Glycidol-modified PEI: a highly selective adsorbent for SO2 in the presence of CO2†
Chanjot
Kaur
 and
Abdelhamid
Sayari
and
Abdelhamid
Sayari
 *
*
Centre for Catalysis Research and Innovation, Department of Chemistry and Biomolecular Sciences, University of Ottawa, Ottawa, Ontario K1N 6N5, Canada. E-mail: abdel.sayari@uottawa.ca
First published on 28th October 2024
Abstract
Amine-containing CO2 adsorbents are highly sensitive to the presence of SO2 in the feed gas, even in minute amounts. It is thus necessary to remove SO2 quantitatively prior to CO2 capture. To this end, we developed a silica-supported polyethylenimine (GD-PEI/S) adsorbent containing only tertiary amines via quantitative glycidol functionalization. The novel material was characterized by infra-red (IR) and nuclear magnetic resonance (NMR) spectroscopy, and by thermogravimetric analysis (TGA). In the presence of a gas mixture containing 5 ppm SO2 and more than a 2 × 104 higher concentration of CO2, the GD-PEI/S material adsorbed SO2 quantitatively until near saturation, with no CO2 uptake, indicating that the adsorbent exhibits 100% SO2 selectivity versus CO2, even in the presence of very high CO2/SO2 ratios. Furthermore, the SO2 uptake of the adsorbent almost doubled in the presence of humidity, possibly due to increased diffusion of SO2. Under recycling conditions, GD-PEI/S showed good reversibility in the presence of both dry and humid SO2 at low and high SO2 concentrations.
1. Introduction
As the main contributor to the greenhouse gas effect, CO2 plays a predominant role in global warming.1 The National Oceanic and Atmospheric Administration (NOAA) reported increasing levels of CO2 in the atmosphere reaching 422 ppm in 2024.2 Increasing levels of CO2 in the atmosphere were correlated with the increase in earth's temperature.3 CO2 capture and sequestration from flue gas and directly from air, is recognized as a key strategy for reducing CO2 emissions, from stationary and distributed sources.4,5Regarding CO2 capture, solid amine adsorbents received significant attention due to their excellent selectivity, high adsorption capacity and low energy regeneration.6–8 Nevertheless, there are challenges in using solid amine adsorbents, including the deleterious effect of harmful acidic gas impurities, such as SOx and NOx, that may occur in flue gas. Such species not only cause environmental and health issues but are detrimental to CO2 capture due to their strongly competitive adsorption.9 Note that, due to the potential health risks and environmental impact associated with elevated concentrations of SO2 in the atmosphere,10 limits are imposed on sulfur emissions from large power facilities. Typical mandatory limits for SO2 in exhaust gases are set to 120 ppm in the US, 75 to 300 ppm in China, and 50 to 250 ppm in Europe.11 However, no matter how low the residual SO2 content in the feed gas, amines in CO2 adsorbents will be irreversibly deactivated.12–14 For example, Jones and co-workers14 reported that when a PEI-impregnated silica support was exposed to a mixture of 20 ppm SO2, 10% CO2 balance N2 at 35 °C, the adsorbent lost 41% of CO2 uptake after the first cycle of adsorption. Therefore, before adsorbing CO2 on amine-containing materials, it is crucial to remove SO2 quantitatively from the feed gas.
Although there are numerous technologies for flue gas desulfurization, particularly liquid phase scrubbing,15 work on selective removal of traces of SO2 in the presence of much higher CO2 partial pressures is more pertinent for carbon capture over amine-containing adsorbents. More specifically, meeting this objective using adsorption would be easier to streamline with processes of CO2 capture by adsorption. Many candidate materials were proposed for SO2 removal, including metal organic frameworks (MOFs),16,17 zeolites,18,19 porous polymers20–22 and activated carbons.23,24 Nonetheless, they have limitations; for example, activated carbons showed very limited selectivity and efficiency25 in the presence of O2 and H2O, as they deactivated, producing H2SO4.26 A number of MOFs with SO2vs. CO2 selectivity between 28 and 44 were reported in the literature.27–29 However, not only are such selectivities not high enough, but the SO2 concentration used was somewhat high (≥2000 ppm).
Adsorbents containing only tertiary amines seem to be the most selective for SO2vs. CO2. Tertiary amines adsorb SO2 readily, but do not interact with CO2, at least under dry conditions.14,30–39 For instance, when Tailor and Sayari32 exposed propyldiethanolamine grafted on a pore-expanded MCM-41 support to a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixture of 0.1% SO2 balance N2 and 20% CO2 balance He at room temperature, they found that the presence of CO2 had no effect on the adsorbent's ability to adsorb SO2. Also, the adsorbent could be fully regenerated after 11 adsorption–desorption cycles, which indicates high stability of the material against heat and SO2.
50 mixture of 0.1% SO2 balance N2 and 20% CO2 balance He at room temperature, they found that the presence of CO2 had no effect on the adsorbent's ability to adsorb SO2. Also, the adsorbent could be fully regenerated after 11 adsorption–desorption cycles, which indicates high stability of the material against heat and SO2.
However, achieving complete functionalization of PEI protic amines into tertiary amines was found to be either difficult33 or tedious.30,35,36,38 Moreover, the majority of reported studies focused on removing high levels of SO2 in gas mixtures,30–32,35,36 and to the best of our knowledge, none of the studies dealt with SO2 concentrations below 50 ppm. Furthermore, in some cases, the working capacity of the adsorbent was found to decrease significantly in the presence of humid SO2.32
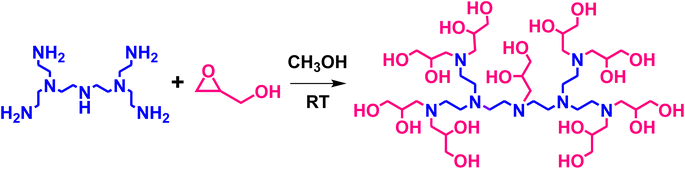
The objective of this work was to develop a novel polytertiary amine adsorbent to selectively and quantitatively remove SO2 at concentrations as low as 5 ppm in the presence of a typical flue gas CO2 concentration of 10–15%. To this end, complete functionalization of PEI with GD was achieved as shown in Scheme 1. In addition to its straightforward preparation, the glycidol-functionalized amine adsorbent exhibited stable working capacity in the presence of both dry and humid SO2 at low and high SO2 concentrations. It is noteworthy that in addition to tertiary amines being highly selective to SO2 adsorption, it was reported that the occurrence of hydroxyethylene groups decreases the energy for regeneration and increases the reversibility of the adsorbent.33,40
2. Experimental section
2.1. Materials
Polyethylenimine (PEI, Mw 1200), glycidol (GD, 96%), fumed silica (Cab-O-Sil, M5), tetramethylammonium hydroxide (TMAOH, 25 wt% solution), cetyltrimethylammonium bromide (CTAB, 98%), N,N-dimethyldodecyl amine (DMDA, 97%), tetraethyl orthosilicate (TEOS, 98%) and deuterium oxide (D2O, 99.9%) were obtained from Sigma-Aldrich. Sodium aluminate (NaAlO2, 92%) was obtained from Strem Chemicals. Anhydrous methanol (99.8%) and ammonia solution (30 wt%) were obtained from Fischer. Chemicals were used as obtained. Ultrahigh purity (99.999%) nitrogen, 15% or 20% CO2 in nitrogen and gas cylinders containing 20, 100 and 1000 ppm SO2 in N2, were purchased from Messer Canada.2.2. Preparation of GD-PEI
A pore-expanded aluminosilica (PE-AlSiO2) support was prepared as reported elsewhere,41 and further details are included in the ESI (Fig. S1).† Functionalization of primary and secondary amines of PEI with GD was carried out as reported by Fan et al.42 with slight modifications. Briefly, 2 g of PEI (44 mmol of N) was dissolved in 20 mL of anhydrous methanol under a nitrogen atmosphere. Then, 3.55 g (47.96 mmol) of GD was added dropwise, and the solution was stirred for 4 h. After removing excess methanol using a rotary evaporator, the product was precipitated with acetone, and then separated and dried in a vacuum oven at 70 °C overnight. The functionalized PEI, denoted as GD-PEI was impregnated onto PE-AlSiO2 as follows. The obtained GD-PEI compound was dissolved in 30 mL of anhydrous methanol and stirred until complete dissolution. After that, 5.5 g of PE-AlSiO2 was slowly added into the solution and the mixture was stirred until the solvent evaporated. The final material was dried in an oven at 80 °C for 3 h and referred to as GD-PEI/S. 0.5 g of PEI was dissolved in 15 mL of methanol. After that, 0.5 g of PE-AlSiO2 was added and the mixture was stirred overnight followed by evaporation of the solvent in an oven at 70 °C for 6 h to obtain PEI/S. The overall loading of PEI/S and GD-PEI/S was fixed to 50 wt% with respect to the adsorbent.2.3. Characterization
The pore structure of PE-AlSiO2 was investigated by N2 adsorption measurements at −196 °C using a 3Flex instrument (Micromeritics). The sample was pretreated in flowing N2 at 120 °C for 4 h. The specific surface area was determined using the Brunauer–Emmett–Teller (BET) method at relative pressures ranging from 0.06 to 0.2. The total pore volume was measured at P/P0 = 0.99, whereas the pore size distribution was calculated using the Kruk–Jaroniec–Sayari method.43PEI and GD-PEI were dissolved in D2O and their 13C nuclear magnetic resonance (NMR) spectra were obtained using an AVIII 600 spectrometer set to 45° pulses, 40 scans, 90 second relaxation delay and an acquisition time of 0.999 seconds. Inverse-gated proton decoupling was used to avoid the nuclear Overhauser effect. Fourier transform infrared spectroscopy (FTIR) spectra were obtained using a Cary 630 FTIR instrument by Agilent.
2.4. Adsorption measurements
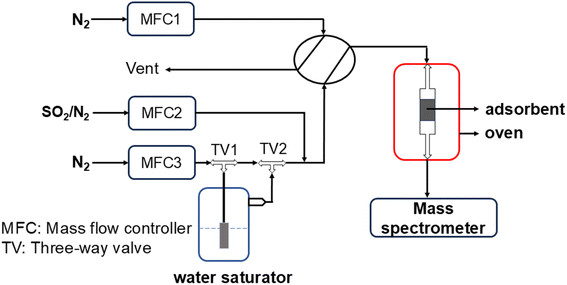
SO2 adsorption measurements were carried out in a fixed bed reactor as shown in Fig. 1. In a typical experiment, 0.5–1 g of the sample was loaded into a 1 cm long stainless steel column with 0.42 cm inner diameter, and placed in a temperature-controlled oven. The material was pretreated under N2 (40 mL min−1) at 110 °C for 2 h. After cooling to 23 °C, it was exposed to SO2 in a N2 gas mixture (40 mL min−1) with different compositions. As for experiments under humid conditions, N2 was bubbled through a water saturator placed in a thermostatic bath maintained at 20 °C, and then combined with the SO2 containing N2. The gas mixture exiting the column was constantly analyzed by using a MKS Cirrus 3 mass spectrometer (MS), and breakthrough curves were obtained using MS data obtained for 64 amu. The SO2 adsorption capacity (mmol g−1) of GD-PEI/S at different partial pressures was calculated using eqn (1):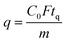 | (1) |
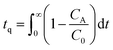 | (2) |
The CO2 uptake and organic content of GD-PEI/S were measured using a thermogravimetric analyzer (TGA 550, TA Instruments). The sample (ca. 20 mg) was pretreated under N2 for 60 min at 110 °C, followed by cooling to 25 °C, and then the gas stream was switched to 15% CO2 balance N2 for 30 min. After removal of adsorbed CO2, if any, at 110 °C for 10 min under flowing N2, the adsorbent was cooled down to 75 °C, and the gas stream was switched to 15% CO2/N2 for 30 min. Then, the temperature was increased to 700 °C at a rate of 10 °C min−1 under flowing N2, before switching the gas to air for 25 min. The organic content was determined as the weight loss of the material beyond 200 °C.
3. Results and discussion
3.1. Characterization of the adsorbent
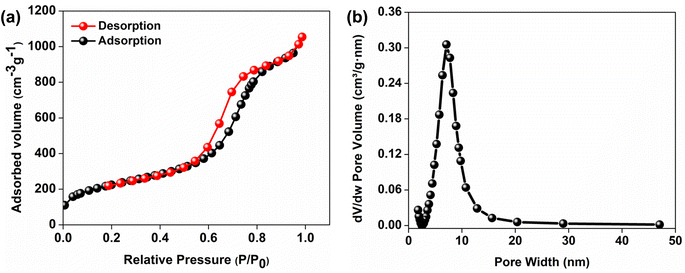
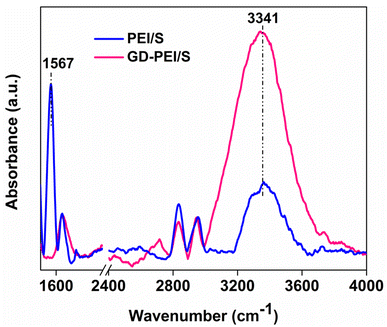
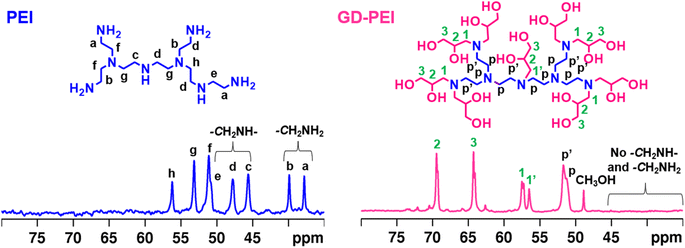
The surface area, pore volume and average pore size of PE-AlSiO2 were found to be 818 m2 g−1, 1.66 cm3 g−1 and 8.13 nm. According to IUPAC nomenclature, the nitrogen adsorption–desorption isotherm of the support (Fig. 2a) was a Type IV isotherm, with an H1 hysteresis loop, indicating that the support is mesoporous. This is further confirmed by the pore size distribution shown in Fig. 2b, where the majority of pores fall within the range of 3–15 nm, with a maximum at 7 nm. The total polymer content of GD-PEI/S was 55 wt% with respect to the weight of the adsorbent (Fig. S2a†).The FTIR spectra in Fig. 3 showed that the band at 1567 cm−1 in PEI/S corresponding to N–H bending44 disappeared completely upon functionalization with GD. Moreover, the higher intensity hydroxyl band at 3347 cm−1 in GD-PEI/S compared to PEI/S is consistent with the significant increase of hydroxyl groups. Functionalization of PEI with GD was further confirmed using 13C NMR measurements as shown in Fig. 4. The carbon peaks were assigned as outlined in the literature.45,46 Using the intensity of NMR peaks of carbon atoms adjacent to primary, secondary and tertiary amines in PEI, the percentage of such amines was found to be 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27. Upon functionalization of PEI with GD, the NMR peaks corresponding to carbons neighboring primary and secondary amines disappeared, and new peaks attributable to carbons neighboring tertiary amines and to added GD, appeared (Scheme 1). This finding confirmed the incorporation of GD with complete conversion of primary and secondary amines into tertiary amines.
27. Upon functionalization of PEI with GD, the NMR peaks corresponding to carbons neighboring primary and secondary amines disappeared, and new peaks attributable to carbons neighboring tertiary amines and to added GD, appeared (Scheme 1). This finding confirmed the incorporation of GD with complete conversion of primary and secondary amines into tertiary amines.
3.2. SO2 adsorption isotherm
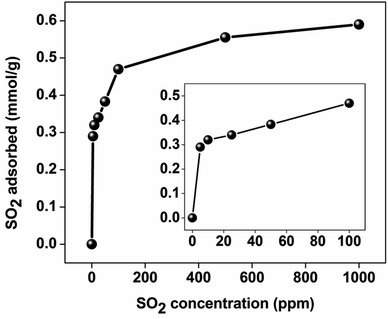
Fig. 5 shows the SO2 uptake of GD-PEI/S at 23 °C at different concentrations. The different gas compositions were achieved by diluting premixed 20, 100 or 1000 ppm SO2/N2 with pure N2, at different flowrates, while maintaining the overall flowrate at 40 mL min−1. The shape of this isotherm is consistent with chemisorption. At low concentrations, the adsorption capacity increases steeply with increasing concentration, indicating the high sensitivity of tertiary amine groups toward SO2. The uptake reaches a plateau at ca. 1000 ppm SO2, presumably because most of the accessible adsorption sites are occupied. The actual SO2 uptakes versus partial pressure are listed in Table S1.† The breakthrough curves of GD-PEI/S under different partial pressures of SO2 are shown in Fig. S3.†3.3. Selectivity towards SO2versus CO2
Fig. 6 depicts the column-breakthrough data over GD-PEI/S in the presence of 5 to 50 ppm SO2 in 10% or 11% CO2, as indicated. The gas compositions were achieved by mixing different concentrations of SO2 in N2 with 15% or 20% premixed CO2 in N2, while maintaining the overall flowrate at 40 mL min−1. Column breakthrough curves showed that CO2 comes out of the column within few seconds of passing the gas mixture. Furthermore, regardless of the presence of CO2, the SO2 uptake (Fig. S4†), breakthrough time, and equilibrium time remained the same at any given SO2 concentration. This is consistent with TGA measurements (Fig. S2b†), which showed that the CO2 uptake of GD-PEI/S was only 0.08 and 0.01 mmol g−1 at 25 and 75 °C, respectively. These results indicate that no CO2 was chemisorbed by GD-PEI/S, which is in line with the fact that under dry conditions, only protic amines interact with CO2 to afford ammonium carbamate.47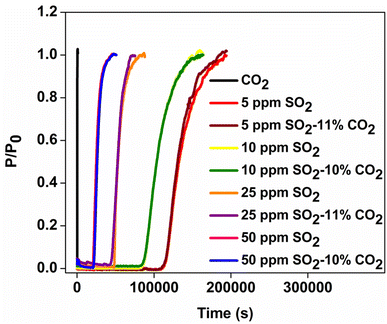 | ||
| Fig. 6 Breakthrough curves of GD-PEI/S in the presence of different concentrations of SO2 with and without CO2. | ||
3.4. Stability of the adsorbent under cycling conditions
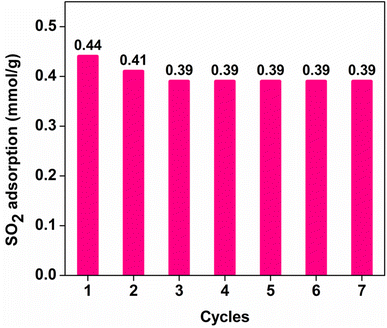
To investigate the stability of SO2 working capacity over GD-PEI/S or the lack thereof, a series of SO2 adsorption–desorption cycles was carried out, with adsorption at 23 °C in the presence of dry 100 ppm SO2 in N2, and desorption at 110 °C in N2. SO2 uptake of GD-PEI/S decreased by 7 and 5% during the first and second cycles, and then remained stable thereafter (Fig. 7). Moreover, breakthrough and equilibrium times remained the same after the second adsorption cycle, as seen in Fig. S5.† Zhu et al.38 observed a similar trend and attributed the decrease in SO2 uptake during the first cycle to the adsorption of SO2 onto basic sites that were not completely regenerated.Adsorption–desorption cycles were also performed under dry and humid 500 ppm SO2 balance N2 as shown in Fig. S6† and 8 respectively. In the absence of moisture, the adsorbent lost 17% SO2 uptake after the first two regeneration cycles, and it remained almost stable in subsequent cycles. However, the adsorbent was deactivated more in 500 ppm SO2 in N2 than 100 ppm SO2 in N2. This could be a result of elevated SO2 concentration, which increases the interaction of SO2 with adsorption sites and subsequently the likelihood of their deactivation. In agreement with other studies,30–32 SO2 adsorption capacity of GD-PEI/S increased by 64% under humid (42% RH) 500 ppm SO2 in N2, from 0.55 to 0.90 mmol g−1, which may be because water can act as a lubricant, reducing the diffusion resistance for incoming SO2. Tailor et al.30 proposed that this improvement may be due to the formation of ammonium bisulfite salt48,49 in a humid environment as shown in eqn (3). Overall, the decrease in SO2 uptake before stabilization was similar under dry and wet conditions i.e. ∼23%. The corresponding breakthrough curves are shown in Fig. S7.†
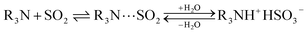 | (3) |
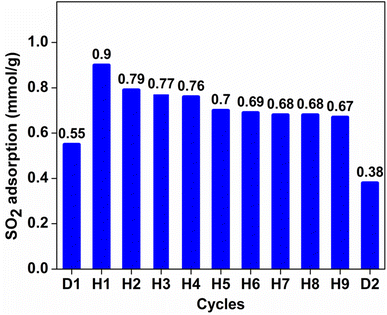 | ||
| Fig. 8 Working capacity over GD-PEI/S of humid 500 ppm SO2 in N2. D1 and D2 indicate data under dry conditions before and after the experiment under humid conditions. | ||
4. Conclusion
GD-PEI/S was synthesized by converting all protic amines in PEI into tertiary amines using glycidol functionalization, followed by impregnation onto pore-expanded AlSiO2. The adsorbent showed a SO2 capacity of 0.29 mmol g−1 when exposed to 5 ppm SO2/N2, with a positive correlation with increasing SO2 concentration. The selectivity of the adsorbent towards SO2 was investigated in the presence of different concentrations of SO2 in CO2/N2 mixtures. GD-PEI/S was found to be 100% selective for SO2 at concentrations as low as 5 ppm in the presence of 11% CO2, corresponding to a CO2/SO2 ratio of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The presence of only tertiary amines with no interaction with CO2 is at the origin of the high selectivity toward SO2versus CO2. Moreover, the adsorbent showed a more than two times increase in SO2 uptake under humid conditions. The adsorbent was also found to be stable during adsorption–desorption cycling in the presence of dry and wet SO2/N2 mixtures. Therefore, GD-PEI/S may be used as a filter for extensive desulfurization before CO2 capture on amine-containing adsorbents.
000. The presence of only tertiary amines with no interaction with CO2 is at the origin of the high selectivity toward SO2versus CO2. Moreover, the adsorbent showed a more than two times increase in SO2 uptake under humid conditions. The adsorbent was also found to be stable during adsorption–desorption cycling in the presence of dry and wet SO2/N2 mixtures. Therefore, GD-PEI/S may be used as a filter for extensive desulfurization before CO2 capture on amine-containing adsorbents.
Data availability
The data supporting this article have been included as part of the ESI.† More data can be provided upon request.Author contributions
Chanjot Kaur: conceptualization, methodology, investigation, formal analysis, data curation, writing – original draft. Abdelhamid Sayari: conceptualization, supervision, writing (review and editing), funding acquisition.Conflicts of interest
There are no competing interests to declare.Acknowledgements
A. S. thanks the Natural Sciences and Engineering Research Council of Canada (NSERC) for a Discovery Grant (RGPIN-2024-06127).References
- E. Kaur, M. Crippa, D. Guizzardi, F. Pagani, M. Banja, M. Muntean, E. Schaaf, W. Becker, F. Monforti-Ferrario, R. Quadrelli, A. Risquez Martin, P. Taghavi-Moharamli, J. Köykkä, G. Grassi, S. Rossi, J. Brandao De Melo, D. Oom, A. Branco and San-Miguel, GHG Emissions of All World Countries, p. 2023 Search PubMed.
- Trends in Atmospheric Carbon Dioxide, https://gml.noaa.gov/ccgg/trends/weekly.html, accessed 18 August 2024 Search PubMed.
- https://www.climate.nasa.gov, accessed 2024-08-18.
- P. Markewitz, W. Kuckshinrichs, W. Leitner, J. Linssen, P. Zapp, R. Bongartz, A. Schreiber and T. E. Müller, Energy Environ. Sci., 2012, 5, 7281–7305 Search PubMed.
- X. Zhu, W. Xie, J. Wu, Y. Miao, C. Xiang, C. Chen, B. Ge, Z. Gan, F. Yang, M. Zhang, D. O'Hare, J. Li, T. Ge and R. Wang, Chem. Soc. Rev., 2022, 51, 6574–6651 Search PubMed.
- M. M. Jaffar, A. Rolfe, C. Brandoni, J. Martinez, C. Snape, S. Kaldis, A. Santos, B. Lysiak, A. Lappas, N. Hewitt and Y. Huang, Carbon Capture Sci. Technol., 2024, 10, 100179 Search PubMed.
- J. M. Kolle, M. Fayaz and A. Sayari, Chem. Rev., 2021, 121, 7280–7345 Search PubMed.
- S. Choi, J. H. Drese and C. W. Jones, ChemSusChem, 2009, 2, 796–854 Search PubMed.
- M. J. Lashaki, S. Khiavi and A. Sayari, Chem. Soc. Rev., 2019, 48, 3320–3405 Search PubMed.
- https://www.epa.gov/criteria-air-pollutants/naaqs-table, accessed 18 August 2024.
- https://thundersaidenergy.com/downloads/air-quality-sulphur-nox-and-particulate-emissions/, accessed 18 August 2024.
- M. Wang, L. Yao, J. Wang, Z. Zhang, W. Qiao, D. Long and L. Ling, Appl. Energy, 2016, 168, 282–290 Search PubMed.
- Y. Belmabkhout and A. Sayari, Energy Fuels, 2010, 24, 5273–5280 Search PubMed.
- F. Rezaei and C. W. Jones, Ind. Eng. Chem. Res., 2013, 52, 12192–12201 Search PubMed.
- L. Kumar and S. K. Jana, Rev. Chem. Eng., 2022, 38, 843–880 Search PubMed.
- W. Li, C. Cheng, G. Gao, H. Xu, W. Huang, Z. Qu and N. Yan, Mater. Horiz., 2024, 11, 1889–1898 Search PubMed.
- P. Brandt, A. Nuhnen, M. Lange, J. Möllmer, O. Weingart and C. Janiak, ACS Appl. Mater. Interfaces, 2019, 11, 17350–17358 Search PubMed.
- H. Yi, H. Deng, X. Tang, Q. Yu, X. Zhou and H. Liu, J. Hazard. Mater., 2012, 203, 111–117 Search PubMed.
- E. Ivanova and B. Koumanova, J. Hazard. Mater., 2009, 167, 306–312 Search PubMed.
- Y. Fu, Z. Wang, S. Li, X. He, C. Pan, J. Yan and G. Yu, ACS Appl. Mater. Interfaces, 2018, 10, 36002–36009 Search PubMed.
- X. C. An, Z. M. Li, Y. Zhou, W. Zhu and D. J. Tao, Chem. Eng. J., 2020, 394, 124859 Search PubMed.
- J. Jia, P. M. Bhatt, S. R. Tavares, E. Abou-Hamad, Y. Belmabkhout, H. Jiang, A. Mallick, P. T. Parvatkar, G. Maurin and M. Eddaoudi, Angew. Chem., Int. Ed., 2024, 63, e202318844 Search PubMed.
- J. H. Jacobs, N. Chou, K. L. Lesage, Y. Xiao, J. M. Hill and R. A. Marriott, Fuel, 2023, 353, 129239 Search PubMed.
- J. G. Bell, M. J. Benham and K. M. Thomas, Energy Fuels, 2021, 35, 8102–8116 Search PubMed.
- A. A. Abdulrasheed, A. A. Jalil, S. Triwahyono, M. A. A. Zaini, Y. Gambo and M. Ibrahim, Renew. Sustain. Energy Rev., 2018, 94, 1067–1085 Search PubMed.
- F. H. Yang and R. T. Yang, Carbon, 2003, 41, 2149–2158 Search PubMed.
- Y. L. Fan, H. P. Zhang, M. J. Yin, R. Krishna, X. F. Feng, L. Wang, M. B. Luo and F. Luo, Inorg. Chem., 2021, 60, 4–8 Search PubMed.
- Y. Zhang, P. Zhang, W. Yu, J. Zhang, J. Huang, J. Wang, M. Xu, Q. Deng, Z. Zeng and S. Deng, ACS Appl. Mater. Interfaces, 2019, 11, 10680–10688 Search PubMed.
- G. L. Smith, J. E. Eyley, X. Han, X. Zhang, J. Li, N. M. Jacques, H. G. W. Godfrey, S. P. Argent, L. J. McCormick McPherson, S. J. Teat, Y. Cheng, M. D. Frogley, G. Cinque, S. J. Day, C. C. Tang, T. L. Easun, S. Rudić, A. J. Ramirez-Cuesta, S. Yang and M. Schröder, Nat. Mater., 2019, 18, 1358–1365 Search PubMed.
- R. Tailor, M. Abboud and A. Sayari, Environ. Sci. Technol., 2014, 48, 2025–2034 Search PubMed.
- R. Tailor, A. Ahmadalinezhad and A. Sayari, Chem. Eng. J., 2014, 240, 462–468 Search PubMed.
- R. Tailor and A. Sayari, Chem. Eng. J., 2016, 289, 142–149 Search PubMed.
- C. Kim, W. Choi and M. Choi, ACS Appl. Mater. Interfaces, 2019, 11, 16586–16593 Search PubMed.
- A. Diaf, J. L. Garcia and E. J. Beckman, J. Appl. Polym. Sci., 1994, 53, 857–875 Search PubMed.
- H. C. Lan, J. Y. Zhang, Q. J. Dai, H. Ye, X. Y. Mao, Y. C. Wang, H. L. Peng, J. Du and K. Huang, Chem. Eng. J., 2021, 409, 127378 Search PubMed.
- H.-C. Lan, Y. T. Zou, Y. C. Wang, N. N. Cheng, W. L. Xu, H. L. Peng, K. Huang, L. Y. Kong and J. Du, Chem. Eng. J., 2021, 422, 129699 Search PubMed.
- L. L. Vasilieva, F. N. Dultsev and A. K. Milekhin, J. Struct. Chem., 1996, 37, 142–145 Search PubMed.
- G. Zhu, J.-M. Y. Carrillo, A. Sujan, C. N. Okonkwo, S. Park, B. G. Sumpter, C. W. Jones and R. P. Lively, J. Mater. Chem. A, 2018, 6, 22043–22052 Search PubMed.
- W. Buijs, Ind. Eng. Chem. Res., 2020, 59, 13388–13395 Search PubMed.
- Y. Zhi, Y. Zhou, W. Su, Y. Sun and L. Zhou, Ind. Eng. Chem. Res., 2011, 50, 8698–8702 Search PubMed.
- H. Ziaei-Azad, J. M. Kolle, N. Al-Yasser and A. Sayari, Microporous Mesoporous Mater., 2018, 262, 166–174 Search PubMed.
- Y. Fan, Y. Q. Cai, X. Bin Fu, Y. Yao and Y. Chen, Polymer, 2016, 107, 154–162 Search PubMed.
- M. Kruk, M. Jaroniec and A. Sayari, Langmuir, 1997, 13, 6267–6273 Search PubMed.
- X. Yan, L. Zhang, Y. Zhang, G. Yang and Z. Yan, Ind. Eng. Chem. Res., 2011, 50, 3220–3226 Search PubMed.
- A. von Harpe, H. Petersen, Y. Li and T. Kissel, J. Control. Release, 2000, 69, 309–322 Search PubMed.
- D. R. Holycross and M. Chai, Macromolecules, 2013, 46, 6891–6897 Search PubMed.
- A. Sayari, Y. Belmabkhout and E. Da’na, Langmuir, 2012, 28, 4241–4247 Search PubMed.
- H. T. Vo, S. H. Cho, U. Lee, J. Jae, H. Kim and H. Lee, J. Ind. Eng. Chem., 2019, 69, 338–344 Search PubMed.
- H. J. Lee, S. R. Lim, K. I. Lee, C. S. Kim, H. S. Kim, J. S. Choi and S. D. Lee, US Pat., US8894956, 2014 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta05829a |
| This journal is © The Royal Society of Chemistry 2024 |