Ultrahigh output energy density of explosive-energy-conversion devices assembled from multilayer ferroelectric films†
Zhengwei
Xiong‡
a,
Zhangyang
Zhou‡
b,
Yi
Liu‡
c,
Zhengqian
Fu
d,
Fangfang
Xu
d,
Leiming
Fang
e,
Xiaoru
Liu
a,
Jun
Li
c,
Ke
Jin
c and
Zhipeng
Gao
 *ac
*ac
aJoint Laboratory for Extreme Conditions Matter Properties, School of Mathematics and Physics, Southwest University of Science and Technology, Mianyang 621010, China. E-mail: z.p.gao@foxmail.com
bCollege of Intelligent Systems Science and Engineering, Hubei Minzu University, Enshi, 445000, China
cInstitute of Fluid Physics, China Academy of Engineering Physics, Mianyang 621900, China
dState Key Laboratory of High Performance Ceramics and Superfine Microstructures, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, China
eInstitute of Nuclear Physics and Chemistry, China Academy of Engineering Physics, Mianyang 621900, China
First published on 24th October 2024
Abstract
Explosive-energy-conversion materials are increasingly utilized in energy, defense, and mining due to their ultra-rapid response, extra-long storage life, and enormous power density. The energy output capability and temperature stability determine the application potential of these materials. Herein, we report 0.25Pb(Mg1/3Nb2/3)O3–0.75Pb(Zr0.4Ti0.6)O3 + 0.2 wt% Li2CO3 (PMN–PZT + 2Li) multilayer films developed by cost-effective low-temperature sintering with ultrahigh output energy density and high temperature stability. The multilayer PMN–PZT + 2Li films with a volume of 0.9 cm3 could generate a current of 3156 A, exceeding that of existing ferroelectric ceramics by two orders of magnitude. The output energy density of the multilayer PMN–PZT + 2Li films is up to 3.059 J cm−3, which is the state-of-the-art value achieved so far. The temperature stability of PMN–PZT + 2Li with the energy output could be stable up to 213 °C, higher than those of most of the ferroelectrics. In situ high-pressure synchrotron X-ray diffraction revealed that the ultrahigh output energy was derived from polar rhombohedral phase (R3m) to non-polar phase (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) shock-induced phase transitions. These findings provide a paradigm of multilayer design for high performance explosive-energy-conversion devices.
c) shock-induced phase transitions. These findings provide a paradigm of multilayer design for high performance explosive-energy-conversion devices.
1. Introduction
Explosive-energy-conversion materials with ultra-rapid response time have continuously growing applications in defense, mining, medical and other fields.1–6 Ferroelectric materials have occupied a crucial position in this field due to their distinctive responses to mechanical stimuli. Under low-pressure mechanical loading, poled ferroelectric materials show a piezoelectric response. High-pressure shock loading can induce depoling behavior of ferroelectric materials within microsecond intervals, thereby generating sharp currents or voltage pulses with megawatt power.7–15 The phenomenon of shock-induced depolarization in ferroelectrics, which effectively converts mechanical energy into electrical energy, is known as the force-electric effect of ferroelectric generators.1–3 Compared to batteries and electrochemical capacitors, ferroelectric generators have high power density, and they store energy in the form of spontaneous polarization, which provides an extra-long storage life and excellent stability.3,7–10 This lays the foundation for the development of explosive-energy-conversion devices.To date, lead zirconate titanate (Pb(Zr0.95Ti0.05)O3, PZT 95/5) ceramics have been the most widely used in the explosive-energy-conversion, which is derived from the ferroelectric (polar) to antiferroelectric (non-polar) phase transformation under high pressures, exhibiting a high output charge density of 32 μC cm−2.7–10,16,17 In recent decades, the development of other ferroelectric ceramics has been one of the most active research topics. Significant progress has been made with materials such as BaTiO3 (BT), Bi0.5Na0.5TiO3 (BNT), BiFeO3 (BFO), and (K,Na)NbO3 (KNN)-based ceramics.3,12–15,18 Nevertheless, several drawbacks have hindered their continued development in the field of explosive-energy-conversion, such as the low Curie temperature of BT and BNT-based ceramics, the high leakage current of BFO-based ceramics, and the low remnant polarization (Pr) of KNN-based ceramics.3,12–15,18 With the development of pulsed power technology, higher requirements have been placed on the energy storage density and volume size of explosive-energy-conversion devices. Due to the limitations of the effective electrode areas and small Pr, bulk ferroelectric ceramics and single crystals show a limited number of surface-bound charges. Consequently, it is difficult for them to achieve an output current exceeding 50 A during the pressure-induced depolarization process.3,4,8–15 By employing the co-fired multilayer film technology, multilayer films could significantly expand the effective electrode area, resulting in a remarkable enhancement of energy storage density.7,19–21 It represents an effective technological solution for realizing the miniaturization of ferroelectric explosive-energy-conversion devices and increasing the output energy.
Among all these ferroelectric materials, xPb(Mg1/3Nb2/3)O3–(1 − x)Pb(Zr0.4Ti0.6)O3 doped with trace amounts of Li2CO3 exhibits a high Pr of 37.2 μC cm−2, a low sintering temperature, and high temperature stability, and shows great potential for designing high-energy miniaturized explosive-energy-conversion devices.22–25 A large Pr is of great importance for the application of explosive-energy-conversion devices, which directly determines the amount of charge stored/released.3,7 To achieve co-firing, a lower sintering temperature is advantageous as it allows the use of low-cost internal electrodes (e.g., Ag, Cu) to replace the currently common noble metals in multilayer ceramic capacitors, while also reducing the volatilization of highly toxic PbO.26 Additionally, compared to commercial PZT 95/5 ceramics, the high temperature stability indicates that it can be used in higher temperature environments.12,14 Based on the intrinsic properties of materials and the performance-cost requirements of devices, the co-fired xPb(Mg1/3Nb2/3)O3–(1 − x)Pb(Zr0.4Ti0.6)O3 + Li2CO3 (PMN–PZT + Li) multilayer films are expected to be well applied in explosive-energy-conversion devices with high energy, high temperature stability, low cost and miniaturized sizes. However, the shock-induced electrical response of co-fired multilayer devices assembled from PMN–PZT + Li materials was rarely reported. The phase transition mechanism under high pressures is still unclear, which seriously hinders the applications.
In this work, 0.25Pb(Mg1/3Nb2/3)O3–0.75Pb(Zr0.4Ti0.6)O3 + 0.2 wt% Li2CO3 (PMN–PZT + 2Li) multilayer films with high Pr and high temperature stability have been prepared at 960 °C. The electrical response of poled PMN–PZT + 2Li multilayer ferroelectric films under shock compression was investigated using the gas-gun technique. The depolarization mechanism of poled PMN–PZT + 2Li films under shock pressures was elucidated.
2. Experimental section
2.1. Preparation of PMN–PZT + 2Li mixed powders
The PMN–PZT + 2Li powders were prepared by a conventional solid-state reaction process.22,23 Starting powders of PbO, MgO, Nb2O5, ZrO2 and TiO2 of a high purity of 99.9% were weighed according to the desired composition. All raw materials were produced by the Sinopharm Chemical Reagent Co., Ltd. The initial powders were milled with anhydrous ethanol and agate balls as the grinding medium for 12 hours in a Teflon jar. The milled mixture was subsequently dried in an oven and then calcined at 850 °C for 6 hours using a sealed Al2O3 crucible. Afterward, approximately 0.2 wt% of Li2CO3 (99.9%) additive was added to the calcined powder, followed by another round of ball milling and calcination. Binder (polyvinyl butyral PVB: 5%) and plasticizer (dibutyl phthalate DBP: 5%) were added to the calcined powders to form mixed powders.2.2. Preparation of multilayer PMN–PZT + 2Li films
Multilayer ferroelectric films were created using a tape-casting slurry containing a mixture of powders, dispersant, solvent, plasticizer, and organic binders. The castable slurry was prepared by ball-milling the mixed powders with a solvent composed of anhydrous ethanol and methyl ethyl ketone in a weight ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, along with a dispersant (corn oil), for 8 hours. The green films were produced employing the doctor blade technique, maintaining a thickness of 45 μm, and later air-dried at ambient temperature.19–21 Following this, Ag/Pd (7
7, along with a dispersant (corn oil), for 8 hours. The green films were produced employing the doctor blade technique, maintaining a thickness of 45 μm, and later air-dried at ambient temperature.19–21 Following this, Ag/Pd (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) paste was applied onto the green films to serve as internal electrodes, with the thickness adjusted to 1.5 μm through the dilution of the Ag/Pd paste and refinement of the screen printing procedure.19–21 The tapes were stacked together to achieve the desired number of layers through warm-pressing at 50 °C. Subsequently, the multilayer stacks were enclosed in Al2O3 packing powder and heated to 550 °C. Finally, the samples were sintered in a sealed Al2O3 crucible at 960 °C for 4 hours. For electrical measurements, outer electrodes were readied by depositing a thin layer of Ag paste on both sides of the actuator, establishing a connection with the inner electrodes. Finally, the samples were poled by subjecting them to an electric field of 30 kV cm−1 at a temperature of 110 °C for 30 minutes in a silicone oil bath.
3) paste was applied onto the green films to serve as internal electrodes, with the thickness adjusted to 1.5 μm through the dilution of the Ag/Pd paste and refinement of the screen printing procedure.19–21 The tapes were stacked together to achieve the desired number of layers through warm-pressing at 50 °C. Subsequently, the multilayer stacks were enclosed in Al2O3 packing powder and heated to 550 °C. Finally, the samples were sintered in a sealed Al2O3 crucible at 960 °C for 4 hours. For electrical measurements, outer electrodes were readied by depositing a thin layer of Ag paste on both sides of the actuator, establishing a connection with the inner electrodes. Finally, the samples were poled by subjecting them to an electric field of 30 kV cm−1 at a temperature of 110 °C for 30 minutes in a silicone oil bath.
2.3. Dynamic compression experiments
The shock compression experiment was conducted using a gas-gun technique, and the experimental details have been previously reported in existing studies.12,14 In brief, a Cu plate was propelled by the gun to generate shock waves that shocked the sample encapsulated at the end of the gun barrel in epoxy resin. Shock wave propagation was perpendicular to the direction of remnant polarization. To eliminate the effects of rarefaction waves, three unpoled ceramics were positioned on both sides of the experimental samples (Fig. S1, ESI†). The transient electrical measurement system consists of the circuit load and oscilloscope (TBS1000, Tektronix Inc., Cleveland, Ohio, USA). The shock compression pressures were controlled by the velocity of the Cu plate. In the single-layer film shock compression experiments, the Cu plate speeds are 225, 322, 475, 657 and 801 m s−1, respectively. For 34-layer films, the Cu plate speeds are 246, 302, 510, 689 and 801 m s−1, respectively. For the 120-layer film, the Cu plate speed is 801 m s−1. The load resistance was set as 0.2 Ω.2.4. Characterization
The crystal structure of the samples was determined by XRD (X'pert Pro MPD, Netherlands). The microstructures of the films were obtained using a scanning electron microscope (SEM, Carl Zeiss Supra VP55, Germany). To visualize the domains, transmission electron microscopy (TEM, JEOL 2100, JEOL, Japan) was employed. The polarization–electric field (P–E) hysteresis loops were measured using a ferroelectric measuring instrument (aixACCT TF Analyzer 2000, Germany) at a frequency of f = 10 Hz. The temperature dependence of the piezoelectric constant (d33) and the dielectric constant (εr) were tested using a Berlincourt meter (ZJ-3A, China) and a precision impedance analyzer (TH2827, Changzhou Tonghui, China), respectively. For checking the temperature stability, the poled films were heated at each temperature for 20 min and then their d33 values were measured. The pyroelectric coefficient was determined using a pyroelectric coefficient test system (PCTS-2000, Wuhan Yanhe Technology Co., Ltd, Wuhan, China) with a heating rate of 2 °C min−1. The Hugoniot curve of the ceramic samples was derived using the impedance matching method (Fig. S2†).27,28High-pressure synchrotron X-ray diffraction experiments were carried out using a symmetric-type diamond anvil cell (DAC) at the Shanghai Synchrotron Radiation Facility (SSRF) in China.29 The incident monochromatic synchrotron radiation beam exhibited a wavelength of 0.6199 Å. The resulting diffraction patterns (Fig. S3†) were subsequently transformed into one-dimensional profiles using the Fit2D software. Pressure measurements were conducted at ambient temperature using the ruby fluorescence method. To effectively transmit pressures, a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume mixture of fully deuterated methanol–ethanol was employed as the pressure medium.
1 volume mixture of fully deuterated methanol–ethanol was employed as the pressure medium.
3. Results and discussion
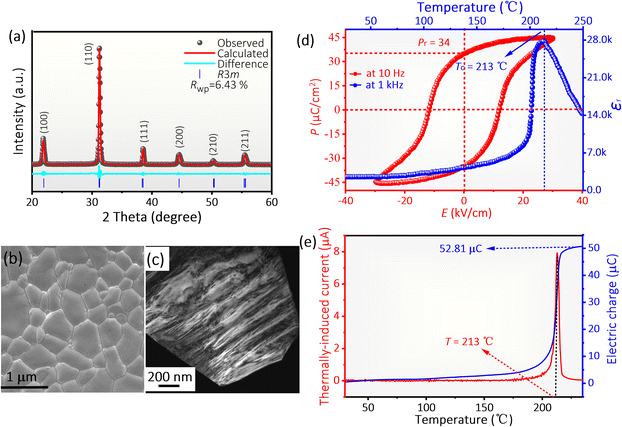
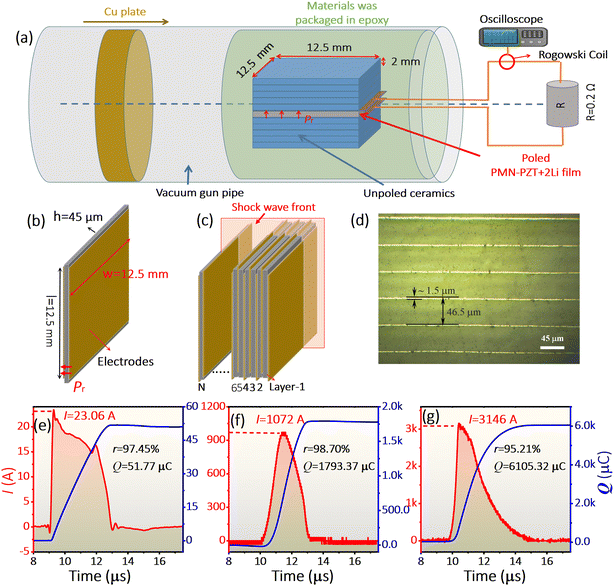
Fig. 1a displays the XRD patterns of polarized PMN–PZT + 2Li films, exhibiting a pure perovskite structure without any secondary impurity phases. The Rietveld refinement results show that the studied specimens are in the ferroelectric rhombohedral phase with the space group of R3m. The absence of a signal for Li2CO3 in the diffraction data is attributed to the low addition. The scanning electron microscopy (SEM, Fig. 1b) and transmission electron microscopy (TEM, Fig. 1c) indicate that the grain size is approximately 0.8 μm and the ferroelectric domain structures are characterized by parallel nanosized stripes, respectively. As shown in Fig. 1d, the poled film shows a saturated square hysteresis loop at 25 °C, suggesting the coercive field and Pr. The typical characteristic of ferroelectrics contains long-range interaction between dipoles.30–33 The Pr value derived from the P–E loop is measured as 34 μC cm−2, which is slightly higher than the 32 μC cm−2 of commercial PZT 95/5 ceramics.8–10 Moreover, the temperature dependence of the εr presents only one peak at 213 °C, corresponding to Curie temperature (Tc) (Fig. 1d). There is not any phase transition below Tc, implying excellent temperature stability. Note that this value is four times the 50 °C of PZT 95/5 associated with the low-temperature to high-temperature ferroelectric phase transition.34,35Fig. 1e shows the thermal depolarization current and released charge of the single-layer PMN–PZT + 2Li film. The sample exhibits a distinct peak at 213 °C, in agreement with the temperature dependence of εr, further indicating the excellent temperature stability of the PMN–PZT + 2Li film. Furthermore, the PMN–PZT + 2Li film exhibits a large d33 value (597 pC N−1) (Fig. S4†), presenting significant advantages in rapid energy transfer.The shock depolarization experiment was conducted using a gas-gun technique (Fig. 2a). Shock waves impact and compress the film along the vertical polarization direction, which induces the release of surface-bound charges, thereby generating currents in the load circuit. Fig. 2b and c show a schematic diagram of multilayer ferroelectric films for energy conversion, prepared by casting and stack technology.19,20 The multilayer film was formed by the polarized ferroelectric films stacked in parallel. The area of the single layer film is 12.5 × 12.5 mm2 (Fig. 2b). The polarization of the films is perpendicular to the direction of shock wave propagation (Fig. 2c). The optical microscopy image of the sintered multilayer film is depicted in Fig. 2d. The thickness of the ceramic layer and inner-electrode layer was ∼45 and 1.5 μm, respectively. The amplitude of the current and the total released charge of PMN–PZT + 2Li films increase with increasing shock pressures (Fig. S5 and S6†). Fig. 2e–g illustrate the observed current I and released charge Q in PMN–PZT + 2Li films with different layers under a higher shock pressure of 7.8 GPa. The Q is estimated based on the current, and the parameter r represents the ratio of the actually released charges to the total bound charges calculated from the Pr value. In Fig. 2e, the single-layer PMN–PZT + 2Li film generates a peak current signal around 23.06 A, exhibiting a square wave shape, and releases a charge of 51.77 μC. 97.45% of the total bound charges suggest complete depolarization of the single-layer PMN–PZT + 2Li film. The released charges do not reach 100%, which may be attributed to localized breakdown occurring under shock compression, resulting in partial charge loss.12,14 The 34-layer film energy-storage devices release 98.70% of the total bound charges at the same shock pressure (Fig. 2f). It is noteworthy that the current waveform of fully depolarized 34-layer ferroelectric films does not exhibit a quasi-rectangular shape as observed in single-layer films due to interferences between layers in the multilayer films.7 The complete depolarization of the 34-layer film energy storage device reveals that interlayer interference does not affect the charge release quantity of multilayer ferroelectric films. Fig. 2g shows the typical current waveform released by a 120-layer ferroelectric film energy storage device with a volume of 0.9 cm3 under the shock pressure of 7.8 GPa. The peak current generated by the module is 3156 A, and the released charge is 6105.32 μC, which is the highest current value achieved in such a small volume at present. The released surface charge density is 32.56 μC cm−2 (r = 95.21%), implying that the module is fully depolarized. Hence, the PMN–PZT + 2Li films with high Pr and high current output stemming from complete depolarization provide a new compound for high-performance explosive-energy-conversion devices.
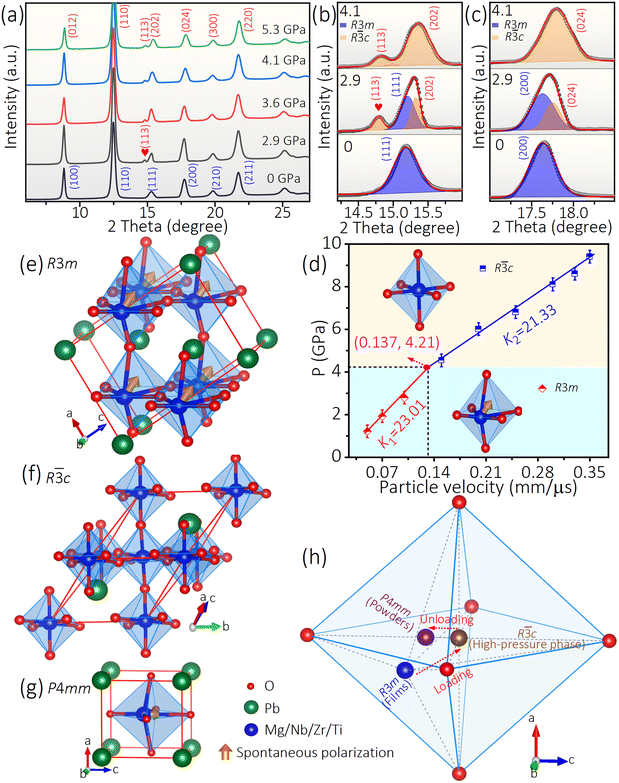
To elucidate the depolarization mechanism of PMN–PZT + 2Li films, in situ high-pressure synchrotron X-ray diffraction measurements were conducted, as shown in Fig. 3a. During pressing, all diffraction peaks shift toward higher angles, indicating a compression of the lattice volume.14 As the pressure escalates from 0 to 2.9 GPa, a fresh reflection materializes at 2θ = 14.8° highlighted by a red spade, signifying a structural phase transition. According to Rietveld refinement of high-pressure diffraction data (Fig. S7†), the structural information and reliability factor (Rwp) for the respective pressures were obtained and are summarized in Table S1.† The low Rwp value (<10%) proves a strong correspondence between the fitted values and the actual experimental data, underscoring the dependability of the findings. The diffraction pattern at 2.9 GPa can be indexed by a combination of the polar R3m structure and non-polar R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c structure. The weight fractions of R3m and R
c structure. The weight fractions of R3m and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c phases are 37.18% and 62.82%, respectively. The significant alterations in the synchrotron X-ray diffraction data as pressure increases can be conspicuously emphasized by focusing on specific regions within the diffraction data (Fig. 3b and c). With the increase of pressures, the intensity of characteristic peaks (111) and (200) in the R3m phase gradually decreases and disappears at 4.1 GPa (Fig. 3b and c), suggesting that the PMN–PZT + 2Li undergoes a structural phase transition from the polar R3m phase to the non-polar R
c phases are 37.18% and 62.82%, respectively. The significant alterations in the synchrotron X-ray diffraction data as pressure increases can be conspicuously emphasized by focusing on specific regions within the diffraction data (Fig. 3b and c). With the increase of pressures, the intensity of characteristic peaks (111) and (200) in the R3m phase gradually decreases and disappears at 4.1 GPa (Fig. 3b and c), suggesting that the PMN–PZT + 2Li undergoes a structural phase transition from the polar R3m phase to the non-polar R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c phase. The polar R3m phase is defined by the alignment of dipoles, which sustains its ferroelectric properties, while the non-polar R
c phase. The polar R3m phase is defined by the alignment of dipoles, which sustains its ferroelectric properties, while the non-polar R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c phase lacks this alignment, resulting in the loss of ferroelectricity. The pressure-induced transformation suggests a loss of ferroelectric polarization in the material.
c phase lacks this alignment, resulting in the loss of ferroelectricity. The pressure-induced transformation suggests a loss of ferroelectric polarization in the material.
Furthermore, the correlation between stress experienced by ferroelectrics during the shock compression process and particle velocity, namely the Hugoniot curve (Fig. 3d), can provide a foundation for understanding phase transitions and energy conversion behaviors.27,28,36 The turning point on the curve signifies the structural phase transition point. The phase transition at a dynamic pressure of 4.21 GPa is revealed through analysis of the Hugoniot curve for the PMN–PZT + 2Li material, which is close to the static transition pressure of 4.1 GPa (Fig. 3a). Therefore, the pressure-induced structural phase transition from R3m (polar) to R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c (non-polar) is responsible for the depolarization mechanism of PMN–PZT + 2Li films during the dynamic shock process.
c (non-polar) is responsible for the depolarization mechanism of PMN–PZT + 2Li films during the dynamic shock process.
After shock unloading, the film undergoes a transformation into powders, influenced by rarefaction waves.9 The XRD analysis of the samples, both before (films) and after (powders) shock loading, shows that the powders contain a substantial amount of the tetragonal phase with the space group P4mm, along with a small quantity of the rhombohedral phase (R3m), as shown in Fig. S8.† The genesis of the P4mm phase is attributed to the shifting of B-site atoms in the ABO3 perovskite structure along the direction of spontaneous polarization, which could be compelled by residual stress during the film breakage process.8,9,37,38 The differences observed in XRD measurements of the film samples before and after breakage suggest the high sensitivity of PMN–PZT + 2Li to external mechanical processing (Fig. S9†). Under shock loading, the PMN–PZT + 2Li undergoes a series of transitions from ambient pressure to high pressure and back to ambient pressure, following a phase transition path of R3m → R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c → P4mm + R3m. Combined with the XRD (Fig. S8†) and unit cells (Fig. 3e–g) of PMN–PZT + 2Li materials, a schematic diagram of the structural changes in the shock depolarization process of PMN–PZT + 2Li films is obtained (Fig. 3h). Under the driving of shock pressures, B-site atoms move towards the O-octahedron symmetry center in the [111] direction, accomplishing a transition from polarity to non-polarity. After shock unloading, residual stress in the powders is generated inevitably, which promotes the movement of B-site atoms in the [−1−10] direction, leading to the transformation of the material into the P4mm structure. Hence, the phase transition process under in situ high-pressure conditions, as well as after pressure unloading, has been confirmed uncontroversially. These findings not only offer a reference for designing pressure-loaded devices but also lay the groundwork for comprehending material phase transition mechanisms.
c → P4mm + R3m. Combined with the XRD (Fig. S8†) and unit cells (Fig. 3e–g) of PMN–PZT + 2Li materials, a schematic diagram of the structural changes in the shock depolarization process of PMN–PZT + 2Li films is obtained (Fig. 3h). Under the driving of shock pressures, B-site atoms move towards the O-octahedron symmetry center in the [111] direction, accomplishing a transition from polarity to non-polarity. After shock unloading, residual stress in the powders is generated inevitably, which promotes the movement of B-site atoms in the [−1−10] direction, leading to the transformation of the material into the P4mm structure. Hence, the phase transition process under in situ high-pressure conditions, as well as after pressure unloading, has been confirmed uncontroversially. These findings not only offer a reference for designing pressure-loaded devices but also lay the groundwork for comprehending material phase transition mechanisms.
The output energy density (Eoutput) of ferroelectric materials can be calculated theoretically using the following equation:
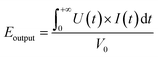 | (1) |
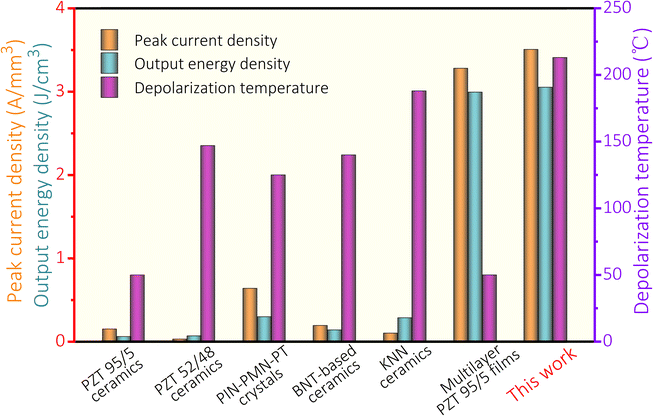 | ||
| Fig. 4 Comparison of peak current density, output energy density and depolarization temperature among ferroelectric ceramics and multilayer ferroelectric films. | ||
The temperature stability of polarized ferroelectrics serves as a crucial indicator in assessing the capability of ferroelectric devices to maintain stable operation under high-temperature conditions. In explosive-energy-conversion, ferroelectric materials with high remanent polarization are commonly used. These materials primarily include PZT 95/5,7,8 PZT 52/48,10,11,34 PIN–PMN–PT,8,9 BNT,3,13 and KNN,14 with stability temperatures of approximately 50 °C,34 147 °C,34 125 °C,8 140 °C,3 and 188 °C,14 respectively, all lower than the 213 °C stability temperature of the synthesized PMN–PZT + 2Li material. While PZT-based ferroelectric materials are widely used in actuators, piezoelectric detectors, sensors, and ultrasonic transducers due to their excellent piezoelectric and ferroelectric properties, their stability temperature is only around 150 °C.34,38 The PMN–PZT + 2Li film also shows excellent stability up to 213 °C, which is four times that of commercial PZT 95/5 (Fig. 4).34,35 Herein, the PMN–PZT + 2Li material, with its stability temperature of 213 °C, presents greater potential for applications in these fields. Obviously, compared with the multilayer PZT 95/5 films,7 the multilayer PMN–PZT + 2Li films not only yield cost reduction due to the use of low-cost internal electrodes and enhanced temperature stability against the high depolarization temperature, but also generates a superior output energy density (Fig. 4). Through material design, we have successfully obtained PMN–PZT + 2Li ferroelectric films with high-temperature stability, and significant energy storage capacity at lower sintering temperatures. Combined with low-temperature co-fired ceramic technology, the multilayer ferroelectric film devices were further assembled, achieving the amazing peak current density of 3.507 A cm−3 and output energy density of 3.059 J cm−3.
4. Conclusions
In summary, a high-performance PMN–PZT + 2Li film has been fabricated at a low sintering temperature, showcasing exceptional potential in the realm of explosive energy conversion. Under shock compression, the PMN–PZT + 2Li film can undergo complete depolarization, thus acquiring high-energy pulses. In situ high-pressure synchrotron X-ray diffraction analysis was employed to decode the depolarization process, involving a pressure-driven structural phase transition from the polar rhombohedral phase (R3m) to the non-polar phase (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c). Furthermore, tape-casting technology has proficiently assembled the multilayer ferroelectric film device. Strikingly, a multilayer film device with a compact volume of 0.9 cm3 produces a current of 3156 A, surpassing the performance of electrochemical capacitors or batteries. The output energy density is slightly higher than that of multilayer PZT 95/5 films, 45 times higher than that of commercially available PZT 95/5 ceramics, and notably exceeds those of other lead-free ferroelectric materials. Importantly, the temperature stability of PMN–PZT + 2Li films extends to 213 °C, four times that of commercial PZT 95/5, demonstrating the effectiveness of PMN–PZT-based pulse power devices in challenging conditions. These findings effectively cater to the demands for miniaturization and integration in the realm of ferroelectric explosive-energy-conversion device applications.
c). Furthermore, tape-casting technology has proficiently assembled the multilayer ferroelectric film device. Strikingly, a multilayer film device with a compact volume of 0.9 cm3 produces a current of 3156 A, surpassing the performance of electrochemical capacitors or batteries. The output energy density is slightly higher than that of multilayer PZT 95/5 films, 45 times higher than that of commercially available PZT 95/5 ceramics, and notably exceeds those of other lead-free ferroelectric materials. Importantly, the temperature stability of PMN–PZT + 2Li films extends to 213 °C, four times that of commercial PZT 95/5, demonstrating the effectiveness of PMN–PZT-based pulse power devices in challenging conditions. These findings effectively cater to the demands for miniaturization and integration in the realm of ferroelectric explosive-energy-conversion device applications.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Zhengwei Xiong, Zhangyang Zhou and Yi Liu: investigation, methodology, data management, writing – original draft. Zhengqian Fu and Fangfang Xu: investigation, validation. Leiming Fang: writing – review & editing. Xiaoru Liu: writing – review & editing. Jun Li and Ke Jin: conceptualization. Zhipeng Gao: conceptualization, resources, funding acquisition, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. U23A20567 and U2230119) and Outstanding Youth Science and Technology Talents Program of Sichuan (22JCQN0005).References
- L. L. Altgilbers, J. Baird, B. Freeman, C. S. Lynch and S. I. Shkuratov, Explosive Pulsed Power, Imperial College Press, London, U.K., 2010 Search PubMed.
- S. I. Shkuratov, Explosive Ferroelectric Generators: from Physical Principles to Engineering, World Scientific Publishing Co., 2019 Search PubMed.
- Z. Gao, W. Peng, B. Chen, S. A. T. Redfern, K. Wang, B. Chu, Q. He, Y. Sun, X. Chen, H. Nie, W. Deng, L. Zhang, H. He, G. Wang and X. Dong, Phys. Rev. Mater., 2019, 3, 035401 CrossRef CAS.
- Z. Liu, T. Lu, F. Xue, H. Nie, R. Withers, A. Studer, F. Kremer, N. Narayanan, X. Dong, D. Yu, L. Chen, Y. Liu and G. Wang, Sci. Adv., 2020, 6, eaba0367 CrossRef CAS PubMed.
- H. Lu, C. W. Bark, D. Ojos, J. Alcala, C. Eom, G. Catalan and A. Gruverman, Science, 2012, 336, 59–61 CrossRef CAS PubMed.
- H. Zhang, M. Krynski, A. D. Fortes, T. G. Saunders, M. Palma, Y. Hao, F. Krok, H. Yan and I. Abrahams, J. Am. Chem. Soc., 2024, 146, 5569–5579 CrossRef CAS PubMed.
- S. I. Shkuratov, J. Baird, V. G. Antipov, S. Zhang and J. Chase, Adv. Mater., 2019, 31, 1904819 CrossRef CAS PubMed.
- S. I. Shkuratov, J. Baird, V. G. Antipov, E. F. Talantsev, J. Chase, W. Hackenberger, J. Luo, H. R. Jo and C. S. Lynch, Sci. Rep., 2017, 7, 46758 CrossRef PubMed.
- S. I. Shkuratov, J. Baird, V. G. Antipov, C. S. Lynch, S. Zhang, J. B. Chase and H. R. Jo, J. Mater. Chem. A, 2021, 9, 12307 RSC.
- S. I. Shkuratov, J. Baird, V. G. Antipov and J. B. Chase, Appl. Phys. Lett., 2019, 115, 262903 CrossRef.
- S. I. Shkuratov and C. S. Lynch, J. Materiomics, 2022, 8, 739–752 CrossRef.
- Z. Zhou, Z. Gao, Z. Xiong, G. Liu, T. Zheng, Y. Shi, M. Xiao, J. Wu, L. Fang, T. Han, H. Liang and H. He, Appl. Phys. Lett., 2022, 121, 113903 CrossRef CAS.
- P. Peng, H. Nie, G. Wang, Z. Liu, F. Cao and X. Dong, Appl. Phys. Lett., 2018, 113, 082901 CrossRef.
- Z. Zhou, L. Fang, Z. Xiong, Y. Zhang, Y. Liu, G. Liu, Y. Liu, R. He, T. Han, J. Li, K. Wang and Z. Gao, Appl. Phys. Lett., 2023, 123, 012904 CrossRef CAS.
- Z. Xiong, G. Liu, H. Nie, Y. Liu, Z. Gao, Q. Liu, X. Chen, J. Li, L. Fang, Q. Yang, X. Zhang, J. Tang, G. Wang and X. Dong, J. Am. Ceram. Soc., 2021, 104, 1169–1177 CrossRef CAS.
- M. Xie, H. Nie, Y. Bao, F. Cao and G. Wang, ACS Appl. Mater. Interfaces, 2024, 16, 4934–4947 CrossRef CAS PubMed.
- M. Xie, H. Nie, Z. Liu, T. Lu, Y. Liu and G. Wang, J. Am. Ceram. Soc., 2023, 106, 4678–4698 CrossRef CAS.
- T. Zheng, H. Wu, Y. Yuan, X. Lv, Q. Li, T. Men, C. Zhao, D. Xiao, J. Wu, K. Wang, J. Li, Y. Gu, Z. Zhu and S. Pennycook, Energy Environ. Sci., 2017, 10, 528–537 RSC.
- K. Fan, Y. Wang and Y. Yang, Energy Harvest. Syst., 2015, 2, 113–117 CrossRef.
- J. Choi, J. Lee, B. Hahn, W. Yoon and D. Park, Mater. Res. Bull., 2008, 43, 483–490 CrossRef CAS.
- R. Zuo, L. Li, Z. Gui, X. Hu and C. Ji, J. Am. Ceram. Soc., 2002, 85, 787–793 CrossRef CAS.
- K. Wen, J. Qiu, H. Ji, K. Zhu, J. Liu, J. Wang, J. Du and F. Zhu, J. Mater. Sci.: Mater. Electron., 2014, 25, 3003–3009 CrossRef CAS.
- J. Yoo, C. Lee, Y. Jeong, K. Chung, D. Lee and D. Paik, Mater. Chem. Phys., 2005, 90, 386–390 CrossRef CAS.
- X. Zhang, L. Yang, H. Ji and J. Qiu, J. Mater. Sci.: Mater. Electron., 2018, 29, 3602–3610 CrossRef CAS.
- G. F. Fan, M. B. Shi, W. Z. Lu, Y. Q. Wang and F. Liang, J. Eur. Ceram. Soc., 2014, 34, 23–28 CrossRef CAS.
- W. Liu, T. Zheng, F. Zhang, X. Ruan, G. Li, Z. Man, Z. Gao, X. Lv and J. Wu, ACS Appl. Mater. Interfaces, 2022, 14, 51113–51121 CrossRef CAS PubMed.
- R. E. Setchell, J. Appl. Phys., 2007, 101, 053525 CrossRef.
- J. C. F. Millett, N. K. Bourne and D. Deas, J. Phys. D: Appl. Phys., 2007, 40, 2948–2953 CrossRef CAS.
- Z. Zhou, Z. Xiong, X. Liu, T. Zeng, W. Liu, J. Wu and Z. Gao, Phys. Rev. B, 2024, 108, 104108 CrossRef.
- K. Xu, J. Li, X. Lv, J. Wu, X. Zhang, D. Xiao and J. Zhu, Adv. Mater., 2016, 38, 8519–8523 CrossRef PubMed.
- Q. Liu, Y. Zhang, J. Gao, Z. Zhou, H. Wang, K. Wang, X. Zhang, L. Li and J. Li, Energy Environ. Sci., 2018, 11, 3531 RSC.
- T. Sluka, A. K. Tagantsev, D. Damjanovic, M. Gureev and N. Setter, Nat. Commun., 2012, 3, 748 CrossRef PubMed.
- G. Du, R. Liang, J. Wang, L. Wang, W. Zhang, G. Wang and X. Dong, Ceram. Int., 2013, 39, 9299–9303 CrossRef CAS.
- S. I. Shkuratov, J. Baird, V. G. Antipov, E. F. Talantsev, H. R. Jo, J. C. Valadez and C. S. Lynch, Appl. Phys. Lett., 2014, 104, 212901 CrossRef.
- J. Handerk and Z. Ujma, J. Phys.: Condens.Matter, 1995, 7, 1721–1728 CrossRef.
- R. E. Setchell, J. Appl. Phys., 2005, 97, 013507 CrossRef.
- S. I. Shkuratov, J. Baird, V. G. Antipov, W. Hackenberger, J. Luo, S. Zhang, C. S. Lynch, J. B. Chase, H. R. Jo and C. C. Roberts, Appl. Phys. Lett., 2018, 112, 122903 CrossRef.
- S. Zhang, F. Li, X. Jiang, J. Kim, J. Luo and X. Geng, Prog. Mater. Sci., 2015, 68, 1–66 CrossRef CAS PubMed.
- D. Jiang, J. Du, Y. Gu and Y. Feng, J. Appl. Phys., 2012, 111, 024103 CrossRef.
- Y. Liu, H. Nie, M. Xie, F. Cao, P. Peng and G. Wang, Appl. Phys. Lett., 2023, 123, 083903 CrossRef.
- S. Zhang, F. Li, N. P. Sherlock, J. Luo, H. J. Lee, R. Xia, R. J. Meyer Jr, W. Hackenberger and T. R. Shrout, J. Cryst. Growth, 2011, 318, 846–850 CrossRef CAS PubMed.
- N. Jha, P. Ramesh, E. Bekyarova, M. E. Itkis and R. C. Haddon, Adv. Energy Mater., 2012, 2, 438–444 CrossRef CAS.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- D. A. Hector, Y. Kiya and J. C. Henderson, Phys. Today, 2008, 61, 43–47 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta06396a |
| ‡ Zhengwei Xiong, Zhangyang Zhou and Yi Liu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |