b) Zeta potential of the membranes and SH-COF particles. (c and d) Surface roughness analysis by AFM. | ||
Additionally, from the AFM images of the SC-IPN membranes, it was observed that the RMS (Sq) roughness values rose to nearly 250 nm, which was much higher than the 114 nm for the neat IPN membranes. This increased roughness and low water contact values further indicate a better wettability of these membranes (Wenzel theory) (Fig. 4c, line profile in S4†).12
The surface charge of the membranes was quantified from zeta potential measurements, using the Helmholtz–Smoluchowski equation,52 which is a direct indication of the interfacial interactions between the membrane and the solvent interface. The membrane charge is a crucial feature for deciding the separation efficiency of the membranes. The zeta potential of the SC-IPN membranes was found to be around −52 mV, which is much higher than that of the neat IPN membranes (Fig. 4b). This drastic improvement or increment in the zeta potential values can be ascribed to the presence of polar groups in the SH-COF. Thus, the SC-IPN membranes containing large amounts of dopamine-tagged SH-COFs and polydopamine moieties could serve as charge-carrying centres and, thus, significantly augment the rejection efficacies of the membrane.
After probing the surface charge and nature, the changes in the membrane pore and surface morphology were analyzed using SEM images and EDAX spectra. The variations brought about by the incorporation of SH-COF moieties were duly studied. It was observed that the SH-COF particles exhibited a combination of flower and ribbon-like architecture. Upon its incorporation, the membrane surface underwent changes with respect to the surface coverage, where the SC-IPN membranes were found to densely occupy the entire matrix, which could be ascribed to the presence of the framework designs of the SH-COF moieties. The pore sizes, as estimated using ImageJ, revealed the pore sizes in the nanometric regime, which were drastically reduced as compared to the neat IPN membranes (300–500 nm).36 This was ratified via pore size distribution measurements in the ESI Section (Fig. S5).† This observation again could be explained by the presence of the large SH-COF moieties, whose presence in the membrane pores led to significant size reduction. From the cross-sectional morphology, a combination of macro voids and network-like structures was revealed, owing to the reduced control over the distribution of pore sizes in the NIPS technique. Such morphological feature is a characteristic of IPNs. Additionally, from the EDAX spectra, the successful inclusion of SH-COF into the IPN matrix was confirmed. The presence of the sulphur peak, along with increased intensities for carbon, nitrogen, and oxygen (as compared to neat IPN membranes), was proof enough for the formation of SC-IPN membranes (Fig. 5). The SEM images and EDAX spectra of the OH-COF moieties are also provided in the ESI Section (Fig. S6).†
Delving deeper into ‘giant COF nanosheets’-controlled pore size in IPN membranes
The porosity of the membranes was measured by the gravimetric method, and it could be directly correlated with the % uptake of the membranes. Using the gravimetric equation and substituting the density of polymers with that of PVDF density (1.78 g cm−3) (since PVDF occupied the major portion of the membrane matrix), the porosity of the membranes was found to be around 0.61 or 61%. Taking a cue from the porosity measurements, the Guerout–Elford–Ferry equation was also employed to calculate the mean pore radius of the membrane in nanometres. The results indicated the average radius to be around ∼2 nm. To calculate the pore size distribution, SEM micrographs were taken from various sections of the membrane, and around 100 pore diameters were measured using the ImageJ software. A histogram employing eqn (3) (provided in the Experimental section of ESI)† was plotted, which deliberates on the pore size data collected. The average pore diameter was found to be around 1.72 nm (Fig. 6a). These observations were further validated by nitrogen adsorption–desorption evaluation (Fig. 6b). According to the IUPAC nomenclature, the isotherm generated for the SC-IPN membrane demonstrated a type IV pattern and had a recognizable hysteresis loop in the multilayer range. Interestingly, the experiments showed that the SC-IPN membrane exhibited pores with an average diameter of 1.7 nm. Consequently, the resulting porous SC-IPN membrane, boasting π-conjugated skeletons, remarkable pore size, and permanent porosity, hold significant promise for advancing the development of next-generation membranes for precise molecular sieving applications. These findings pave new pathways for constructing highly efficient and selective membranes with immense potential in diverse separation processes.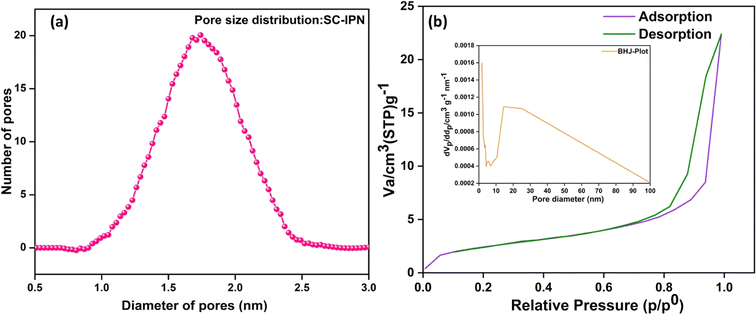 | ||
| Fig. 6 Pore size analysis. (a) Pore size distribution by imaging 100 pore diameters using SEM. (b) N2 adsorption–desorption isotherm for the pore size evaluation of SC-IPN membranes. | ||
Evaluating the membrane performance
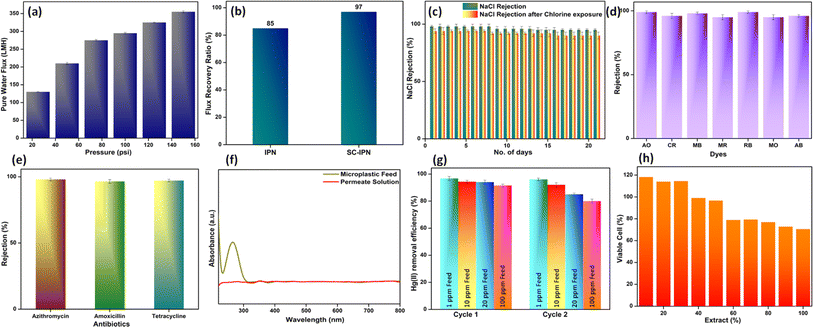
Effective filtration performance can be ideally optimized by fine-tuning a thin, highly selective, and mechanically robust polymeric membrane. The highly homogeneous distribution of the covalently attached SH-COF nanosheets enabled via ultrasonication, stirring, and consequent blending with the compatible IPN matrix helps in bringing together a system that is endowed with enhanced transport efficiency and selectivity.To realize the practical applicability of the water-remediating membranes, water permeation through the membrane under the influence of transmembrane pressure plays a major role. More importantly, with the lurking trade-off between high selectivity and permeability, the quantification of water permeation or water flux, in general, assumes great importance. The SC-IPN membranes were tested under operating pressures ranging between 25 psi and 150 psi (Fig. 7a). The absolute values of pure water flux demonstrated a proportional increment with transmembrane pressure (130 LMH at 25 psi, 210 LMH at 50 psi, 275 LMH at 75 psi, 295 LMH at 100 psi, 325 LMH at 125 psi, and 355 LMH at 150 psi). Such increment can be directly ascribed to the changes in the pore architecture, which are caused by the changing transmembrane pressure. Moreover, the exfoliation of the SH-COF nanosheets (via ultrasonication)53,54 before incorporation into the IPN matrix ensured the successful elimination of non-covalent interactions between the nanosheets, which, in turn, further corroborated the homogeneous distribution of the high-aspect ratio nanosheets having shorter diffusion pathways. Such an arrangement allowed for enhanced transportation efficiency within the framework structure.20,55 However, the flux values were drastically reduced when compared with the neat IPN membranes (900 LMH at a transmembrane pressure of 50 psi). This was further suggestive of the possible pore size reduction caused by the introduction of the COF frameworks into the IPN matrix.
The stability of the membranes against high operating pressures was ascertained by continuing the water flux experiments for a sustained duration of 3 weeks (Fig. S7†). At a transmembrane pressure of 125 psi, the pure water flux values were found to deviate negligibly, thus confirming the longevity of the membranes.
Immediately after the quantification of pure water flux, the feed was replaced with a 1000 ppm solution of BSA, which served the purpose of model protein foulant. This test is essentially crucial for gauging the antifouling performance of the fabricated membranes. Commercial state-of-the-art membranes suffered from the issues of fouling and cake formation, which impedes their performance. In this work, it was observed that the fabricated SC-IPN membranes had a flux recovery ratio of 97%, which was higher than the 85% of neat IPN membranes (Fig. 7b). These augmented antifouling features could result from a synergism of high hydrophilicity and surface charge of the SC-IPN membranes. With a very low water contact angle, i.e., 40°, the membranes augmented the formation of an almost impermeable and thick hydration layer, which prevented the BSA foulant molecules from settling onto the membrane surface, thus bypassing the cake formation process. Additionally, the exceptionally high negative zeta potential values of the SC-IPN membrane surface further ensured the efficient elimination of negatively charged BSA molecules.
After successful evaluation of the water transport properties, the designed membranes were tested for effective ion rejection. The presence of polydopamine and SH-COF-tagged dopamine aided the generation of massive charge-carrying centres. The charge-carrying capacity of the membranes was adjudged from the zeta potential values. The high amounts of charges could be a direct reflection of the presence of highly charged nanochannels of the SH-COF moieties along with the membrane surface, which was highly populated with negative charge-carrying functional groups. Feed solutions having 2000 ppm of monovalent NaCl salt were used for conducting the rejection experiments (Fig. 7c). NaCl was chosen as the model salt owing to the fact that its ions (i.e., Na+ and Cl−) possess the least possible hydrated radius, and thus its rejection would inherently qualify for the efficient removal of other divalent and monovalent salts. The SC-IPN membranes could successfully reject nearly 98% of NaCl salt. The salt rejection efficiency was validated by continuing the evaluations for a period of 21 days. Even after such continuous operational cycles, the salt rejection efficiency was maintained at about 95%. A probable mechanism for such high rejection values could stem from the high surface charge and the small pore sizes of the membranes, along with the charges present in the small nanochannels of the SH-COF moieties.56 All such factors worked in tandem to yield such rejection values. In general, from the perspective of charge exclusion sieving mechanism, the Cl− ions experienced heavy repulsive forces from the negative charge carrying centers of the membrane, and the Na+ ions, however, faced cation–π interactions with the sp2 hybridized domains of the dopamine-tagged SH-COF moieties.57,58 Thus, an effective interplay of charge and size-based interactions aided the rejection performance while retaining electrical neutrality on both sides of the SC-IPN membrane.
Since most membranes are subjected to cleaning treatments using bleach, the chlorine tolerance is a very important parameter to judge the stability of the membranes under exposure to harsh chemical environments. The membranes capable of withstanding chlorine attacks without incurring much damage to their inherent architecture are indeed suitable for real-time deployment. In addition, the ones whose structure deteriorates under chlorine exposure call for periodic replacements, thus increasing operational costs and decreasing sustainability. The SC-IPN membranes were exposed to 2000 ppm of NaOCl solutions for a substantial period and were again put to test with respect to their salt rejection performance. The lesser the deviation in the salt rejection efficiency before and after the chlorine treatment, the more stable the membrane. The SC-IPN membranes retained their NaCl rejection with an efficiency of 94% even after withstanding NaOCl treatment. The slight deviation in the value can, however, be ascribed to the presence of some secondary amines in polydopamine moieties (Fig. 7c).
The separation efficiency of the membranes in terms of charge-based and pore-based sieving was further evaluated using a series of organic dyes and antibiotics. Organic dyes are usually released into water streams from industrial effluents, and as they are highly toxic, they have the potential to wreak havoc in the ecosystem. Both cationic and anionic dyes were used as model foulants, and an accelerated concentration of 20 ppm was used for the evaluation studies. The SC-IPN membranes could reject nearly 99% of the cationic dyes and nearly 95% of the anionic dyes. The rejection efficacies were evaluated using a UV-vis spectrophotometer. The mere absence, presence, or intensity reduction of the dye's absorption maxima in the UV spectra dictated the separation performance of the SC-IPN membranes (Fig. S8†). The selectivity of the membranes in removing the cationic dyes can be explained by the highly negative zeta potential values of the SC-IPN membranes (ca. −52 eV). The cationic dyes (i.e., Acrydine Orange, Methylene Blue, and Rhodamine B) suffered from strong electrostatic attraction and were hence strongly adsorbed onto the membrane surface, thus accounting for their enhanced removal efficiency. The anionic dyes (i.e., Congo Red, Methyl Red, Methyl Orange, and Amido Black) being co-charged faced steric hindrance and electrostatic repulsion and, thus, could not make their way into the permeate side (Fig. 7d). The membranes could be reused via simple water backflushing, and they were found to maintain their rejection efficiency for a minimum of 15 operational cycles (Fig. S9†).
With the ever-increasing deterioration in human health owing to various factors, including the current pandemic situation, the use of antibiotics continues to remain uphill. Containment of most diseases requires the use of these medicines, and being non-biodegradable in nature, they tend to remain in the eco cycle for many years. However, beyond a certain prescribed limit, their presence in water bodies, even at parts per billion levels, leads to the production of resistant superbugs. Thus, the SC-IPN membranes were also evaluated for their antibiotic removal efficiency keeping in mind the current scenario of indiscriminate antibiotic use. Most antibiotics such as negatively charged Amoxicillin and neutral Azithromycin, and Tetracycline were used as model foulants. The SC-IPN membranes could effectively remove nearly 97–98% of all the model antibiotics (Fig. 7e). This observation could be explained by the fact that the removal of the neutral antibiotics, viz. Azithromycin and Tetracycline, was driven by the small pore size of the membrane, and the negatively charged antibiotic removal was due to the electrostatic interplay wherein the Amoxicillin moieties suffered heavy repulsion from the high negative charge centers of the membrane. The UV-vis spectra for the feed and permeate of the antibiotics are given in Fig. S10.†
The pore size-based screening of the membranes was further ratified by their microplastic removal studies. Microplastics have been emerging pollutants, with increasing consumer products in the market. These microplastics enter the water stream, and their removal becomes extremely tedious owing to their sheer size. Their dimensions range between 1 and 1000 μm, and hence, the SC-IPN membranes having much smaller pores can be potential candidates for the successful removal of these microplastics from the wastewater streams. As a representative of microplastic-contaminated water, PVC powder (from sanitary pipelines) (Fig. S11†) was spiked with water samples and used for the evaluations. The removal efficiency was measured using a dead-end setup. With the aid of DLS (dynamic light scattering) experiments, the sizes of particles present in the feed and permeate side were evaluated. Moreover, the EDAX mapping of the membranes was performed after microplastic removal (Fig. S12†). The DLS studies (Fig. S13†) were backed up by recorded the UV-vis spectra of the feed and permeate samples. It was observed that the permeate sample did not exhibit a prominent peak at 280 nm (Fig. 7f), which was present in the feed samples, indicating the effective removal of microplastics, as demonstrated by the SC-IPN membranes.59 The membranes maintained similar removal efficiencies for the next 10 operational cycles, with each cycle consisting of 100 ml of feed PVC-spiked water sample (Fig. S14†).
With the removal of salts, dyes, and antibiotics in place, the SC-IPN membranes were also tested for Hg(II) removal. Mercury removal has gained a huge worldwide impetus right from 1956.60,61 It can easily bioaccumulate and biomagnify in the food chain, thus producing detrimental effects. Battery industries continue to be the top contributor toward the release of mercury into water streams, and once released, they undergo various transformations, especially methylation. Methylation leads to the formation of highly toxic methylmercury, which is very much persistent in nature. Strict regulations have been imposed on the mercury concentrations in water, and hence, it is of utmost importance to find sustainable measures for mercury removal. To this end, the affinity between thiol and mercury ions is well established. Thiol functional groups, being soft ligands, easily react with soft Lewis acids, i.e., Hg(II), thereby forming strong complexes. Thus, thiol groups can act as a toolbox for the removal of mercury ions from wastewater streams. In this work, the synthesized covalent organic framework was carefully chosen and functionalized with thiol (–SH) groups, so that they can easily capture Hg(II) ions from the feed streams efficiently. Four different concentrations of HgCl2 solutions were used as the model heavy metal foulants (1, 10, 20, and 100 ppm, respectively), and the SC-IPN membranes were used to evaluate Hg(II) removal efficiency. EDAX elemental mappings of the SC-IPN membranes indicated the presence of Hg(II) on their surface after the removal studies were performed, thus confirming successful Hg(II) adsorption (Fig. S15†). The concentration of the corresponding permeate solutions was tested by ICP-OES (Fig. S16†). It was observed that the membranes could efficiently remove up to 97% of Hg(II) from water (Fig. 7g). The concentration of Hg(II) ions in the feed solution was found to have a deep impact on the removal efficiency. Moreover, from the FTIR spectra of the SC-IPN membranes after Hg(II) removal studies (Fig. S17†), it was observed that the absence of the thiol –SH peak in the spectra strategically corroborated the formation of complexes between the thiol groups and the incoming Hg(II) ions from the feed side, providing further validation. Notably, the removal efficiency was significantly influenced by the concentration of Hg(II) ions in the feed solution, with higher concentrations leading to increased utilization of complexation sites and diminished rejection efficiency. The higher the concentration of Hg(II) (100 > 20 > 10 > 1 ppm), the greater the number of complexation sites used up and the lower the rejection efficiency.
Toxicity assessment and sustainability
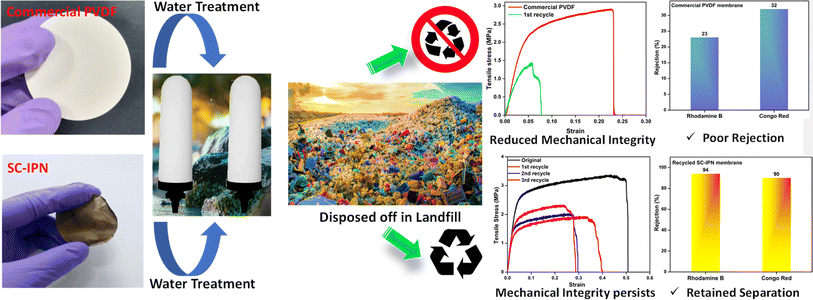
Water-remediating membranes are supposed to adhere to the established prerequisites set for systems compatible with mammalian cell lines since these membranes come in direct contact during real-time use. Thus, the substances with fundamental toxicity are deemed unsuitable for water purification applications. As a result, evaluating the suitability of the developed system and comprehending the potential risks linked with implementing the created membrane heavily rely on conducting cytotoxic assessments using mammalian cell lines. To gauge the inherent compatibility or potential harm of the membranes towards cells, the MTT assay was conducted using L929 (NCCS/1469) cell lines. The metric employed to quantify the potential cytotoxic effects was the percentage of cell line viability. When the cell viability percentage drops below 70%, it is considered unfit and has potential cytotoxic effects. The SC-IPN membranes were found to demonstrate a viability percentage of 70.56% for the 100% test sample and the viability increased as the concentrations of the test samples were lowered (118% for the 10% test sample) (Fig. 7h and S18†). The presence of metabolically thriving and active cells was indicated by the steady increment of the cell viability%. This observation further established the fact that no leaching of the SH-COF moieties took place from the membrane matrix. In general, thiol is supposed to be highly toxic, and since these results validated the non-leaching phenomenon hinting at the obvious cytocompatibility feature, the potential use of these membranes was recognized for water treatment applications.After conducting the toxicity assessment of the fabricated membranes, rigorous efforts were made toward studying the sustainability of the fabricated membranes. Nowadays, most of the commercial state-of-the-art membranes are riddled and pestered with fouling issues, and once cake formation sets in, it becomes increasingly difficult to overcome the strong adhesive forces acting between the membrane and the foulants. This, in turn, gives a huge blow toward the sustainable use of the membranes and calls for regular replacements and replenishments, thus incurring greater operational costs and leaving behind a higher carbon footprint in the environment. As a remedy, sustainable membranes with scope for reuse and recycling have managed to become the research focus of the current century.62,63 A smart and easily adoptable practical technique for the reuse of membranes marks the global demand today. The presence of plenty of exchangeable imine bonds in the SC-IPN membrane structure and the ester bonds (high temperatures and long reaction times can call for catalyst-free transesterification)64 endow the SC-IPN membranes with a recyclable property. Imine exchange reactions usually proceed at temperatures lower than 100 °C, which leaves enough scope for imine exchange to occur while reprocessing the SC-IPN membranes since they were performed at a temperature of 140 °C.65–70 This phenomenon was further substantiated by dissolving the fouled SC-IPN membranes in a DMF solvent. The foulants such as dyes and salts could be easily recovered via solvent extraction (using DCM water mixture), and the dissolved polymer could be regained via solvent evaporation followed by membrane casting using the NIPS technique. The process was repeated for 3 cycles, and the chemical environment of the recycled membranes was evaluated by performing FTIR studies. It was observed that the recycled membranes did not show much deviation even after repeated recycling operations. The pure water flux seemed to increase negligibly, practically owing to the changes in the pore architecture. However, the rejection performance of the SC-IPN recycled membranes remained comparable to the neat ones, an observation that can be directly correlated with the unchanged zeta potential values. The mechanical properties of the recycled membranes were evaluated using tensile tests, and it was observed that after each cycle of reuse and refabrication, the strength decreased, thus making processing difficult after 3 cycles. Moreover, this refabrication process does not guarantee the maintenance of the IPN architecture, however providing a scalable pathway for sustaining the membrane use and reducing the carbon footprint generated once the used membranes are incinerated (Fig. 8).
In order to validate the importance of the exchangeable bonds in facilitating the recycling process, the same experimental procedure was repeated with fouled commercial PVDF membranes (containing no exchangeable bonds, Fig. 9). It was observed that although membrane re-casting was possible, the rejection efficiencies of the recycled membranes deteriorated significantly, and the mechanical integrity was also sacrificed heavily, which prevented re-processing and re-casting after the 1st cycle itself.
Conclusion
This study introduced a novel COF material synthesized specifically for water remediation applications. The methodology involved in fabricating a thiol-terminated COF (SH-COF) from a hydroxyl-terminated COF (OH-COF) involves a straightforward esterification reaction, ensuring reproducibility. By incorporating SH-COF moieties into the IPN dope solution, exchangeable imine bond formation occurs, imparting recyclable features to the designed membranes and addressing the gaps in membrane-based closed-loop economy.The inclusion of novel COF moieties enhances structural and thermal stabilities of the membrane, providing a toolbox (specifically, thiol functional groups) for efficient mercury removal. This addition also contributes to reduced pore sizes and highly charged surfaces, improving separation performance. Covalent attachment to dopamine molecules ensures negligible leaching of COF species, as confirmed by cytotoxicity assessments.
SH-COF moieties exhibit dual attachment to the IPN matrix via covalent and electrostatic interactions with dopamine molecules, resulting in a network architecture upon sequential polymerization. Surface hydrophilicity and electrostatic interactions drive enhanced separation performance. These membranes demonstrate long-term stable water permeation, and efficient dye, antibiotic, and mercury removal, while maintaining inherent chlorine tolerance and antifouling characteristics.
The presence of imine exchange bonds throughout the matrix aids in maintaining the mechanical integrity and performance over continuous recycling operations. In summary, these novel SH-COF-embedded IPN membranes hold promise for revolutionizing membrane technology, offering simple methods for regeneration and facilitating a transition from linear to circular approaches in product life cycles.
Experimental section
All the materials used and methods employed are thoroughly discussed in the ESI Section† of the article.Data availability
The data supporting this article are included in the manuscript and as part of ESI.† For data details contact E-mail: sbose@iisc.ac.in.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Sursayarathi Bose is thankful for the Swarnajayanti Fellowship from DST-SERB. Ria Sen Gupta and Amit Malakar are grateful to MHRD for the PMRF fellowship. Sk. Safikul Islam is thankful to SERB for the NPDF fellowship (PDF/2021/000629). The authors would like to acknowledge CENSE and IPC departments for the characterization facilities, the Material Analysis and Research Centre for BET measurements, Biotech Testing Services for the help with biological studies. Finally, the authors would like to express their gratitude to Ramaiah Advanced Testing Laboratory for helping out with the heavy metal analysis.References
- S. Olivera, H. B. Muralidhara, K. Venkatesh, V. K. Guna, K. Gopalakrishna and Y. Kumar, Carbohydr. Polym., 2016, 153, 600–618 CrossRef CAS PubMed.
- L. Lin, H. Yang and X. Xu, Front. Environ. Sci., 2022, 10, 880246 CrossRef.
- P. K. Samantaray, S. Baloda, G. Madras and S. Bose, J. Mater. Chem. A, 2018, 6, 16664–16679 RSC.
- S. Pathan, S. S. Islam, R. Sen Gupta, B. Maity, P. R. Reddy, S. Mandal, K. Anki Reddy and S. Bose, ACS Nano, 2023, 17(8), 7272–7284 CrossRef CAS PubMed.
- P. K. S. Mural, A. Banerjee, M. S. Rana, A. Shukla, B. Padmanabhan, S. Bhadra, G. Madras and S. Bose, J. Mater. Chem. A, 2014, 2, 17635–17648 RSC.
- R. Sen Gupta, P. K. Samantaray and S. Bose, ACS Omega, 2023, 8(28), 24695–24717 CrossRef CAS PubMed.
- E. van Voorthuizen, A. Zwijnenburg, W. van der Meer and H. Temmink, Water Res., 2008, 42, 4334–4340 CrossRef CAS PubMed.
- R. Zhang, Y. Liu, M. He, Y. Su, X. Zhao, M. Elimelech and Z. Jiang, Chem. Soc. Rev., 2016, 45, 5888–5924 RSC.
- W. Lawler, Z. Bradford-Hartke, M. J. Cran, M. Duke, G. Leslie, B. P. Ladewig and P. Le-Clech, Desalination, 2012, 299, 103–112 CrossRef CAS.
- T. Rasheed, S. Khan, T. Ahmad and N. Ullah, Chem. Rec., 2022, 22, e202200062 CrossRef CAS PubMed.
- B. Li, S. Wang, X. J. Loh, Z. Li and T.-S. Chung, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2301009120 CrossRef CAS PubMed.
- S. Dutta, R. S. Gupta, K. Manna, S. S. Islam and S. Bose, Chem. Eng. J., 2023, 145008 CrossRef CAS.
- A. Malakar, S. Mandal, R. Sen Gupta, K. Manna, S. Parasuram, A. Misra and S. Bose, SPE Polym., 2023, 4(3), 83–92 CrossRef CAS.
- S. Maiti, S. S. Islam and S. Bose, Environ. Sci.: Water Res. Technol., 2023, 9, 249–264 RSC.
- A. R. Bagheri, N. Aramesh, F. Sher and M. Bilal, Chemosphere, 2021, 270, 129523 CrossRef CAS PubMed.
- X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC.
- P. J. Waller, F. Gándara and O. M. Yaghi, Accounts Chem. Res., 2015, 48, 3053–3063 CrossRef CAS PubMed.
- S. Kandambeth, K. Dey and R. Banerjee, J. Am. Chem. Soc., 2018, 141, 1807–1822 CrossRef PubMed.
- S. Karak, S. Kandambeth, B. P. Biswal, H. S. Sasmal, S. Kumar, P. Pachfule and R. Banerjee, J. Am. Chem. Soc., 2017, 139, 1856–1862 CrossRef CAS PubMed.
- S. Wang, X. Wei, Z. Li, Y. Liu, H. Wang, L. Zou, D. Lu, F. H. Akhtar, X. Wang and C. Wu, Sep. Purif. Technol., 2022, 122004 CrossRef CAS.
- K. Duan, J. Wang, Y. Zhang and J. Liu, J. Membr. Sci., 2019, 572, 588–595 CrossRef CAS.
- C. J. Kloxin and C. N. Bowman, Chem. Soc. Rev., 2013, 42, 7161–7173 RSC.
- N. Mokhtari, M. M. Khataei, M. Dinari, B. H. Monjezi and Y. Yamini, Mater. Lett., 2020, 263, 127221 CrossRef CAS.
- M. S. Islam, R. J. Vogler, S. M. Abdullah Al Hasnine, S. Hernández, N. Malekzadeh, T. P. Hoelen, E. S. Hatakeyama and D. Bhattacharyya, ACS Omega, 2020, 5, 22255–22267 CrossRef CAS PubMed.
- D. Upreti, A. Rajendran, N. Lenka, R. Srivastava, R. S. Gupta, B. Maiti, S. Bose and T. U. Patro, Chem. Eng. J., 2023, 464, 142738 CrossRef CAS.
- Y. Li, T. Hu, R. Chen, R. Xiang, Q. Wang, Y. Zeng and C. He, Chem. Eng. J., 2020, 398, 125566 CrossRef CAS.
- L. Merí-Bofí, S. Royuela, F. Zamora, M. L. Ruiz-González, J. L. Segura, R. Muñoz-Olivas and M. J. Mancheño, J. Mater. Chem. A, 2017, 5, 17973–17981 RSC.
- Y. Tian, J. Cheng, X. Han, Y. Li, T. Yang, M.-L. Chen, J. Ma and J.-H. Wang, J. Anal. At. Spectrom., 2022, 37, 157–164 RSC.
- H. Bessbousse, T. Rhlalou, J.-F. Verchère and L. Lebrun, J. Membr. Sci., 2008, 325, 997–1006 CrossRef CAS.
- S. Jana, A. Saikia, M. Purkait and K. Mohanty, Chem. Eng. J., 2011, 170, 209–219 CrossRef CAS.
- A. M. Alosaimi, Polymers, 2021, 13, 2792 CrossRef CAS PubMed.
- M. D. Hanwell, D. E. Curtis, D. C. Lonie, T. Vandermeersch, E. Zurek and G. R. Hutchison, J. Cheminf., 2012, 4, 1–17 Search PubMed.
- T. Das, B. V. Merinov, M. Y. Yang and W. A. Goddard III, J. Mater. Chem. A, 2022, 10, 16319–16327 RSC.
- M. Šimšíková, M. Antalík, M. Kaňuchová and J. Škvarla, Appl. Surf. Sci., 2013, 282, 342–347 CrossRef.
- K. Bakhmutsky, N. L. Wieder, M. Cargnello, B. Galloway, P. Fornasiero and R. J. Gorte, ChemSusChem, 2012, 5, 140–148 CrossRef CAS PubMed.
- R. S. Gupta, N. Padmavathy, P. Agarwal and S. Bose, Chem. Eng. J., 2022, 136997 CrossRef.
- T.-Y. Kuo and Y.-C. Chung, Mater. Adv., 2021, 2, 5686–5690 RSC.
- D. N. Nguyen, U. Sim and J. K. Kim, Polymers, 2020, 12, 912 CrossRef CAS PubMed.
- L. C. Almeida, T. Frade, R. D. Correia, Y. Niu, G. Jin, J. P. Correia and A. S. Viana, Sci. Rep., 2021, 11, 2237 CrossRef CAS PubMed.
- R. Batul, M. Bhave, P. J. Mahon and A. Yu, Molecules, 2020, 25, 2090 CrossRef CAS PubMed.
- S. Chandra, D. Roy Chowdhury, M. Addicoat, T. Heine, A. Paul and R. Banerjee, Chem. Mater., 2017, 29, 2074–2080 CrossRef CAS.
- Q. Lu, Y. Ma, H. Li, X. Guan, Y. Yusran, M. Xue, Q. Fang, Y. Yan, S. Qiu and V. Valtchev, Angew. Chem., 2018, 130, 6150–6156 CrossRef.
- Z. Xu, Y. Liu, Z. Wu, R. Wang, Q. Wang, T. Li, J. Zhang, J. Cheng, Z. Yang and S. Chen, Chem. Eng. J., 2020, 387, 124071 CrossRef CAS.
- S. Karak, K. Dey, A. Torris, A. Halder, S. Bera, F. Kanheerampockil and R. Banerjee, J. Am. Chem. Soc., 2019, 141, 7572–7581 CrossRef CAS PubMed.
- M. Pantano, E. Missale, L. Gazzato, R. Pilot, F. Sedona, G. Speranza and M. Frasconi, Mater. Today Chem., 2022, 26, 101007 CrossRef CAS.
- Q. Fang, C. Sui, C. Wang, T. Zhai, J. Zhang, J. Liang, H. Guo, E. Sandoz-Rosado and J. Lou, Matter, 2021, 4, 1017–1028 CrossRef CAS.
- R. S. Gupta, S. Mandal, S. Arya, S. Dutta, K. Manna, S. S. Islam, S. Pathan and S. Bose, Chem. Eng. J., 2023, 461, 141949 CrossRef.
- M.-Y. Chao, W.-H. Zhang and J.-P. Lang, Molecules, 2018, 23, 755 CrossRef PubMed.
- H. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortés, A. P. Côté, R. E. Taylor, M. O'Keeffe and O. M. Yaghi, Science, 2007, 316, 268–272 CrossRef CAS PubMed.
- A. M. Evans, M. R. Ryder, W. Ji, M. J. Strauss, A. R. Corcos, E. Vitaku, N. C. Flanders, R. P. Bisbey and W. R. Dichtel, Faraday Discuss., 2021, 225, 226–240 RSC.
- P. K. Samantaray, R. Sen Gupta and S. Bose, Adv. Sustainable Syst., 2023, 2200385 CrossRef CAS.
- E. M. Egorova, Electrophoresis, 1994, 15, 1125–1131 CrossRef CAS PubMed.
- S. Wu, L. Qin, K. Zhang, Z. Xin and S. Zhao, RSC Adv., 2019, 9, 9386–9391 RSC.
- Z. Kang, Y. Peng, Y. Qian, D. Yuan, M. A. Addicoat, T. Heine, Z. Hu, L. Tee, Z. Guo and D. Zhao, Chem. Mater., 2016, 28, 1277–1285 CrossRef CAS.
- Y. Song, M. Wei, F. Xu and Y. Wang, Phys. Chem. Chem. Phys., 2019, 21, 26591–26597 RSC.
- X. You, L. Cao, Y. Liu, H. Wu, R. Li, Q. Xiao, J. Yuan, R. Zhang, C. Fan and X. Wang, ACS Nano, 2022, 16, 11781–11791 CrossRef CAS PubMed.
- F. Sheng, B. Wu, X. Li, T. Xu, M. A. Shehzad, X. Wang, L. Ge, H. Wang and T. Xu, Adv. Mater., 2021, 33, 2104404 CrossRef CAS PubMed.
- P. Sun, M. Zhu, K. Wang, M. Zhong, J. Wei, D. Wu, Z. Xu and H. Zhu, ACS Nano, 2013, 7, 428–437 CrossRef CAS PubMed.
- M. Hasan, R. Kumar, M. Barakat and M. Lee, RSC Adv., 2015, 5, 14393–14399 RSC.
- S. Hernández, M. S. Islam, S. Thompson, M. Kearschner, E. Hatakeyama, N. Malekzadeh, T. Hoelen and D. Bhattacharyya, Ind. Eng. Chem. Res., 2019, 59, 5287–5295 CrossRef PubMed.
- Y. Huang, J. R. Du, Y. Zhang, D. Lawless and X. Feng, Sep. Purif. Technol., 2015, 154, 1–10 CrossRef CAS.
- R. S. Gupta, S. S. Islam and S. Bose, Chem. Eng. J., 2024, 488, 150909 CrossRef.
- R. S. Gupta, S. Mandal, A. Malakar, S. Rege, S. S. Islam, K. Samanta, A. Misra and S. Bose, J. Mater. Chem. A, 2024, 12, 321–334 RSC.
- F. Cuminet, D. Berne, S. Lemouzy, É. Dantras, C. Joly-Duhamel, S. Caillol, É. Leclerc and V. Ladmiral, Polym. Chem., 2022, 13, 2651–2658 RSC.
- H. Memon, H. Liu, M. A. Rashid, L. Chen, Q. Jiang, L. Zhang, Y. Wei, W. Liu and Y. Qiu, Macromolecules, 2020, 53, 621–630 CrossRef CAS.
- M. E. Belowich and J. F. Stoddart, Chem. Soc. Rev., 2012, 41, 2003–2024 RSC.
- H. Nabipour, X. Wang, L. Song and Y. Hu, Green Chem., 2021, 23, 501–510 RSC.
- M. Ciaccia and S. Di Stefano, Org. Biomol. Chem., 2015, 13, 646–654 RSC.
- H. Zhang, D. Wang, W. Liu, P. Li, J. Liu, C. Liu, J. Zhang, N. Zhao and J. Xu, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2011–2018 CrossRef CAS.
- R. L. Snyder, C. A. Lidston, G. X. De Hoe, M. J. Parvulescu, M. A. Hillmyer and G. W. Coates, Polym. Chem., 2020, 11, 5346–5355 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta03171g |
| This journal is © The Royal Society of Chemistry 2024 |