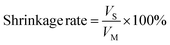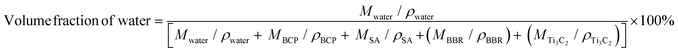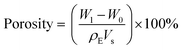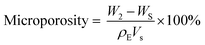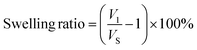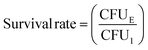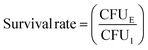Study on 3D printed MXene-berberine-integrated scaffold for photo-activated antibacterial activity and bone regeneration†
Yi
Tan
a,
Huan
Sun
b,
Yuanchen
Lan
c,
Haider Mohammed
Khan
d,
Hui
Zhang
c,
Linli
Zhang
c,
Fengying
Zhang
e,
Yujia
Cui
f,
Lan
Zhang
a,
Dingming
Huang
a,
Xinmei
Chen
a,
Changchun
Zhou
 b,
Jianxun
Sun
*a and
Xuedong
Zhou
a
b,
Jianxun
Sun
*a and
Xuedong
Zhou
a
aState Key Laboratory of Oral Disease & National Center for Stomatology & National Clinical Center for Oral Diseases & Department of Cariology and Endodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan, China. E-mail: jxsun@scu.edu.cn
bNational Engineering Research Centre for Biomaterials, College of Biomedical Engineering, Sichuan University, Chengdu 610041, China
cState Key Laboratory of Oral Disease & National Center for Stomatology & National Clinical Center for Oral Diseases & Department of Orthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan, China
dDepartment of Orthopedics, West China Hospital, Sichuan University, Chengdu 610041, China
eWest China Hospital/West China School of Nursing, Sichuan University, Chengdu 610041, China
fState Key Laboratory of Oral Disease & National Center for Stomatology & National Clinical Center for Oral Diseases & Department of Paediatric Dentistry, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan, China
First published on 25th January 2024
Abstract
The repair of mandibular defects is a challenging clinical problem, and associated infections often hinder the treatment, leading to failure in bone regeneration. Herein, a multifunctional platform is designed against the shortages of existing therapies for infected bone deficiency. 2D Ti3C2 MXene and berberine (BBR) are effectively loaded into 3D printing biphasic calcium phosphate (BCP) scaffolds. The prepared composite scaffolds take the feature of the excellent photothermal capacity of Ti3C2 as an antibacterial, mediating NIR-responsive BBR release under laser stimuli. Meanwhile, the sustained release of BBR enhances its antibacterial effect and further accelerates the bone healing process. Importantly, the integration of Ti3C2 improves the mechanical properties of the 3D scaffolds, which are beneficial for new bone formation. Their remarkable biomedical performances in vitro and in vivo present the outstanding antibacterial and osteogenic properties of the Ti3C2-BBR functionalized BCP scaffolds. The synergistic therapy makes it highly promising for repairing infected bone defects and provides insights into a wide range of applications of 2D nanosheets in biomedicine.
1. Introduction
Jaw bone defects commonly result from maxillofacial infection, trauma, tumors, and/or malformation.1,2 Therapeutic strategies for the reconstruction of critical-sized mandibular defects remain a clinical challenge owing to the irregular shape and large range of bone defects.3,4 An autologous graft is the current gold standard for repairing bone defects; however, donor site injuries and the limited supply of donors restrict its clinical application and thus, call for feasible alternatives.5 Additionally, a high infection rate and maxillofacial surgery also hinder bone repair and complicates the treatment.6 In the presence of practical requirements for reconstructing massive mandibular defects and minimizing the infection risk during the repairing process, an investigation of artificial substitutes should be put forward to optimize bone regeneration capacity and antibacterial activity.Coupled with computer-aided design and manufacture, 3D printing could realize the precise patterning of scaffolds for bone regeneration with personalized constructs based on medical imaging.7,8 For this application, calcium phosphates are considered among the most promising biomaterials owing to their similar chemical constituents to bone tissue and considerable bioactivity.9 In contrast to hydroxyapatite (HA) and beta-tricalcium phosphate (β-TCP), biphasic calcium phosphate (BCP) allows for the preferable adjustment of mechanical properties and biodegradability through manipulating the HA/β-TCP ratio, which is critical to the growth of new bone.10–12 In this respect, 3D printed BCP scaffolds have been applied in various studies for bone tissue engineering and may be potential basic materials for mandible reconstruction.13
Clinically, antibiotics are widely employed and regarded as the primary therapeutic approach for treating bacterial infection.14 However, an imbalance between antibiotic usage and bacterial resistance has become a crucial problem threatening human health, and there is an urgent need to explore efficient antibacterial agents without resistance.15,16 MXene, as a new type of 2D material, has recently gained increasing attention with a focus on its specific nanosheet structure, surface characteristics, conductivity, and other properties.17,18 The MXene formula Mn+1XnTx involves transition metal carbides, nitrides, and carbonitrides, where M denotes transition metal atoms, X denotes carbon or nitrogen, and T represents surface terminations (e.g. –OH and –F).19,20 Apart from a broad range of applications in water purification, catalysis, and energy storage, the ultrathin structure of MXene endows it with great prospects in versatile fields of biomedicine.21–24 Specifically, Ti3C2 MXene has been extensively applied in photothermal therapy (PTT) owing to its high extinction coefficient and photothermal conversion efficiency in the near-infrared region and excellent biocompatibility.25,26 On account of minimal invasiveness, spatiotemporal control, and strong bactericidal effect, Ti3C2 MXene-mediated PTT has shown great advantages in bacterial infections.27,28 In addition, the sharp edge structure of the MXene nanosheets facilitates physical damage to the bacterial membrane, which is applicable for bacteria elimination in combination with thermoablation.29,30 Furthermore, with the potential release of titanium-based substances during the degradation process, Ti3C2 MXene has demonstrated satisfactory performance in restoring bone defects.17,31 Therefore, an antibacterial nanoplatform based on Ti3C2 MXene is highly feasible in applications of infected bone defects.
Considering the transient effect of PTT during laser irradiation, residual bacteria in situ may keep multiplying and result in persistent infection.32 Although traditional antibiotics could compensate for the inadequate antibacterial efficiency of single-mode PTT at a low concentration,33 the combined treatment strategy goes against the original intention of PTT in disrupting bacterial resistance.34,35 Berberine (BBR) is a natural isoquinoline alkaloid extracted from various species of medicinal herbs, such as Coptidis rhizoma, Hydrastis, Berberis vulgaris, and Berberis aristata,36,37 and has been clinically utilized in treating infection and cancer.38 Through increasing the membrane permeability, BBR could realize effective interaction with bacteria membranes and amino acids, thus performing antibacterial activity, while reducing the propensity towards drug resistance.39,40 Regarding the overexpression of osteoclastic cytokines caused by the bacterial infection that disturbs the bone healing process,41–43 BBR has the promise to weaken the inflammatory reaction and inhibit bone destruction.44 As MXene nanosheets possess a large surface area with negative groups, positive-charged BBR can be strongly anchored through electrostatic interactions, which is desirable for the controlled release of antibacterial drugs.25,26
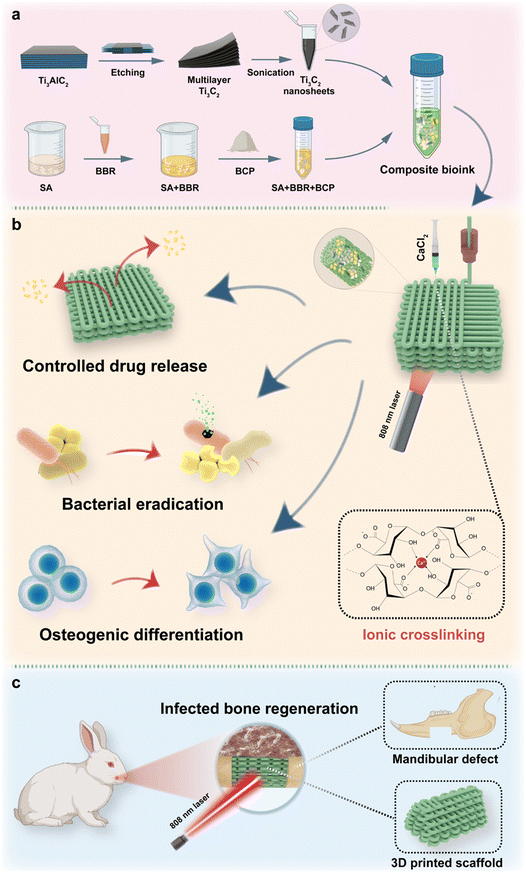
In this study, Ti3C2 MXene nanosheets are fabricated. Together with BBR, the Ti3C2 MXene nanosheets are encapsulated into 3D printed BCP scaffolds. Solidum alginate (SA) with controllable viscosity is applied to integrate different components and form the bioink for 3D printing.45 The specific crosslinking property of SA and loaded MXene nanosheets bring about strong mechanical properties of scaffolds, which could sustain the bone reconstruction. Based on the photothermal property of Ti3C2, the controllable hyperthermia is employed for bacterial elimination and mediating BBR release, while BBR would further enhance the antibacterial and osteogenic effect. The specifically designed MXene-BBR-integrated scaffold and the synergistic therapy are expected to sequentially perform the antibacterial and osteogenic activities, providing a novel therapeutic strategy for tissue engineering in infected mandibular defects (Fig. 1).
2. Materials and methods
2.1. Materials
BCP powders were purchased from National Engineering Research Center for Biomaterials (Chengdu, China). SA was purchased from Sigma-Aldrich (USA). Lithium fluoride (LiF, purity of 99%), hydrochloric acid (HCl, 37%) and berberine (BBR, purity of 99%) were purchased from Aladdin (Shanghai, China). Ti3AlC2 powders (−400 mesh) were purchased from Xinxi Technology Co., Ltd (Foshan, China).2.2. Synthesis and characterization of Ti3C2
0.8 g LiF was dissolved in HCl solution (12 M, 10 mL) and stirred at 40 °C for 10 min (500 rpm). After the above reaction, 0.5 g Ti3AlC2 was gradually added into the mixed solution and stirred at 40 °C for 48 h (500 rpm). The final products were washed with HCl solution (1 M), followed by deionized water via centrifuging at 3500 rpm multiple times until pH ≈ 6. Under the protection of nitrogen, the precipitates were collected and dispersed in deionized water through bath sonication for 2 h. Afterwards, the mixtures were centrifuged at 3500 rpm for 15 min, and the supernatants were collected. Ti3C2 was obtained after the freeze-drying process in an LGJ-10 freeze-dryer (Song Yuan Freeze Dryer, China).25Scanning electron microscope (SEM) images were measured on a S48000 scanning electron microscope (Hitachi, Japan). Transmission electron microscopy (TEM) analysis was performed on a Tecnai G2 F20 S-TWIN transmission electron microscope (FEI, USA). Atomic force microscopy (AFM) images were obtained from an MPF-3D-BIO atomic force microscope (Oxford Instruments, UK). X-ray powder diffraction (XRD) was carried out on an X’Pert 1 X-ray diffractometer (Philips, Netherlands).
2.3. Preparation and characterization of the composite bioinks
Briefly, 3 g SA was dissolved in 45 mL deionized water and stirred at 35 °C for 24 h. Meanwhile, 0.1 mL BBR solution (200 mg mL−1) was added during the stirring process. After the uniform slurry was formed, 30 g BCP powders were gradually added to the slurry in 3 steps by an ARE-310 defoamer (THINKY, Japan). Next, 5 mL Ti3C2 aqueous solution at a concentration of 7.5 mg mL−1 was added to obtain the final bioink (Bioink BBTS).Bioink BS, Bioink BSl, Bioink BSh, Bioink BBS, Bioink BTS, Bioink BBTSl, and Bioink BBTSh were prepared through the same process as that for Bioink BBTS. The formulations of different bioinks are listed in Table 1.
| Bioink | Water/mL | SA/g | BCP/g | BBR/mg | Ti3C2/mg |
|---|---|---|---|---|---|
| Bioink BSl | 50.1 | 3 | 25 | 0 | 0 |
| Bioink BS | 50.1 | 3 | 30 | 0 | 0 |
| Bioink BSh | 50.1 | 3 | 35 | 0 | 0 |
| Bioink BBS | 50.1 | 3 | 30 | 20 | 0 |
| Bioink BTS | 50.1 | 3 | 30 | 0 | 37.5 |
| Bioink BBTSl | 50.1 | 3 | 30 | 20 | 12.5 |
| Bioink BBTS | 50.1 | 3 | 30 | 20 | 37.5 |
| Bioink BBTSh | 50.1 | 3 | 30 | 20 | 62.5 |
For the initial evaluation of fiber formation, different bioinks were transferred into the printing tube attached with the 500 μm radius needle. Pressure was exerted on the plunger until the bioinks were continuous extruded. The photographs of the fiber extrusion were captured by a portable microscope. The photographs of the fiber stacking were recorded by a digital camera.
The tubes filled with bioinks were placed in a water bath at the temperature of 30 °C for 30 min to make sure the temperature of the bioinks was around 30 °C. The tubes were then taken out for rheological characterization to quantify the flow performance and printability of the bioinks using a MCR302 rheometer (Anton Paar, Austira). The shear thinning behavior was measured at shear rates from 0.1 to 100 s−1. The recovery behavior was performed by applying a shear rate of 100 s−1 for 100 s, followed by applying a shear rate of 0.01 s−1 for 100 s.
2.4. Fabrication and characterization of the composite scaffolds
Bioink BS, Bioink BBS, Bioink BTS, Bioink BBTSl, Bioink BBTS, and Bioink BBTSh were further applied in the following 3D printing process to fabricate BCP-SA scaffolds (BS), BCP-SA-BBR scaffolds (BBS), BCP-SA-Ti3C2 (7.5 mg mL−1) scaffolds (BTS), BCP-SA-BBR-Ti3C2 (2.5 mg mL−1) scaffolds (BBTSl), BCP-SA-BBR-Ti3C2 (7.5 mg mL−1) scaffolds (BBTS), and BCP-SA-BBR-Ti3C2 (12.5 mg mL−1) scaffolds (BBTSh), respectively.The scaffolds were fabricated through the extrusion 3D printing by a Bio-Architect®-Pro 3D printer (Regenovo Biotechnology Co. Ltd, China). A tapered nozzle with inner diameter at 500 μm was selected. The extrusion pressure was set at 0.4–0.5 MPa, and the printing speed ranged from 1.1–1.3 mm s−1. The nozzle temperature was maintained at 30 °C and the temperature of the building platform was set at 15 °C. The composite scaffolds (10 × 10 × 2 mm, pore size: 350 μm) were printed with a layer thickness of 0.4 mm. After printing, the scaffolds were immediately immersed in saturated calcium chloride (CaCl2) solution for 24 h. The scaffolds were washed with deionized water 3 times, and then freeze-dried.
For MXene-coated BCP-SA scaffolds (MX@BS), BS was immersed in 1 mL Ti3C2 aqueous solution (5 mg mL−1) and placed in a drying oven at 35 °C for 24 h. The scaffolds were then flipped over, and the procedure was repeated to obtained MX@BS. The content ratio of the coated Ti3C2 on MX@BS was calculated through a 5100 SVDV inductively coupled plasma-optical emission spectrometer (ICP-OES) (Agilent, USA).
Then, BCP-SA-Ti3C2 (6.89 ± 0.39 mg mL−1) scaffolds (BMS) were fabricated with the same content ratio of Ti3C2 (1.03 ± 0.06%) in MX@BS through the same process as that for BTS.
The photographs of different scaffolds were captured by a digital camera. SEM images, element mapping and energy-dispersive spectrometer (EDS) characterization were measured on a scanning electron microscope. XRD was carried out on an X-ray diffractometer. Thermogravimetric (TGA) analysis was performed by a TGA/DSC2 system (Mettler Toledo, Switzerland) at the heating rate of 15 °C min−1, with the temperature ranging from 20 °C to 1000 °C. X-ray photoelectron spectroscopy (XPS) was operated on an Axis Supra X-ray photoelectron spectroscope (Kratos, UK).
2.5. Evaluation of the crosslinking structure and mechanical properties
After freeze-drying, the diameter and height of the scaffold were measured, and the shrinkage rate was tested by the equation:where VS is the volume of the dried scaffold, and VM is the volume of the designed model.
The volume fraction of water was measured by the definition:
The porosity was measured based on the Archimedes principle. The microporosity was measured using a modified method based on the liquid displacement method.46 Briefly, the scaffold was immersed in ethanol in a bottle, then ethanol was compelled into micropores of the scaffold through evacuation process. The scaffold was then suspended in the bottle and the bottle was weighed (W1). Next, the scaffold was taken out and the ethanol on the surface of scaffold was removed, the ethanol-impregnated scaffold was weighed (W2). The porosity, microporosity and macroporosity were calculated by the formulas:
| Macroporosity = (Porosity − Microporosity) × 100% |
To evaluate the swelling ability of the scaffold, the scaffold was immersed in PBS and incubated for 1 month. At each test time, the scaffold was taken out and the diameter was measured. For BTS and BBTS, the swelling capacities under laser stimuli were investigated. After laser irradiation (1 W cm−2) for 15 min, the diameter and height of the scaffold were immediately measured. The swelling ratio was further calculated based on the equation:
The micro mechanical property of the scaffold (10 × 10 × 2 mm) was measured on a Q-800 dynamic mechanical analyzer (TA instruments, USA) at the loading force of 0.1 N min−1. The Young's modulus was calculated according to the stress–strain curves. The mechanical properties of the integral scaffold (10 × 10 × 10 mm) were performed on an AGS-500NX electronic universal testing machine (SHIMADZU, Japan) at the loading speed of 3 mm min−1, and the compressive strength and young's modulus were measured.
2.6. Photothermal properties investigation
UV-vis spectra were recorded by a UV-1900PC spectrometer (AOE Instruments, China).The photothermal properties were evaluated using an 808 nm laser. To compare the photothermal ability of MX@BCP and BMS, MX@BS and BMS were exposed to laser irradiation (0.3 W cm−2). The temperature was monitored by a FLIR T460 infrared thermal image camera (FLIR Systems, USA).
Next, different scaffolds (BS, BBS, BTS, BBTS) were exposed to laser irradiation (0.5 W cm−2) for 10 min. The temperature was monitored by an infrared thermal image camera. Analogously, the photothermal performances of BBTSh and BBTSl at a power density of 0.5 W cm−2, BBTS at different power densities (0.4, 0.5, 0.6 W cm−2) in air and BBTS at different power densities (0.8, 0.9, 1.0 W cm−2) in moisture environment were detected. The photothermal stability of BBTS was further assessed through five circles of laser-on–off procedure (0.5 W cm−2) in air.
2.7. Drug release assay
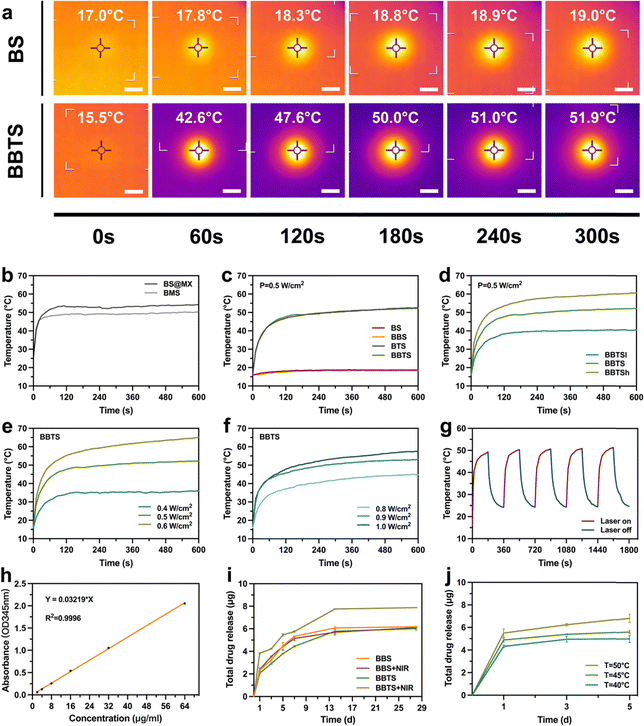
Absorbances at λ = 345 nm of different concentrations of BBR solution were measured by an Epoch 2 microplate reader (Biotek, USA) to establish the standard curve. To evaluate the drug release properties, BBS and BBTS were immersed in 2 mL PBS. At given time intervals, 0.2 mL of releasing solution was extracted, and the absorbance at λ = 345 nm was measured. The amount of the released drug was calculated based on the standard curve. After measuring, 0.2 mL PBS was added to compensate for the extracted solution. Similarly, for assessment of the NIR-responsive drug release, BBS and BBTS were exposed to laser irradiation (1 W cm−2) for 15 min before extraction. The extracted solution was then measured, followed by the compensation of 0.2 mL PBS. Next, BBTS was exposed to laser irradiation at different power densities (1 W cm−2, 1.1 W cm−2, 1.2 W cm−2) for 15 min, and the drug releasing was measured.To record the pH value, different scaffolds (BS, BBS, BTS, BBTS) were immersed in PBS and incubated at 37 °C. At the given time intervals, pH values of PBS immersed with scaffolds were detected by a FiveEasy Plus pH-meter (METTLER TOLEDO, USA).
2.8. In vitro antibacterial assay
The antibacterial properties were evaluated using Staphylococcus aureus (S. aureus, ATCC 25923) and Escherichia coli (E. coli, ATCC 25922). Different scaffolds (BS, BBS, BTS, BBTS) were prepared under ultraviolet sterilization for 3 h.Inhibition zone: initially, 2 mL bacterial suspension (S. aureus, 107 CFU mL−1) was added to BS, BBS, and BBTS and cultured at 37 °C for 12 h to cover the scaffolds with bacteria. Next, 200 μL bacterial suspension (107 CFU mL−1) was spread onto LB agar plates. As-prepared BS, BBS, and BBTS were then placed onto the plates, and BBTS was subjected to an 808 nm laser irradiation (0.5 W cm−2) for 15 min. After culturing at 37 °C for 24 h, the inhibition zone was captured by a digital camera.
Growth curve and survival rate of bacteria: 2 mL bacterial suspension (105 CFU mL−1) was cultured with different scaffolds in an incubator (37 °C, 200 rpm). For BTS + NIR and BBTS + NIR, scaffolds were subjected to an 808 nm laser irradiation (1.2 W cm−2) for 15 min. At given time intervals, the absorbance of the bacterial suspension at λ = 600 nm was measured. After being cultured for 12 h, the bacterial suspension in different groups was diluted, and 100 μL diluted suspension was spread onto LB agar plates. The plates were captured by a digital camera after being incubated at 37 °C for 12 h. Afterwards, the plate counting method was applied to determine the survival rate of bacteria through the following formula:
Live/dead staining: 2 mL bacterial suspension (107 CFU mL−1) was added to different scaffolds, and cultured at 37 °C for 48 h. The LB medium was refreshed at 24 h. For BTS + NIR and BBTS + NIR, scaffolds were subjected to an 808 nm laser irradiation (1.2 W cm−2) for 15 min. After culturing, scaffolds were taken out and a Live/Dead BacLight viability kit (Thermo Fisher, USA) was employed to stain the bacteria on different scaffolds for 20 min. Finally, the scaffolds were rinsed with deionized water and the fluorescence images of bacteria were captured by a SM 800 confocal laser scanning microscope (CLSM) (Zeiss, Germany).
SEM observation: 2 mL bacterial suspension (107 CFU mL−1) was added to different scaffolds, and cultured at 37 °C for 24 h. For BTS + NIR and BBTS + NIR, scaffolds were subjected to an 808 nm laser irradiation (1.2 W cm−2) for 15 min. After culturing, scaffolds were rinsed with PBS and then fixed with 4% paraformaldehyde for 30 min, followed by a dehydration process with graded ethanol solutions (20%, 40%, 60%, 70%, 80%, 90%, 100% for 15 min each). Afterwards, the specimens were dried using an EM CPD300 critical point dryer (Leica, Germany) and sputter-coated with gold for SEM observation.
2.9. In vitro cytotoxicity assay
Mouse pre-osteoblast MC3T3-E1 cells were used for evaluating cell proliferation and adhesion on composite scaffolds. The cells were cultured in a-MEM medium (Gibco, USA) supplemented with 10% FBS (Gibco, USA) and 1% penicillin–streptomycin (100 U mL−1) at 37 °C in an atmosphere containing 5% CO2. Briefly, cells were seeded on different scaffolds (BS, BBS, BTS, BBTS) at a density of 105 mL−1 in 24-well plates. After culturing for 1 and 7 days, cells were stained with fluorescein diacetate/propidium iodide (FDA/PI) (Sigma, USA) and observed by a CLSM. In the next step, at day 7, the cells were fixed with 4% paraformaldehyde and transparentized by 0.1% Triton X-100 in PBS. The cells were then stained with 4′,6-diamidino-2-phenylindole (DAPI) and rhodamine phalloidin (red) (Sigma, USA) for CLSM observation. The proliferation of cells was measured by the cell counting kit-8 (CCK-8) assay on days 1, 3, and 7. RAW 264.7 cells were treated with LPS (100 ng mL−1) for 48 h, and then cultured with different scaffolds for 24 h. The inflammatory activities of cells were test using Mouse TNF-α Elisa kit and Mouse IL-6 Elisa kit (ZCI BIO, China) according to the manufacturer's instruction.2.10. In vitro osteogenic differentiation
Rabbit bone mesenchymal stem cells (rBMSCs) were extracted from the long bones of experimental rabbits. After culturing with scaffolds for 1 day, the culture medium was replaced with a-MEM medium supplemented with 10% FBS, 1% penicillin–streptomycin (100 U mL−1), 50 μM mL−1 ascorbic acid (Sigma, USA), 0.1 μM dexamethasone (Sigma, USA), and 10 mM β-glycerophosphate (Sigma, USA). The osteogenic medium was refreshed every 3 days. After osteogenic induction for 7 days, the total cellular RNA was extracted by the Molpure Cell/Tissue Total RNA Kit (Yeasen, China), and was then transcribed into cDNA with a PrimeScript RT reagent kit (Takara, Japan). The qRT-PCR was conducted using a TB Green Premix Ex Taq II reagent kit (Takara, Japan). The relative expression of the target genes (OCN, OPN, ALP) was normalized to glyceraldehyde-3-phosphate dehydrogenase (GADPH). The forward and reverse primers are listed in Table 2.| Gene | Primer sequence |
|---|---|
| GADPH-F | GCCGCTTCTTCTCGTGCAGTGCTAG |
| GADPH-R | AGCCTTGACCGTGCCGTGGAACTT |
| OCN-F | CTCACTCTTGTCGCCCTGCTG |
| OCN-R | TCGCTGCCCTCCCTCTTGG |
| OPN-F | ACCGCAGAATGCTATGTCCTAG |
| OPN-R | GTGGTCATCGTCCTCATCCTCATC |
| ALP-F | TTGTCGGTGCGGTCGTGAAG |
| ALP-R | GCCTCGTGCGTGCTCTCG |
2.11. Animal surgical procedures
The scaffolds (10 × 5 × 5 mm) for animal experiments were fabricated through the same procedures above (Fig. S19, ESI†). BS and BBTS were selected for the following experiments. The scaffolds were prepared under ultraviolet sterilization for 3 h.The animal experiments were approved by the Animal Ethics Committee of Sichuan University. The New Zealand white rabbits (weight: 2.5–3 kg) were used in the following study. After 7 days of acclimation, general anesthesia was performed by intravenous injection of 2% pentobarbital sodium (30 mg kg−1). The mandibular defect (10 × 5 mm) was built penetrating the corpora mandibulae (Fig. S20, ESI†).
For infection models, scaffolds were prepared immersed in bacterial suspension (S. aureus, 105 CFU mL−1) at 37 °C for 1 min. The scaffolds carrying S. aureus were dried at 37 °C for 15 min and implanted to the defects. The incisions were carefully closed after the surgery. In particular, laser irradiation (0.5 W cm−2) was employed in BS + NIR and BBTS + NIR for 30 min (due to the different shapes between the laser spot and the scaffold, the laser was applied to focus on the partial scaffold at one time for 15 min, and was applied to focus on the remaining part of the scaffold for another 15 min, with a total time of 30 min) at the first 2 days. The temperature was monitored by an infrared thermal image camera. Moreover, the infection sites were captured by a digital camera.
For osteogenesis models, scaffolds were implanted to the mandibular defects and the incisions were carefully closed after the surgery.
2.12. In vivo antibacterial assay
After 1 week of implantation, rabbits were sacrificed with an overdose of anesthesia. The scaffolds were collected and the mandible tissue surrounding the scaffolds was harvested. Different scaffolds were cultured in Luria–Bertani (LB) medium in an incubator (37 °C, 200 rpm) for 12 h, and 100 μL diluted bacterial suspension was spread onto LB agar plates for culturing for another 12 h. Photographs of LB agar plates were acquired by a digital camera, and the plate counting method mentioned above was applied to determine the survival rate of bacteria through the following formula:where CFU1 represents the colony forming units in BS + NIR, and CFUE represents the colony forming units in other experimental groups.
Histological analysis: the harvested tissue was fixed with 4% paraformaldehyde, followed by decalcification with 10% EDTA solution for 1.5 months. The specimens were then embedded in paraffin and cut into proper sections. Later, Gram staining and hematoxylin and eosin (H&E) staining were performed, and the staining sections were evaluated using a Pannoramic MIDI digital scanner (3DHISTECH, Hungary) and a CaseViewer software (3DHISTECH, Hungary).
2.13. In vivo osteogenesis assay
After 1 and 4 months of implantation, rabbits were sacrificed with an overdose of anesthesia and the mandibles implanted with scaffolds were harvested. The specimens were fixed with 4% paraformaldehyde prior to further assessments.Micro-CT analysis: the specimens were assessed by a VivaCT 80 Micro-CT system (SCANCO Medical AG, Switzerland) at a spatial resolution of 22 μm to detect the formation of new bone within the defects. The mandibles were reconstructed and the fundamental parameters were quantitively analyzed with a Mimics software (Materialise, Belgium).
Histological analysis: after being decalcified with 10% EDTA solution for 1.5 months, the specimens were embedded in paraffin and cut into proper sections. Later, H&E staining, Masson's trichrome staining and immunohistochemical staining were performed, and the stained sections were observed. The semi-quantitative analysis was conducted by ImageJ software (NIH, Germany).
2.14. Statistical analysis
The data were expressed as mean ± standard deviation. Student's t-test (unpaired), one-way or two-way ANOVA test were executed through GraphPad Prism Software (GraphPad Software Inc, USA) to perform the statistical comparisons. The value of p < 0.05 was considered statistically significant (*p < 0.05, **p < 0.01, and ***p < 0.001).3. Results and discussion
3.1. Synthesis and characterization of Ti 3 C 2 nanosheets
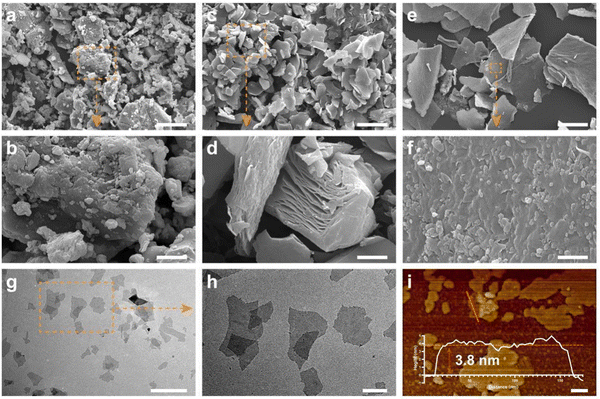
Ultrathin Ti3C2 nanosheets were synthesized by a modified two-step exfoliation process from Ti3AlC2 MAX ceramics, which endowed MXene with large surface areas and few defective structures.47,48 According to the Tyndall effect, the bright ‘pathway’ in the colloid demonstrated the great dispersion of Ti3C2 in aqueous solution (Fig. S1a, ESI†), enabling the even mixture of Ti3C2 into bioink.17Initially, Ti3AlC2 ceramics (Fig. 2a and b) were immersed in HCL/LiF etching solution for 48 h to selectively extract Al from Ti3AlC2, in which the multilayer Ti3C2 (Fig. 2c and d) was fabricated in a relatively mild etching condition.30 The accordion structure indicated the successful removal of Al. Subsequently, multilayer Ti3C2 was delaminated through sonication under the protection of nitrogen to obtain monolayer Ti3C2 nanosheets (Fig. 2e and f). As shown in the TEM images (Fig. 2g and h), the Ti3C2 nanosheets revealed the typical 2D morphology with a smooth surface. In addition, the standing Ti3C2 (Fig. S1b, ESI†) exhibited the nanoscale thickness around 4 nm, which was further confirmed in the AFM image (Fig. 2i). The XRD result (Fig. S8, ESI†) showed that the (002) peak of Ti3AlC2 at around 10° was shifted to 6.30° in Ti3C2 with increased lattice parameters, suggesting the increased layer space in Ti3C2 after the etching process, and the XRD pattern of Ti3C2 (in agreement with previous reports) demonstrated the successful fabrication of the Ti3C2 nanosheets.25,27,49
3.2. Preparation and characterization of composite bioinks
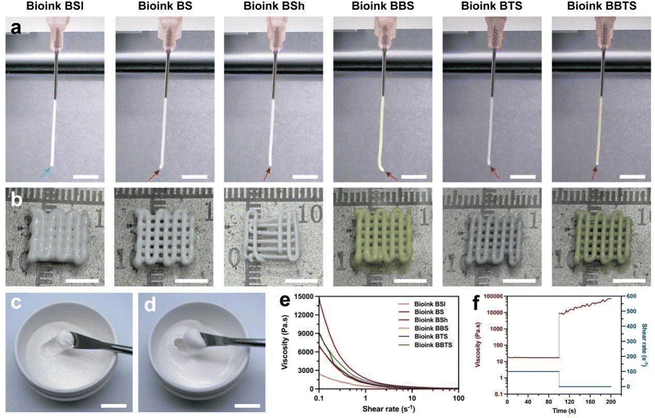
Briefly, BCP (Fig. S2a and b, ESI†) was mixed with SA (Fig. S2c and d, ESI†) solution to obtain the bioink for BCP-SA scaffolds (BS). Due to the irregular shape and spontaneous agglomeration, BCP powders would aggregate when over-added into SA solution at one time, which hindered the formation of a homogeneous bioink (Fig. 3c). Therefore, the total BCP powders were divided into several portions, and gradually added into the SA solution with repetitive stirring to prepare the bioink for BS, through which nearly no agglomeration appeared in the bioink (Fig. 3d), beneficial to 3D printing. Furthermore, BBR, Ti3C2 (7.5 mg mL−1), BBR + Ti3C2 (2.5 mg mL−1), BBR + Ti3C2 (7.5 mg mL−1), and BBR + Ti3C2 (12.5 mg mL−1) were added during the mixing process to prepare the bioinks for BCP-SA-BBR scaffolds (BBS), BCP-SA-Ti3C2 (7.5 mg mL−1) scaffolds (BTS), BCP-SA-BBR-Ti3C2 (2.5 mg mL−1) scaffolds (BBTSl), BCP-SA-BBR-Ti3C2 (7.5 mg mL−1) scaffolds (BBTS), and BCP-SA-BBR-Ti3C2 (12.5 mg mL−1) scaffolds (BBTSh), respectively. Bioinks with different content ratios of BCP were prepared through the same process. The formulations of different bioinks are listed (Table 1).To detect the printability of bioinks, the fiber formation of composite bioinks was evaluated. As shown in Fig. 3a and b, Bioink BSl with the low content ratio of BCP tended to form the droplet shape (blue arrow) during the extrusion, and the fiber showed dispersion when layering on the platform. With the increased BCP content ratio, Bioink BS maintained the shape of the tip (red arrow) of fibers with a flat cross-section when extruded, while the increased BCP powders further stabilized the fibers and inhibited the fiber breaking after layering. When continuing to add BCP into the bioink, although enabling the formation of solid fibers, Bioink BSh displayed a discontinuous extrusion that tended to plug up the printing needle, hindering the consistency of printing. Furthermore, BBR and Ti3C2 showed a negligible effect on the fiber formation of bioinks, indicating that the modified bioinks performed similarly with Bioink BS.
Rheological measurements were then performed. The viscosity of different bioinks gradually dropped with the increasing shear rate (Fig. 3e). Specifically, at low shear rates, the viscosity was proportional to the content ratio of BCP, and Bioink BTS and Bioink BBTS exhibited higher viscosities compared to BS and BBS, indicating the role of BCP and Ti3C2 in determining the viscosity of bioinks. As the shear rate increased, the viscosity of different bioinks decreased to nearly the same level. Notably, concluding from the extrusion behavior, while Ti3C2 resulted in more robust bioinks, Ti3C2-modified bioinks still possessed satisfactory fiber extrusion required for printability. During the 3D printing, the shear thinning behavior decreased the viscosity of bioinks subjected to high shear stress that was conducive to the smooth extrusion process. To mimic the printing process, a constant shear was forced, and the bioink revealed a stable viscosity throughout the test. Subsequently, a tiny shear was applied to simulate the after-printing condition, and the viscosity recovered to the initial state of the bioink (Fig. 3f), indicating the rapid recovery of the bioink that would prevent the fibers from spreading on the platform.
The above analysis displayed the favorable printability of composite bioinks with a suitable content ratio of BCP, for which BBR and Ti3C2 revealed no adverse effects to the rheological properties of bioinks.
3.3. Fabrication and characterization of composite scaffolds
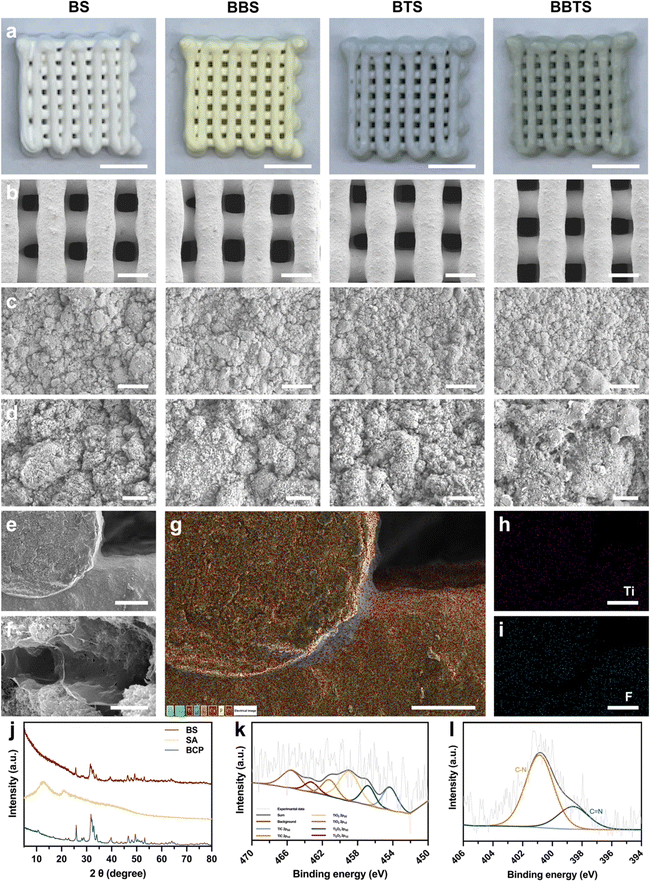
The as-prepared bioinks were applied in the extrusion 3D printing to fabricate the 3D porous scaffolds, followed by the ionic crosslinking and freeze-drying process.Fig. 4a shows the photographs of different scaffolds. Composite scaffolds were precisely printed with porous structures, and BS, BBS, BTS, and BBTS appeared in white, yellow, grey, and green, respectively, by the colors of loaded substances. The minimal color variation in the cross section of the composite scaffolds (Fig. S3, ESI†) demonstrated the excellent homogeneity and stability of bioinks during the 3D printing. With the increase in loaded Ti3C2 concentration, the scaffolds exhibited a deeper green color (Fig. S4, ESI†). As observed under SEM (Fig. 4b), all of the scaffolds revealed a uniformly granular surface and possessed well-arranged macropores ranging from 230–380 μm. The magnified SEM images of the surface and cross-section (Fig. 4c–f) displayed the microporous structure and crosslinked network of scaffolds, which were not compromised by BBR and Ti3C2. Ascribed to the model design, ionic crosslinking, and freeze-drying process, hierarchical pores (<400 μm) were formed in 3D scaffolds, which were beneficial to bone regeneration and controlled drug release.46,50,51 The corresponding elemental distribution of the cross-section demonstrated the uniform dispersion of Ca, P, Na, C, and O in BS (Fig. S5, ESI†), while BBTS exhibited extra signals of Ti and F (Fig. 3g–i), indicating the existence of Ti3C2 in BBTS. EDS further confirmed the successful loading of Ti3C2 (Fig. S6, ESI†).
The results of the thermogravimetric analysis are depicted (Fig. S7, ESI†). Apart from the evaporation of solution around 100 °C, the mass loss of BBR occurred within the temperature range of 190–220 °C and 300–450 °C, which could be ascribed to the decomposition of chemical bonds to generate gas. Because of the high thermal stability, weight loss of Ti3C2 at 5% could be ignored in scaffolds under 800 °C.52,53 The thermogram of BBTS almost coincided with that of BS, which both were determined by the stability of SA.51 Two curves began to diversify around 200 °C due to the mass loss of BBR in BBTS. Although nearly no mass loss of BBR was observed from 230–300 °C, the decomposition products of BBR restricted in the crosslinked network were released with the decomposition of SA within the range of 230–270 °C,54,55 leading to the increased difference of mass loss between BS and BBTS. Once the obstacle of gas release was removed after the collapse of the SA hydrogel, the mass loss of BBR within the 300–400 °C range turned into the disparity of the thermograms. These suggested the presence of BBR in BBTS.
XRD was performed to analyze the components of the scaffolds. Because of the highest content ratio of BCP in scaffolds, the conspicuous peaks of BS matched well with those of the BCP powders. The main peaks of SA in BS were less obvious due to its lower content. In contrast, the pattern of BS became disorderly compared to BCP, which was by the characteristic pattern of SA (Fig. 4j), indicating the inclusion of SA in the composite scaffolds. In contrast, BBR and Ti3C2 did not show diffraction peaks in BBTS (Fig. S8, ESI†) due to the minimal contents in the scaffolds. However, BBTS exhibited a stronger peak intensity after modification, implying the potential influence of the extra-loaded substances on the crystallinity of BCP in scaffolds.51
The XPS spectra (Fig. S9, ESI†) revealed the significant peaks of BS, which were assigned to O (O 1s), Ca (Ca 2p), C (C 1s), P (P 2p), and Na (Na 1s). The new peaks that appeared in BBTS corresponded to Ti (Ti 2p) and N (N 1s), verifying the components of BS and BBTS. Furthermore, in the high-resolution Ti 2p spectra (Fig. 4k), peaks of TixOy were observed. These were attributed to the oxidation of Ti3C2 during printing. Meanwhile, the C–N bond and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond detected in the N 1s spectra (Fig. 4l) conformed to the typical bonds of BBR, which could be the solid evidence of the BBR existence in the scaffolds.56
N bond detected in the N 1s spectra (Fig. 4l) conformed to the typical bonds of BBR, which could be the solid evidence of the BBR existence in the scaffolds.56
3.4. Crosslinking and mechanical properties
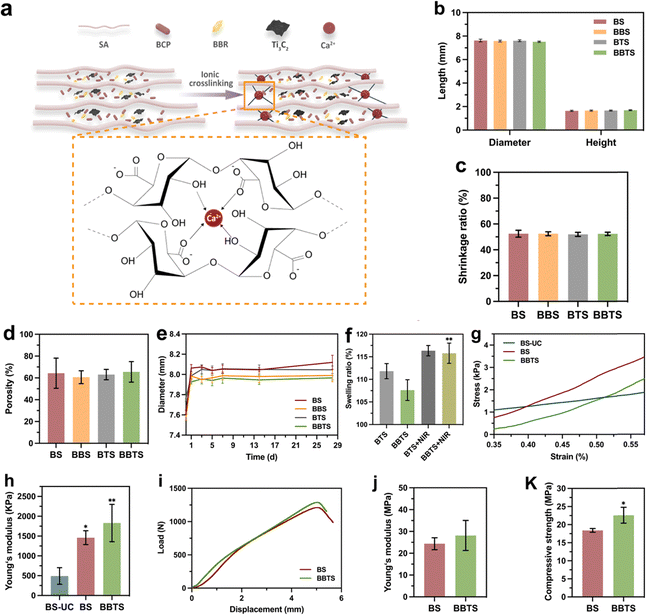
In addition to the controllable viscosity of SA utilized in 3D printing, the crosslinking property was further investigated. In the presence of Ca2+, alginate composed of β-D-mannuronic acid and α-L-glucuronic acid units would form the gels through ionic chelation (Fig. 5a). Benefiting from the porous structure of 3D scaffolds, a full crosslinking reaction throughout the scaffolds was realized in a short time. The cross-section image (Fig. 3f) showed the typical film-like networks inside the scaffolds.57 Because of the association of alginate polymers during the crosslinking process,57 a quick shrinkage of scaffolds immersed in CaCl2 solution was observed (Fig. S10, ESI†). After freeze-drying, the dimensions of the scaffolds were measured, in which different groups showed no significant difference (Fig. 5b). Based on the designed models of scaffolds (10 × 5 × 5 mm), the diameter and height of the composite scaffolds were decreased to around 7.58 ± 0.09 mm and 1.66 ± 0.05 mm, respectively, and the shrinkage rate was 52.29 ± 1.82% (Fig. 5c). Although the crosslinking process reduced the volume of scaffolds, the shrinkage rate was still lower than the volume fraction of water (81.23 ± 0.02%) lost in freeze drying. The as-formed SA hydrogel provided the structural integrity that connected the BCP particles and maintained the intervals, thus preventing the deformation and collapse of the scaffolds.51,58Notably, the microporous structure was formed along with the loss of water in the scaffolds. Different scaffolds revealed a similar microporosity (Fig. S11a, ESI†) around 17.88 ± 9.07%. Combined with the designed macropores (45.5 ± 8.68%) (Fig. S11b, ESI†), the hierarchical porous structure with satisfactory porosity (63.38 ± 8.06%) (Fig. 5d) would further promote cell proliferation and nutrient exchange, which are favorable for bone regeneration.59,60 The swelling behavior of different scaffolds (Fig. 5e) exhibited the same regularity independent of drug release. Attributed to the absorption property of the hydrogel in aqueous media, the scaffolds swelled on the first day immersed in PBS. After 24 h, the scaffolds reached the equilibrium state in solution and kept the scaffolds’ dimension within the stable fluctuation. Upon laser irradiation, BBTS exhibited a higher swelling ratio (Fig. 5f). As indicated in previous reports, SA and the alginate hydrogel possessed high thermostability below 60 °C.54,55,60,61 Thereby, the enhanced swelling capacity mainly depended on the heat expansion of the scaffold matrix. Evidently, with or without thermal stimuli, the composite scaffolds were kept at low swelling ratios for a long period, indicating the high structural stability of the composite scaffolds in a physiological environment, which was crucial for tissue engineering.46
To investigate the micromechanical property, the dynamic mechanical analysis (DMA) was performed on the single fiber of scaffolds (Fig. 5g). The scaffolds without crosslinking (BS-UC) revealed elastic modules of 491.8 ± 209.9 kPa. After ionic crosslinking, the elastic modules of BS and BBTS were increased to 1460.0 ± 176.2 kPa and 1829.0 ± 471.0 kPa, respectively (Fig. 5h). The strong chelation and the hydrogel bonds formed between BCP and SA made the interaction between these particles more stable, and the stiff hydrogel structure contributed to the increased mechanical property, while Ti3C2 revealed no effect to the elastic modules of the single fiber.57,62,63 The elastic modulus of the microstructures around 1600 kPa could further guarantee the stability of cellular activity.46 The mechanical feature of scaffolds was then detected (Fig. S12 and Fig. 5i, ESI†). The Young's modulus of the integral scaffolds was increased to BS of 24.37 ± 2.75 MPa, and BBTS of 28.16 ± 6.90 MPa (Fig. 5j) due to the integrity of fibers and the enhancement of the 3D structure. BS and BBTS exhibited compressive strength at 18.41 ± 0.52 MPa and 22.55 ± 2.19 MPa, respectively (Fig. 5k), which was endowed by the high strength of the BCP ceramics and the reinforcement of the SA hydrogel.51 Furthermore, similar to graphene oxide,64 as the MXene nanosheets got closer in the compacted scaffolds during the compression test, the interlayered π–π interaction uniformly distributed the stress between SA hydrogel, BCP, and Ti3C2 nanosheets. This avoided the stress concentration and delayed the crack of scaffolds. The increased compressive strength of BBTS compared to that of BS demonstrated the enhancement of Ti3C2 nanosheets to the strength of scaffolds. With the mechanical properties comparable to that of natural bone, the composite scaffolds were of promising potential in bone reconstruction.65
3.5. In vitro photothermal performance
The essential property of nano-agents for PTT is based on the strong light-absorption capability and high photothermal conversion efficiency at a specific bio-window.17 According to the optical absorption spectrum in the NIR region, Ti3C2 presented the typical absorption around 800 nm at different concentrations (Fig. S13, ESI†), indicating the remarkable advantages of Ti3C2 applied in PTT.26As described previously, Ti3C2 tended to agglomerate due to the high surface energy and van der Waals forces, which impaired its electrochemical performance.49,66 Inspired by the combination between the nanoparticles and hydrogel,67 Ti3C2 nanosheets were involved in the bioinks through the 3D printing and coating method to detect the influence of different methods of modification on the photothermal properties. Composite scaffolds were modified with the same content ratio of Ti3C2 through the coating process and 3D printing process, which were named MX@BS and BMS, respectively (Fig. S14, ESI†). As shown in Fig. 6b, under laser irradiation at the identical power density, BMS exhibited a lower equilibrium temperature compared to MX@BS. Although BMS avoids the large-scale agglomeration of Ti3C2, the light absorption of Ti3C2 in the core of the fiber was affected by other components of scaffolds, leading to a weaker heating ability. However, through 3D printing, Ti3C2 was evenly mixed in the scaffolds, which was expected to delay the degradation of Ti3C2 compared to the coating method. This was of great prospect to investigate the long-term photothermal scaffolds in further research.
Subsequently, the effects of the content ratio of Ti3C2, laser power density, surrounding environment, and loaded BBR on the photothermal performance of scaffolds were systematically assessed under NIR-I laser illumination (Fig. S15, ESI†). Exposed to 808 nm laser irradiation at a power density of 0.5 W cm−2, the temperature of BTS was increased from 16 °C to 46.9 °C in 2 min. The equilibrium state was reached at 52.5 °C in 10 min, while no significant rise of temperature was detected in BS. In the presence of BBR with the changed colors of the scaffolds, the equilibrium temperatures of BBS (18.4 °C) and BBTS (52.1 °C) were not affected compared to those of BS (18.7 °C) and BTS (52.5 °C), respectively (Fig. 6a and c). The alteration of temperature demonstrated the strong photothermal conversion efficiency of BTS and BBTS with Ti3C2 modification, and the negligible influence of the loaded BBR on the photothermal performance. In addition, the photothermal performance relied on the content ratio of Ti3C2 and laser power density. The equilibrium temperature increased with the increasing content ratio of Ti3C2 (Fig. 6d), and the elevation of power density (Fig. 6e) indicated that the temperature of BBTSh (0.5 W cm−2) and BBTS (0.6 W cm−2) had peaked at over 60 °C. Because of the endothermic reaction of the surrounding liquid, BBTS in a moist environment required a higher laser power density (0.8 W cm−2) to reach the equivalent equilibrium temperature in a dry environment (Fig. 6f).
Next, the photothermal stability of BBTS was investigated under five cycles of laser-on–off process (laser on for 3 min followed by laser off for 3 min). After periodic laser irradiation, no significant deterioration of the final temperature was observed. The photothermal property of BBTS was preserved during the cycles (Fig. 6g), implying that BBTS was a prominent photothermal platform for continuous photothermal treatment with high stability.
3.6. Controlled release of BBR
Based on the microporous structure of composite scaffolds and large surface area with plentiful anchoring sites of Ti3C2 nanosheets,51,68 BBS and BBTS were further developed as drug-delivery platforms. BBR was loaded into the scaffolds simply by stirring BBR into the SA solution. The procedure ensured the even distribution of BBR in scaffolds, and the drug-loading capacity was further enhanced through electrostatic interaction between Ti3C2 and BBR.26 The standard concentration curve of BBR was established with a fit determination coefficient of 0.9996 (Fig. 6h), which was precise enough for the conversion between the OD value and drug concentration.As shown in Fig. 6i, the efficient release of BBR was detected at the initial stage. This was because a great amount of drug on the surface of the scaffolds could easily be released with no obstruction. Over time, the release rate was gradually decreased, which was mainly ascribed to the following: (i) the drug stored inside the scaffolds required migration to the surface before releasing. (ii) The released drug reduced the concentration difference between the scaffolds and the surrounding environment. However, the microporous penetration throughout the scaffolds could still sustain the relatively slow release of the drug in a long period. Compared to BBS, BBTS revealed a slower speed of drug release, confirming the interaction between BBR and Ti3C2, which further promoted the controlled drug release system. When exposed to laser irradiation for 15 min, BBS + NIR showed no difference with BBS. Comparatively, a significant increase in BBR release was detected in BBTS + NIR. This is because the thermal stimuli mediated by Ti3C2 and laser irradiation enlarged the pore structure during the swelling process. Furthermore, the electrostatic interaction was weakened, which contributed to the acceleration of drug release.68 In addition, the BBR concentration was correlated with the power density, such that the releasing speed and the amount of released drug were increased with the elevation of the equilibrium temperature (Fig. 6j). More importantly, the controlled BBR release of BBTS exhibited a dual action to the infected bone defects. Upon laser irradiation, the mass release of BBR could realize a strong antibacterial effect on infections at the early and middle stages. Afterward, BBTS maintained a slow release of BBR in long-term therapy. The continuous release of BBR at a low concentration is expected to further promote osteogenesis.69 Changes in pH values (Fig. S16, ESI†) revealed the gradually decreased pH values of the solution immersed with the composite scaffolds to around 4.7 after 21 days, and slight changes were observed afterward. In addition, the pH values were not affected by the release of BBR and degradation products of Ti3C2.
3.7. In vitro antibacterial activity
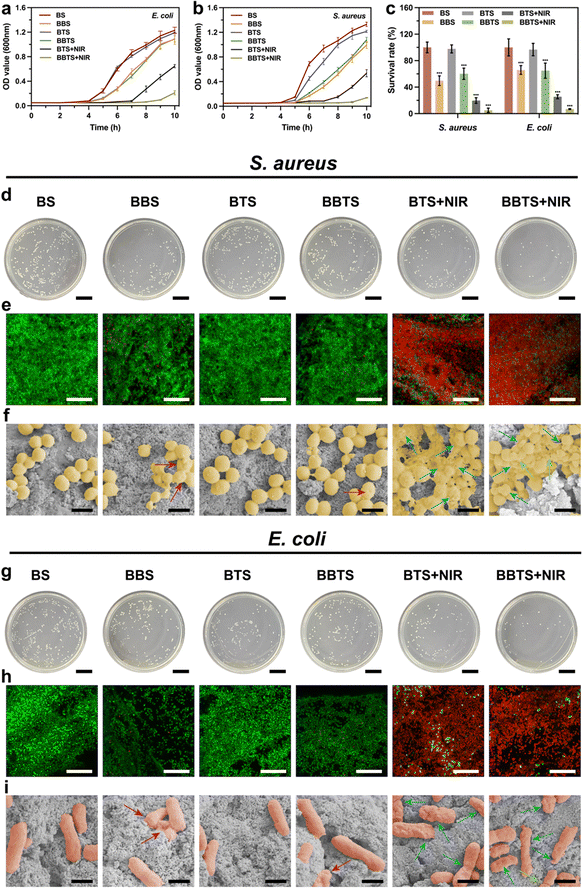
As the main pathogens that caused infection in the human body, S. aureus and E. coli were used as models for investigation in antibacterial properties.67 To generally understand the antibacterial activity of BBR and PTT, an agar diffusion assay was used to record the inhibition zone (Fig. S17, ESI†). BS displayed a high concentration of bacteria surrounding the scaffold, without showing an inhibition zone. In contrast, a relatively lower concentration of bacteria was shown surrounding BBS because of the bacteriostatic action of the released BBR. After laser irradiation, hyperthermia, and BBR, an extremely low concentration of bacteria surrounding BBTS was observed. The inhibition zone presented a general illustration of the bacteriostatic effect of BBR and the bactericidal effect of PTT that could be a satisfactory combination for bacterial infection.Bacterial growth curves are displayed in Fig. 7a and b. Bacteria seeded on BS steadily grew without impact over the observation time. Modified with Ti3C2, BTS did not show an apparent inhibition effect on the bacterial growth. Although the individual Ti3C2 solution has been proven effective in killing bacteria based on the sharp edges of nanosheets, a few studies demonstrated the negligible antibacterial action of Ti3C2 and Ti3C2-modified substances. The conflicting results could be ascribed to differences in the concentration and states of Ti3C2, such that the effect of sharp edges was minimal whether Ti3C2 is at low concentration or turns into a solid after the drying process, explaining the poor antibacterial performance of BTS with a low content ratio of Ti3C2.25,27,70 Due to the release of BBR, BBS and BBTS reduced the growth rate of bacteria and sustained the inhibition activity for more than 10 h. As the inhibition effect of BBR at different concentrations revealed little difference when below minimum inhibitory concertation (MIC), BBS and BBTS showed similar influence on bacterial growth.71 Given their excellent photothermal properties, BTS and BBTS were further studied for PTT in bacterial eradication upon laser irradiation. After exposure to laser irradiation for 15 min, the growth curves of BTS and BBTS turned into straight lines at the early stage, indicating that a great number of bacteria was killed by thermal stimuli. However, residual bacteria cultivated with BTS would gradually grow after temporal irradiation. In contrast, BBTS exhibited an obvious inhibition effect on residual bacteria, which kept the reduced quantity of bacteria, confirming the satisfactory antibacterial activity of PTT combined with BBR.
Bacteria cultivated in different groups were further evaluated by plate counting approach. As presented in Fig. 7d and g, BS and BTS possessed the largest number of bacteria in the absence of antibacterial function. BBS and BBTS displayed a survival ratio of 49.71 ± 7.25% and 60.18 ± 8.57% for S. aureus, and 63.61 ± 6.46% and 62.64 ± 11.04% for E. coli, respectively, which was consistent with results in growing curves. The small difference between BBS and BBTS was because BBR in BBS released more rapidly at the beginning, which slightly enhanced the inhibition effect. Next, under laser irradiation, hyperthermia killed a great number of bacteria, and the survival ratio was decreased to 19.78 ± 4.27% for S. aureus, and 24.59 ± 2.89% for E. coli, respectively. Moreover, when combining PPT with BBR, BBTS significantly reduced the survival ratio to 5.14 ± 3.18% for S. aureus, and 6.60 ± 0.64% for E. coli, respectively (Fig. 7c). The results demonstrated the excellent antibacterial action of the synergistic effect of BBTS.
Additionally, live/dead staining assay was performed for the characterization of the bactericidal effect (Fig. 6e and h). The live bacteria were stained in green, while the dead bacteria presented red fluorescence. Compared to the nearly entire green fluorescence in BS and BTS, BBS and BBTS loaded with BBR revealed slight red signals, demonstrating the mild antibacterial effect of BBR to both Gram-positive bacteria and Gram-negative bacteria. In addition, BBR inhibited the growth of bacteria, such that the bacterial density on the scaffolds was reduced in BBS and BBTS. Upon laser irradiation, the red fluorescence intensity was significantly increased in BTS and BBTS, and almost no live bacteria were found in BBTS, corroborating the appreciable antibacterial activity of hyperthermia and the synergistic treatment.
To explore the antibacterial process of BBTS under laser irradiation, bacteria under different treatments were observed using SEM (Fig. 7f and i). Both S. aureus and E. coli cultured on BS and BTS maintained the normal structure, and presented an intact and smooth cell wall. Previous studies have demonstrated that the antibacterial activity of MXene relied on the direct contact of nanosheets to bacterial membranes.26,27 In this case, it was found that an extremely low ratio of Ti3C2 mingled with BCP made it hard to realize the contact with bacteria. Thus, BTS showed no damage to the bacterial structure. Moreover, most bacteria in BBS and BBTS remained in their normal shape, and few bacterial surfaces showed shrinkage (red arrows), suggesting the relatively weak antibacterial effect of BBR. In contrast, bacteria in the BTS + NIR group were injured, the bacterial shape was distorted (blue arrows), and the leakage of the internal matrix was observed due to the damage of hyperthermia. More importantly, in the presence of BBR, the membrane permeability was increased,40 the morphology of the bacteria cultured in BBTS + NIR was disrupted more severely or even ruptured (green arrows) after thermal stimuli. Combing BBR with phototherapy, BBTS possessed an outstanding antibacterial property upon laser irradiation.
3.8. In vitro cytocompatibility and osteogenesis
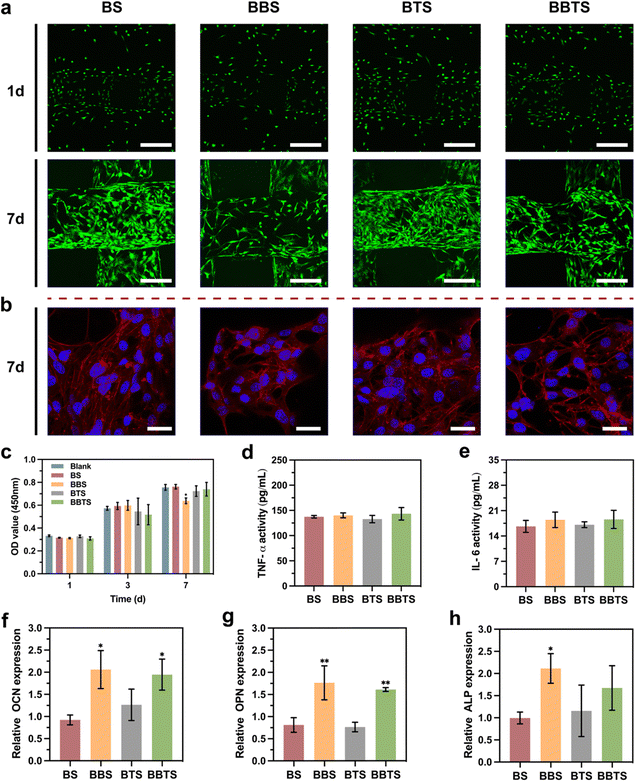
Cytocompatibility plays a crucial role in the antibacterial application of implanted biomaterials.6,67 In this assessment, MC3T3-E1 cells and rBMSCs were employed to investigate the cytotoxicity and osteogenic differentiation. MC3T3-E1 cells were seeded on different scaffolds. As shown in Fig. 8a, after culturing for 1 day, negligible dead cells were seen on the scaffolds. Live cells stained in green appeared on the first and second layers of scaffolds, indicating the uniform distribution of cells over the upper and side surfaces of the fibers outlining the scaffolds. Notably, as the cell density of BBS was relatively lower, the fast release of BBR in BBS at the initial stage was likely to affect the adhesion and proliferation of the cells. On day 7 after seeding, significant growth of cells was observed, and the fibers were covered with extended cells. Same as that observed on day 1, BBS without the controlled release of BBR revealed fewer cells on the scaffolds. Fig. 8b displays the cytoskeleton and nucleus of the cells in red and blue, respectively. It was noticed that the cells spread well on different groups, showing typical cell morphology and the good shape of the nucleus. On account of the coarse surface of porous structures, numerous pseudopods were formed which further contributed to the great adhesion and stretch of cells. Subsequently, a CCK-8 assay was performed (Fig. 8c). Overall, continuous cell proliferation was presented in all of the scaffolds. Compared to the control groups, no significant toxicity was shown in BS and BTS, implying the excellent biocompatibility of the calcium phosphate scaffolds and Ti3C2. In agreement with the fluorescence images, BBS with the sustained mass release of BBR exhibited mild toxicity to cells. Conversely, the slow release of BBR contributed to a better biocompatibility of BBTS. ELISA was then conducted to measure the activities of inflammatory cytokines. No significant changes in the TNF-α activity and IL-6 activity were seen in BBS, BTS, and BBTS compared to BS (Fig. 8d and e), demonstrating that Ti3C2 and BBR would not cause inflammation. Considering the cytotoxicity of BBR at a relatively high concentration, BBTS was expected to inhibit the proliferation of cells when exposed to laser irradiation, which would promote the drug release. Nevertheless, the mass release of BBR appeared in the earlier stage of bone infection, which conformed the requirement for antibacterial ability rather than bone regeneration. After the initial release of BBR, the release speed was reduced and the drug concentration at the defect sites was maintained at sustainably low levels under drug metabolism, so BBR would show no toxicity to osteoblasts, beneficial to bone repair.69As verified in a previous study, BBR can activate Runt-related transcription factor 2 (Runx2), which is regarded as the key osteogenic transcription factor through β-catenin, P38 MAPK, PKA, and AMPK pathways, and will ultimately increase the expression of osteogenic genes (osteogenesis-related gene expressions covering osteocalcin (OCN), osteopontin (OPN), and alkaline phosphatase (ALP)), promoting osteogenesis. Moreover, induced by receptor activating factor ligand (RANKL), BBR can suppress NF-κB and Akt activation, resulting in the inhibition of osteoclastogenesis and osteoclast survival.72 Given that observation, OCN, OPN, and ALP were further evaluated. Fig. 8f–h reveals no significant difference in gene expressions between BS and BTS. Due to the limited content ratio of Ti3C2 on the surface of scaffolds that would release osteogenic substances, Ti3C2 did not show a distinct effect on osteogenesis. The OCN expression and OPN expression were increased in BBS and BBTS, and ALP expression was upregulated in BBS as compared to BS, which confirmed the osteoinduction of BBR. Thanks to the prominent bioactivity of composite scaffolds and the sustained release of BBR which would promote osteogenic differentiation, BBS and BBTS revealed a great prospect in the applications of bone tissue engineering.
3.9. In vivo anti-Infection properties
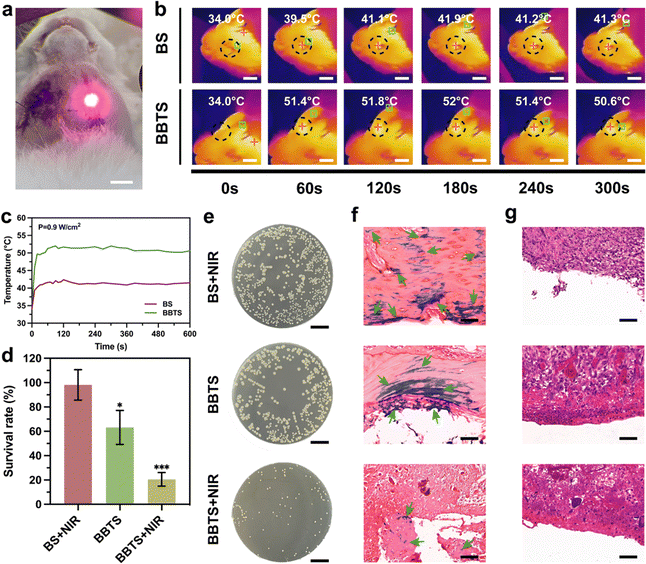
Encouraged by the prominent antibacterial performance in vitro, the anti-infection property in vivo was further evaluated in the infected mandibular defects of rabbits. The experimental models were divided into 3 groups (BS + NIR, BBTS, BBTS + NIR). Upon laser irradiation, local hyperthermia with precise localization at the irradiation points was shown in Fig. 9a and b. The temperature of BS was increased merely from 34.0 °C to 41.3 °C. Conversely, for BBTS, the temperature soared from 34.0 °C to 50.6 °C (Fig. 9c), indicating the strong photothermal ability of BBTS in vivo. After 1 week of implantation, the infection sites were observed. Visually, obvious abscesses and tissue swelling were observed in BS + NIR. In contrast, BBTS + NIR possessed minor swelling without showing secretion (Fig. S18, ESI†), suggesting the fast control and eradication of bacterial infection. Subsequently, the extracted scaffolds were immersed in the LB medium, and the plate counting method was applied to determine the survival rate. Fig. 9e displays the great number of bacteria that remained in BS + NIR. By contrast, the growth of bacteria was inhibited in the presence of BBR in BBTS, and the thermal stimuli resulted in the smallest number of bacteria remaining in BBTS + NIR. The survival rate of bacteria sharply decreased from 98.15 ± 12.54% of BS + NIR to 20.54 ± 5.62% of BBTS + NIR (Fig. 9d), verifying the extraordinary curative effect of synergistic treatments for bacterial elimination.To further reveal the degree of bacterial infection and inflammatory reaction, after 1 week of implantation, the tissue surrounding the scaffolds was harvested and measured by the Gram staining and H&E staining. Fig. 9f shows the bacteria (green arrows) colored in purple widely distributed throughout the tissue surrounding the defects. It was noted that bacteria were out of the range of defect sites and migrated to the surrounding intact bone. With the lack of antibacterial properties, the infection was not suppressed in BS + NIR and tended to aggravate the bone injury. On account of the inhibition effect of BBR, BBTS restricted the growth of bacteria and resulted in fewer bacteria in tissue. In contrast, upon laser irradiation, hyperthermia killed most of the bacteria at infection sites, BBTS + NIR significantly reduce the number of bacteria, and less bacteria were observed surrounding the defects after the synergistic PPT/BBR treatment.
Additionally, the inflammation phase in the initial stage of tissue defects would potentially result in secondary damage to tissue.73 The transition from inflammation to the anti-inflammation phase was required for establishing an environment for tissue regeneration.74 As shown in Fig. 9g, BS + NIR revealed a great amount of inflammatory cells in the surrounding tissue caused by bacterial infection and scaffold implantation.6 Because of the reduced infection, fewer inflammation cells were shown in BBTS. Combining PTT with BBR, BBTS + NIR distinctly reduced the number of inflammation cells. The remarkable reduction in tissue infection and inflammation confirmed BBTS as a promising therapeutic platform with excellent antibacterial properties for infected bone defects.
3.10. In vivo bone regeneration
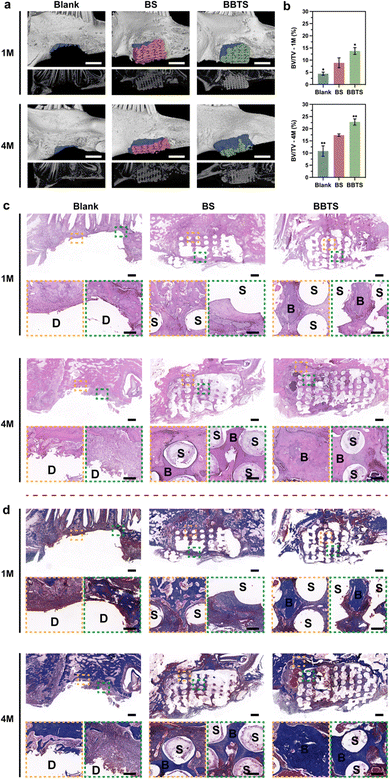
Inspired by the prominent performance of the 3D scaffolds in tissue regeneration,17,24,51 the large mandibular defects in New Zealand white rabbits were constructed and the personalized 3D scaffolds (Fig. S19, ESI†) were fabricated to further evaluate the capability of composite scaffolds in inducing bone regeneration (Fig. S20, ESI†). With respect to hyperthermia above the threshold during PTT for bacterial infection, osteoblasts would be injured along with the bacterial eradication.25 Nevertheless, osteoblasts required for bone regeneration are mainly present in peripheral circulation that were minimally impacted by local hyperthermia.75 Meanwhile, inflammation caused by PTT met with the early stage of bone repair, which was crucial for cell recruitment.76 Hence, in a long-term repairing process, transient hyperthermia with limited influence on osteoblasts in situ had negligible effects on bone tissue regeneration.77To visualize the osteogenic performance, micro-computed tomography (micro-CT) images and the corresponding reconstructed bone defects implanted with 3D scaffolds are presented (Fig. 10a). Overall, all groups exhibited time-dependent bone repair. Without the support of the bioactive scaffolds, it was difficult for new bone in the large defects. Only a small amount of new bone was shown at the edge of mandibular defects at month 1 in Blank groups. In comparison, BS-providing struts and multilevel pores demonstrated the induction of bone regeneration in the margin of mandibular defects. Moreover, loaded with BBR and Ti3C2, more tissue was observed in the pores of BBTS, confirming the better osteogenic properties of BBTS. At month 4, the defects in Blank groups were slightly reduced and the defect surfaces became smooth due to the self-healing process. Compared to month 1, more bone tissue was formed in BS and partial degradation was shown in the scaffolds. Conversely, the degradation in BBTS was less obvious and a great number of residual scaffolds maintained the osteogenic effect in the long term. With the sustained release of BBR, bone regeneration was distinctly promoted throughout the scaffolds. The fundamental parameters of micro-CT were further quantitively analyzed (Fig. 10b). The bone volume/tissue volume (BV/TV), which represents the percentage of newborn bone volume accounting for the mandibular defect space were superior in BBTS, implying the excellent osteoinduction of BBTS.
For the histological analysis of bone regeneration in mandibular defects, H&E and Masson's trichrome staining were conducted on tissue harvested at month 1 and month 4 after implantation to trace the formation of new bone in vivo (Fig. 10c and d). For the Blank groups, there was a lack of support for the ingrowth of tissue, and nearly no tissue repair was observed in the large mandibular defect. In clear contrast, the scaffolds-implanted groups displayed the formation of fibrous tissue and osseous tissue around the fibers of the scaffolds, while the mineralized bone tissue was more evident in BBTS, demonstrating the critical role of porous scaffolds in inducing bone regeneration. After 4 months of implantation, the defect sites were minimally repaired with fibrous tissue in peripheral areas close to the original bone tissue in the Blank groups. Although there was a rich blood supply, the self-healing process of the mandible was still insufficient for repairing large mandibular defects. Moreover, without external support, the tissue formation was restricted in the marginal sites of the defect. As expected, the proportion of bone tissue was increased in BS, and new bone was also formed in the central areas of the defect. Remarkably, BBTS exhibited stronger regenerative capacity compared to BS on which more new bone was observed, and the tissue regeneration reached the inferior margin of the mandibular bone. Furthermore, the ingrowth of new blood vessels was seen in BS and BBTS, which significantly improved the quality of mandibular reconstruction.
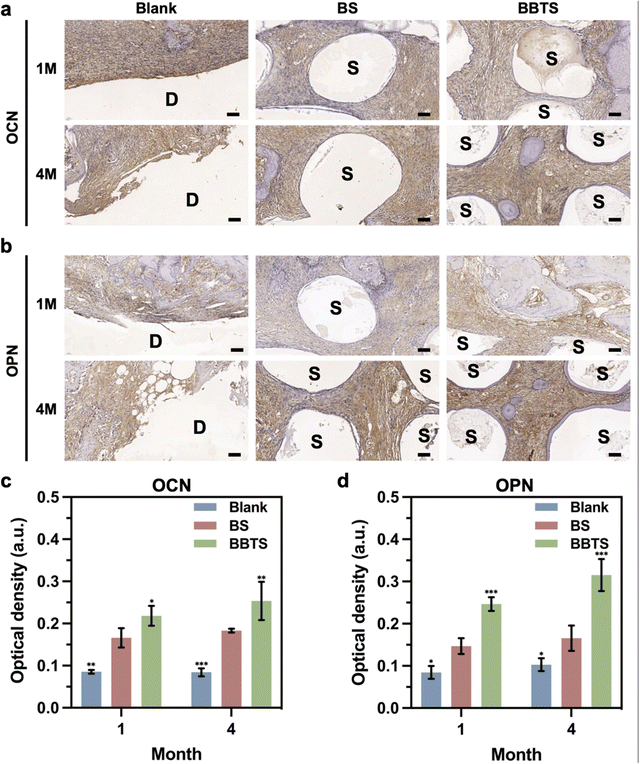
In addition, immunohistochemical staining and the corresponding semi-quantitative analysis were performed for investigating osteogenic activities. The images (Fig. 11a and b) showed the enhanced expression of OCN and OPN in BS compared to Blank groups, while BBTS stimulated the highest expression of OCN and OPN. The results were further verified by semi-quantitative analysis (Fig. 11c and d). With the combination of the multilevel porous structure, enhanced mechanical properties, and sustained released of BBR, BBTS exhibited great potential in facilitating bone regeneration for large mandibular defects.
Based on the synergistic strategy for bacterial elimination and bone repair, Mxene-berberine-integrated scaffold revealed great prospect in treating mandibular defect with high infection rate. For the antibacterial aspect, by activating PTT for bactericidal effect and promoting the release of BBR for bacteriostatic effect, NIR is expected to be applied after the implantation to minimize the risk of infection, and throughout the healing process to control the infection when it occurs. While the microenvironment is stable for bone repair, BBR is allowed to release in a relatively slow speed without laser stimuli, which is less toxic and will promote bone regeneration along with 3D scaffolds.
Despite the satisfactory outcome in this work, several issues of responsive scaffolds and MXene still need to be addressed in the future. (1) Biodegradability of the scaffold is critical to clinical translation, and should be further studied along with the deterioration of photothermal properties, which is related to the degradation rate of the scaffold.78 (2) The parameters of NIR stimuli need to be optimized to achieve the balance among the antibacterial effect, and the damage to surrounding tissue and bone repair.79 (3) The top-down synthesis method used in this work revealed poor control of the size distribution, structure, defect and surface terminations. The alternative bottom-up method can avoid the usage of strong acids, and allow for better control of the morphology and physiochemical properties of MXene. However, the bottom-up fabrication still remains a challenge in the monolayer MXene stack, and its development is urgently needed to obtain high-quality MXene with predetermined properties.30,80 (4) Although MXene has been regarded as “practically nontoxic” according to the rating scale by the Fish and Wildlife Service (FWS), more studies are needed to engage on its genotoxicity and reproductive toxicity.26 In addition, as few studies have detected the biodistribution of MXene, further investigation in the cellular uptake, accumulation, organ distribution and clearance pathway of MXene should be carried out before practical applications.81
4. Conclusions
In summary, a multifunctional 3D printed scaffold embedded with Ti3C2 nanosheets and BBR was rationally designed and fabricated for mandibular defects, which frequently suffer from bacterial infection. The composite scaffold provided the NIR-responsive biological function, and the integrated components satisfied the demand for bacterial elimination and bone regeneration. Mechanistically, Ti3C2 endowed the scaffold with great photothermal capacity. Under laser stimulation, local hyperthermia contributed to the strong bactericidal performance and the precise control of BBR release. The controllable release of BBR further enhanced the antibacterial and osteogenic effects. Moreover, the crosslinking process and the integrated Ti3C2 significantly strengthened the mechanical properties, enabling the scaffolds to maintain bone formation, which is beneficial to the repair of large bone defects. Overall, the well-designed scaffold and the synergistic therapy demonstrated satisfactory antibacterial and osteogenic activities, revealing great prospects in treating infected mandibular defects.Author contributions
Yi Tan: writing – original draft; methodology; data analysis; and conceptualization. Huan Sun: methodology; and visualization. Yuanchen Lan: methodology; and visualization. Haider Mohammed Khan: methodology. Hui Zhang: data analysis. Linli Zhang: data analysis. Fengying Zhang: methodology. Yujia Cui: methodology. Lan Zhang: methodology. Dingming Huang: methodology. Xinmei Chen: methodology; and supervision. Changchun Zhou: methodology; supervision; and funding acquisition. Jianxun Sun: writing – review & editing; supervision; and funding acquisition. Xuedong Zhou: conceptualization; and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
All authors listed, have made substantial, direct, and intellectual contribution to the work, and approved it for publication. We thank Dr Guolong Meng from NERCB for microstructure characterization. Li Chen from Analytical and Testing Center, Sichuan University for in vivo biological Characterization. This work was partially supported by Sichuan Science and Technology Program (2022YFS0115). National Key Research and Development Program of China (No. 2019YFA0110600). National Natural Science Foundation of China (31971251). Tibet Autonomous Region Science and Technology Program (XZ202202YD0013C). Sichuan Science and Technology Program (2023YFQ0053). The interdisciplinary Innovation Fund, West China Hospital, Sichuan University (0040206107025).References
- D. Mehrabani, A. Khodakaram-Tafti, H. Shaterzadeh-Yazdi, B. Zamiri and M. Omidi, Dent. Traumatol., 2018, 34, 413–420 CrossRef CAS PubMed.
- A. Cianciosi, M. Costantini, S. Bergamasco, S. Testa, E. Fornetti, J. Jaroszewicz, J. Baldi, A. Latini, E. Choinska, M. Heljak, C. Zoccali, S. Cannata, W. Swieszkowski, A. Diaz Lantada, C. Gargioli and A. Barbetta, ACS Appl. Bio. Mater., 2019, 2, 5077–5092 CrossRef CAS PubMed.
- M. Bartnikowski, C. Vaquette and S. Ivanovski, Clin. Oral. Implants Res., 2020, 31, 431–441 CrossRef PubMed.
- X. Ye, J. He, S. Wang, Q. Han, D. You, B. Feng, F. Zhao, J. Yin, M. Yu, H. Wang and H. Yang, Int. J. Oral Sci., 2022, 14, 31 CrossRef CAS PubMed.
- M. Zhou, X. Peng, C. Mao, F. Xu, M. Hu and G. Y. Yu, Biomaterials, 2010, 31, 4935–4943 CrossRef CAS PubMed.
- Y. Wu, Q. Liao, L. Wu, Y. Luo, W. Zhang, M. Guan, H. Pan, L. Tong, P. K. Chu and H. Wang, ACS Nano, 2021, 15, 17854–17869 CrossRef CAS PubMed.
- P. C. Chang, H. T. Luo, Z. J. Lin, W. C. Tai, C. H. Chang, Y. C. Chang, D. L. Cochran and M. H. Chen, J. Periodontol., 2021, 92, 428–435 CrossRef CAS PubMed.
- F. Pati, J. Gantelius and H. A. Svahn, Angew. Chem., Int. Ed., 2016, 55, 4650–4665 CrossRef CAS PubMed.
- B. Q. Lu, T. Willhammar, B. B. Sun, N. Hedin, J. D. Gale and D. Gebauer, Nat. Commun., 2020, 11, 1546 CrossRef CAS PubMed.
- N. Zhao, Y. Wang, L. Qin, Z. Guo and D. Li, RSC Adv., 2017, 7, 43186–43196 RSC.
- S. Mistry, R. Roy, A. K. Jha, N. Pandit, S. Das, S. Burman and M. Joy, J. Controlled Release, 2022, 346, 180–192 CrossRef CAS.
- M. N. Collins, G. Ren, K. Young, S. Pina, R. L. Reis and J. M. Oliveira, Adv. Funct. Mater., 2021, 31 Search PubMed.
- N. Beheshtizadeh, M. Azami, H. Abbasi and A. Farzin, J. Adv. Res., 2022, 40, 69–94 CrossRef CAS PubMed.
- N. Li, G. Wu, L. Tang, W. Zhou, S. Yang, Q. Pan, M. Wang, P. Wu, H. Xiao, Y. He, X. Tan and Q. Yang, ACS Appl. Mater. Interfaces, 2022, 14, 46362–46373 CrossRef CAS PubMed.
- K. Zhu, S. Qian, H. Guo, Q. Wang, X. Chu, X. Wang, S. Lu, Y. Peng, Y. Guo, Z. Zhu, T. Qin, B. Liu, Y. W. Yang and B. Wang, ACS Nano, 2022, 16, 11136–11151 CrossRef CAS.
- M. J. Hajipour, A. A. Saei, E. D. Walker, B. Conley, Y. Omidi, K. B. Lee and M. Mahmoudi, Adv. Sci., 2021, 8, e2100556 CrossRef.
- S. Pan, J. Yin, L. Yu, C. Zhang, Y. Zhu, Y. Gao and Y. Chen, Adv. Sci., 2020, 7, 1901511 CrossRef CAS.
- G. Liu, J. Zou, Q. Tang, X. Yang, Y. Zhang, Q. Zhang, W. Huang, P. Chen, J. Shao and X. Dong, ACS Appl. Mater. Interfaces, 2017, 9, 40077–40086 CrossRef CAS PubMed.
- P. P. Michalowski, M. Anayee, T. S. Mathis, S. Kozdra, A. Wojcik, K. Hantanasirisakul, I. Jozwik, A. Piatkowska, M. Mozdzonek, A. Malinowska, R. Diduszko, E. Wierzbicka and Y. Gogotsi, Nat. Nanotechnol., 2022, 17, 1192–1197 CrossRef CAS.
- B. Akuzum, K. Maleski, B. Anasori, P. Lelyukh, N. J. Alvarez, E. C. Kumbur and Y. Gogotsi, ACS Nano, 2018, 12, 2685–2694 CrossRef CAS.
- L. Ding, Y. Wei, Y. Wang, H. Chen, J. Caro and H. Wang, Angew. Chem., Int. Ed., 2017, 56, 1825–1829 CrossRef CAS PubMed.
- J. Ran, G. Gao, F. T. Li, T. Y. Ma, A. Du and S. Z. Qiao, Nat. Commun., 2017, 8, 13907 CrossRef CAS.
- C. J. Zhang, B. Anasori, A. Seral-Ascaso, S. H. Park, N. McEvoy, A. Shmeliov, G. S. Duesberg, J. N. Coleman, Y. Gogotsi and V. Nicolosi, Adv. Mater., 2017, 29 CAS.
- Q. Yang, H. Yin, T. Xu, D. Zhu, J. Yin, Y. Chen, X. Yu, J. Gao, C. Zhang, Y. Chen and Y. Gao, Small, 2020, 16, e1906814 CrossRef PubMed.
- J. Yin, Q. Han, J. Zhang, Y. Liu, X. Gan, K. Xie, L. Xie and Y. Deng, ACS Appl. Mater. Interfaces, 2020, 12, 45891–45903 CrossRef CAS PubMed.
- H. Lin, Y. Chen and J. Shi, Adv. Sci., 2018, 5, 1800518 CrossRef PubMed.
- X. Wang, M. Yao, L. Ma, P. Yu, T. Lu, L. Zhang, X. Yuan, Y. Zhang and J. Ye, Adv. Healthcare Mater., 2021, 10, e2100392 CrossRef.
- L. Yang, S. Chen, H. Wei, Y. Luo, F. Cong, W. Li, L. Hong and J. Su, ACS Appl. Mater. Interfaces, 2022, 14, 45178–45188 CrossRef CAS PubMed.
- C. Yang, Y. Luo, H. Lin, M. Ge, J. Shi and X. Zhang, ACS Nano, 2021, 15, 1086–1099 CrossRef CAS PubMed.
- M. Soleymaniha, M. A. Shahbazi, A. R. Rafieerad, A. Maleki and A. Amiri, Adv. Healthcare Mater., 2019, 8, e1801137 CrossRef.
- Y. Bai, Y. Deng, Y. Zheng, Y. Li, R. Zhang, Y. Lv, Q. Zhao and S. Wei, Mater. Sci. Eng., C, 2016, 59, 565–576 CrossRef CAS.
- Y. Yang, X. Zhou, Y. K. Chan, Z. Wang, L. Li, J. Li, K. Liang and Y. Deng, Small, 2022, 18, e2105988 CrossRef PubMed.
- Z. Guo, J. X. He, S. H. Mahadevegowda, S. H. Kho, M. B. Chan-Park and X. W. Liu, Adv. Healthcare Mater., 2020, 9, e2000265 CrossRef PubMed.
- J. Li, Z. Li, X. Liu, C. Li, Y. Zheng, K. W. K. Yeung, Z. Cui, Y. Liang, S. Zhu, W. Hu, Y. Qi, T. Zhang, X. Wang and S. Wu, Nat. Commun., 2021, 12, 1224 CrossRef CAS.
- B. Sun, Z. Ye, M. Zhang, Q. Song, X. Chu, S. Gao, Q. Zhang, C. Jiang, N. Zhou, C. Yao and J. Shen, ACS Appl. Mater. Interfaces, 2021, 13, 42396–42410 CrossRef CAS.
- H. H. Guo, C. L. Feng, W. X. Zhang, Z. G. Luo, H. J. Zhang, T. T. Zhang, C. Ma, Y. Zhan, R. Li, S. Wu, Z. Abliz, C. Li, X. L. Li, X. L. Ma, L. L. Wang, W. S. Zheng, Y. X. Han and J. D. Jiang, Nat. Commun., 2019, 10, 1981 CrossRef PubMed.
- W. H. Chueh and J. Y. Lin, J. Agric. Food Chem., 2011, 59, 8021–8027 CrossRef CAS PubMed.
- K. B. Wang, J. Dickerhoff and D. Yang, J. Am. Chem. Soc., 2021, 143, 16549–16555 CrossRef CAS PubMed.
- H. Sun, Z. Z. Li, P. Jeyakkumar, Z. L. Zang, B. Fang and C. H. Zhou, J. Agric. Food Chem., 2022, 70, 12320–12329 CrossRef CAS.
- G. Porras, F. Chassagne, J. T. Lyles, L. Marquez, M. Dettweiler, A. M. Salam, T. Samarakoon, S. Shabih, D. R. Farrokhi and C. L. Quave, Chem. Rev., 2021, 121, 3495–3560 CrossRef CAS PubMed.
- K. Ishihata, C. H. Seong, T. Kibe, K. Nakazono, F. Mardiyantoro, R. Tada, M. Nishimura, T. Matsuguchi and N. Nakamura, Int. J. Mol. Sci., 2022, 23 Search PubMed.
- Y. H. Wang, C. Z. Zhao, R. Y. Wang, Q. X. Du, J. Y. Liu and J. Pan, Stem Cell Res. Ther., 2022, 13, 511 CrossRef.
- L. Claes, S. Recknagel and A. Ignatius, Nat. Rev. Rheumatol., 2012, 8, 133–143 CrossRef CAS PubMed.
- S. Mohammadian Haftcheshmeh and A. A. Momtazi-Borojeni, J. Cell. Mol. Med., 2021, 25, 11333–11337 CrossRef CAS PubMed.
- L. Y. Meng, A. S. Ivanov, S. Kim, X. Zhao, N. Kumar, A. Young-Gonzales, T. Saito, W. Bras, K. Gluesenkamp and V. Bocharova, ACS Appl. Polym. Mater., 2022, 4, 6563–6571 CrossRef CAS.
- Y. Zhao, X. Peng, D. Wang, H. Zhang, Q. Xin, M. Wu, X. Xu, F. Sun, Z. Xing, L. Wang, P. Yu, J. Xie, J. Li, H. Tan, C. Ding and J. Li, Adv. Sci., 2022, 9, e2204535 CrossRef PubMed.
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CrossRef CAS.
- M. Alhabeb, K. Maleski, B. Anasori, P. Lelyukh, L. Clark, S. Sin and Y. Gogotsi, Chem. Mater., 2017, 29, 7633–7644 CrossRef CAS.
- N. Kong, K. Lv, W. Chen, J. Guan, P. Zhao, J. Tao and J. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 56877–56885 CrossRef CAS PubMed.
- C. M. Murphy, M. G. Haugh and F. J. O'Brien, Biomaterials, 2010, 31, 461–466 CrossRef CAS.
- H. Sun, C. Zhang, B. Zhang, P. Song, X. Xu, X. Gui, X. Chen, G. Lu, X. Li, J. Liang, J. Sun, Q. Jiang, C. Zhou, Y. Fan, X. Zhou and X. Zhang, Chem. Eng. J., 2022, 427 Search PubMed.
- P. Barmann, R. Nolle, V. Siozios, M. Ruttert, O. Guillon, M. Winter, J. Gonzalez-Julian and T. Placke, ACS Nano, 2021, 15, 3295–3308 CrossRef PubMed.
- Z. Huo, Y. Liao, Y. He, Y. Zhang, X. Liao, Q. Zhang, H. Wu, J. Shi, G. Wen, H. Su and S. Yao, Front. Chem., 2022, 10, 865847 CrossRef CAS.
- S. Liu, Y. Li and L. Li, Carbohydr. Polym., 2017, 160, 62–70 CrossRef CAS.
- A. Naeem, C. Yu, W. Zhu, X. Chen, X. Wu, L. Chen, Z. Zang and Y. Guan, Molecules, 2022, 27 Search PubMed.
- A. K. Singh, S. S. Singh, A. S. Rathore, S. P. Singh, G. Mishra, R. Awasthi, S. K. Mishra, V. Gautam and S. K. Singh, ACS Biomater. Sci. Eng., 2021, 7, 3737–3753 CrossRef CAS.
- A. Al-Sabah, S. E. A. Burnell, I. N. Simoes, Z. Jessop, N. Badiei, E. Blain and I. S. Whitaker, Carbohydr. Polym., 2019, 212, 242–251 CrossRef CAS.
- P. C. Shanto, S. Park, M. Park and B. T. Lee, Biomater. Adv., 2023, 145, 213239 CrossRef CAS PubMed.
- M. K. Haider, D. Kharaghani, L. Sun, S. Ullah, M. N. Sarwar, A. Ullah, M. Khatri, Y. Yoshiko, M. Gopiraman and I. S. Kim, Biomater. Adv., 2023, 144, 213203 CrossRef CAS.
- Y. Zhao, Y. Li, T. Lan, B. Wang, M. Huang, H. Huang, C. Qiao and J. Sun, Gels, 2022, 8 Search PubMed.
- C. C. Piras, P. Slavik and D. K. Smith, Angew. Chem., Int. Ed., 2020, 59, 853–859 CrossRef CAS PubMed.
- X. Guo, H. Zhao, X. Qiang, C. Ouyang, Z. Wang and D. Huang, Int. J. Biol. Macromol., 2023, 227, 297–306 CrossRef CAS PubMed.
- L. Xu, K. Zhao, J. Miao, Z. Yang, Z. Li, L. Zhao, H. Su, L. Lin and Y. Hu, Int. J. Biol. Macromol., 2022, 220, 267–279 CrossRef CAS PubMed.
- Y. Zhao, J. D. Chen, L. Zou, G. Xu and Y. S. Geng, Chem. Eng. J., 2019, 378 CAS.
- H. Lin, S. Shi, X. Lan, X. Quan, Q. Xu, G. Yao, J. Liu, X. Shuai, C. Wang, X. Li and M. Yu, Small Methods, 2021, 5, e2100536 CrossRef PubMed.
- W. Gao, W. Zhang, H. Yu, W. Xing, X. Yang, Y. Zhang and C. Liang, Front. Bioeng. Biotechnol., 2022, 10, 996177 CrossRef PubMed.
- X. Qi, Y. Huang, S. You, Y. Xiang, E. Cai, R. Mao, W. Pan, X. Tong, W. Dong, F. Ye and J. Shen, Adv. Sci., 2022, 9, e2106015 CrossRef PubMed.
- X. Han, J. Huang, H. Lin, Z. Wang, P. Li and Y. Chen, Adv. Healthcare Mater., 2018, 7, e1701394 CrossRef PubMed.
- K. Tao, D. Xiao, J. Weng, A. Xiong, B. Kang and H. Zeng, Toxicol. Lett., 2016, 240, 68–80 CrossRef CAS PubMed.
- H. Cheng, J. Wang, Y. Yang, H. Shi, J. Shi, X. Jiao, P. Han, X. Yao, W. Chen, X. Wei, P. K. Chu and X. Zhang, Small, 2022, 18, e2200857 CrossRef PubMed.
- X. Huang, P. Wang, T. Li, X. Tian, W. Guo, B. Xu, G. Huang, D. Cai, F. Zhou, H. Zhang and H. Lei, ACS Appl. Mater. Interfaces, 2020, 12, 227–237 CrossRef CAS PubMed.
- Y. Zhang, J. Ma and W. Zhang, J. Ethnopharmacol., 2021, 277, 114249 CrossRef CAS PubMed.
- P. Chen, C. Zhang, P. He, S. Pan, W. Zhong, Y. Wang, Q. Xiao, X. Wang, W. Yu, Z. He, X. Gao and J. Song, Int. J. Nanomed., 2022, 17, 5165–5186 CrossRef CAS PubMed.
- F. S. C. Sczepanik, M. L. Grossi, M. Casati, M. Goldberg, M. Glogauer, N. Fine and H. C. Tenenbaum, Periodontol, 2020, 84, 45–68 CrossRef PubMed.
- D. Baksh and R. S. Tuan, J. Cell. Physiol., 2007, 212, 817–826 CrossRef CAS PubMed.
- M. N. Knight and K. D. Hankenson, Adv. Wound Care, 2013, 2, 306–316 CrossRef PubMed.
- H. Ma, J. Luo, Z. Sun, L. Xia, M. Shi, M. Liu, J. Chang and C. Wu, Biomaterials, 2016, 111, 138–148 CrossRef CAS PubMed.
- H. Wei, J. Cui, K. Lin, J. Xie and X. Wang, Bone Res., 2022, 10, 17 CrossRef CAS PubMed.
- J. Zhang, Y. Zhuang, R. Sheng, H. Tomas, J. Rodrigues, G. Yuan, X. Wang and K. Lin, Mater. Horiz., 2023, 11 Search PubMed.
- K. Huang, Z. Li, J. Lin, G. Han and P. Huang, Chem. Soc. Rev., 2018, 47, 5109–5124 RSC.
- M. S. Kang, H. J. Jang, H. J. Jo, I. S. Raja and D. W. Han, Nanoscale Horiz., 2023, 9, 93–117 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3tb02306k |
| This journal is © The Royal Society of Chemistry 2024 |