Bifunctional interface stabilizer for promoting preferential crystal face adsorption and inducing planar Zn growth†
Tiancun
Liu
 *a,
Song
Lu
*a,
Song
Lu
 a,
Ronghan
Jiang
a,
Ling
Chen
b,
Xusheng
Wang
a,
Ronghan
Jiang
a,
Ling
Chen
b,
Xusheng
Wang
 d,
Yong
Wang
d,
Yong
Wang
 *b and
Zhixin
Yu
ac
*b and
Zhixin
Yu
ac
aInstitute of New Energy, School of Chemistry and Chemical Engineering, Shaoxing University, Shaoxing 312000, Zhejiang, People's Republic of China. E-mail: liutc@usx.edu.cn
bDepartment of Chemical Engineering, School of Environmental and Chemical Engineering, Shanghai University, Shanghai 200444, People's Republic of China. E-mail: yongwang@shu.edu.cn
cDepartment of Energy and Petroleum Engineering, University of Stavanger, 4036 Stavanger, Norway
dSchool of Materials Science and Engineering, Zhejiang Sci-Tech University, Hangzhou 310018, People's Republic of China
First published on 2nd September 2024
Abstract
Aqueous Zn-ion batteries (AZBs) with high safety and low cost have been considered as one of the most promising energy storage devices, but the related application is always impeded by the uneven Zn deposition and uncontrollable dendrite formation. Herein, a unique kind of organic small molecule with two zincophilic sites (O-containing and amino groups), 2-aminoethanesulfonic acid, has been used as a bifunctional interface stabilizer (BIS) for modifying the common electrolyte of AZBs. Interestingly, O-containing groups in BIS prefer to tightly adsorb on the (100) and (101) planes of Zn metal, selectively exposing the (002) plane that can guide planarized Zn growth. Meanwhile, amino groups play significant roles in attracting Zn ions and promoting homogenous distribution. According to the theoretical calculation and in/ex situ experimental observations, dendrite-free plating and reduced by-product performances can be determined. AZBs adopting functional electrolytes with BIS display improved electrochemical results. An extremely high CE value over 99.5% for 2000 cycles, stable and ultralong lifespan of 4000 h and high capacity retention of 73.8% after 700 cycles for the full cell loading MnO2 can be obtained. The exploration of BIS can provide beneficial progress for constructing stable and long-life AZBs.
1. Introduction
To curb climate change and save resources, the exploration and application of new clean energy, such as wind and solar energy, are necessary for the development of the society. Meanwhile, the important issue of energy storage should be seriously considered. Owing to their low cost and high safety, aqueous Zn-ion batteries (AZBs) employing a nonflammable electrolyte and Zn metal electrode possess great potential in large-scale energy storage.1,2 Regarding the Zn metal anode, a reasonable specific capacity (820 mA h g−1 in theory) and low redox potential (−0.76 V vs. SHE) make AZBs more attractive when matched with common cathode materials (MnO2, V2O5 and organic polymer).3–5 However, some difficulties are associated with the use of AZBs, similar to those of Li-ion and Li/K metal batteries.6–8 Specifically, the long-term cycling stability and high-capacity retention of cathode materials are always hindered by dissolution and structure collapse during the continuous charge/discharge process.9,10 In addition, fatal dendrite generation, poor interface stability, hydrogen evolution and by-product accumulation on Zn metal anodes result in irreversible electrolyte consumption, active Zn loss and internal short circuit.11–14 Therefore, it is important to enhance the electrode stability, particularly of Zn metal anodes, in the AZBs.To date, some typical strategies have proved that random Zn deposition, uncontrollable migration of free water, inevitable interface corrosion and passivation production can be reduced. Constructing an artificial protective layer on Zn electrode, such as nanoporous CaCO3, faceted TiO2, ZnWO4 and SnO coatings, is highly effective for preventing dendrite formation and direct electrolyte contact.15–18 Preparing current collectors with massive zincophilic sites and large specific surface area is also helpful for uniformly guiding dispersed Zn nucleation and growth, thus promoting dendrite-free Zn metal anodes and avoiding fatal side reactions.19–21 Exploring the fabrication of functional separators shows beneficial effects for suppressing dendrite formation and prolonging the lifespan of Zn metal anodes in aqueous electrolytes.22–24 In addition, electrolyte engineering including gel electrolyte preparation and aqueous electrolyte modification have been strongly verified to enhance the stability of the Zn plating/stripping behavior.25–27 In particular, some functional additives are added into the aqueous electrolyte for improving the wetting property, accelerating Zn ion transport, affecting the solvation structure, restraining the activity of free water and even producing a protective layer on Zn metal anodes. Common inorganic salts such as LiCl, ammonium acetate and L-ascorbic acid sodium have been applied in suppressing the tip effect.28–30 It has been determined that organic solvents (acetonitrile, ethanol, dimethyl sulfoxide, glycerol, NMP and DME) play a critical role in reshaping the hydrogen bond environment and adjusting the internal solvation structure for boosting the reversibility of Zn metal anodes.31–36 Moreover, some organic compounds have displayed the excellent ability of inducing directional deposition along the (002) plane of Zn, causing flat Zn nucleation and development. Among them, soluble organic small molecules are more attractive and advantageous as the electrolyte additive due to their cheapness and ready availability. It has been reported that some typical components, such as thiourea, urea, sucrose, glucose and amino acids, are beneficial for binding free water molecules and promoting the quick desolvation of Zn ions.37–42 However, for these additives of organic small molecules, the fundamental mechanism guiding dendrite-free Zn deposition and reducing the formation of side-products has not been fully explicated and summarized.
In the present work, a new kind of organic small molecules with functional groups (different polarization), 2-aminoethanesulfonic acid, has been applied as a bifunctional interface stabilizer (BIS) in electrolyte modification. The unique BIS with sulfonic acid and amino group shows the excellent performance for suppressing the hydrogen evolution behavior and dendrite-like development of deposited Zn metal. Based on the theoretical calculation, compared to water molecule, BIS will be preferentially and vertically adsorbed on the Zn metal interface (inner sulfonic acid and outer amino group), especially the (100) and (101) planes. During the desolvation process of hydrated Zn ions, other water molecules would be attracted by the amino group, resulting in the effective suppression of hydrogen evolution. Meanwhile, Zn ions would be guided by the sulfonic acid group to transfer near the Zn metal interface and are then plated along the exposed (002) plane. According to the in/ex situ tests, dendrite-free, hydrogen-inhibition and reduced by-products performances can be determined by the results obtained. In addition, because of the directional arrangement of taurine molecules on the electrode, massive Zn ions would be strongly hooked by the sulfonic acid groups and uniformly dispersed between the regular channels, causing homogenous Zn deposition. Therefore, as shown in the obtained electrochemical results containing coulombic efficiency (CE) and long-term cycling tests, the Zn ion battery with the taurine-based electrolyte can show an extremely high CE value over 99.5% for 2000 cycles with a slow increase rate (1.9‰ per cycle) of the voltage polarization at 5 mA cm−2 and 3 mA h cm−2 (64.2 mV at the 50th cycle to 98.6 mV at the 2000th cycle), a long and stable lifespan of 4000 h at 1 mA cm−2 and 1 mA h cm−2 and the excellent rate performance from 1 to 10 mA cm−2. In addition, for the full cell loading MnO2 cathode, a high capacity retention of 73.8% can be also delivered after 700 cycles at 1 A g−1. Consequently, the significant exploration and application of the multifunctional taurine additive in aqueous electrolyte modification will promote the practical procedure of high-performance AZBs.
2. Results and discussion
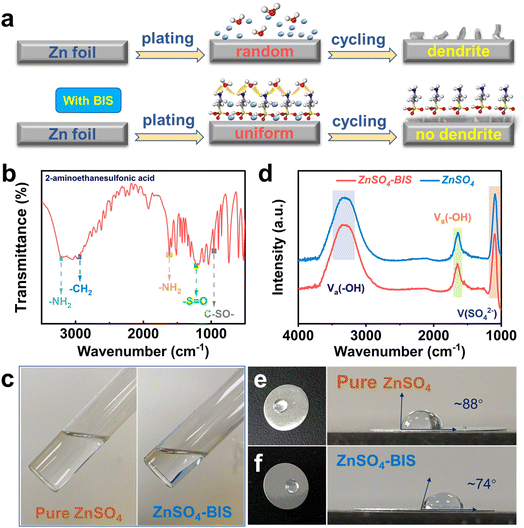
The distinctive difference in Zn plating and growth behavior in pristine and BIS-modified electrolyte can be directly observed from Fig. 1a. Due to the random distribution of Zn ions on the electrode interface, disordered Zn nucleation and subsequent dendrite formation will take place in ZnSO4 electrolyte, easily causing the side reactions and accelerating cell failure. In comparison, uniform and stable Zn development can be achieved in the electrolyte with 2-aminoethanesulfonic acid as the bifunctional interface stabilizer (BIS). Specifically, functional groups (sulfonic acid and amino group) in BIS play significant roles in inducing uniform Zn-ion dispersion and promoting dendrite-free Zn deposition and growth. Sulfonic acid groups will be preferentially adsorbed and connected with the electrode surface, selectively shielding the (100) and (101) planes and exposing the (002) plane of Zn metal. Amino groups not only guide the migration of Zn ions but also facilitate ion distribution during the Zn plating/stripping process.As shown in Fig. S1,† the detailed structure can be clearly show that BIS (white powder) molecule contains one amino group and sulfonic acid group. To determine the functional structure of the selected electrolyte additive, the related FT-IR spectra was obtained, as shown in Fig. 1b, from which typical peaks located at 3211.4 and 1616.3 cm−1 should be attributed to the amino group, and other signals at 1215.1 and 960.5 cm−1 are ascribed to the sulfonic acid group.43 Interestingly, Fig. 1c shows the as-prepared pristine ZnSO4 solution and functional electrolyte with BIS. Both electrolytes are clarified and transparent, which means that there are no intuitive differences between these two solutions due to the superior solubility of BIS in water. As displayed in Fig. 1d, the detailed information on the two electrolytes can be determined by liquid-state FT-IR measurement. Specifically, BIS addition causes a slight blue shift for ν(O–H) and ν(SO42−), which changed from 3314.5 to 3322.8 cm−1 and from 1092.5 to 1095.9 cm−1, respectively. The variation confirms the interaction enhancement between BIS and Zn ions. Meanwhile, the wettability for the two solutions was briefly examined, as shown in Fig. 1e and f, where a contact angle of ∼74° is exhibited by the ZnSO4-BIS electrolyte, lower than ∼88° of the pristine ZnSO4 solution. This may be because BIS partially changes the internal solvation structure, destroys the hydrogen-bond effect, and decreases the surface tension of the functional electrolyte. Moreover, the professional contact angle test was conducted and is shown in Fig. S3,† from which similar and expected result (better electrolyte wettability) has been obtained for ZnSO4-BIS compared to pristine ZnSO4.
To verify the positive effects of BIS additive in promoting uniform Zn deposition, the CA test was conducted. Specifically, a typical 2D diffusion demonstrates that Zn ions diffuse laterally along the electrode surface and accumulate on the local tips, leading to dendrite evolution. Additionally, 3D diffusion represents that Zn ions absorbed on the surface would be locally reduced, promoting flat and dense Zn deposition.44 As exhibited in Fig. 2a, the curve indicated rapid Zn-ion diffusion on the electrode surface and then achieved stable 3D development in the ZnSO4-BIS electrolyte. In contrast, the delayed planar Zn dispersion and large nucleation barrier (high current density) can be obviously observed from the conducted result, manifesting the comparatively inhomogeneous Zn deposition in the ZnSO4 electrolyte.45 As observed from Fig. 2b and c, the different surface morphologies of the plated Zn metal (1 mA h cm−2) in the two electrolytes further determine the superior ability of the BIS component. Relatively compact and flat Zn growth appeared in the electrolyte with BIS, differing much from the locally concentrated and irregular Zn formation in the pristine electrolyte. The typical observation of continuous Zn deposition was conducted by an in situ optical microscope. Fig. 2d shows the evolution of Zn growth in ZnSO4 electrolyte at 3 mA cm−2, from which there was an unevenly dispersed Zn accumulation at 0.5 h and then large Zn bulks affected by various growth rates are gradually generated on the local zone of the electrode after a plating time of 1 h. However, due to BIS addition, the stable and ordered Zn development can be promoted by the uniform Zn-ion guidance and moderate nucleation rate and excellent interface modification (Fig. 2e). Moreover, the expected condition of hydrogen generation caused by the quick local pH deterioration and enhancement of side reactions can be observed at a higher current density of 5 mA cm−2. Notably, large amounts of hydrogen bubbles are produced in the ZnSO4 electrolyte (Fig. S4†). In sharp contrast, merely few bubbles escape near the electrode surface, strongly verifying the effects of improving Zn plating and maintaining the pH balance of the functional BIS.
Fig. 2f exhibits a related brief diagram of Zn plating in ZnSO4-BIS electrolyte for a dendrite-free process. The significant mechanism of the dendrite-free and uniform Zn deposition behavior in ZnSO4-BIS electrolyte was also investigated by the in situ XRD test. Fig. 2g displays the spectra at different plating capacities for the system with BIS. Five typical characteristic peaks of Ti foil can be recognized at 35.3°, 38.6°, 40.4°, 53.2° and 63.2° respectively, which is in good accordance with the XRD result in Fig. S5.† In addition, the intensity of the (002) plane for fresh Zn metal becomes gradually increased, which can be noticed from the peaks at 36.1° in Fig. 2h, indicating the preferentially horizontal orientation during the plating process.46,47 Moreover, the intensity of the characteristic peaks attributed to the (100) and (101) planes shows almost no change (Fig. S6†). Specifically, for Zn deposition in pristine electrolyte, extremely different plane selectivity can be obtained. In Fig. S7,† the preferential (101) plane of Zn metal shows continuous enhancement from 0.5 to 2 mA h cm−2. However, the peak for the (002) plane almost disappears in the spectrum, which demonstrates the vertical orientation and easily encountered tip effect for generating Zn dendrites. The intuitive comparison of in situ XRD measurement highlights the significance of the added BIS component.
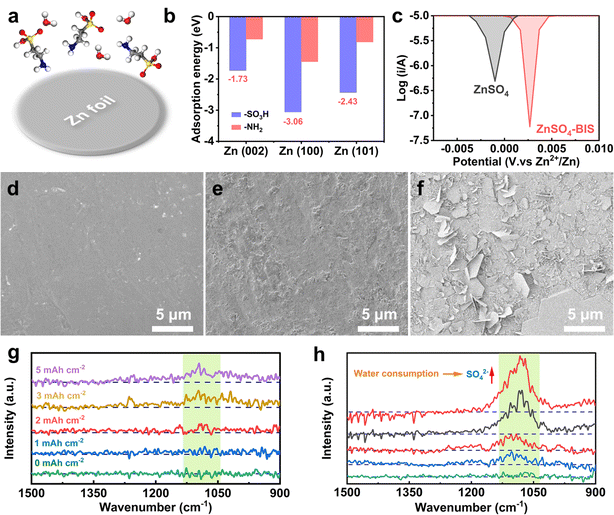
It is necessary to make out the anti-corrosion effect and the corresponding mechanism of the added BIS for the electrode (Fig. 3a). As exhibited in Fig. S8 and S9,† the theoretical calculation of BIS on Zn metal was conducted. Fig. 3b shows the adsorption energies between the functional groups of BIS and various crystal planes of Zn metal, among which the sulfonic acid group shows higher adsorption ability, compared to the amino group. Moreover, there are obvious adsorption effects for two functional groups and the (100) and (101) planes with high activity prior to the (002) plane. According to this result, it can be inferred that the sulfonic acid groups of BIS are attached to the Zn electrode surface easily and preferential adsorption will be provided by the (100) and (101) planes. Therefore, the Zn electrode interface adsorbed with sulfonic acid groups may greatly resist the intense corrosion by free water. As displayed in Fig. 3c, a slightly high corrosion potential (0.003 V) was obtained by the electrode in ZnSO4 with BIS than the pristine electrolyte (−0.001 V) in the symmetric cell. To examine the practical effect of BIS addition, cleaned Zn electrodes were immersed in various electrolytes for 7 days to explore the morphology variation. For the electrode in ZnSO4 with BIS, the processed sample changed from the flat state (Fig. 3d) and showed some roughness, although some by-products were generated (Fig. 3e). In sharp contrast, there were numerous and obvious flakes on the surface, which should be determined as the by-products of basic zinc sulfate (Fig. 3f). As we know, during the formation of by-products, HER and OH− ions will be promoted, thereby leading to the unavoidable consumption of free water.48,49 Due to the protection of BIS, the interface corrosion and side reactions will be effectively inhibited. The rational hypothesis has also been verified by the in situ FT-IR spectra in two electrolytes. As displayed in Fig. 3g, with an increase in the Zn deposition capacity, the typical peak at about 1080 cm−1 also encountered a gradual density enhancement from 3 to 5 mA h cm−2 in the FT-IR plots, which should be caused by the concentration increase of SO42− for free water loss in the electrolyte.22,50 Expectedly, the inevitable water consumption also appeared in the ZnSO4 electrolyte and seemed more exaggerated during Zn plating. A typical enhancement in the peak intensity of SO42− was observed after merely the deposition of 1 mA h cm−2, followed by continuous density improvement (Fig. 3h), obviously indicating more rapid concentration change and severe production of side reactions, compared to the electrolyte with BIS. The distinct comparison of the in situ FT-IR spectra further confirms the directional shielding of the active crystal planes and superior interface protection for adding the BIS component.
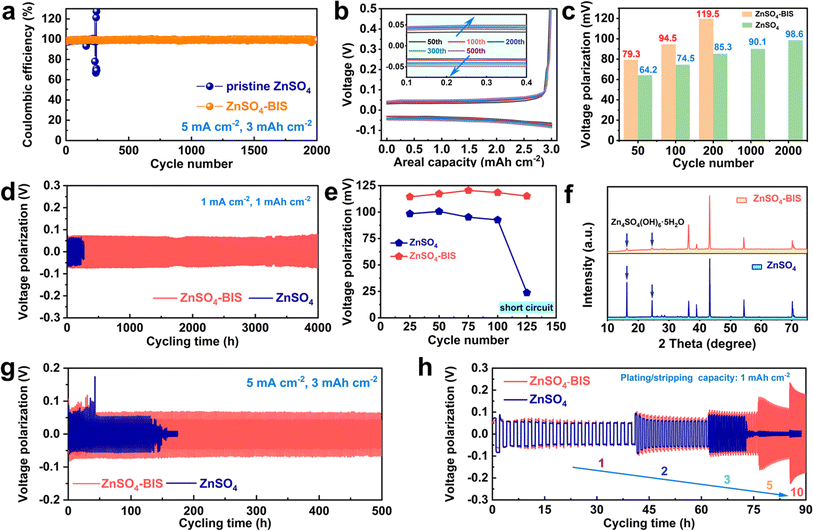
The related electrochemical performances for evaluating the cycling lifespan and effective utilization ratio of Zn plating/stripping were studied in different electrolytes. As shown in Fig. S10,† the results in these electrolytes demonstrate that the optimized BIS additive amount should be controlled at 0.1 M. Fig. 4a exhibits coulombic efficiency at 5 mA cm−2 and 3 mA h cm−2, from which a long-term of 2000 cycles, high efficiency retention over 99.5% and only mild curve fluctuation can be demonstrated in the electrolyte with BIS. But for the pristine electrolyte, there was an intense change at the 230th cycle and then rapid battery failure, which could be caused by electrolyte loss and the generation of dead Zn. In addition, the excellent maintenance of polarization throughout the 2000 cycles can be well delivered by the electrolyte with BIS (Fig. 4b). In Fig. 4c, due to the assistance of BIS, the polarization values of the modified electrolyte were always inferior to those of pristine ZnSO4 under the same cycling conditions, which also demonstrated the good inhibition of side reactions.51,52 Specifically, a relatively slow polarization rise from the initial 64.2 mV (50th) to the final 98.6 mV (2000th) could be observed, far lower than that in the pristine electrolyte (from 79.3 mV at 50th to 119.5 mV at 200th). Moreover, for further investigating the continuous Zn deposition/dissolution, symmetric cells were assembled using different electrolytes. As shown in Fig. 4d, an effective and stable lifespan of 4000 h was exhibited by the cell with ZnSO4-BIS electrolyte at 1 mA cm−2, extremely better than that of some reported works on electrolyte modification (Table S1†). However, the cell injected with pristine ZnSO4 encountered a clear short circuit after 220 h, which should be related to inhomogeneous Zn dissolution and growth. Fig. 4e displays a brief comparison of voltage polarization between the two cells. For the pristine ZnSO4 electrolyte, there is a rapid Zn reduction on the bare electrode interface during the plating/stripping process. However, the interface shielding of BIS on the electrode promotes a slight increase in the energy barrier for Zn reduction, causing larger voltage polarization than ZnSO4. Because of the interface shielding of BIS on the electrode, the voltage polarization was always higher in the modified electrolyte. However, it can lead to the benefit of reducing the interfacial by-products of basic ZnSO4, which was confirmed by the XRD patterns obtained for the cycled electrodes (Fig. 4f). More intense typical peaks were detected in the XRD pattern of the electrode in ZnSO4. In addition, there is a calculated intensity ratio of 3.2 for the (002) and (100) planes in the BIS-containing electrolyte, much higher than 1.3 in ZnSO4. The obvious difference suggests the enhanced capacity of the guiding planar Zn plating and stabilizing interface with the help of BIS. As observed from the SEM image of the Zn electrode after 50 cycles, random sediment accumulation further highlights the unfavorable induced flat deposition and promoted growth uniformity for ZnSO4 (Fig. S11†). However, the cycled electrode can still maintain the flat morphology with the help of BIS (Fig. S12†). Similarly, when tested at another condition of 5 mA cm−2 and 3 mA h cm−2, the cell with ZnSO4-BIS can exhibit a superior lifetime of 500 h, which is 4 times longer than that in pristine ZnSO4 (Fig. 4g). In addition, as exhibited in Fig. 4h, the vital rate capability from 1 to 10 mA cm−2 further demonstrates the superior effects of promoted uniform Zn deposition and no dendrite formation provided by BIS. Notably, the cell in ZnSO4 is unable to withstand high current density (>3 mA cm−2) and then undergoes a rapid short circuit.
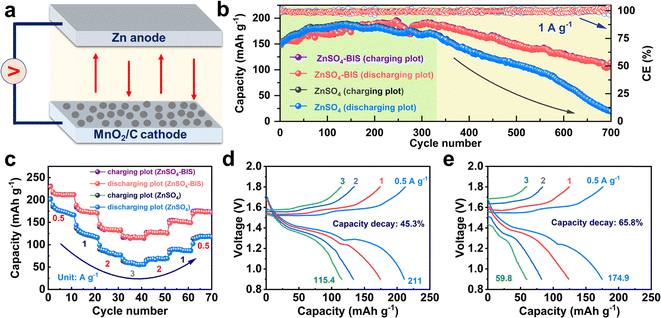
To examine the practical application of electrolyte modification, it is necessary to explore the Zn-ion batteries with cathode materials such as MnO2 (Fig. 5a). The detailed electrochemical results for the full cells with the two electrolytes have been exhibited in Fig. 5b–e. For the cell based on ZnSO4-BIS, when tested at 1 A g−1, there was a slow capacity rise before 325 cycles due to the gradual activation of the MnO2 material.53,54 Subsequently, a long-term capacity decline near 360 cycles was encountered, and a final capacity of 112.4 mA h g−1 was achieved at the 700th cycle. In sharp contrast, a relatively short capacity improvement of 150 cycles and an extremely rapid capacity loss was obtained by the cell with pristine ZnSO4. Moreover, compared to the initial capacity of 150.4 mA h g−1, there was merely a low specific capacity of 18.8 mA h g−1 after 700 cycles, demonstrating an ultra-low capacity retention of 12.5%. The difference in the results for the two electrolytes can also verify the positive capability of relieving the dissolution and promoting the capacity of the active material. As shown in Fig. S13,† the optical images of MnO2 electrodes after 150 cycles have been provided, from which more loss of the active material occurred in the ZnSO4 electrolyte. In comparison, the GF separator simply displayed light yellow color in ZnSO4-BIS, indicating reduced Mn dissolution. Fig. 5c exhibits related rate performances. For the cell with BIS in the stage of current increase, the average specific capacities of 211.7, 175.2, 133.3 and 116.8 mA h g−1 were delivered at 0.5, 1, 2 and 3 A g−1, respectively, which all are superior to those in ZnSO4. In particular, for both the cells in the reverse process, the displayed capacities are also lower than those under the same conditions. In addition, Fig. 5d and e show the charge–discharge plots at various current densities in the initial 40 cycles. The cell with ZnSO4-BIS could maintain a slow capacity decay of 45.3% from 211 (0.5 A g−1) to 115.4 mA h g−1 (3 A g−1), better than 65.8% for the cell using ZnSO4. These performances can further reflect the positive effect of BIS in inhibiting the side reaction and slowing down the capacity loss.
3. Conclusion
In this work, a unique kind of organic small molecules, BIS with two zincophilic sites of O-containing and amino groups, is demonstrated to effectively improve the cycling stability of AZBs. Except for the homogenous Zn distribution induced by the zincophilic groups, the strong adsorption of BIS on the (100) and (101) planes of Zn metal can cause the selective exposure of the (002) plane, thereby achieving planar Zn growth and suppressing the dendrite formation. DFT calculation and various advanced tests, such as in situ XRD and in situ FT-IR measurement, further confirmed the positive abilities of promoting dendrite-free deposition and relieving unnecessary electrolyte loss. Therefore, AZBs adopting functional electrolytes with BIS display improved electrochemical results. An extremely high CE value over 99.5% for 2000 cycles, stable and ultralong lifespan of 4000 h and high capacity retention of 73.8% after 700 cycles for the full cell loading MnO2 could be obtained. BIS exploration can provide beneficial progress in the construction of stable and long-life AZBs.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors gratefully thank Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ23E030016) and China Postdoctoral Science Foundation (No. 2023M733154) for their financial support.References
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210–1211 CrossRef CAS PubMed.
- Z. Chang, Y. Yang, M. Li, X. Wang and Y. Wu, J. Mater. Chem. A, 2014, 2, 10739–10755 RSC.
- X. Wang, Z. Zhang, B. Xi, W. Chen, Y. Jia, J. Feng and S. Xiong, ACS Nano, 2021, 15, 9244–9272 CrossRef CAS PubMed.
- J. Zhou, Q. Li, X. Hu, W. Wei, X. Ji, G. Kuang, L. Zhou, L. Chen and Y. Chen, Chin. Chem. Lett., 2024, 35, 109143 CrossRef CAS.
- M. L. Li, F. B. Zeng, S. L. Li, S. L. Li, S. L. Hu, Q. M. Liu, T. F. Zhang, J. Zhou and C. P. Han, Energy Mater. Devices, 2023, 1, 9370007 CrossRef.
- Y. N. Cao, Y. Sun, C. F. Guo, W. W. Sun, Y. Wu, Y. Xu, T. C. Liu and Y. Wang, Angew. Chem., Int. Ed., 2024, 63, e202316208 CrossRef CAS PubMed.
- Y. N. Cao, Q. Xu, Y. Sun, J. X. Shi, Y. Xu, Y. F. Tang, X. D. Chen, S. Yang, Z. Jiang, H. D. Um, X. P. Li and Y. Wang, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2315407121 CrossRef CAS PubMed.
- Y. F. Liu, Y. T. Shi, C. T. Gao, Z. D. Shi, H. B. Ding, Y. H. Feng, Y. M. He, J. W. Sha, J. Zhou and B. A. Lu, Angew. Chem., Int. Ed., 2023, 62, e202300016 CrossRef CAS PubMed.
- A. Zhou, R. Chi, Y. Shi, X. Zhao, X. Li, Z. Kou, Z. Zhang, X. Zhang and G. Nie, Mater. Today Chem., 2023, 27, 101294 CrossRef CAS.
- Y. Ding, L. Zhang, X. Wang, L. Han, W. Zhang and C. Guo, Chin. Chem. Lett., 2023, 34, 107399 CrossRef CAS.
- J. Yang, B. Yin, Y. Sun, H. Pan, W. Sun, B. Jia, S. Zhang and T. Ma, Nano-Micro Lett., 2022, 14, 42 CrossRef CAS PubMed.
- X. Zhang, J.-P. Hu, N. Fu, W.-B. Zhou, B. Liu, Q. Deng and X.-W. Wu, InfoMat, 2022, 4, e12306 CrossRef CAS.
- W. Du, E. H. Ang, Y. Yang, Y. Zhang, M. Ye and C. C. Li, Energy Environ. Sci., 2020, 13, 3330–3360 RSC.
- G. Li, L. Sun, S. Zhang, C. Zhang, H. Jin, K. Davey, G. Liang, S. Liu, J. Mao and Z. Guo, Adv. Funct. Mater., 2024, 34, 2301291 CrossRef CAS.
- L. Kang, M. Cui, F. Jiang, Y. Gao, H. Luo, J. Liu, W. Liang and C. Zhi, Adv. Energy Mater., 2018, 8, 1801090 CrossRef.
- Q. Zhang, J. Luan, X. Huang, Q. Wang, D. Sun, Y. Tang, X. Ji and H. Wang, Nat. Commun., 2020, 11, 3961 CrossRef CAS PubMed.
- J. Cao, H. Wu, D. Zhang, D. Luo, L. Zhang, X. Yang, J. Qin and G. He, Angew. Chem., Int. Ed., 2024, 63, e202319661 CrossRef CAS PubMed.
- Y. Zhang, P. Chen, M. Li, S. Li, Y. Yue, Y. Wang, S. Xie and W. Zhou, J. Mater. Chem. A, 2023, 11, 14333–14344 RSC.
- Z. Hao, Y. Zhang, Y. Lu, J. Hou, X. Liu, Z. Yan and J. Chen, Adv. Funct. Mater., 2024, 34, 2315726 CrossRef CAS.
- T. Liu, Y. Xu, H. Fang, L. Chen, J. Ying, M. Guo, Y. Wang, Q. Shen, X. Wang, Y. Wang and Z. Yu, J. Mater. Chem. A, 2024, 12, 2283–2293 RSC.
- O. Blumen, G. Bergman, K. Schwatrzman, S. Harpaz, S. H. Akella, M. S. Chae, N. Bruchiel-Spanier, N. Shpigel and D. Sharon, J. Mater. Chem. A, 2023, 11, 19970–19980 RSC.
- T. Liu, J. Hong, J. Wang, Y. Xu and Y. Wang, Energy Storage Mater., 2022, 45, 1074–1083 CrossRef.
- Y. Fang, X. Xie, B. Zhang, Y. Chai, B. Lu, M. Liu, J. Zhou and S. Liang, Adv. Funct. Mater., 2022, 32, 2109671 CrossRef CAS.
- H. Lee, J. Kang, H. W. Kang, M. Lee, J. Han, M. Lim, J. Lee, W. Kwon, D.-H. Nam, B. G. Kim and H. Lee, J. Mater. Chem. A, 2024, 12, 3623–3632 RSC.
- C. Li, W. Wang, J. Luo, W. Zhuang, J. Zhou, S. Liu, L. Lin, W. Gong, G. Hong, Z. Shao, J. Du, Q. Zhang and Y. Yao, Adv. Mater., 2024, 36, 2313772 CrossRef CAS PubMed.
- B. Qiu, K. Y. Liang, W. Huang, G. Q. Zhang, C. X. He, P. X. Zhang and H. W. Mi, Adv. Energy Mater., 2023, 13, 202301193 Search PubMed.
- S. Dilwale, P. P. Puthiyaveetil, A. Babu and S. Kurungot, Small, 2024, 20, 2311923 CrossRef CAS PubMed.
- C. Lin, X. Yang, P. Xiong, H. Lin, L. He, Q. Yao, M. Wei, Q. Qian, Q. Chen and L. Zeng, Adv. Sci., 2022, 9, 2201433 CrossRef CAS PubMed.
- A. Bayaguud, X. Luo, Y. Fu and C. Zhu, ACS Energy Lett., 2020, 5, 3012–3020 CrossRef CAS.
- X. Guo, Z. Zhang, J. Li, N. Luo, G.-L. Chai, T. S. Miller, F. Lai, P. Shearing, D. J. L. Brett, D. Han, Z. Weng, G. He and I. P. Parkin, ACS Energy Lett., 2021, 6, 395–403 CrossRef CAS.
- Y. Dong, N. Zhang, Z. Wang, J. Li, Y. Ni, H. Hu and F. Cheng, J. Energy Chem., 2023, 83, 324–332 CrossRef CAS.
- K. Wang, F. Liu, Q. Li, J. Zhu, T. Qiu, X.-X. Liu and X. Sun, Chem. Eng. J., 2023, 452, 139577 CrossRef CAS.
- Y. Zhang, M. Zhu, K. Wu, F. Yu, G. Wang, G. Xu, M. Wu, H.-K. Liu, S.-X. Dou and C. Wu, J. Mater. Chem. A, 2021, 9, 4253–4261 RSC.
- Z. Wu, Y. Li, A. Amardeep, Y. Shao, Y. Zhang, J. Zou, L. Wang, J. Xu, D. Kasprzak, E. J. Hansen and J. Liu, Angew. Chem., Int. Ed., 2024, 63, e202402206 CrossRef CAS PubMed.
- K. Bao, M. Wang, Y. Zheng, P. Wang, L. Yang, Y. Jin, H. Wu and B. Sun, Nano Energy, 2024, 120, 109089 CrossRef CAS.
- D. Feng, F. Cao, L. Hou, T. Li, Y. Jiao and P. Wu, Small, 2021, 17, 2103195 CrossRef CAS PubMed.
- F. Ilyas, J. Chen, Y. Zhang, Y. Huang, H. Ma and J. Wang, Energy Storage Mater., 2023, 55, 566–574 CrossRef.
- C. Wang, J. Hou, Y. Gan, L. Xie, Y. He, Q. Hu, S. Liu and S. Chan Jun, J. Mater. Chem. A, 2023, 11, 8057–8065 RSC.
- P. Sun, L. Ma, W. Zhou, M. Qiu, Z. Wang, D. Chao and W. Mai, Angew. Chem., Int. Ed., 2021, 60, 18247–18255 CrossRef CAS PubMed.
- Y. Wang, T. Wang, Y. Mao, Z. Li, H. Yu, M. Su, K. Ye, D. Cao and K. Zhu, Adv. Energy Mater., 2024, 14, 2400353 CrossRef CAS.
- S.-H. Huh, Y. J. Choi, S. H. Kim, J.-S. Bae, S.-H. Lee and S.-H. Yu, J. Mater. Chem. A, 2023, 11, 19384–19395 RSC.
- Y. Hu, J. Fu, H. Hu, D. Ho and H. Hu, Energy Storage Mater., 2023, 55, 669–679 CrossRef.
- G. Duan, Y. Wang, B. Luo, L. Sun, S. Zheng, J. Huang and Z. Ye, Energy Storage Mater., 2023, 61, 102882 CrossRef.
- Z. Y. Jiao, X. X. Cai, X. X. Wang, Y. R. Li, Z. Bie and W. X. Song, Adv. Energy Mater., 2023, 13, 202302676 Search PubMed.
- Q. Guo, G. Teri, W. Mo, J. Huang, F. Liu, M. Ye and D. Fu, Energy Environ. Sci., 2024, 17, 2888–2896 RSC.
- B. Lv, Q. Zong, Y. Yu, C. Liu, A. Pan, J. Wang, D. Tao, J. Zhang, Q. Zhang and G. Cao, Adv. Funct. Mater., 2024, 34, 2316535 CrossRef CAS.
- Y. Cheng, Y. Jiao and P. Wu, Energy Environ. Sci., 2023, 16, 4561–4571 RSC.
- X. Bai, Y. Nan, K. Yang, B. Deng, J. Shao, W. Hu and X. Pu, Adv. Funct. Mater., 2023, 33, 2307595 CrossRef CAS.
- Y. Mao, Z. Li, Y. Li, D. Cao, G. Wang, K. Zhu and G. Chen, Chem. Eng. J., 2023, 461, 141707 CrossRef CAS.
- X. Yu, M. Chen, Z. Li, X. Tan, H. Zhang, J. Wang, Y. Tang, J. Xu, W. Yin, Y. Yang, D. Chao, F. Wang, Y. Zou, G. Feng, Y. Qiao, H. Zhou and S.-G. Sun, J. Am. Chem. Soc., 2024, 146, 17103–17113 CrossRef CAS PubMed.
- H. Meng, Q. Ran, T.-Y. Dai, J.-H. Jia, J. Liu, H. Shi, G.-F. Han, T.-H. Wang, Z. Wen, X.-Y. Lang and Q. Jiang, Adv. Mater., 2024, 36, 2403803 CrossRef CAS PubMed.
- D. Li, Y. Tang, S. Liang, B. Lu, G. Chen and J. Zhou, Energy Environ. Sci., 2023, 16, 3381–3390 RSC.
- S. J. Kim, D. Wu, N. Sadique, C. D. Quilty, L. Wu, A. C. Marschilok, K. J. Takeuchi, E. S. Takeuchi and Y. Zhu, Small, 2020, 16, 2005406 CrossRef CAS PubMed.
- W. Li, X. Gao, Z. Chen, R. Guo, G. Zou, H. Hou, W. Deng, X. Ji and J. Zhao, Chem. Eng. J., 2020, 402, 125509 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta04973j |
| This journal is © The Royal Society of Chemistry 2024 |