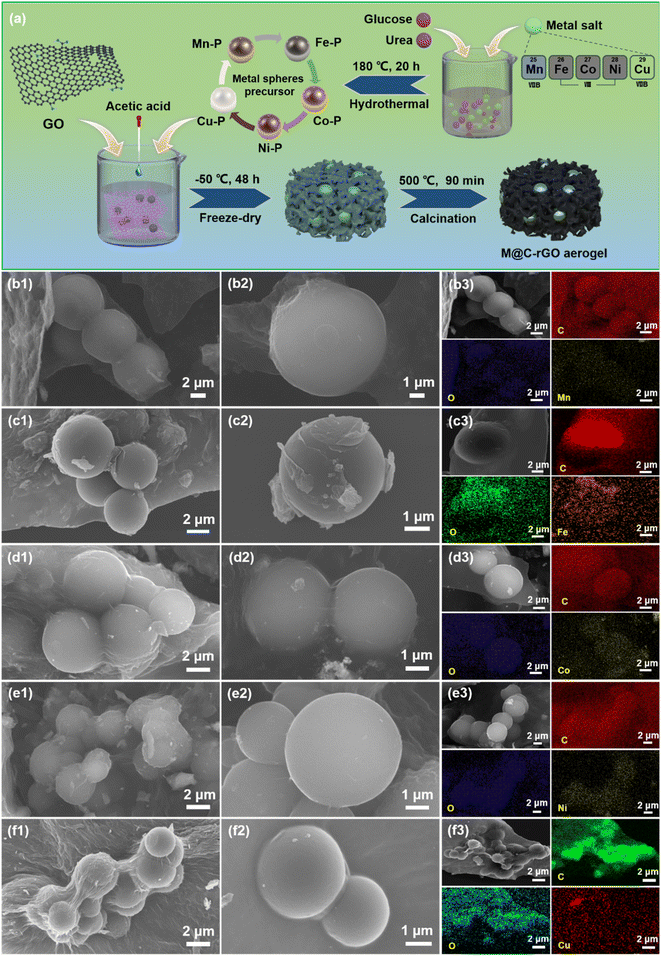
Porous metal microsphere M@C-rGO (metal = Mn, Fe, Co, Ni, Cu) aerogels with high low-frequency microwave absorption, strong thermal insulation and superior anticorrosion performance†
Junfeng
Qiu
a,
Xin
Liu
a,
Chunyi
Peng
a,
Sihan
Wang
a,
Rongchen
Wang
a and
Wei
Wang
 *ab
*ab
aDepartment of Physics and Electronics, School of Mathematics and Physics, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: wangwei@mail.buct.edu.cn
bBeijing Key Laboratory of Environmentally Harmful Chemical Analysis, Beijing University of Chemical Technology, Beijing 100029, China
First published on 11th July 2024
Abstract
Nowadays, the development of multifunctional electromagnetic wave absorbers to improve the survivability of military equipment in complex environments is becoming a significant and unavoidable challenge. Here, three-dimensional porous metal microsphere@chitosan reduced graphene oxide (M@C-rGO, M = Mn, Fe, Co, Ni, Cu) aerogels are constructed via a simple ice template method and subsequent pyrolysis. The embedding of different metal microspheres realizes the modulation of the electromagnetic parameters of the synthesized aerogel, achieving a balance between attenuation capability and impedance matching. As expected, the synthesized Cu@C-rGO aerogel exhibits strong microwave absorption in the C-band with a minimum reflection loss (RLmin) of −59.28 dB, which also features an effective absorption bandwidth (EAB) of 6.32 GHz in the high-frequency band. Furthermore, the synthesized aerogels have superior radar stealth, thermal insulation and anticorrosion properties. Typically, the thermal conductivity of Cu@C-rGO is 0.0339 W m−1 K−1, and the charge transfer resistance (Rct) is 1.421 × 105 Ω cm2. Impressively, more than 90% of the EAB could be retained after 7 days of corrosion in NaCl solution. Consequently, this work provides valuable guidance and insight into the development of multifunctional electromagnetic wave absorbers.
1. Introduction
With the rapid development of fifth-generation (5G) wireless technology, electronic devices and artificial intelligence technology have brought great convenience to our lives, but have also caused serious problems of electromagnetic radiation and electromagnetic pollution.1–3 Developing efficient microwave absorbers is an effective way to reduce and eliminate electromagnetic wave radiation.4–7 In contrast to traditional high-frequency radar signals, 5G wireless signals are mainly located in the C-band and lower frequencies.8–11 However, the absorption frequencies of most current microwave absorbers are concentrated in the high-frequency range (X and Ku bands).12,13 Therefore, it is urgent to develop microwave absorption materials with strong absorption in low-frequency bands.High magnetic loss has been considered as one of the critical factors for obtaining strong absorption at low-frequencies in microwave absorption materials.14–16 Magnetic materials represented by Fe, Co and Ni and their alloys have attracted much attention in the field of microwave absorption due to their high saturation magnetization strength and excellent magnetic loss performance.17,18 However, due to the lack of dielectric loss, a single magnetic metal is usually inadequate to obtain excellent microwave absorption performance owing to impedance mismatch.19 To realize the effective matching of complex permittivity and complex permeability, many scholars have researched on composites of magnetic metals and light dielectric materials such as carbon materials. Che et al. synthesized FeCo@C hollow microspheres through a confined diffusion strategy, obtaining a minimum reflection loss (RLmin) of −35.9 dB and an effective absorption bandwidth (EAB) covering almost the entire C-band.20 Also, Liu et al. synthesized size-tunable Co/CoO nanoparticles by disassembling the cubic zeolitic imidazolate framework (ZIF-67) and subsequent hydrogen reduction, which resulted in an RLmin of −90.3 dB at 4.4 GHz.19 Jiang et al. constructed MXene/CoNi/N-CNT three-dimensional (3D) heterostructures by electrostatic assembly, which exhibited an RLmin value of −52.64 dB at 3.12 GHz.14
Recently, achieving multifunctional applications has been an important research direction of advanced materials. However, magnetic metals usually suffer from disadvantages such as high density, poor dispersibility and susceptibility to corrosion, which seriously restrict their multifunctional applications. Graphitic carbon is considered to be a perfect atom-scale barrier that prolongs the path of penetration of corrosive media (e.g., H2O, O2 and Cl−) into the metal substrate, due to its impermeability and chemical stability.18 As a typical two-dimensional carbon material, reduced graphene oxide (rGO) with low density, high specific surface area, abundant surface defects, and strong conductive loss has become a representative candidate for high-performance microwave absorption materials.21,22 However, the impedance mismatch and skinning effect caused by the high conductivity of rGO hinder its further development in the field of microwave absorption. In fact, combining rGO with magnetic metals can balance the impedance matching and attenuation ability to enhance the microwave absorption performance of the materials. Meanwhile, rGO can also provide an anticorrosion barrier to improve the long-term stability of the material in a corrosive environment. For instance, Xu et al. synthesized a three-dimensional NiAl layered double hydroxide/graphene (NiAl-LDH/G) composite as an anticorrosion microwave absorber with an RLmin of −41.5 dB and an EAB of 4.4 GHz by atomic layer deposition. Remarkably, the composite exhibits long-term corrosion resistance due to the synergetic effect of the superior impermeability of graphene and the chloridion-capture capacity of the NiAl-LDH.23 Yao et al. applied the rare earth lanthanum (La) pinning effect to FeCo/rGO composites to achieve a corrosion-resistant and highly efficient microwave absorber. As a result, the FeCo@rGO composites exhibit excellent microwave absorption properties covering a frequency range of 6.11 GHz at 1.55 mm. Meanwhile, the electromagnetic properties of the absorber remained stable even after prolonged exposure to 3.5 wt% NaCl solution for at least 15 days.24 In addition, the unique two-dimensional structure of rGO enables its excellent energy storage and thermal management capabilities.25,26 Thus, rational component modulation and innovative structural design are key to the development of multifunctional composites.
Since the intrinsic mechanism and parametric conditions of low-frequency microwave absorption are still unclear enough, this study tries to find out the parametric conditions of low-frequency microwave absorption using numerical simulation. Subsequently, magnetic and nonmagnetic metal microspheres were embedded into chitosan-rGO aerogels by a simple ice template method and pyrolysis to modulate the complex permittivity and magnetic permeability of the absorber. Remarkably, the as-obtained composites exhibit strong absorption at low-frequency and a wide effective absorption bandwidth in the high-frequency band, based on excellent dielectric loss and good impedance matching. Furthermore, the composite aerogels exhibit excellent thermal insulation and anticorrosion capabilities due to the unique 3D porous structure. Therefore, this work provides theoretical and experimental guidance for the preparation of multifunctional materials for low-frequency microwave absorption with strong thermal insulation and excellent anticorrosion performance.
2. Experimental section
2.1. Materials
Sulfuric acid (H2SO4), phosphoric acid (H3PO4), hydrogen peroxide (H2O2), potassium permanganate (KMnO4), graphite powder (325 mesh), manganese chloride tetrahydrate (MnCl2·4H2O), ferric chloride hexahydrate (FeCl3·6H2O), cobalt chloride hexahydrate (CoCl2·6H2O), nickel chloride hexahydrate (NiCl2·6H2O), copper sulfate pentahydrate (CuSO4·5H2O), glacial acetic acid, glucose, chitosan, urea paraffin and ethanol were directly used after purchase from Sinopharm Chemical Reagent Co., Ltd, China without further purification. Graphene oxide was synthesized according to the modified Hummers' method.2.2. Synthesis of the metal microsphere precursor
Firstly, 0.01 mol of metal ion, 0.015 mol glucose and 0.02 mol urea were dissolved into 60 mL deionized water. After ultrasonic treatment for 30 min, the resulting mixture was transferred into a 100 mL Teflon-lined stainless-steel autoclave, and heated at 180 °C for 20 h. The obtained black precipitate was washed three times with water and ethanol, and then, oven-dried overnight at 60 °C to obtain the precursor. Here, the obtained samples are denoted as Mn–P, Fe–P, Co–P, Ni–P and Cu–P according to the added metal salts, MnCl2·4H2O, FeCl3·6H2O, CoCl2·6H2O, NiCl2·6H2O, and CuSO4·5H2O, respectively.2.3. Synthesis of M@C-rGO aerogels
0.1 g of metal precursor, 50 mg of GO and 0.2 g of chitosan were added to 15 mL of deionized water and sonicated for 1 h to form a homogeneous suspension. After that, 0.4 mL of glacial acetic acid was quickly dripped to form a gel. Then, the gel was poured into molds and freeze-dried at −50 °C for 48 h. Finally, M@C-rGO aerogels were obtained after 500 °C calcination for 90 min under a nitrogen atmosphere. The synthesized aerogels were named Mn@C-rGO, Fe@C-rGO, Co@C-rGO, Ni@C-rGO and Cu@C-rGO according to the metal microsphere precursors. Similarly, all steps were repeated without the metal sphere precursor to obtain the sample C-rGO.2.4. Characterization
The morphology of the samples was characterized using a scanning electron microscope (SEM, Hitachi S-3500N). The crystal structure and crystallinity of the samples were analyzed with an XRD-6000 diffractometer using Cu Kα radiation at a scanning rate of 5° min−1. Raman spectra at room temperature were recorded using a LabRAM ARAMIS spectrometer (HORIBA Jobin-Yvon) with an excitation of 532 nm. The bonding characteristics and chemical composition of the samples were studied with a PHI Quantera II XPS Scanning Microprobe. The infrared images were taken with a thermographic camera (HM-TPK20-3AQF/W) and the thermal conductivity was measured using a thermal constant analyzer (Hot-Disk TPS2200).2.5. Electromagnetic wave absorption measurements
First, the as-synthesized aerogels and paraffin with a mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 were added into 4 mL of hexane solution. Then, the as-obtained mixture was sonicated for 30 min until a homogeneous solution formed, and then the mixed solution was dried at 60 °C overnight. The as-formed black waxy solid was placed in a metal mold and pressed as a hollow cylinder with outer and inner diameters of 7.0 mm and 3.04 mm, respectively. Subsequently, the electromagnetic performance and S parameters of the samples were characterized using a vector network analyzer (VNA, Agilent E8362B) using the coaxial-line method in the frequency range of 2–18 GHz.
8 were added into 4 mL of hexane solution. Then, the as-obtained mixture was sonicated for 30 min until a homogeneous solution formed, and then the mixed solution was dried at 60 °C overnight. The as-formed black waxy solid was placed in a metal mold and pressed as a hollow cylinder with outer and inner diameters of 7.0 mm and 3.04 mm, respectively. Subsequently, the electromagnetic performance and S parameters of the samples were characterized using a vector network analyzer (VNA, Agilent E8362B) using the coaxial-line method in the frequency range of 2–18 GHz.
2.6. Anticorrosion performance measurements
The corrosion resistance of the samples was measured using the classical three-electrode system of an electrochemical workstation (CHI660E). The platinum sheet electrode, Ag/AgCl electrode and glassy carbon electrode were used as the counter electrode, reference electrode and working electrode respectively. The open-circuit potential (OCP), linear sweep voltammetry (LSV) curves and electrochemical impedance spectroscopy (EIS) spectra of the samples were recorded under acidic (0.5 M H2SO4), neutral (3.5 wt% NaCl) and alkaline (1.0 M NaOH) conditions. To prepare the working electrode, 3 mg of sample was mixed with 510 μL of solvent (5 wt% Nafion, isopropanol and deionized water in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) and sonicated for 1 h to obtain a homogeneous suspension. 10 μL of the above suspension was dropped onto a glassy carbon electrode with a radius of 2.5 mm and dried at room temperature. The LSV curve is measured at a scan rate of 1 mV s−1 with a voltage range of OCP ± 0.6 V. EIS was performed in the 105–10−2 Hz range and fitted with ZView software.
30) and sonicated for 1 h to obtain a homogeneous suspension. 10 μL of the above suspension was dropped onto a glassy carbon electrode with a radius of 2.5 mm and dried at room temperature. The LSV curve is measured at a scan rate of 1 mV s−1 with a voltage range of OCP ± 0.6 V. EIS was performed in the 105–10−2 Hz range and fitted with ZView software.
2.7. Radar cross-section (RCS) simulation
The RCS values of the samples were simulated using a CST Studio Suite 2020. The simulation model was a double-layered square (100 × 100 mm) with a wave-absorbing layer of 4 mm thickness in the upper layer and a (perfect electric conductor) PEC layer of 5 mm thickness in the lower layer. And then, the simulation model was placed in the X–O–Y plane, with the electromagnetic wave in the linear polarization plane incident along the negative direction of the z-axis, and the electric polarization direction incident along the x-axis. Furthermore, open boundary conditions are applied in all directions and the electromagnetic wave frequency is set to 7 GHz.3. Results and discussion
3.1. Numerical simulation
Now, we propose a numerical simulation method to reveal the effect of the change in complex permittivity and complex permeability on the low-frequency microwave absorption performance of the absorbers. Normally, the microwave absorption performance of an absorber is evaluated by the reflection loss (RL) value. According to the transmission line theory, the RL of a material is closely related to its complex permittivity (εr = ε′ − jε′′) and complex permeability (μr = μ′ − jμ′′),27,28 where the real and imaginary parts of the electromagnetic parameters are related to the storing and attenuating ability of electromagnetic wave energy, respectively.29 Meanwhile, the dielectric loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε = ε′′/ε′) and magnetic loss tangent (tan
δε = ε′′/ε′) and magnetic loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ = μ′′/μ′) values reflect the strength of the dielectric loss and magnetic loss, respectively. Furthermore, according to the transmission line theory, the corresponding RL values can be obtained from the following equations:30
δμ = μ′′/μ′) values reflect the strength of the dielectric loss and magnetic loss, respectively. Furthermore, according to the transmission line theory, the corresponding RL values can be obtained from the following equations:30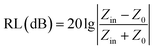 | (1) |
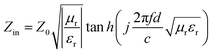 | (2) |
The numerical simulations are divided into the nonmagnetic and magnetic cases. First, for nonmagnetic materials, μ′ and μ′′ are defined as 1 and 0, respectively. After reviewing the dielectric constants of an abundance of microwave-absorbing materials, ε′ is defined from 4 to 12 (interval of 2), ε′′/ε′ is defined from 0.2 to 0.8 (interval of 0.2), and the thickness is fixed from 2 to 5 mm (interval of 0.5 mm). In Fig. S1,† when the thickness and ε′′ are fixed, the matching frequency of the RL peak is related to ε′, i.e., as ε′ increases, the RL peak shifts to a lower frequency. At the same time, when the ε′ increases it also leads to a slow shift of the RL peak towards lower frequency. In addition, ε′′/ε′ affects the magnitude of the RL value, and a smaller ε′′/ε′ yields a smaller RL value when the ε′ increases. The variation of the RL peak corresponding to the frequency (RLmin@Frequency) with the complex dielectric constant can be clearly seen in Fig. S3a.† It can be seen that the RLmin@Frequency moves to lower frequencies as ε′ increases. Therefore, for non-magnetic materials, an increase in ε′ and an appropriate decrease in ε′′/ε′ are required to achieve strong absorption at low frequencies.
Similarly, for a magnetic absorber, ε′ and ε′′/ε′ are defined as 6 and 0.4, while μ′ is defined from 1 to 4 (interval of 1), μ′′/μ′ is defined from 0.2 to 0.8 (interval of 0.2), and the thickness is fixed from 2 to 5 mm (interval of 0.5 mm). As in Fig. S2,† the RLmin@Frequency moves to a lower frequency with increasing μ′, while μ′′/μ′ determines the magnitude of RL value. It is worth noting that an increase in μ′ and μ′′/μ′ also significantly lead to an increase of EAB, which can cover 2–18 GHz at its maximum. As shown in Fig. S3b,† It is clearly observed that as both μ′ and μ′′ increase, RL peaks shift to lower frequency, and the shift with increasing μ′ is more pronounced. Therefore, for magnetic absorbers, larger μ′ and μ′′ not only favor the shift of RL to a lower frequency, but also result in a wider EAB.
Moreover, the RL peak of the absorber also shifts to a lower frequency with increasing thickness, which can be explained by the quarter-wavelength model as follows:31,32
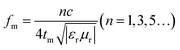 | (3) |
In summary, there are two ways to achieve low-frequency absorption: (1) sufficiently large ε′ and small ε′′/ε′ values; (2) introduction of strong magnetic losses. So, in our experiments, the rGO aerogel with high electrical conductivity is selected as the substrate to provide the ε′ values. Furthermore, classical magnetic metals (Fe, Co, and Ni) and their neighboring nonmagnetic metals (Mn and Cu) are introduced to introduce magnetic losses or reduced ε′′/ε′ values to achieve strong absorption at low frequency.
3.2. Multifunctional M@C-rGO aerogels
The phase and crystal structure of the samples are determined by X-ray diffraction (XRD) as shown in Fig. 2a. It is clearly observed that the wide peaks around 25° in all samples belong to the (002) crystal plane of rGO, indicating that GO is reduced to rGO in the calcination.33 Similarly, the metal sphere precursors Co–P, Ni–P and Cu–P are reduced to Co, Ni and Cu, respectively. In particular, the temperatures do not reach the reduction temperatures of Fe and Mn, thus the XRD patterns of Mn@C-rGO and Fe@C-rGO have peaks of Fe3O4 and MnO.4,34 Moreover, X-ray photoelectron spectroscopy (XPS) analysis of the samples can further reveal their chemical composition and elemental valence states. In Fig. 2b, the XPS survey spectrum of sample C-rGO has only peaks for C 1s, N 1s and O 1s, while the peaks of Mn 2p, Fe 2p, Co 2p, Ni 2p and Cu 2p are also observed for the M@C-rGO aerogels with the addition of the corresponding metal microspheres. Specifically, the high-resolution spectrum of C 1s as in Fig. S7a† can be fitted to four characteristic peaks 284.7, 285.3, 286.2 and 288.5 eV belonging to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N, C–O and C
C, C–N, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.34,35 Here, chitosan is rich in nitrogen, and the doped nitrogen atoms exist in the form of pyridine N (398.7 eV), pyrrole N (400.5 eV) and graphite N (403.4 eV) as shown in Fig. S7b.36,37† The difference in electronegativity between carbon and nitrogen atoms alters the distribution of the electron clouds, which promotes the generation and vibration of electric dipoles. This helps to induce dipole polarization and contributes to the attenuation of electromagnetic waves.4 In the O 1s high-resolution spectrum of C-rGO (Fig. S7c†), the peaks at 532.2 eV and 532.5 eV belong to C–O bonds and oxygen in adsorbed water, respectively.38 Noteworthily, after loading the metal microspheres, the synthesized M@C-rGO aerogels show an obvious peak of metal–oxygen at about 530.3 eV.39 Furthermore, as shown in Fig. S8,† 2p2/3 and 2p2/1 of the metals Mn, Fe, Co, Ni, and Cu are clearly observed, indicating the successful loading of the metal microspheres.
O bonds, respectively.34,35 Here, chitosan is rich in nitrogen, and the doped nitrogen atoms exist in the form of pyridine N (398.7 eV), pyrrole N (400.5 eV) and graphite N (403.4 eV) as shown in Fig. S7b.36,37† The difference in electronegativity between carbon and nitrogen atoms alters the distribution of the electron clouds, which promotes the generation and vibration of electric dipoles. This helps to induce dipole polarization and contributes to the attenuation of electromagnetic waves.4 In the O 1s high-resolution spectrum of C-rGO (Fig. S7c†), the peaks at 532.2 eV and 532.5 eV belong to C–O bonds and oxygen in adsorbed water, respectively.38 Noteworthily, after loading the metal microspheres, the synthesized M@C-rGO aerogels show an obvious peak of metal–oxygen at about 530.3 eV.39 Furthermore, as shown in Fig. S8,† 2p2/3 and 2p2/1 of the metals Mn, Fe, Co, Ni, and Cu are clearly observed, indicating the successful loading of the metal microspheres.
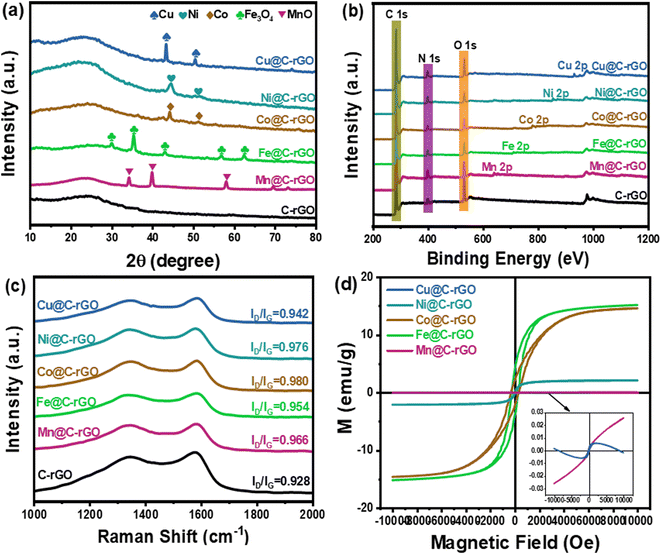 | ||
| Fig. 2 Compositional characterization: (a) the XRD patterns, (b) XPS survey spectra, (c) Raman spectra and (d) the hysteresis loop of the samples. | ||
Besides, the degree of graphitization of the composites is characterized by the Raman spectrum. The characteristic band at ∼1350 cm−1 (D-band) is attributed to disordered carbon, while the band at ∼1590 cm−1 (G-band) belongs to the in-plane stretching vibration of sp2 hybrid carbon atoms.29,40 Thus, the intensity ratio between the D-band and G-band (ID/IG) can reveal the degree of graphitization of the material.41 As illustrated in Fig. 2c, the ID/IG of the C-rGO aerogel is only 0.928, while those of Mn@C-rGO, Fe@C-rGO, Co@C-rGO, Ni@C-rGO and Cu@C-rGO aerogels are 0.966, 0.954, 0.980, 0.976 and 0.942, respectively. It is clear that the loading of metal microspheres introduces more defects into the M@C-rGO aerogels and decreases the degree of graphitization of the carbon material. The decrease in graphitization decreases the electrical conductivity of the composites leading to a lower ε′′, while the increase in defects results in more dipole polarization loss to the samples. Furthermore, different metal microspheres are crucial for modulating the magnetic properties of M@C-rGO aerogels.
Theoretically, μr is closely related to the saturation magnetization (Ms) strength and coercivity (Hc) which can be expressed as follows:42
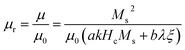 | (4) |
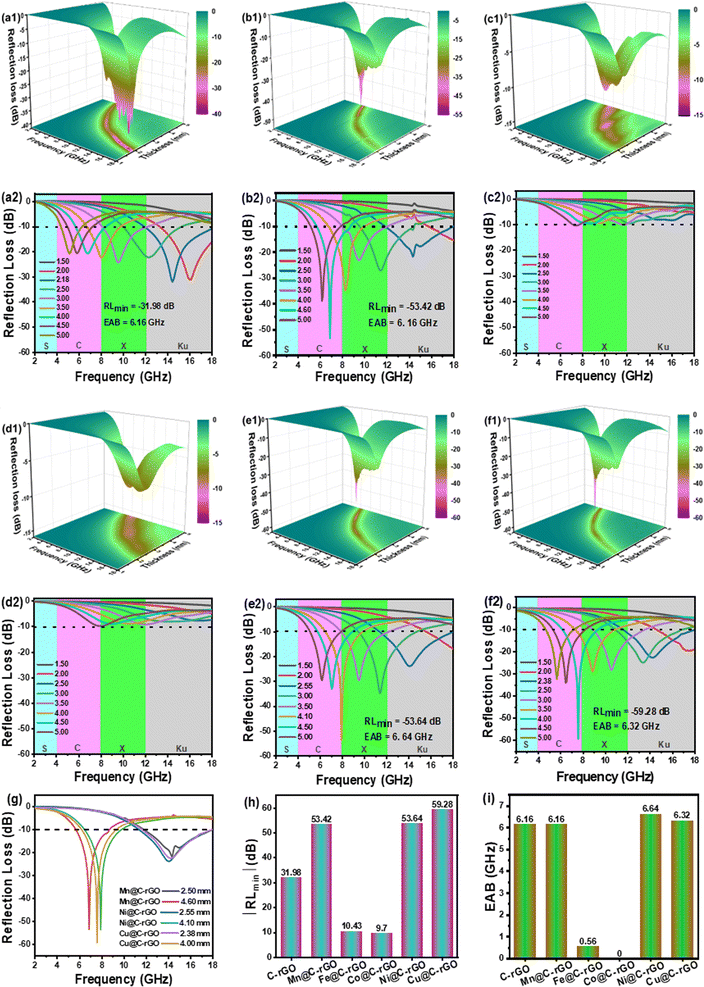 | ||
| Fig. 3 The 3D RL contour plots and RL values of (a) C-rGO, (b) Mn@C-rGO, (c) Fe@C-rGO, (d) Co@C-rGO, (e) Ni@C-rGO and (f) Cu@C-rGO. The comparison of (g) RL values, (h) RLmin and (i) EAB for samples. | ||
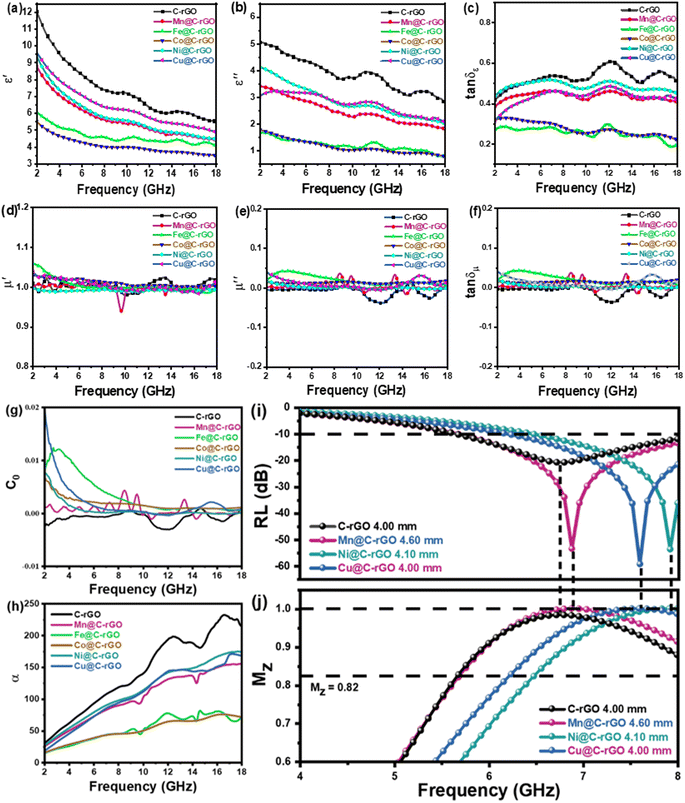
The microwave absorption properties of the synthesized aerogels and other rGO-based absorbers were further analyzed and compared as shown in Fig. 4 and Table S1.† Mn@C-rGO, Ni@C-rGO, and Cu@C-rGO exhibit a wide EAB of 6.16, 6.64 and 6.32 GHz, respectively, outperforming most of the rGO-based absorbers. Furthermore, Mn@C-rGO, Ni@C-rGO, and Cu@C-rGO show strong microwave absorption (RLmin = −53.42, −53.64 and −59.28 dB) in the lower frequency bands of 6.88, 7.92 and 7.6 GHz with thicknesses of 4.60, 4.10, and 4.00 mm, respectively. In fact, an absorber with a wide bandwidth and a strong absorption capacity has a huge potential for applications. Thus, the synthesized aerogels can be among the advanced microwave absorbers.
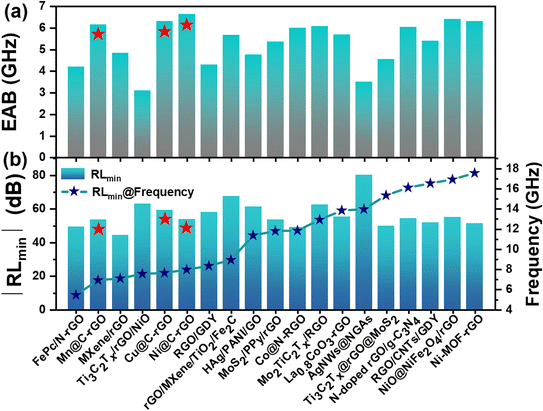 | ||
| Fig. 4 Comparison of microwave absorption properties: (a) EAB and (b) RLminversus f of reported graphene-based absorbers. | ||
It is well known that the real (ε′) and imaginary (ε′′) parts of the complex dielectric constant reflect the storage and loss ability of electrical energy, respectively.45 As seen from Fig. 5a and b, the decreasing trend of the permittivity with increasing frequency for all samples is due to the dispersion effect, which is caused by the fact that the orientation of the electric dipole lags behind the periodical variation of the electromagnetic field.29 In particular, C-rGO shows the highest ε′ value decreasing from 12.03 to 5.53 due to the higher conductivity of the carbon material. Then, with the addition of metal microspheres, the complex permittivity of M@C-rGO aerogels decreases to different degrees in both the real and imaginary parts. In detail, the values of ε′ decrease from 8.80 to 4.42, 6.06 to 4.05, 5.50 to 3.50, 9.41 to 4.46 and 9.62 to 4.87 for the Mn@C-rGO, Fe@C-rGO, Co@C-rGO, Ni@C-rGO and Cu@C-rGO, respectively. Similarly, a decrease of ε′′ could be observed with the increase in the frequency in the range of 3.40–1.80, 1.66–0.80, 1.77–0.76, 4.11–2.01 and 3.03–2.06 for the five samples, respectively. Furthermore, the tangent of permittivity (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε = ε′′/ε′) can further reveal the dielectric loss properties of the material. As shown in Fig. 5c, the tan
δε = ε′′/ε′) can further reveal the dielectric loss properties of the material. As shown in Fig. 5c, the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε values of the samples exhibit similar variations in the order of C-rGO > Ni@C-rGO > Cu@C-rGO > Mn@C-rGO > Co@C-rGO > Fe@C-rGO. According to the previous numerical simulations, C-rGO with the maximum ε′ favors the shift of the RL peak to a lower frequency, but the larger ε′′/ε′ prevents it from achieving strong microwave absorption at lower frequency. Obviously, the composite of metal microspheres can effectively modulate the dielectric constant of the synthesized aerogel. In particular, Cu@C-rGO maintains a large ε′ along with a small ε′′/ε′, thus it exhibits a minimum RLmin at the C-band, which is in accordance with the results of the above numerical simulations for nonmagnetic materials.
δε values of the samples exhibit similar variations in the order of C-rGO > Ni@C-rGO > Cu@C-rGO > Mn@C-rGO > Co@C-rGO > Fe@C-rGO. According to the previous numerical simulations, C-rGO with the maximum ε′ favors the shift of the RL peak to a lower frequency, but the larger ε′′/ε′ prevents it from achieving strong microwave absorption at lower frequency. Obviously, the composite of metal microspheres can effectively modulate the dielectric constant of the synthesized aerogel. In particular, Cu@C-rGO maintains a large ε′ along with a small ε′′/ε′, thus it exhibits a minimum RLmin at the C-band, which is in accordance with the results of the above numerical simulations for nonmagnetic materials.
In addition, the Cole–Cole curve can describe the relationship between the real and imaginary parts of the complex permittivity. According to the Debye theory, the complex permittivity can be expressed by the following equations:46
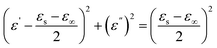 | (5) |
As shown in Fig. 5d and e, the real part (μ′) and imaginary part (μ′′) of the magnetic permeability are close to 1 and 0, respectively. Moreover, as presented in Fig. 5c and f, the dielectric loss is higher than the magnetic loss for all samples, indicating that the dielectric loss is the dominant factor in the microwave absorption of electromagnetic waves. Generally, the magnetic loss of an absorber is mainly due to hysteresis loss, domain wall resonance, eddy current loss, natural resonance and exchange resonance. Meanwhile, the domain wall resonance and hysteresis loss can be neglected at the frequency range of 2.0–18.0 GHz.46 Herein, the C0 curve, defined as C0 = μ′′(μ′)−2f−1, can be analyzed for the contribution of eddy current losses to the magnetic loss.38 If C0 is constant at different frequencies, the magnetic loss of the absorber is dominated by eddy current loss.8,50Fig. 5g shows the C0–f curve, where a significant fluctuation of C0 within 2–8 GHz can be observed, indicating that the magnetic loss mainly results from the natural resonance. Simultaneously, C0 remains constant from 10 to 18 GHz, indicating that the magnetic loss is mainly determined by eddy current loss in this frequency range. Here, the natural resonance in the low-frequency band of Fe@C-rGO and Co@C-rGO, which have strong magnetic properties, is more significant compared to the other samples. According to previous numerical simulation results, strong magnetic loss will shift the peak reflection loss towards lower frequencies. However, the magnetic metal microspheres provide negligible magnetic loss to the composite aerogel, which differs significantly from the numerical simulation of the magnetic absorber. Meanwhile, the poorer electrical conductivity of the magnetic metal microspheres also leads to a significant decrease in the ε′ value. Therefore, Fe@C-rGO and Co@C-rGO failed to achieve superior low-frequency microwave absorption properties.
Typically, the attenuation constant and the impedance matching coefficient are the key parameters that affect the microwave absorption performance of the absorber. In particular, the attenuation constant reflects the ability of the absorber to attenuate the electromagnetic wave, while the impedance matching determines whether the electromagnetic wave can enter the absorber. Specifically, the impedance matching coefficient (MZ) and attenuation constant (α) are calculated using the following equations:31,51
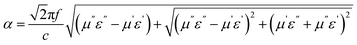 | (6) |
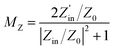 | (7) |
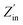 is the real part of the input impedance. As shown in Fig. 5h, C-rGO shows the largest attenuation constant, and the attenuation constants of Mn@C-rGO, Ni@C-rGO, and Cu@C-rGO decrease with the addition of metal microspheres. However, C-rGO does not display good microwave absorption performance owing to its poor impedance matching. Here, the impedance matching of the absorber is revealed by the MZ value, where the closer the MZ value is to 1 the better impedance matching of the microwave absorber is. Moreover, when MZ >0.82, the corresponding RL value is lower than −10 dB. Obviously, Mn@C-rGO, Ni@C-rGO, and Cu@C-rGO show excellent impedance matching in the C-band, and the frequency of MZ = 1 corresponds exactly to RLmin, proving that the absorber impedance matching is closely related to the microwave absorption performance, as shown in Fig. 5i, and j. In contrast, although the RL peak of C-rGO is shifted to lower frequencies at the same thickness, it fails to achieve strong microwave absorption due to its poor impedance matching, i.e., most of the electromagnetic wave is reflected at the surface of the absorber. Remarkably, the EABs of C-rGO, Mn@C-rGO, Ni@C-rGO and Cu@C-rGO in the C-band are 2.32, 2.32, 1.52 and 1.76 GHz, respectively. Therefore, regulating the electromagnetic parameters of the material to achieve a balance between the attenuation constant and impedance matching is crucial to obtaining an absorber with superior microwave absorption performance.
is the real part of the input impedance. As shown in Fig. 5h, C-rGO shows the largest attenuation constant, and the attenuation constants of Mn@C-rGO, Ni@C-rGO, and Cu@C-rGO decrease with the addition of metal microspheres. However, C-rGO does not display good microwave absorption performance owing to its poor impedance matching. Here, the impedance matching of the absorber is revealed by the MZ value, where the closer the MZ value is to 1 the better impedance matching of the microwave absorber is. Moreover, when MZ >0.82, the corresponding RL value is lower than −10 dB. Obviously, Mn@C-rGO, Ni@C-rGO, and Cu@C-rGO show excellent impedance matching in the C-band, and the frequency of MZ = 1 corresponds exactly to RLmin, proving that the absorber impedance matching is closely related to the microwave absorption performance, as shown in Fig. 5i, and j. In contrast, although the RL peak of C-rGO is shifted to lower frequencies at the same thickness, it fails to achieve strong microwave absorption due to its poor impedance matching, i.e., most of the electromagnetic wave is reflected at the surface of the absorber. Remarkably, the EABs of C-rGO, Mn@C-rGO, Ni@C-rGO and Cu@C-rGO in the C-band are 2.32, 2.32, 1.52 and 1.76 GHz, respectively. Therefore, regulating the electromagnetic parameters of the material to achieve a balance between the attenuation constant and impedance matching is crucial to obtaining an absorber with superior microwave absorption performance.
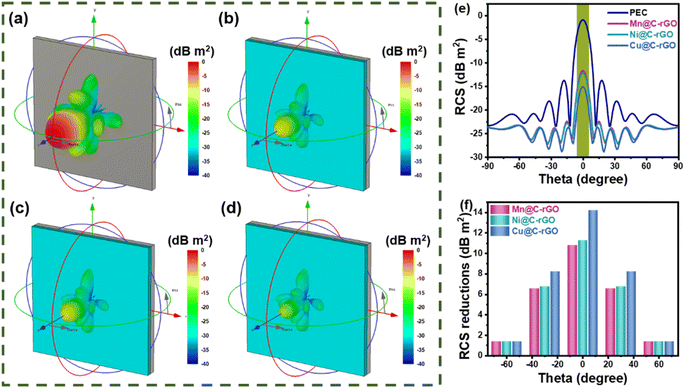
Most of the traditional military equipment contains metal components with strong electromagnetic scattering characteristics, which are easily recognized by radar detection. The coating of microwave-absorbing materials on the surface of military equipment can effectively reduce the RCS value to improve the stealth capability. Typically, a certain scattering direction is determined by theta and phi in spherical coordinates, and the RCS value (σ) is calculated as follows:2,17
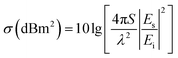 | (8) |
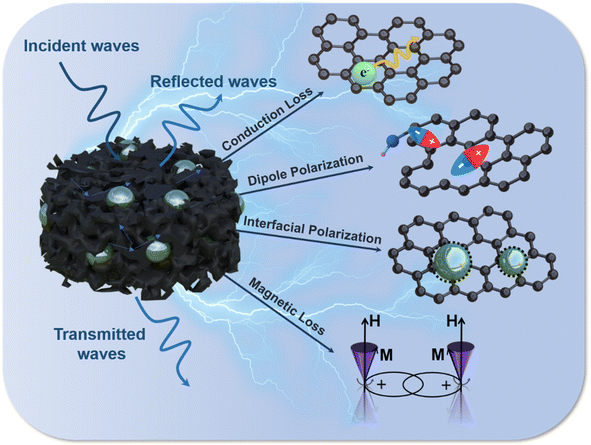
Therefore, based on the above analysis, the possible microwave absorption mechanism of the synthesized M@rGO aerogels is presented in Fig. 7, which can be described as follows. (1) Multiple reflection and scattering. The sublimation of ice crystals builds a unique 3D porous structure for the M@rGO aerogels, which facilitates multiple reflections and scattering of electromagnetic waves within it, thus promoting multiple attenuation of electromagnetic waves. (2) Conduction loss. The 3D conductive network formed by cross-linking between reduced graphene oxide nanosheets provides a transmission channel for electron migration and hopping, which can effectively accelerate the electron transfer rate. In the transmission process of electrons, the electric field energy will be converted into thermal energy, and then the attenuation of the electromagnetic wave takes place. Moreover, the high electrical conductivity of the material results in a high complex dielectric constant, which leads to the efficient absorption of low-frequency electromagnetic waves. (3) Polarization loss. The abundance of defects and polar functional groups in the composites facilitates the formation of polarization centers, which induces dipole polarization. Furthermore, under the action of alternating electromagnetic field, due to the difference in conductivity between the metal sphere and rGO, the high-density positive and negative charges tend to accumulate at the interface of the metal sphere and rGO, forming a double capacitance structure, which can produce interface polarization and improve the dielectric loss. (4) Magnetic loss. The magnetic metal microspheres can provide magnetic loss to the synthesized aerogel, especially the natural resonance at low-frequency.
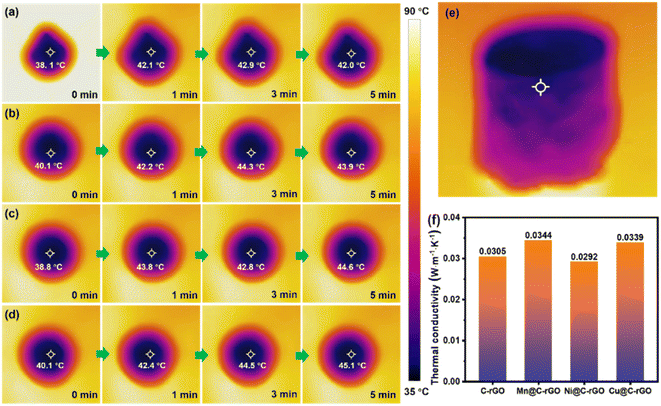 | ||
| Fig. 8 The infrared images of (a) C-rGO, (b) Mn@C-rGO, (c) Ni@C-rGO and (d) Cu@C-rGO placed on the heat source at 90 °C. (e) The side-view infrared image of Cu@C-rGO and (f) thermal conductivity. | ||
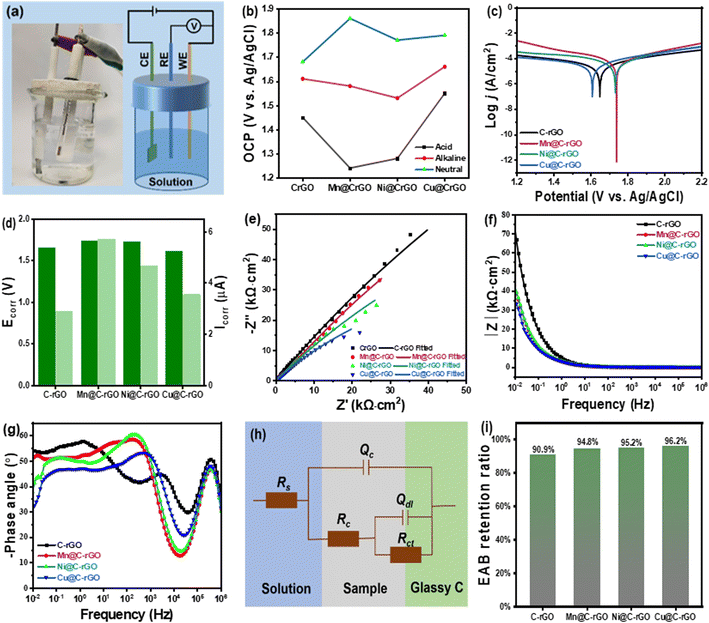
Furthermore, the anticorrosive properties of the composite aerogel are investigated using electrochemical measurements with a three-electrode system in Fig. 9a. Generally, a higher open-circuit potential (OCP) indicates that the sample has a weak self-corrosion tendency.55,56 As shown in Fig. 9b, the corrected OCP shows a trend of OCPneutral > OCPalkaline > OCPacid, indicating that the samples show the strongest corrosion resistance under neutral conditions and the weakest under acidic conditions. Fig. 9c and d display the Tafel curves of composite aerogels in a neutral 3.5 wt% NaCl solution and the related corrosion parameters, including the corrosion voltage (Ecorr) and the corrosion current (Icorr). Notably, the C-rGO, Mn@C-rGO, Ni@C-rGO and Cu@C-rGO demonstrate excellent anticorrosion ability due to their high Ecorr values (1.65, 1.74, 1.73 and 1.61 V) and low Icorr values (2.88, 5.71, 4.68 and 3.55 μA). The fluctuations in OCP, Ecorr and Icorr for different absorbers may be related to the activity of the inner metal microspheres. Moreover, the anticorrosion resistance of M@C-rGO can be further revealed by electrochemical impedance spectroscopy (EIS) measurement, as shown in Fig. 9e–g. The big radii of the capacitive arcs of the four absorbers in the Nyquist plots suggest their large charge transfer resistances. Typically, the impedance modulus at 0.01 Hz (|Z|0.01 Hz) is considered a semi-quantitative indicator for evaluating the system impedance and ability to shield corrosive media.57 As shown in Fig. 9f, the |Z|0.01 Hz values of the four samples reach 104 Ω cm2 under neutral conditions, where C-rGO has the largest |Z|0.01 Hz value of 7.52 × 104 Ω cm2, indicating excellent anticorrosion properties. In addition, the high-frequency phase angle is a crucial indicator for evaluating the corrosion resistance of materials. The large high-frequency phase angle of the composite aerogels indicates their excellent anticorrosion properties (Fig. 9g).58 To investigate further the corrosion mechanism, the EIS data are fitted using ZView software with the equivalent circuit of Fig. 9h, where Rs, Rc and Rct represent the solution resistance, coating capacitance and charge transfer resistance, respectively, and Qc and Qdl are constant phase components. Generally, the Rct value is a crucial indicator for evaluating the anticorrosion performance of materials.59 Remarkably, the Rct values of C-rGO, Mn@C-rGO, Ni@C-rGO and Cu@C-rGO are 5.841 × 105, 3.971 × 105, 2.885 × 105 and 1.421 × 105 Ω cm2 respectively as shown in Table S2,† indicating that the synthesized aerogels have excellent corrosion resistance. Similarly, the samples also have superior corrosion resistance under acidic and alkaline conditions, as shown in Fig. S10 and S11.† Thus, the excellent corrosion resistance of synthetic aerogels is mainly attributed to the unique porous structure that can extend the penetration path of corrosive media through the “maze effect”.55
In order to visually investigate the effect of corrosion on the microwave absorption performance of the samples, the samples are soaked in 3.5 wt% NaCl solution for 7 days. As shown in Fig. S12,† after 7 days of corrosion, the microwave absorption performance of the samples decreased but still demonstrated low-frequency absorption properties. Notably, all samples retained more than 90% of the EAB after corrosion. Therefore, excellent thermal insulation and anticorrosion protection properties favor the application of M@CrO aerogels in complex environments.
4. Conclusion
In summary, the M@C-rGO aerogel as a multifunctional nanomaterial was prepared via a facile ice template method and subsequent pyrolysis. Notably, the synthesized aerogels exhibit strong absorption in the low-frequency band and high-frequency band, where Mn@C-rGO, Ni@C-rGO, and Cu@C-rGO exhibit strong microwave absorption in the C-band with RL up to −53.42, −53.64 and −59.28 dB, respectively. More importantly, Mn@C-rGO, Ni@C-rGO, and Cu@C-rGO also have a wide EAB in the high-frequency band up to 6.16, 6.64 and 6.32 GHz, respectively. Moreover, the synthesized aerogels also exhibit superior radar stealth, thermal management and anticorrosion capability, wherein the thermal conductivities of Mn@C-rGO, Ni@C-rGO, and Cu@C-rGO are 0.0344, 0.0292 and 0.0339 W m−1 K−1. Meanwhile, the Rct values of Mn@C-rGO, Ni@C-rGO, and Cu@C-rGO are up to 3.971 × 105, 2.885 × 105 and 1.421 × 105 Ω cm2, respectively. This work provides a new thought to design low-frequency microwave absorbers with good thermal management and anticorrosion capability. Also, it offers new insights into the preparation of a new generation of multifunctional materials in the field of microwave absorption, thermal insulation and anticorrosion, and the extended applications of the corresponding materials will be achieved.Data availability
All data required to understand and verify the research in our manuscript have been made available on submission, and can be made available in this way, as part of the article (ESI),† or both.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 52071009, 12011530067 and 11774020).References
- X. Li, D. Xu, D. Zhou, S. Pang, C. Du, M. A. Darwish, T. Zhou and S. K. Sun, Vertically Stacked Heterostructures of MXene/rGO Films with Enhanced Gradient Impedance for High-Performance Microwave Absorption, Carbon, 2023, 208, 374–383 CrossRef CAS.
- X. Chen, Y. Li, S. Cheng, K. Wu, Q. Wang, L. Liu, F. Yang, A. Xie, H. Pang and C. Yu, Liquid Metal-MXene-Based Hierarchical Aerogel with Radar-Infrared Compatible Camouflage, Adv. Funct. Mater., 2024, 34, 2308274 CrossRef CAS.
- L. Wang, R. Mao, M. Huang, H. Jia, Y. Li, X. Li, Y. Cheng, J. Liu, J. Zhang, L. Wu and R. Che, Heterogeneous Interface Engineering Of High-Density MOFs-Derived Co Nanoparticles Anchored on N-Doped RGO toward Wide-Frequency Electromagnetic Wave Absorption, Mater. Today Phys., 2023, 35, 101128 CrossRef CAS.
- S. Wang, X. Zhang, S. Hao, J. Qiao, Z. Wang, L. Wu, J. Liu and F. Wang, Nitrogen-Doped Magnetic-Dielectric-Carbon Aerogel for High-Efficiency Electromagnetic Wave Absorption, Nano-Micro Lett., 2024, 16, 16 CrossRef CAS PubMed.
- H. Liu, Y. Yang, N. Tian, C. You and Y. Yang, Foam-Structured Carbon Materials and Composites for Electromagnetic Interference Shielding: Design Principles and Structural Evolution, Carbon, 2024, 217, 118608 CrossRef CAS.
- H. Wei, W. Li and K. Bachagha, Component Optimization and Microstructure Design of Carbon Nanotube-Based Microwave Absorbing Materials: A review, Carbon, 2024, 217, 118651 CrossRef CAS.
- X. Chen, Y. Li, S. Cheng, K. Wu, Q. Wang, L. Liu, F. Yang, A. Xie, H. Pang and C. Yu, Liquid Metal-MXene-Based Hierarchical Aerogel with Radar-Infrared Compatible Camouflage, Adv. Funct. Mater., 2024, 34, 2308274 CrossRef CAS.
- Y. Guo, Y. Duan, X. Liu, H. Zhang, T. Yuan, N. Wen, C. Li, H. Pan, Z. Fan and L. Pan, Boosting Conductive Loss and Magnetic Coupling Based on “Size Modulation Engineering” toward Lower-Frequency Microwave Absorption, Small, 2023, 20, 2308809 CrossRef PubMed.
- M. Yuan, H. Lv, H. Cheng, B. Zhao, G. Chen, J. Zhang and R. Che, Atomic and Electronic Reconstruction in Defective 0 D Molybdenum Carbide Heterostructure for Regulating Lower-Frequency Microwaves, Adv. Funct. Mater., 2023, 33, 2302003 CrossRef CAS.
- Z. Li, H. Zhu, L. Rao, M. Huang, Y. Qian, L. Wang, Y. Liu, J. Zhang, Y. Lai and R. Che, Wrinkle Structure Regulating Electromagnetic Parameters in Constructed Core–shell ZnFe2O4@PPy Microspheres as Absorption Materials, Small, 2024, 20, 2308581 CrossRef CAS PubMed.
- J. Xiao, B. Zhan, M. He, X. Qi, X. Gong, J. Yang, Y. Qu, J. Ding, W. Zhong and J. Gu, Interfacial Polarization Loss Improvement Induced by the Hollow Engineering of Necklace-like PAN/Carbon Nanofibers for Boosted Microwave Absorption, Adv. Funct. Mater., 2024, 2316722 CrossRef.
- Q. Su, Y. He, D. Liu, K. Jia, L. Xia, X. Huang and B. Zhong, Facile Fabrication of Ultra-Light N-Doped-rGO/g-C3N4 for Broadband Microwave Absorption, J. Colloid Interface Sci., 2023, 650, 47–57 CrossRef CAS PubMed.
- C. Wu, Z. Chen, M. Wang, X. Cao, Y. Zhang, P. Song, T. Zhang, X. Ye, Y. Yang, W. Gu, J. Zhou and Y. Huang, Confining Tiny MoO2 Clusters into Reduced Graphene Oxide for Highly Efficient Low Frequency Microwave Absorption, Small, 2020, 16, 2001686 CrossRef CAS PubMed.
- J. Zhou, F. Guo, J. Luo, G. Hao, G. Liu, Y. Hu, G. Zhang, H. Guo, H. Zhou and W. Jiang, Designed 3D Heterostructure with 0D/1D/2D Hierarchy for Low-Frequency Microwave Absorption in the S-band, J. Mater. Chem. C, 2022, 10, 1470–1478 RSC.
- L. Chang, Y. Wang, X. Zhang, L. Li, H. Zhai and M. Cao, Toward High Performance Microwave Absorber by Implanting La0.8CoO3 Nanoparticles on rGO, J. Mater. Sci. Technol., 2024, 174, 176–187 CrossRef CAS.
- S. Wu, D. Chen, W. Han, Y. Xie, G. Zhao, S. Dong, M. Tan, H. Huang, S. Xu, G. Chen, Y. Cheng and X. Zhang, Ultralight and Hydrophobic Mxene/Chitosan-Derived Hybrid Carbon Aerogel with Hierarchical Pore Structure for Durable Electromagnetic Interference Shielding and Thermal Insulation, Chem. Eng. J., 2022, 446, 137093 CrossRef CAS.
- L. Rao, L. Wang, C. Yang, R. Zhang, J. Zhang, C. Liang and R. Che, Confined Diffusion Strategy for Customizing Magnetic Coupling Spaces to Enhance Low-frequency Electromagnetic Wave Absorption, Adv. Funct. Mater., 2023, 33, 2213258 CrossRef CAS.
- C. Zheng, M. Ning, Z. Zou, G. Lv, Q. Wu, J. Hou, Q. Man and R. Li, Two Birds with One Stone: Broadband Electromagnetic Wave Absorption and Anticorrosion Performance in 2-18 GHz for Prussian Blue Analog Derivatives Aimed for Practical Applications, Small, 2023, 19, 2208211 CrossRef CAS PubMed.
- J. Li, Q. Wu, X. Wang, B. Wang and T. Liu, Metal-Organic Framework-Derived Co/Coo Nanoparticles with Tunable Particle Size for Strong Low-Frequency Microwave Absorption in the S and C Bands, J. Colloid Interface Sci., 2022, 628, 10–21 CrossRef CAS PubMed.
- L. Rao, L. Wang, C. Yang, R. Zhang, J. Zhang, C. Liang and R. Che, Confined Diffusion Strategy for Customizing Magnetic Coupling Spaces to Enhance Low-Frequency Electromagnetic Wave Absorption, Adv. Funct. Mater., 2023, 33, 2213258 CrossRef CAS.
- M. Ling, P. Liu, F. Wu and B. Zhang, Three-dimensional RGO/CNTs/GDY Assembled Microsphere: Bridging-Induced Electron Transport Enhanced Microwave Absorbing Mechanism, Carbon, 2023, 214, 118351 CrossRef CAS.
- H. Liu, Y. Yang, N. Tian, C. You and Y. Yang, Foam-Structured Carbon Materials and Composites for Electromagnetic Interference Shielding: Design Principles and Structural Evolution, Carbon, 2024, 217, 118608 CrossRef CAS.
- X. Xu, S. Shi, Y. Tang, G. Wang, M. Zhou, G. Zhao, X. Zhou, S. Lin and F. Meng, Growth of NiAl-Layered Double Hydroxide on Graphene toward Excellent Anticorrosive Microwave Absorption Application, Adv. Sci., 2021, 8, 2002658 CrossRef CAS PubMed.
- J. Yao, J. Zhou, L. Lu, F. Yang, Z. Yao, B. Ouyang, E. Kan, Y. Zuo, R. Che and F. Wu, Rare Earth Lanthanum Pinning Effect for Corrosion Resistance Ultraefficient Microwave Absorption FeCo@rGO Composites, J. Mater. Sci. Technol., 2024, 177, 181–190 CrossRef CAS.
- L. Yao, Y. Wang, J. Zhao, Y. Zhu and M. Cao, Multifunctional Nanocrystalline-Assembled Porous Hierarchical Material and Device for Integrating Microwave Absorption, Electromagnetic Interference Shielding, and Energy Storage, Small, 2023, 19, 2208101 CrossRef CAS PubMed.
- J. Zhang, L. Chen, X. Li, H. Cao, W. Chen and X. Wang, Regulation Dipole Moments of N-Doped Graphene Coordinated with FePc Toward Highly Efficient Microwave Absorption Performance in C Band, Small, 2024, 2308459 CrossRef CAS PubMed.
- Z. Tang, L. Xu, C. Xie, L. Guo, L. Zhang, S. Guo and J. Peng, Synthesis of CuCo2S4@Expanded Graphite with Crystal/Amorphous Heterointerface and Defects for Electromagnetic Wave Absorption, Nat. Commun., 2023, 14, 5951 CrossRef CAS PubMed.
- H. Jin, J. Zhou, J. Tao, Y. Gu, C. Yu, P. Chen and Z. Yao, Commonly Neglected Ester Groups Enhanced Microwave Absorption, Small, 2023, 19, 2304536 CrossRef CAS PubMed.
- J. Qiu, C. Peng, R. Wang, C. Yao, X. Liu, Q. Wang and W. Wang, One-step In-Situ Preparation Of C/TiO2@rGO Aerogel Derived from Ti3C2T MXene for Integrating Microwave Absorption, Electromagnetic Interference Shielding and Catalytic Degradation of Antibiotics, Carbon, 2024, 217, 118610 CrossRef CAS.
- C. Peng, R. Wang, C. Yao, J. Qiu, X. Liu, Q. Wang and W. Wang, Nest-like Hollow Cu-Doped Co/CoO/C Microspheres Derived from Spherical Co-ZIF with Controllable Microwave Absorption Performance, Appl. Surf. Sci., 2023, 641, 158496 CrossRef CAS.
- Z. L. Hou, X. Gao, J. Zhang and G. Wang, A Perspective On Impedance Matching and Resonance Absorption Mechanism for Electromagnetic Wave Absorbing, Carbon, 2024, 222, 118935 CrossRef CAS.
- D. Yang, S. Dong, T. Cui, J. Xin, X. Xu, J. Chen, Y. Xie, G. Chen, C. Hong and X. Zhang, Multifunctional Carbon Fiber Reinforced C/SiOC Aerogel Composites for Efficient Electromagnetic Wave Absorption, Thermal Insulation, and Flame Retardancy, Small, 2023, 2308145 Search PubMed.
- T. Zhu, S. Chang, Y. F. Song, M. Lahoubi and W. Wang, PVP-Encapsulated CoFe2O4/rGO Composites with Controllable Electromagnetic Wave Absorption Performance, Chem. Eng. J., 2019, 373, 755–766 CrossRef CAS.
- M. Qin, L. Zhang, X. Zhao and H. Wu, Defect Induced Polarization Loss in Multi-Shelled Spinel Hollow Spheres for Electromagnetic Wave Absorption Application, Adv. Sci., 2021, 8, 2004640 CrossRef CAS PubMed.
- T. Zhu, W. Shen, X. Wang, Y. F. Song and W. Wang, Paramagnetic CoS2@MoS2 Core–Shell Composites Coated by Reduced Graphene Oxide as Broadband and Tunable High-Performance Microwave Absorbers, Chem. Eng. J., 2019, 378, 122159 CrossRef CAS.
- X. Wang, J. Liao, R. Du, G. Wang, N. Tsidaeva and W. Wang, Achieving Super-Broad Effective Absorption Bandwidth with Low Filler Loading for Graphene Aerogels/Raspberry-Like CoFe2O4 Clusters by N Doping, J. Colloid Interface Sci., 2021, 590, 186–198 CrossRef CAS PubMed.
- P. Liu, Y. Zhang, J. Yan, Y. Huang, L. Xia and Z. Guang, Synthesis of Lightweight N-Doped Graphene Foams with Open Reticular Structure for High-Efficiency Electromagnetic Wave Absorption, Chem. Eng. J., 2019, 368, 285–298 CrossRef CAS.
- J. Qiu, H. Cao, J. Liao, R. Du, K. Dou, N. Tsidaeva and W. Wang, 3D Porous Coral-Like Co1.29Ni1.71O4 Microspheres Embedded into Reduced Graphene Oxide Aerogels with Lightweight and Broadband Microwave Absorption, J. Colloid Interface Sci., 2022, 609, 12–22 CrossRef CAS PubMed.
- F. Zhang, S. Shang, Y. Li, B. Fan, R. Zhang, B. Zhao, H. Lu and C. Ma, Tunable Electromagnetic Properties of Ti3C2Tx/rGO Foams Decorated with NiO Particles, CrystEngComm, 2022, 24, 5949–5957 RSC.
- T. Hou, J. Wang, T. Zheng, Y. Liu, G. Wu and P. Yin, Anion Exchange of Metal Particles on Carbon-Based Skeletons for Promoting Dielectric Equilibrium and High-Efficiency Electromagnetic Wave Absorption, Small, 2023, 19, 2303463 CrossRef CAS PubMed.
- M. Ling, F. Ge, F. Wu, L. Zhang, Q. Zhang and B. Zhang, Effect of Crystal Transformation on the Intrinsic Defects and the Microwave Absorption Performance of Mo2TiC2Tx/RGO Microspheres, Small, 2024, 20, 2306233 CrossRef CAS PubMed.
- M. Yuan, B. Zhao, C. Yang, K. Pei, L. Wang, R. Zhang, W. You, X. Liu, X. Zhang and R. Che, Remarkable Magnetic Exchange Coupling via Constructing Bi–Magnetic Interface for Broadband Lower-Frequency Microwave Absorption, Adv. Funct. Mater., 2022, 32, 2203161 CrossRef CAS.
- C. Wang, Y. Feng, J. Zhou, G. Wen and L. Xia, Numerical Analysis, Experimental Verification and Criterion Establishment of Non-Magnetic Microwave Absorbing Material, J. Colloid Interface Sci., 2022, 613, 256–264 CrossRef CAS PubMed.
- L. Guo, Q. D. An, Z. Y. Xiao, S. R. Zhai and L. Cui, Inherent N-Doped Honeycomb-like Carbon/Fe3O4 Composites with Versatility for Efficient Microwave Absorption and Wastewater Treatment, ACS Sustain. Chem. Eng., 2019, 7, 9237–9248 CrossRef CAS.
- X. Wang, Y. Lu, T. Zhu, S. Chang and W. Wang, CoFe2O4/N-Doped Reduced Graphene Oxide Aerogels for High-Performance Microwave Absorption, Chem. Eng. J., 2020, 388, 124317 CrossRef CAS.
- X. Wang, T. Zhu, S. Chang, Y. Lu, W. Mi and W. Wang, 3D Nest-Like Architecture of Core–Shell CoFe2O4@1T/2H-MoS2 Composites with Tunable Microwave Absorption Performance, ACS Appl. Mater. Interfaces, 2020, 12, 11252–11264 CrossRef CAS PubMed.
- T. Yu, J. Qiu, J. Liao, X. Wang, W. Chen, Y. Cheng, W. Wang and Y. F. Song, Topological Transformation Strategy for Layered Double Hydroxide@Carbon Nanofibers as Highly Efficient Electromagnetic Wave Absorber, J. Alloys Compd., 2021, 867, 159046 CrossRef CAS.
- F. Hu, H. Tang, F. Wu, P. Ding, P. Zhang, W. Sun, L. Cai, B. Fan, R. Zhang and Z. Sun, Sn Whiskers from Ti2SnC Max Phase: Bridging Dual-Functionality in Electromagnetic Attenuation, Small Methods, 2024, 2301476 CrossRef PubMed.
- J. Qiu, J. Liao, G. Wang, R. Du, N. Tsidaeva and W. Wang, Implanting N-doped CQDs into rGO Aerogels With Diversified Applications in Microwave Absorption and Wastewater Treatment, Chem. Eng. J., 2022, 443, 136475 CrossRef CAS.
- C. Peng, H. Cao, R. Wang, J. Qiu and W. Wang, Construction of ZIF/CS and ZIF/CS-Derivatives with High-Efficiency Adsorption for Dyes/Antibiotics and Strong Microwave Absorption Performance, Colloids Surf., A, 2004, 695, 134210 CrossRef.
- F. Pan, K. Pei, G. Chen, H. Guo, H. Jiang, R. Che and W. Lu, Integrated Electromagnetic Device with On–Off Heterointerface for Intelligent Switching Between Wave-Absorption and Wave-Transmission, Adv. Funct. Mater., 2023, 33, 2306599 CrossRef CAS.
- Z. Zhao, D. Lan, L. Zhang and H. Wu, A Flexible, Mechanically Strong, and Anti-Corrosion Electromagnetic Wave Absorption Composite Film with Periodic Electroconductive Patterns, Adv. Funct. Mater., 2022, 32, 2111045 CrossRef CAS.
- L. Cai, H. Jiang, F. Pan, H. Liang, Y. Shi, X. Wang, J. Cheng, Y. Yang, X. Zhang, Z. Shi, H. Wu and W. Lu, Linkage Effect Induced by Hierarchical Architecture in Magnetic MXene-based Microwave Absorber, Small, 2024, 20, 2306698 CrossRef CAS PubMed.
- F. Wu, P. Hu, F. Hu, Z. Tian, J. Tang, P. Zhang, L. Pan, M. Barsoum, L. Cai and Z. Sun, Multifunctional MXene/C Aerogels for Enhanced Microwave Absorption and Thermal Insulation, Nano-Micro Lett., 2023, 15, 194 CrossRef CAS PubMed.
- C. Zheng, M. Ning, Z. Zou, G. Lv, Q. Wu, J. Hou, Q. Man and R. Li, Two Birds with One Stone: Broadband Electromagnetic Wave Absorption and Anticorrosion Performance in 2-18 GHz for Prussian Blue Analog Derivatives Aimed for Practical Applications, Small, 2023, 19, 2208211 CrossRef CAS PubMed.
- J. Xiao, B. Zhan, M. He, X. Qi, X. Gong, J. Yang, Y. Qu, J. Ding, W. Zhong and J. Gu, Interfacial Polarization Loss Improvement Induced by the Hollow Engineering of Necklace-like PAN/Carbon Nanofibers for Boosted Microwave Absorption, Adv. Funct. Mater., 2024, 2316722 CrossRef.
- S. Chen, Y. Meng, X. Wang, D. Liu, X. Meng, X. Wang and G. Wu, Hollow tubular MnO2/MXene (Ti3C2, Nb2C, and V2C) Composites as High-Efficiency Absorbers with Synergistic Anticorrosion Performance, Carbon, 2024, 218, 118698 CrossRef CAS.
- Y. Zhang, L. Zhang, L. Tang, R. Du and B. Zhang, S-NiSe/HG Nanocomposites with Balanced Dielectric Loss Encapsulated in Room-Temperature Self-Healing Polyurethane for Microwave Absorption and Corrosion Protection, ACS Nano, 2024, 18, 8411–8422 CrossRef CAS PubMed.
- K. Li, L. Han, X. Wang, F. Gao, J. Zhang and J. Cheng, MOF-derived CoNC@rGO/Amine-Rich@rGO/Fluorinated-Epoxy Nanocomposites with EMI Shielding, Mechanical Robustness, Superamphiphobicity and Long-Term Anticorrosion Properties, Chem. Eng. J., 2023, 455, 140542 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta04051a |
| This journal is © The Royal Society of Chemistry 2024 |