Intramolecular assembly of dinitromethyl and bistetrazole: a strategy for constructing advanced and environmentally friendly high-energy density materials†
Xuezhi
Yu
a,
Jie
Tang
a,
Caijin
Lei
a,
Chungui
Xue
a,
Guangbin
Cheng
 *a,
Chuan
Xiao
*b and
Hongwei
Yang
*a,
Chuan
Xiao
*b and
Hongwei
Yang
 *a
*a
aSchool of Chemistry and Chemical Engineering, Nanjing University of Science and Technology, Xiaolingwei 200, Nanjing, Jiangsu, China. E-mail: hyang@mail.njust.edu.cn; gcheng@mail.njust.edu.cn
bChina Northern Industries Group Co., Ltd. (NORINCO GROUP), Beijing 100089, P. R. China. E-mail: 47785121@qq.com
First published on 27th June 2024
Abstract
Bistetrazoles have received extensive attention from researchers in the field of energetic materials due to their high heat of formation (ΔHf = 4.06 kJ g−1) and high nitrogen content (N% = 81.14%). In this work, target product 2,2′-bis(dinitromethyl)-2H,2′H-5,5′-bistetrazole (BDNBT), composed of dinitromethyl and bistetrazole, could significantly improve the dilemma of high sensitivity of bistetrazole-derived energetic materials. The two polymorphic forms (α and β) of BDNBT exhibit differences in density and detonation performance among other aspects. The polymorph α of BDNBT with near-zero oxygen balance (OB% = −4.62%) has a crystal density of up to 1.917 g cm−3 (193 K) and excellent detonation properties (D = 9480 m s−1 and P = 40.2 GPa), superior to those of polymorph β of BDNBT (ρ = 1.899 g cm−3 (193 K), D = 9306 m s−1, and P = 38.5 GPa). In this study, five monovalent cationic salts (4–8) and two divalent cationic salts (9–10) of BDNBT were synthesised and discussed. Among them, energetic 2,4,6-triamino-1,3-dihydroxy-1,3,5-triazine-1,3-diium salts 10 exhibits superior detonation performance to RDX (D = 8795 m s−1 and P = 34.9 GPa) and HMX (D = 9144 m s−1 and P = 39.3 GPa), with a density of 1.904 g cm−3, a detonation velocity of 9612 m s−1, an impact sensitivity of 10 J and a friction sensitivity of 120 N. This work provides guidance for future researchers to develop high-energy density materials and enhance material properties through crystal phase transitions.
Introduction
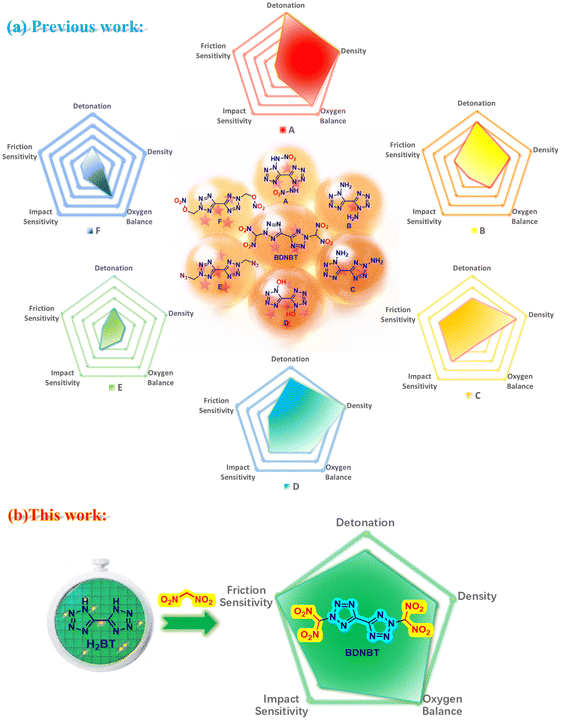
In the development of energetic materials, bistetrazole (H2BT), as an early high energy material, has attracted much attention due to its high nitrogen content (N% = 81.14%) and high heat of formation (ΔHf = 4.06 kJ g−1). Energetic materials with high nitrogen content typically exhibit excellent detonation performance and environmental friendliness.1–3 Therefore, a series of reactions based on bistetrazole have emerged in the past decade, leading to the synthesis of many high-performance bistetrazole derivatives, such as compounds A–F shown in Fig. 1.1,4–8 The detonation performance is further improved by the introduction of two nitroamino groups on the N–H bond of bistetrazole in N,N′-(1H,1′H-[5,5′-bistetrazole]-1,1′-diyl)dinitramide (A), with a detonation velocity of up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 055 m s−1.3 Amino groups were introduced at different positions of bistetrazole to yield a group of structural isomers 1H,1′H-[5,5′-bistetrazole]-1,1′-diamine (B) and 1H,2′H-[5,5′-bistetrazole]-1,2′-diamine (C), which achieve a breakthrough in nitrogen content (N% = 83.31%). 1H,1′H-[5,5′-Bistetrazole]-1,1′-diol (D) has a high density of 1.953 g cm−3 due to the presence of hydroxyl groups.6 Although all these compounds further leverage the advantages of high detonation velocity of bistetrazoles, they exhibit poor sensitivity and oxygen balance performance. The impact sensitivities of the above derivatives A to F are all less than 3 J, and friction sensitivities are all less than 5 N. Compared with RDX (FS = 120 N) and HMX (FS = 120 N), these derivatives exhibit a more significant disadvantage in terms of friction sensitivity.6 Even with a high detonation velocity, highly sensitive bistetrazole derivatives cannot be practically utilized due to the significant safety risks.9–12
055 m s−1.3 Amino groups were introduced at different positions of bistetrazole to yield a group of structural isomers 1H,1′H-[5,5′-bistetrazole]-1,1′-diamine (B) and 1H,2′H-[5,5′-bistetrazole]-1,2′-diamine (C), which achieve a breakthrough in nitrogen content (N% = 83.31%). 1H,1′H-[5,5′-Bistetrazole]-1,1′-diol (D) has a high density of 1.953 g cm−3 due to the presence of hydroxyl groups.6 Although all these compounds further leverage the advantages of high detonation velocity of bistetrazoles, they exhibit poor sensitivity and oxygen balance performance. The impact sensitivities of the above derivatives A to F are all less than 3 J, and friction sensitivities are all less than 5 N. Compared with RDX (FS = 120 N) and HMX (FS = 120 N), these derivatives exhibit a more significant disadvantage in terms of friction sensitivity.6 Even with a high detonation velocity, highly sensitive bistetrazole derivatives cannot be practically utilized due to the significant safety risks.9–12
 | ||
| Fig. 1 (a) The structures of bistetrazole and its derivatives. (b) Target energy materials for the current study. | ||
In order to achieve a better balance between energy and safety of energetic materials in the design of high-energy and low-sensitivity energetic materials today, three strategies are generally adopted to improve their overall performance: (1) introducing stabilizing groups containing hydrogen, such as amino groups or different hydrogen-containing nitrogen heterocyclic energetic groups, into the energetic framework to form highly stable multiple hydrogen bonding network structures; (2) optimizing the crystal structure of energetic compounds through microstructural control methods to create more stable and ordered packing forms; (3) constructing energetic ionic salts based on high-energy energetic cations and anions to enhance the stability of energetic materials. The above three efficient strategies were attempted to be applied in this study. Firstly, the introduction of dinitromethyl, a hydrogen-containing nitrogen heterocyclic high-energy group, is a highly efficient means of improving the overall performance of novel high-energy density materials (HEDMs).13–16 In addition, introducing the planar dinitromethyl anion into nitrogen-containing rings facilitates the formation of a conjugated structure, thereby effectively increasing the density, oxygen balance, and detonation performance of energetic compounds.17–19 Intramolecular assembly of dinitromethyl and bistetrazoles has been a major highlight of this work. Secondly, crystal phase transformation, as an efficient method to enhance the overall performance of energetic materials, involves orderly arrangement of molecules at the microscopic level to increase compound density and intermolecular forces. This in turn impacts properties such as detonation performance and thermal stability of materials. In this study, the polymorphic transformation from one crystal form into another was discussed for the target compound BDNBT. Thirdly, to fully leverage the advantage of a dinitromethyl anion in forming a conjugated structure to effectively enhance the stability of energetic compounds, five monovalent and two divalent cationic energetic salts were synthesized to study the differences in their comprehensive performance changes. Multinuclear NMR (1H and 13C), infrared spectra, elemental analysis, and differential scanning calorimetry (DSC) have been used to characterize each compound. Compounds BDNBT, 4, 5, 7, and 9 underwent crystal X-ray diffraction analysis.
Results and discussion
Synthesis
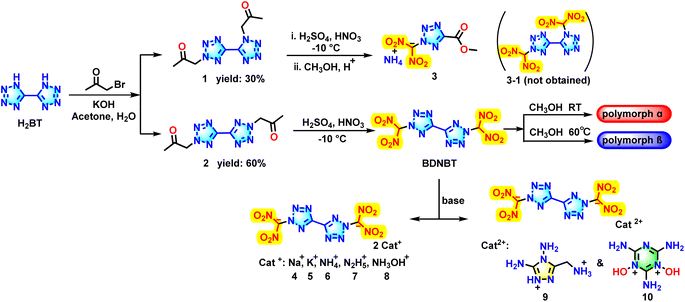
The synthetic route for preparing these energetic materials is illustrated in Scheme 1. The starting material, 1H,1′H-5,5′-bis(tetrazole) (H2BT), was synthesized following literature methods.20 Firstly, H2BT forms a potassium salt in aqueous potassium hydroxide solution. Then, it undergoes a substitution reaction with bromoacetone in the N–H position under acetone reflux conditions. After completion of the reaction, filtration and washing gave bright white product 2 with a 2,2-substituent position. The filtrate was concentrated under reduced pressure, and filtered and washed with water to give a beige solid product 1 with 1,2-substitution. Interestingly, it was found that the yield of compound 2 (yield: 60%) with a 2,2-substituent position was nearly twice that of compound 1 (yield: 30%) with 1,2-substitution after several experiments. It is worth noting that compounds 1 and 2 are isomers with similar onset decomposition temperatures (1: Td = 240 °C; 2: Td = 235 °C) but radically different onset melting points (1: Tm = 128 °C; 2: Tm = 220 °C) (DSC plot in ESI Fig. S2†). This may be attributed to the symmetrical structure of compound 2, which exhibits greater molecular polarity, a more compact molecular arrangement and higher lattice energy compared with compound 1. Unexpectedly, dinitromethyl-ammonium salt 3, the nitration decomposition product of compound 1, was synthesized instead of the desired nitration product, 1,2′-bis(dinitromethyl)-1H,2′H-5,5′-bitetrazole (3-1) under nitration conditions (nitric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sulfuric acid = 1
sulfuric acid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Subsequently, different nitration systems were tested (100% nitric acid, fuming nitric acid, acetic anhydride
1). Subsequently, different nitration systems were tested (100% nitric acid, fuming nitric acid, acetic anhydride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitric acid = 1
nitric acid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, fuming sulfuric acid
1, fuming sulfuric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fuming nitric acid = 1
fuming nitric acid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), but none of them successfully yielded the desired product 3-1.
1), but none of them successfully yielded the desired product 3-1.
In contrast to compound 1, the nitration of compound 2 proceeded successfully, yielding the target compound 2,2′-bis(dinitromethyl)-2H,2′H-5,5′-bistetrazole (BDNBT). Like compounds E and F, the larger size of the dinitromethyl group makes the overall steric hindrance of the compound at the 2-position less than that at the 1-position. As a result, the 2-position bistetrazole skeleton is easier to form. Surprisingly, different crystal types α-BDNBT and β-BDNBT can be obtained by using different temperatures to incubate the crystals. BDNBT forms α-type cubic crystals when a saturated methanol solution is allowed to slowly evaporate at room temperature, but β-type spike-like crystals are obtained when the saturated methanol solution is rapidly evaporated at 60 °C. Furthermore, heating the α-form crystals in saturated methanol solution to 60 °C and then stopping the heating will cause the precipitated crystals to transform into β-form crystals. Similarly, β-form crystals dissolved in saturated methanol and slowly evaporated at room temperature will also transform into α-form crystals. To further investigate the detonation performance of BDNBT as an anionic salt, the corresponding energetic salts 4–10 were synthesized with different ratios of cations to anions (compounds 4–8, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or compounds 9, 10, 1
1 or compounds 9, 10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Addition of sodium hydroxide, potassium hydroxide, ammonia, hydrazine hydrate, and hydroxylamine to the acetonitrile solution of BDNBT gives compounds 4 (yield: 79%), 5 (yield: 88%), 6 (yield: 94%), 7 (yield: 72%), and 8 (yield: 84%), respectively. Considering that 3-(aminomethyl)-4,5-diamino-1,2,4-triazole could provide melting point for potential molten-cast explosives and that 2,4,6-triamino-1,3,5-triazine-1,3-dioxide exhibits a high density, the energetic 3-(aminomethyl)-4,5-diamino-1,2,4-triazol-1-ium salt 9 and energetic 2,4,6-triamino-1,3-dihydroxy-1,3,5-triazine-1,3-diium salt 10 were attempted to be synthesized. BDNBT and 3-(aminomethyl)-4,5-diamino-1,2,4-triazole or 2,4,6-triamino-1,3,5-triazine-1,3-dioxide were stirred in deionised water and the two corresponding divalent high-energy salts were prepared to give a yellow solid 9 (yield: 92%) and a white solid 10 (yield: 94%), respectively.
1). Addition of sodium hydroxide, potassium hydroxide, ammonia, hydrazine hydrate, and hydroxylamine to the acetonitrile solution of BDNBT gives compounds 4 (yield: 79%), 5 (yield: 88%), 6 (yield: 94%), 7 (yield: 72%), and 8 (yield: 84%), respectively. Considering that 3-(aminomethyl)-4,5-diamino-1,2,4-triazole could provide melting point for potential molten-cast explosives and that 2,4,6-triamino-1,3,5-triazine-1,3-dioxide exhibits a high density, the energetic 3-(aminomethyl)-4,5-diamino-1,2,4-triazol-1-ium salt 9 and energetic 2,4,6-triamino-1,3-dihydroxy-1,3,5-triazine-1,3-diium salt 10 were attempted to be synthesized. BDNBT and 3-(aminomethyl)-4,5-diamino-1,2,4-triazole or 2,4,6-triamino-1,3,5-triazine-1,3-dioxide were stirred in deionised water and the two corresponding divalent high-energy salts were prepared to give a yellow solid 9 (yield: 92%) and a white solid 10 (yield: 94%), respectively.
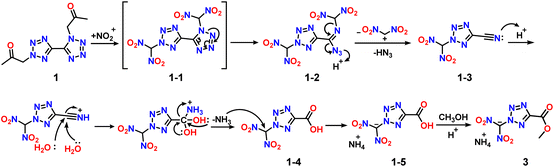
The occurrence of the decomposition product dinitromethylammonium salt 3 attracted our attention. Possible reaction processes for the synthesis of compound 3 from compound 1 with the 1,2-substituent group are illustrated in Scheme 2. First, the methylene group at the 1,2-substituent position of compound 1 is attacked by nitronium to form an unstable dinitromethyl intermediate 1-1. Initially, the N–N bond at the 1-substituted position on the tetrazole ring breaks due to its low bond energy, which results in the formation of intermediates 1-2. Next, a series of intramolecular electron transfers occurred, leading to the removal of the dinitromethyl cation and azide. The azide ends up in the form of azidic acid under acidic conditions. 2-(Dinitromethyl)-2H-tetrazole-5-carbonitrile 1-3 is thus formed. Then, cyano is attacked twice by water molecules to undergo a hydrolysis reaction in a strong acid system. The hydroxyl group introduced during hydrolysis undergoes electron transfer, which leads to the removal of ammonia and the formation of 2-(dinitromethyl)-2H-tetrazole-5-carboxylic acid 1-4. The stripped ammonia reacted with the dinitromethyl group to form an ammonium salt 1-5. The final ammonium salt 1-5 is heated and stirred in a weak acid solution in methanol to undergo an esterification reaction, resulting in the formation of decomposition product dinitromethylammonium salt 3. Since the environment for the esterification reaction is not very acidic, the ammonium ion is retained.
Single crystal X-ray structure analysis
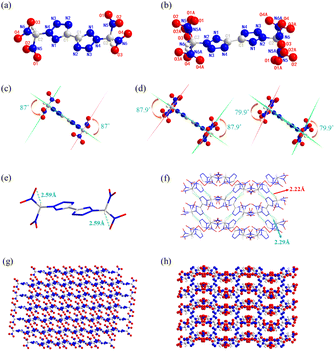
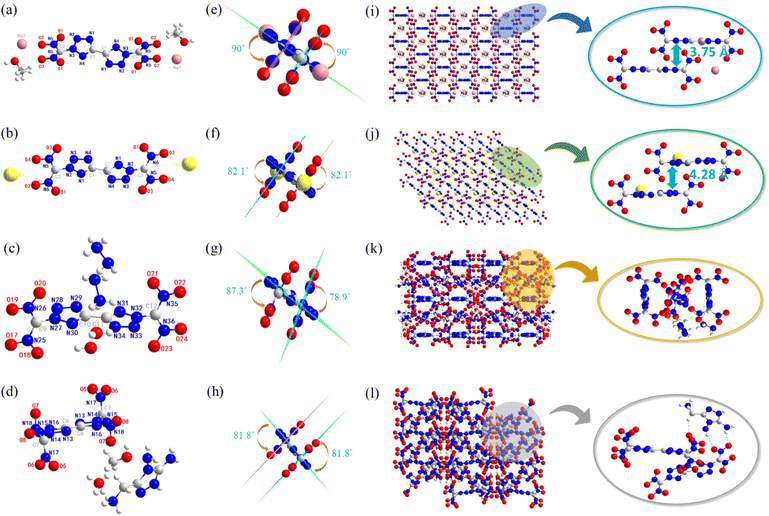
Two polymorphs of BDNBT were confirmed by single-crystal X-ray diffraction and the differences in the molecular structure, packing styles and intermolecular interactions were investigated. α-BDNBT crystals (CCDC: 2336263 (193 K) and 2336254 (298 K)) and β-BDNBT crystals (CCDC: 2336247 (193 K)) were obtained by gradual evaporation of methanol at room temperature and 64 °C, respectively. The polymorph α of BDNBT belongs to the monoclinic space group P21/n with two molecules per unit cell (Z = 2) and a crystal density of 1.917 g cm−3 at 193 K (Fig. 2a). The polymorph β of BDNBT crystallizes in the monoclinic space group P21/c with four molecules per lattice cell (Z = 4) and has a crystal density of 1.899 g cm−3 at 193 K (Fig. 2b). As shown in Fig. 2c or Fig. 2d, the bistetrazole planar structure is also supported by torsion angles (N1–C1–C1a–N1a 180.00(1)° and C1–N2–N3–N4 −0.12(1)° in α-BDNBT; N1–N2–N3–N4 −0.4(7)° and N1–C1–C1_a–N1_a −180.0(5)° in β-BDNBT). In α-BDNBT, the dihedral angle between the dinitromethyl plane through N5–C2–N6 and the plane through the tetrazole ring is 87°. In β-BDNBT, the angles between the tetrazole and the two sets of dinitromethyl planes through N11–C4–N12 and N11A−C4−N12A are 87.9° and 79.9°, respectively. The structure of α-BDNBT is mainly dominated by intramolecular hydrogen bonding (C2–H2⋯O3) with a bond length of 2.59 Å (Fig. 2e). As shown in the green dashed portion of Fig. 2f, there is no intramolecular hydrogen bonding interaction in the structure of β-BDNBT, which is mainly dominated by intermolecular hydrogen bonding (C2–H2A⋯N10 and C4–H4A⋯N4), with bond lengths of 2.22 Å and 2.29 Å, respectively. In Fig. 2g and h, both α-BDNBT and β-BDNBT are stacked in a haphazard manner, but α-BDNBT is relatively neater than β-BDNBT. This also demonstrates why the α-BDNBT crystal density (1.917 g cm−3) is higher than the β-BDNBT crystal density (1.899 g cm−3).Four molecules of the crystal 4·CH3OH fit into each unit cell as it crystallizes in the monoclinic C2/m space group, where its measured density is 1.892 g cm−3 (193 K). Unlike the crystal 4·CH3OH, crystal 5 crystallizes in the monoclinic P21/c space group with two molecules per unit and a measured density of 1.941 g cm−3 (223 K). As shown in Fig. 3e, the dinitromethyl plane through N5–C2–N5_d is perpendicular to the bistetrazole plane. Similar to 4·CH3OH, the two dinitromethyl groups of crystal 5 are almost perpendicular to the bistetrazole plane at an angle of 82.1° (Fig. 3f). It is possible that the large atomic radius of the potassium ion has deflected the dinitromethyl group. As shown in Fig. 3i or Fig. 3j, the crystal stacking systems of both crystals 4·CH3OH and crystals 5 exhibit layer-by-layer stacking with layer distances of 3.75 Å and 4.28 Å, respectively.
The crystal 7·2H2O was measured at 193 K with a density of 1.761 g cm−3. It crystalized in the monoclinic system and C2/c space group. There are two molecules in one single unit cell (Z = 2). The crystal 9·CH3OH H2O also belongs to the monoclinic C2/c space group, but has four molecules per unit cell. Its crystal density at 218 K is 1.687 g cm−3. Similar to the previous crystals, the two tetrazoles in crystal 7·2H2O also remain in the same plane (N9–N10–C3–C2 −176.8(5) and N4–N5–C2–C3 −179.4(5)). However, the two dinitromethyl planes through N25–C9–N26 and N35–C12–N36 in crystal 7·2H2O are not parallel. Different from 7·2H2O, the two dinitromethyl planes in crystal 9·CH3OH H2O are parallel and the dinitromethyl group has an angle of 81.8° with the bistetrazole plane (Fig. 3h). As shown in Fig. 3g, the angles between these two planes and the bistetrazole plane are 87.3° and 78.9°, respectively. In addition, 7·2H2O exhibits a composite stacking and intermolecular hydrogen bonds involving N41–H41A⋯O14, N38–H38A⋯N29 and N45–H45B⋯O22 are observed (Fig. 3k). Similarly, crystal 9·CH3OH H2O exhibits a composite stacking and intermolecular hydrogen bonds (N8–H8⋯O1, O9–H9A⋯N12, and C8–H8A⋯O9).
Physicochemical and energetic properties
The physicochemical and energetic properties of α-BDNBT, β-BDNBT and energetic salts 4–10 are summarized in Table 1.| Comp. | ρ (g cm−3) | ΔfHmb (kJ mol−1/kJ g−1) | D (m s−1) | P (GPa) | OBCO2e (%) | N + Of (%) | T m (°C) | T d (°C) | ISi (J) | FSj (N) |
|---|---|---|---|---|---|---|---|---|---|---|
| a Measured densities using a gas pycnometer at 25 °C. b Calculated molar enthalpy of formation. c Calculated detonation velocity. d Calculated detonation pressure. e Oxygen balance (based on CO2). f Nitrogen and oxygen contents in %. g Melting temperature (onset). h Decomposition temperature (onset). i Impact sensitivity. j Friction sensitivity. k Crystal density at 298 K. l Ref. 23. m Ref. 24. | ||||||||||
| α-BDNBT | 1.880k | 838.8/2.42 | 9480 | 40.2 | −4.62 | 85.54 | — | 91 | 4 | 40 |
| β-BDNBT | 1.863 | 838.8/2.42 | 9306 | 38.5 | −4.62 | 85.54 | — | 84 | 3 | 28 |
| 4 | 1.915 | 343.4/0.88 | 7882 | 27.1 | −4.10 | 75.90 | — | 142 | 3 | 32 |
| 5 | 1.922 | 223.1/0.19 | 7955 | 28.6 | −3.79 | 70.11 | — | 222 | <1 | <5 |
| 6 | 1.813 | 484.4/1.27 | 8966 | 35.2 | −16.83 | 85.24 | — | 121 | 6 | 60 |
| 7 | 1.822 | 759.1/1.85 | 9224 | 37.2 | −19.50 | 85.83 | — | 145 | 5 | 60 |
| 8 | 1.891 | 617.4/1.50 | 9503 | 41.1 | −7.76 | 86.38 | — | 124 | 5 | 60 |
| 9 | 1.824 | 1815.1/3.82 | 9413 | 39.3 | −37.10 | 80.15 | 97 | 138 | 9 | 108 |
| 10 | 1.904 | 1639.9/3.25 | 9612 | 42.8 | −25.38 | 81.73 | — | 146 | 10 | 120 |
| RDXl | 1.805 | 80.0/0.36 | 8795 | 34.9 | −21.6 | 81.06 | — | 204 | 7.4 | 120 |
| HMXm | 1.905 | 104.8/0.25 | 9144 | 39.2 | −21.6 | 81.06 | — | 280 | 7.4 | 120 |
The densities of all compounds were measured using a gas hydrometer at 25 °C in a helium (He) atmosphere. Compounds were dried in an oven at 30 °C to remove the solvents completely, as evidenced by DSC curves. The densities of BDNBT and its derivatives ranged from 1.813 to 1.922 g cm−3, which are higher than those of RDX (1.805 g cm−3) (Table 1). The Gaussian 09 program was used to calculate the heats of formation (ΔHf) for BDNBT and its derivatives, which ranged from 0.19 to 3.82 kJ g−1. The heat of formation for all target compounds, except for the potassium salt 7 (ΔHf = 0.19 kJ g−1), was much greater than that of RDX (ΔHf = 0.36 kJ g−1) and HMX (ΔHf = 0.25 kJ g−1). Detonation velocities and pressures were predicted using EXPLO5 v6.01 based on measured densities and the calculated heat of formation of BDNBT and its derivatives.21α-BDNBT maintains a high density of 1.880 g cm−3 and outstanding detonation performance (D = 9480 m s−1 and P = 40.2 GPa), with a total nitrogen and oxygen content of 85.54% and a near-zero oxygen balance (OB = −4.62%). Although β-BDNBT has slightly inferior performance compared with α-BDNBT, it still exhibits higher detonation performance (D = 9306 m s−1 and P = 38.5 GPa) than the commonly used explosives RDX (D = 8795 m s−1 and P = 34.9 GPa) and HMX (D = 9144 m s−1 and P = 39.3 GPa). Energetic ionic salts 4 and 5 are metal salts with advantages in density, measuring 1.915 g cm−3 and 1.922 g cm−3, respectively. Energetic potassium salts 5 shows a significant improvement in thermal decomposition temperature (Td = 222 °C), which is the highest among that of all salt compounds. Compared with α-BDNBT (D = 9480 m s−1, P = 40.2 GPa; Td = 91 °C; IS = 4 J, and FS = 40 N), the energetic ammonium salt 6 shows low sensitivity (IS = 6 J and FS = 60 N), the energetic hydrazine salt 7 displays a high thermal decomposition temperature (Td = 145 °C), and the energetic hydroxylamine salt 8 exhibits a high detonation performance (D = 9503 m s−1 and P = 41.1 GPa). Divalent high-energy salts 9 and 10 have achieved breakthroughs in detonation performance (9: D = 9413 m s−1 and P = 39.3 GPa; 10: D = 9612 m s−1 and P = 42.8 GPa) and sensitivity (9: IS = 9 J and FS = 108 N; 10: IS = 10 J and FS = 120 N). Aside from the energy properties, the friction sensitivity (FS) and impact sensitivity (IS) measured using the standard BAM methods are also of great interest.22 Compound 10 (FS = 120 N) exhibits friction sensitivity similar to that of HMX and RDX, while its impact sensitivity (IS = 10 J) is superior to that of HMX (IS = 7.4 J) and RDX (IS = 7.4 J).
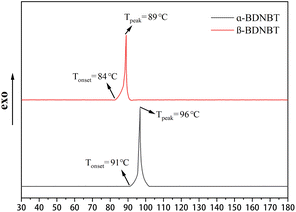
To investigate the difference between the thermal stability of α-BDNBT and β-BDNBT, differential scanning calorimetry (DSC) measurements were carried out in a nitrogen atmosphere at a linear heating rate of 5 °C min−1. The onset decomposition temperature of β-BDNBT is 84 °C with a peak temperature of 89 °C (Fig. 4). The onset decomposition temperature of α-BDNBT is 91 °C, which is slightly higher than that of β-BDNBT (Tonset = 84 °C). It is noteworthy that the decomposition peak widths of α-BDNBT and β-BDNBT are almost the same at 10 °C, which confirms the rapid energy release rate and strong detonation performance of BDNBT.
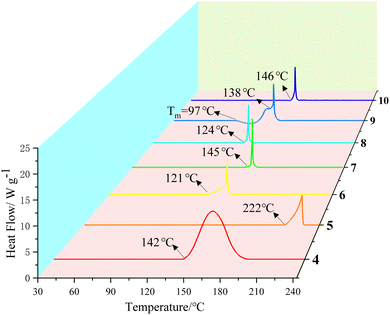
Similarly, monovalent cationic salts of BDNBT, such as sodium salt 4, potassium salt 5, ammonium salt 6, hydrazine salt 7, hydroxylamine salt 8, and divalent cationic salts, such as 4,5-diamino-3-(aminomethyl)-4H-1,2,4-triazolium salt 9, and 2,4,6-triamino-1,3-dihydroxy-1,3,5-triazinane-1,3-diium salt 10, were also characterized with regard to their thermal decomposition temperatures. The decomposition temperature profile waterfall plots are shown in Fig. 5. The decomposition temperatures of these salts range from 121–146 °C, except for the energetic potassium salt 5, with a decomposition temperature of 222 °C. This is generally consistent with our common perception that dinitromethyl compounds have low decomposition temperatures. Energetic salt 9 has melting point due to the presence of a methylene component in its cation with a starting melting temperature of 97 °C.
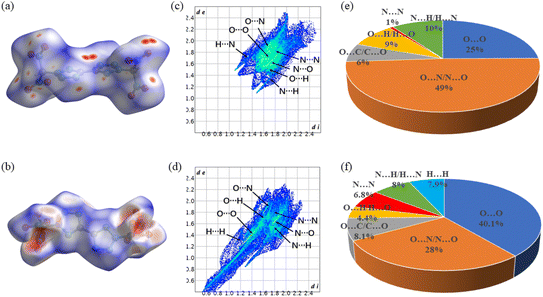
As shown in Table 1, α-BDNBT exhibits lower sensitivity than β-BDNBT, which may be related to intermolecular forces. To gain more insight into the intermolecular interactions, the two-dimensional (2D) fingerprints of α-BDNBT and β-BDNBT crystals and the associated Hirshfeld surfaces were analyzed using Crystalexplorer 17.5 (Fig. 6).25 The red and blue regions on the Hirshfeld surfaces represent the high and low contact groups, respectively.26 As shown in Fig. 6a and b, the red dots in α-BDNBT are scattered on various parts of the molecule, in which N⋯O and O⋯N interactions are the strongest, while the red dots in β-BDNBT are scattered on the molecule mainly close to the dinitromethyl group, with the largest proportion of O⋯O bonds. The proportion of O⋯O bonds in β-BDNBT is 40.1%, higher than that of α-BDNBT (25%), which might be related to the fact that β-BDNBT is more sensitive than α-BDNBT.
Conclusions
In conclusion, two polymorphic forms (α and β) of bistetrazole and seven energetic salts were synthesized and characterized using a strategy involving the co-assembly of dinitro and bistetrazole. This strategy effectively addresses the issue of high sensitivity in bistetrazole derivatives. Among them, the structures of α-BDNBT, β-BDNBT, 4·CH3OH, 5, 7·2H2O, and 9·CH3OH H2O are confirmed by single crystal X-ray diffraction. The neutral compound α-BDNBT has a crystal density of up to 1.880 g cm−3 (298 K) and excellent detonation properties (D = 9480 m s−1 and P = 40.2 GPa), superior to those of the conventional explosives RDX (D = 8795 m s−1 and P = 34.9 GPa) and HMX (D = 9144 m s−1 and P = 39.3 GPa). Furthermore, α-BDNBT shows superior oxygen balance (OB% = −4.62%) and lower friction sensitivity (FS = 40 N) compared with other tetrazole derivatives (FS < 5 N). Within the energetic salts (4–10) derived from BDNBT, energetic potassium salts 5 boast the highest density and thermal decomposition temperature (ρ = 1.922 g cm−3 and Td = 222 °C), energetic 2,4,6-triamino-1,3-dihydroxy-1,3,5-triazine-1,3-diium salts 10 display superior detonation performance (D = 9612 m s−1 and P = 42.8 GPa) and the lowest sensitivity (IS = 10 J and FS = 120 N). The results demonstrate that BDNBT and its energetic salts have high densities (1.813–1.922 g cm−3) and exhibit excellent detonation performance (7882–9612 m s−1), indicating their potential as a novel type of high-energy density material (HEDM).Data availability
All data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22075143 and 22205110), the Natural Science Foundation of Jiangsu Province (BK20220958), and the Fundamental Research Funds for the Central Universities (30922010404).References
- T. M. Klapötke, M. Kofen and J. Stierstorfer, N-Functionalisation of 5,5′-bistetrazole providing 2,2′-di(azidomethyl)bistetrazole: a melt-castable metal-free green primary explosive, Dalton Trans., 2021, 50, 13656–13660 RSC.
- N. Fischer, D. Izsák, T. M. Klapötke, S. Rappenglück and J. Stierstorfer, Nitrogen-rich 5,5′-bistetrazolates and their potential use in propellant systems: a comprehensive study, Chem.–Eur. J., 2012, 18, 4051–4062 CrossRef CAS PubMed.
- D. Fischer, T. M. Klapötke, J. Stierstorfer and N. Szimhardt, 1,1′-Nitramino-5,5′-bitetrazoles, Chem.–Eur. J., 2016, 22, 4966–4970 CrossRef CAS PubMed.
- T. M. Klapötke, D. G. Piercey and J. Stierstorfer, Amination of energetic anions: high-performing energetic materials, Dalton Trans., 2012, 41, 21–31 RSC.
- M. Benz, T. M. Klapötke and J. Stierstorfer, Krapcho Decarboxylation of ethyl-carbazate: synthetic approach toward 1,1′-diamino-5,5′-bistetrazole and its utilization as a high-performing metal-free initiator, Org. Lett., 2022, 24, 1747–1751 CrossRef CAS PubMed.
- N. Fischer, L. Gao, T. M. Klapötke and J. Stierstorfer, Energetic salts of 5,5′-bis(tetrazole-2-oxide) in a comparison to 5,5′-bis(tetrazole-1-oxide) derivatives, Polyhedron, 2013, 51, 201–210 CrossRef CAS.
- X. Wang, S. Jin, C. Zhang, L. Li, S. Chen and Q. Shu, Preparation, Crystal structure and properties of a new crystal form of diammonium 5,5′-bistetrazole-1,1′-diolate, Chin. J. Chem., 2015, 33, 1229–1234 CrossRef CAS.
- N. Fischer, D. Fischer, T. M. Klapötke, D. G. Piercey and J. Stierstorfer, Pushing the limits of energetic materials – the synthesis and characterization of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate, J. Mater. Chem. A, 2012, 22, 38–50 RSC.
- W. Hu, J. Tang, X. Ju, Z. Yi, H. Yang, C. Xiao and G. Cheng, An efficient one-step reaction for the preparation of advanced fused bistetrazole-based primary explosives, ACS Cent. Sci., 2023, 9, 742–747 CrossRef CAS PubMed.
- T. Yan, H. Yang, C. Yang, Z. Yi, S. Zhu and G. Cheng, An advanced and applicable heat-resistant explosive through controllable regiochemical modulation, J. Mater. Chem. A, 2020, 8, 23857–23865 RSC.
- C. Lei, G. Cheng, Z. Yi, Q. Zhang and H. Yang, A facile strategy for synthesizing promising pyrazole-fused energetic compounds, Chem. Eng. J., 2021, 416, 129190 CrossRef CAS.
- J. Singh, R. J. Staples and J. M. Shreeve, Manipulating nitration and stabilization to achieve high energy, Sci. Adv., 2023, 9, eadk3754 CrossRef CAS PubMed.
- Q. Yu, P. Yin, J. Zhang, C. He, G. H. ImLer, D. A. Parrish and J. M. Shreeve, Pushing the limits of oxygen balance in 1,3,4-oxadiazoles, J. Am. Chem. Soc., 2017, 139, 8816–8819 CrossRef CAS PubMed.
- M. A. Kettner and T. M. Klapötke, 5,5′-Bis-(trinitromethyl)-3,3′-bi-(1,2,4-oxadiazole): a stable ternary CNO-compound with high density, Chem. Commun., 2014, 50, 2268–2270 RSC.
- X. X. Zhao, S. H. Li, Y. Wang, Y. C. Li, F. Q. Zhao and S. P. Pang, Design and synthesis of energetic materials towards high density and positive oxygen balance by N-dinitromethyl functionalization of nitroazoles, J. Mater. Chem. A, 2016, 4, 5495–5504 RSC.
- N. V. Muravyev, K. Y. Suponitsky, I. V. Fedyanin, I. V. Fomenkov, A. N. Pivkina and I. L. Dalinger, Bis-(2-difluoroamino-2,2-dinitroethyl) nitramine – energetic oxidizer and high explosive, Chem. Eng. J., 2022, 449, 137816 CrossRef CAS.
- L. Hu, P. Yin, G. Zhao, C. He, G. H. ImLer, D. A. Parrish, H. Gao and J. M. Shreeve, Conjugated energetic salts based on fused rings: insensitive and highly dense materials, J. Am. Chem. Soc., 2018, 140, 15001–15007 CrossRef CAS PubMed.
- C. He and J. M. Shreeve, Potassium 4,5-bis(dinitromethyl)furoxanate: a green primary explosive with a positive oxygen balance, Angew. Chem., Int. Ed., 2016, 55, 772–775 CrossRef CAS PubMed.
- J. Zhang, S. Dharavath, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, Energetic salts based on 3,5-bis(dinitromethyl)-1,2,4-triazole monoanion and dianion: controllable preparation, characterization, and high performance, J. Am. Chem. Soc., 2016, 138, 7500–7503 CrossRef CAS PubMed.
- E. Oliveri-Mandala and T. Passalacqua, Bistetrazole and isomeric derivatives of tetrazole, Gazz. Chim. Ital., 1914, 43, 465–475 CAS.
- C. Li, T. Zhu, C. Lei, G. Cheng, C. Xiao and H. Yang, Construction of p-nitropyrazole-1,3,4-triazole framework energetic compounds: towards a series of high-performance heat-resistant explosives, J. Mater. Chem. A, 2023, 11, 12043–12051 RSC.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, Revealing noncovalent interactions, J. Am. Chem. Soc., 2010, 132, 6498–6506 CrossRef CAS PubMed.
- C. Li, T. Zhu, J. Tang, G. Yu, Y. Yang, H. Yang, C. Xiao and G. Cheng, Trinitromethyl groups-driven fused high energy compound featuring superior comprehensive performances, Chem. Eng. J., 2024, 479, 147355 CrossRef CAS.
- J. Li, Y. Liu, W. Ma, T. Fei, C. He and S. Pang, Tri-explosophoric groups driven fused energetic heterocycles featuring superior energetic and safety performances outperforms HMX, Nat. Commun., 2022, 11, 2837 CrossRef PubMed.
- M. A. Spackman and J. J. McKinnon, Fingerprinting intermolecular interactions in molecular crystals, CrystEngComm, 2002, 4, 378–392 RSC.
- J. Zhang, Q. Zhang, T. T. Vo, D. A. Parrish and J. M. Shreeve, J. Am. Chem. Soc., 2015, 137, 1697–1704 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2336247, 2336254–2336256, 2336263, 2336277, 2339222 and 2339223. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ta03537b |
| This journal is © The Royal Society of Chemistry 2024 |