Fabrication of anti-freezing and self-healing nanocomposite hydrogels based on phytic acid and cellulose nanocrystals for high strain sensing applications†
Dongqi
Yue
,
Shaoning
Shi
,
Hou
Chen
 *,
Liangjiu
Bai
*,
Wenxiang
Wang
*,
Liangjiu
Bai
*,
Wenxiang
Wang
 ,
Huawei
Yang
,
Huawei
Yang
 ,
Lixia
Yang
and
Donglei
Wei
,
Lixia
Yang
and
Donglei
Wei
School of Chemistry and Materials Science, Ludong University, Key Laboratory of High Performance and Functional Polymer in the Universities of Shandong Province, Collaborative Innovation Center of Shandong Province for High Performance Fibers and Their Composites, Yantai 264025, China. E-mail: chenhou@ldu.edu.cn; bailiangjiu@ldu.edu.cn
First published on 4th December 2023
Abstract
For hydrogel-based flexible sensors, it is a challenge to enhance the stability at sub-zero temperatures while maintaining good self-healing properties. Herein, an anti-freezing nanocomposite hydrogel with self-healing properties and conductivity was designed by introducing cellulose nanocrystals (CNCs) and phytic acid (PA). The CNCs were grafted with polypyrrole (PPy) by chemical oxidation, which were used as the nanoparticle reinforcement phase to reinforce the mechanical strength of hydrogels (851.8%). PA as a biomass material could form strong hydrogen bond interactions with H2O molecules, endowing hydrogels with prominent anti-freezing properties. Based on the non-covalent interactions, the self-healing rate of the hydrogels reached 92.9% at −15 °C as the content of PA was 40.0 wt%. Hydrogel-based strain sensors displayed high sensitivity (GF = 0.75), rapid response time (350 ms), good conductivity (3.1 S m−1) and stability at −15 °C. Various human movements could be detected by using them, including small (smile and frown) and large changes (elbow and knee bending). This work provides a promising method for the development of flexible wearable sensors that work stably in frigid environments.
1 Introduction
Hydrogels with three-dimensional network structures have been considered as promising candidates for flexible sensors, thanks to their outstanding flexibility, tunable mechanical properties and ion transport.1–5 Hydrogel-based flexible sensors can convert mechanical deformations continuously into detectable electrical signals, for instance, resistance, capacitance and current signals, and collecting signals in time for human movement and physiological monitoring,6 which show immense prospects in the fields of medical health diagnosis, soft robots, smart devices, and many others.7 The mechanical strength, self-healing and anti-freezing properties also characterize the importance of hydrogel-based flexible sensors.8–12 Nevertheless, most traditional hydrogels with water as a solvent would freeze at sub-zero temperatures and lose self-healing and mechanical properties, thereby hindering their practical applications in frigid environments.When the ambient temperature is lower than the freezing point, ice crystals appear due to strong hydrogen bonds between H2O molecules in traditional hydrogels, which are composed of polymer networks in the solid phase and pure water systems in the liquid phase.13 Therefore, the introduction of cryogenic protective agents such as inorganic salts, organic alcohols and ionic liquids into hydrogels to design anti-freezing hydrogels has been widely researched.14–19 Pan et al.20 designed a PVA/glycerol/NaCl ionic hydrogel sensor. Due to the presence of glycerol and NaCl, the hydrogel sensors had the characteristics of frost resistance, and the sensor could still maintain good mechanical and strain sensitivity at −20 °C. However, the introduction of organic alcohols usually leads to the decrease of the conductivity and mechanical properties.21 A high concentration of inorganic salts in hydrogels makes them prone to crystallization, which affects the stability of hydrogels. Hydrogel-based flexible sensors cannot be directly attached to the human skin due to the toxicity of ionic liquids. Therefore, it is great significance to develop biocompatible anti-freezing agents to meet the needs for the practical application of hydrogel-based flexible sensors.
As another emerging biomass-derived material, phytic acid (PA) is a non-toxic biomacromolecular material extracted from plant seeds with good biocompatibility and effortless raw material supply.22–24 As a non-toxic bioorganic acid, naturally extracted PA is an environmentally friendly material and has been widely used as a crosslinking agent, gel and food preservative.25 Moreover, PA containing six phosphate groups provides abundant donor and acceptor positions of hydrogen bonds, which can combine with H2O molecules through hydrogen bond interactions, effectively preventing crystallization and enhancing the anti-freezing properties of hydrogels.26,27 Gao et al.26 prepared a highly conductive hydrogel with remarkable frost resistance and moisturizing ability by incorporating PA into a polyacrylamide/chitosan hydrogel. Based on the strong hydrogen bond between PA and water molecules, the hydrogel exhibits good flexibility and good electrical conductivity (0.041 S cm−1) at sub-zero temperatures while still performing well after 15 days of storage under ambient conditions. As an electrolyte, after being dissolved in water, PA can improve the conductivity of hydrogels by generating affluent free hydrogen ions (H+), which is the momentous performance of hydrogel-based flexible sensors.28 Therefore, PA as a bio-antifreezing agent would effectively improve the anti-freezing, self-healing, and electrical conductivity of hydrogels while eliminating the negative effects of traditional anti-freezing agents on hydrogels, enabling a promising development for the environmental stability of flexible wearable devices.
Cellulose nanocrystals (CNCs) are rigid rod-shaped nanoparticles isolated from natural cellulose. Meanwhile, CNCs have high crystallinity, strong rigidity and excellent biocompatibility, making them suitable nanofillers in composite manufacturing.29,30 Zhang et al. developed a skin-like self-healing elastomeric material with dynamic structural colors by introducing CNCs to assemble liquid crystal structures and designing multiple interfacial hydrogen bonds.31 Polypyridine (PPy) is a common conductive polymer that has attracted a lot of attention for its excellent conductivity, low cost and biocompatibility.32 Guo et al.33 prepared a self-healing conductive elastic flexible multifunctional hydrogel based on β-CD, NIPAM, CNT and PPy, which has great potential for application in smart electronic devices. Therefore, the simultaneous introduction of CNCs, PPy, and PA into hydrogels is expected to impart a variety of satisfactory properties to hydrogels, including enhanced mechanical properties, high electrical conductivity, and frost resistance. Unfortunately, few attempts have been made to use the synergistic effects of these three biomass-derived materials to toughen and functionalize traditional hydrogels.
Herein, an anti-freezing nanocomposite hydrogel with self-healing properties and conductivity was designed by introducing CNCs and PA. The CNCs were grafted with PPy by chemical oxidation, which were used as nanoparticle reinforcement phases to strengthen the tensile strength of hydrogels. Thanks to the intense non-covalent interactions between PA and H2O molecules, the hydrogels exhibited remarkable anti-freezing properties and long-term stability at −15 °C. The hydrogen bonding interactions between PA, cellulose nanocrystals-g-polypyrrole (CNCs-g-PPy) and poly(vinyl alcohol) (PVA), and the electrostatic interactions between PA and poly(diallyldimethylammonium chloride) (PDDA) endowed the hydrogels with excellent self-healing properties, which enhance the durability of the flexible sensors in sub-zero environments. The hydrogels still possessed remarkable conductivity at sub-zero temperatures, which provide the possibility of stable operation of the flexible sensors in sub-zero environments. Furthermore, the hydrogel-based flexible sensors could still maintain good sensitivity, stability, and even good durability at −15 °C. It is effective to detect human motion signals. This work broadens the use of flexible sensors and provides greater potential for flexible sensors to be applied in sub-zero environments.
2 Experimental section
2.1 Preparation of CNCs and CNCs-g-PPy
CNCs were obtained from MCC by sulfuric acid hydrolysis. Briefly, MCC (3.0 g) was added to a three-mouth flask, and 250.0 mL 61.0 wt% sulfuric acid solution was slowly added under agitation (400 rpm) and reacted at 45 °C for 4 h. After centrifugation and dialysis, CNCs were finally obtained. Firstly, Fe(III) was adsorbed onto the surface of CNCs by electrostatic interaction. The Fe(III) solution (50.0 mL, 80 mM) was added to a flask containing CNC suspension (10.0 mL, 2.5 wt%) with stirring at 200 rpm, and the stirring was continued at 25 °C for 24 h. Then the unabsorbed Fe(III) was removed by centrifugation about 2 times (9000 rpm, 1 min). After that, PPy was grafted onto the surface of CNCs by chemical oxidation. The pyrrole (10.0 mL) was dispersed in deionized water and stirred at 0–10 °C for 17 h. The reaction product was repeatedly washed three times with deionized water and alcohol and separated by centrifugation to obtain CNCs-g-PPy.2.2 Fabrication of anti-freezing nanocomposite hydrogels
PVA (0.8 g) and deionized water (4.1 mL) were added into a three-necked flask and heated at 90 °C for 0.5 h. PA (9.2 g) was then mixed into the solution under agitation (70 rpm) and reacted for 1 h. PDDA (2.0 g) was mixed into the reaction system and stirred at 90 °C for 1 h. Eventually, the solution was transferred to the mold and frozen at −30 °C for 24 h. It was placed at room temperature until it thawed. A total of 4 freeze–thaw cycles were performed (Table S2, ESI†).2.3 In vitro biocompatibility evaluation
All human experiments were approved by the Ethics Committee of Ludong University. Informed consent was obtained from the human participants involved in this experiment. The hydrogels were adhered to the wrists, fingers and elbows of the volunteers and the skin condition was observed after 24 h and 48 h, respectively. The biocompatible properties of the hydrogels were evaluated according to the skin irritation.3 Results and discussion
3.1 Synthesis and characterization of CNCs-g-PPy
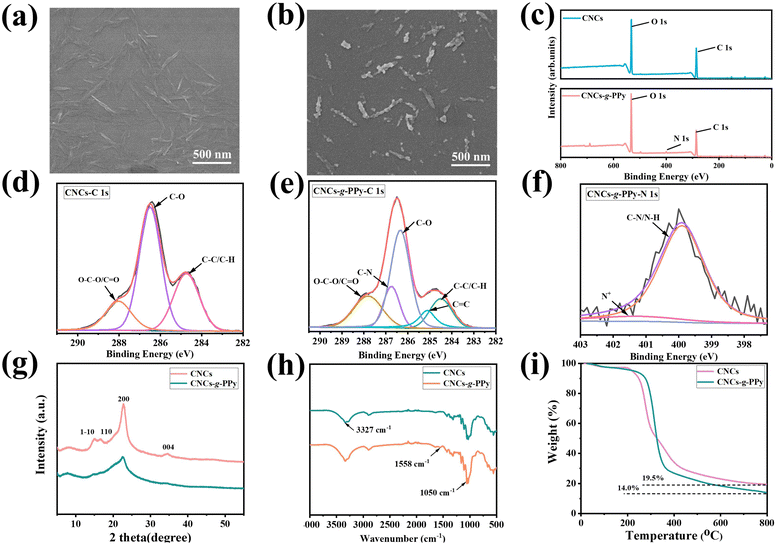
The microscopic morphology of CNCs and CNCs-g-PPy was observed by SEM. In Fig. 1(a), pure CNCs have a rod-like structure and are relatively finely dispersed in water. From Fig. 1(b), it is obvious that spherical PPy particles grow along the rod-like structure of CNCs and the surface of the modified CNCs is granular, which contributes to enhancing the mechanical properties and electrical conductivity of the hydrogel. In Fig. S1(a) and (b) (ESI†), it can be seen that the average diameters of CNCs and CNCs-g-PPy are 57.3 ± 5.7 nm and 65.6 ± 6.4 nm, respectively. The particle size increased to a certain extent, indicating that the preparation of functionalized nanomaterials was successful. XPS is used as a significant means to analyze the chemical composition of the substance surface. The XPS spectrum of CNCs and CNCs-g-PPy is demonstrated in Fig. 1(c). By comparison to CNCs, a new characteristic peak of N 1s appears at 399.1 eV. The XPS spectra of CNCs and CNCs-g-PPy were recorded and are shown in Fig. 1(d and e). The C 1s peak of CNCs could be classified into C–C/C–H (284.7 eV), C–C (286.5 eV), and O–C–O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.1 eV) (Fig. 1(d)). In Fig. 1(e), the emerging peaks of C–N (286.7 eV) and C
O (288.1 eV) (Fig. 1(d)). In Fig. 1(e), the emerging peaks of C–N (286.7 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (285.1 eV) were attributed to PPy, indicating that PPy had been successfully grafted onto CNC surfaces. As shown in Fig. 1(f), the peak at 399.9 eV was assigned to C–N/N–H. Crystallinity has been of remarkable concern for nanocomposite materials.34 Typical XRD spectra of CNCs are shown in Fig. 1(g). The peaks at the 2θ values of 14.6°, 16.4°, 22.5° and 34.0° were assigned to the (1–10), (110) (200) and (004) reflection planes, respectively, of CNCs. As can be seen from Fig. 1(g), the peak strength of the CNCs has decreased after grafting PPy, which is due to the amorphous structure of PPy.35 In Fig. 1(h), pure CNCs display emblematic bands at 3327 and 2901 cm−1, which are ascribed to the O–H stretching and asymmetric C–H stretching vibrations, respectively. The band around 1414 cm−1 originates from the –CH2 stretching vibration. The peak at 1050 cm−1 is attributed to the C–O–C stretching vibration. For the spectrum of CNCs-g-PPy, compared with pure CNCs, the peak intensity of CNCs-g-PPy at 3327 cm−1 is slightly increased, which is attributed to the existence of N–H stretching vibration. Additionally, the peak at 1558 cm−1 was ascribed to the C
C (285.1 eV) were attributed to PPy, indicating that PPy had been successfully grafted onto CNC surfaces. As shown in Fig. 1(f), the peak at 399.9 eV was assigned to C–N/N–H. Crystallinity has been of remarkable concern for nanocomposite materials.34 Typical XRD spectra of CNCs are shown in Fig. 1(g). The peaks at the 2θ values of 14.6°, 16.4°, 22.5° and 34.0° were assigned to the (1–10), (110) (200) and (004) reflection planes, respectively, of CNCs. As can be seen from Fig. 1(g), the peak strength of the CNCs has decreased after grafting PPy, which is due to the amorphous structure of PPy.35 In Fig. 1(h), pure CNCs display emblematic bands at 3327 and 2901 cm−1, which are ascribed to the O–H stretching and asymmetric C–H stretching vibrations, respectively. The band around 1414 cm−1 originates from the –CH2 stretching vibration. The peak at 1050 cm−1 is attributed to the C–O–C stretching vibration. For the spectrum of CNCs-g-PPy, compared with pure CNCs, the peak intensity of CNCs-g-PPy at 3327 cm−1 is slightly increased, which is attributed to the existence of N–H stretching vibration. Additionally, the peak at 1558 cm−1 was ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C ring stretching vibration due to the quinonoid structure.36,37 To investigate the thermal stability of CNCs and CNCs-g-PPy, TGA analysis was performed and the results are demonstrated in Fig. 1(i). The pure CNCs experienced weightlessness between 204 °C and 800 °C, with a residual weight of 19.5% at 800 °C. When PPy was grafted onto the CNC surface, significant mass loss occurred between 270 °C and 800 °C, and the final residual mass at 800 °C was 14.0%, slightly lower than that of CNCs. In addition, it can be observed that the change curve of CNCs-g-PPy is almost the same as that of CNCs, which proves that the thermal decomposition behavior of CNCs is not changed by loading of PPy.38
C ring stretching vibration due to the quinonoid structure.36,37 To investigate the thermal stability of CNCs and CNCs-g-PPy, TGA analysis was performed and the results are demonstrated in Fig. 1(i). The pure CNCs experienced weightlessness between 204 °C and 800 °C, with a residual weight of 19.5% at 800 °C. When PPy was grafted onto the CNC surface, significant mass loss occurred between 270 °C and 800 °C, and the final residual mass at 800 °C was 14.0%, slightly lower than that of CNCs. In addition, it can be observed that the change curve of CNCs-g-PPy is almost the same as that of CNCs, which proves that the thermal decomposition behavior of CNCs is not changed by loading of PPy.38
3.2 Synthesis and characterization of the hydrogels
The natural non-toxic organic acid PA derived from plants contained six symmetrical phosphate groups in its molecular structure, which made PA have rich hydrogen bond sites and could interact with PVA-based hydrogels. The CNCs obtained from natural cellulose were able to produce interfacial interactions with the conducting polymer PPy, resulting in the fabricated CNCs-g-PPy nanoparticles with higher flexibility and conductivity. The preparation process and self-healing mechanism of nanocomposite hydrogels are shown in Fig. 2. PA was used as the anti-freezing agent and crosslinking agent, and CNCs-g-PPy was added into PDDA and PVA double network hydrogels as the nonporous particle enhancement phase. The nanocomposite hydrogels with anti-freezing properties were successfully acquired from facile freeze–thaw cycles. The interaction between PA and the polymer chain could make the hydrogels undergo the corresponding energy dissipation when they were deformed. In addition, the hydrogen bonds between PA and H2O molecules made the hydrogels have anti-freezing properties at −15 °C. Based on the hydrogen-bonding interactions between PVA, CNCs-g-PPy and PA and the electrostatic interactions between PA and PDDA, hydrogels had excellent self-healing properties. Therefore, hydrogels based on PA and CNCs-g-PPy as flexible sensors had good temperature and environmental adaptability.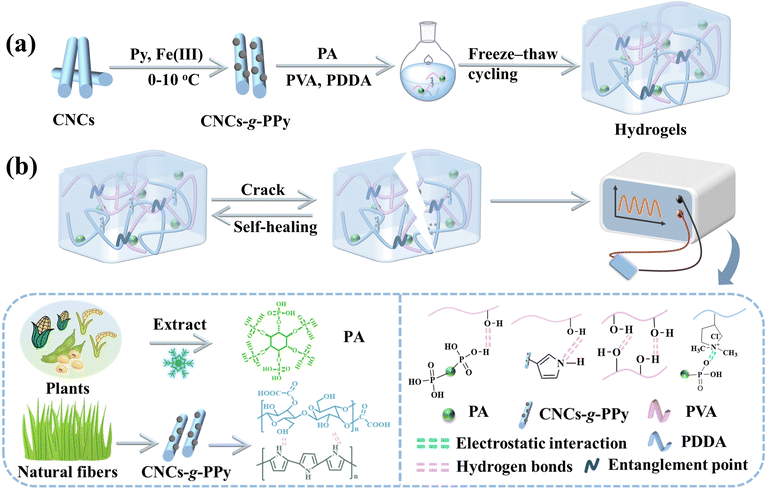 | ||
| Fig. 2 (a) Diagrammatical illustration of the synthesis of CNCs-g-PPy and hydrogels. (b) The self-healing process of hydrogels and their application in anti-freezing sensors. | ||
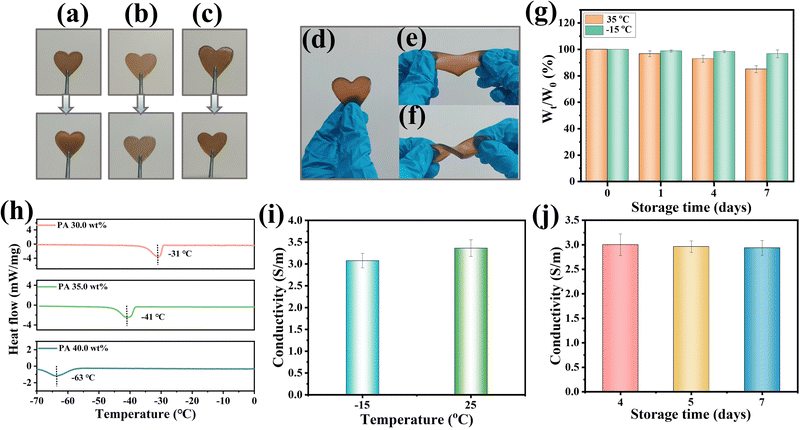
The internal network structure of the freeze-dried hydrogel was investigated by SEM. Due to the presence of PA, the hydrogels could not be totally freeze-dried, and the hydrogel (PA 0.0 wt%) was selected to study the internal structure of the hydrogels. From Fig. S2 (ESI†), it can be observed that the hydrogels have typical three-dimensional network structures, which act as an ion transport channel and are beneficial to the improvement of the conductivity of hydrogels. PA can combine with water molecules through hydrogen bonds to prevent the crystallization of water and ensure that the hydrogels have the anti-freezing ability. The hydrogel samples containing varying PA contents were stored at −15 °C for 1 day, and the morphology of the hydrogels is shown in Fig. 3(a)–(c). In Fig. 3(a), the hydrogels (PA 30.0 wt%) had obvious ice crystals, and the hydrogels (PA 35.0 wt%) showed partial crystallization (Fig. 3(b)), while the hydrogels (PA 40.0 wt%) had no ice crystals and remained consistent with the initial state (Fig. 3(c)), which still had excellent flexibility to be twisted and stretched (Fig. 3(d–f)). To verify this phenomenon, the effects of PA contents on the freezing resistance of hydrogels were investigated by DSC experiments. In Fig. 3(h), by increasing the PA content, the freezing points of the hydrogels were effectively lowered. The freezing point of the hydrogels reached −63 °C with 40.0 wt% PA, indicating that phytic acid can significantly improve the freezing resistance of the hydrogels (unless otherwise specified, the hydrogels (40.0 wt% PA) were used in the following studies). Besides, the conductive ability of the hydrogels has played an important role in flexible sensor applications. The abundant hydroxyl groups in PA molecules showed good ionization properties for ionized hydrogen protons (H+).39 Therefore, the conductivity of the hydrogels was effectively improved by the incorporation of water-soluble PA and conductive CNCs-g-PPy nanomaterials in the process of PVA/PDDA sol–gel formation. As shown in Fig. S3 (ESI†), to verify the conductivity of the hydrogels, a LED light bulb was connected to a conductive loop consisting of the hydrogel and wire, and the light bulb could be observed to brighten and change with different amounts of stretching deformation. Next, the electrical conductivity of the hydrogel at different temperatures was investigated. As shown in Fig. 3(i), due to the influence of ion transport resistance, the conductivity of the hydrogels decreased as the temperatures reduced, and the conductivity of the hydrogels could achieve 3.1 S m−1 even at −15 °C. To explore their environmental stability, hydrogels were deposited at 35 °C and −15 °C for various days (0, 1, 4 and 7 days). Slight water loss in the hydrogels was observed at 35 °C, and the weight loss was 16.9% after 7 days. Importantly, the hydrogels remained relatively stable at −15 °C, and it was only 1.2% after 7 days at −15 °C (Fig. 3(g)). Next, the stability of the hydrogels at −15 °C was further verified by conductivity. As depicted in Fig. 3(j), after being placed at −15 °C for different time periods, the conductivity of the hydrogels remained relatively stable. Even after being placed for 7 days, the conductivity of the hydrogels still reached 2.9 S m−1, which provides the possibility of long-term stable employment of flexible sensors in sub-zero environments.
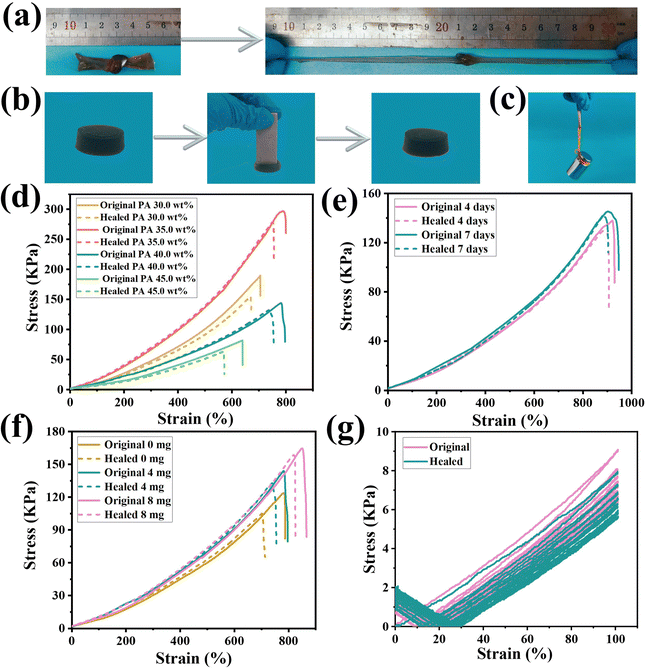
In order to explore the service life of the hydrogel-based flexible sensor under sub-zero conditions, the self-healing and mechanical properties of the hydrogel were evaluated by stress–strain curves. The hydroxyl groups in the PVA chain were abundant, and strong hydrogen bonds could be formed with the inside, thereby giving the hydrogel skeleton excellent self-healing and mechanical properties.40–42 Especially, when CNCs-g-PPy was introduced as a crosslinking agent, PVA exhibited better mechanical properties and flexibility because PVA and nanomaterials could produce a series of interactions in hydrogel systems, which were conducive to the preparation of nanocomposite hydrogels with mechanical strength and self-healing properties.43–45 In addition, the electrostatic interactions generated between PDDA and PA could significantly improve the mechanical strength of the hydrogels. Meanwhile, the entanglement and hydrogen bonding interactions between PVA and PDDA polymer chains have resulted in more tightly crosslinked polymer networks, which greatly improved the self-healing and mechanical properties of hydrogels.46 Tensile tests were performed to explore the effects of different amounts of PA and CNCs-g-PPy and self-healing time on the mechanical properties of hydrogels. In Fig. 4(a) and (b), hydrogels could withstand knotting and stretching without fracture and could recover after being squeezed by heavy objects at −15 °C, indicating that the hydrogels have good mechanical properties. In addition, the weight (200.0 g) could be lifted easily by the hydrogels without external aid (Fig. 4(c)). The tensile test was carried out to further explore the effects of the amount of PA and CNCs-g-PPy on the mechanical and self-healing properties of hydrogels. As illustrated in Fig. 4(d), with the increase of PA content, the stress showed a downward trend. The fracture stress was 144.5 kPa and the fracture strain was 782.8% since the content of PA was 40.0 wt%. At the same time, based on the non-covalent interactions between PA and each matrix inside the hydrogels, the self-healing efficiency reached 92.9% after being placed at −15 °C for 3 h. As we can see, the hydrogels were placed at −15 °C for 4 and 7 days, the mechanical properties of the hydrogels did not change significantly (145.3 kPa), further verifying the excellent environmental stability of the hydrogels. With the enhancement of the non-covalent interactions between PA and CNCs-g-PPy and the matrix of hydrogels, the self-healing rate increased and the self-healing efficiency reached 97.3% after 7 days of storage (Fig. 4(e)). Next, the effect of CNCs-g-PPy content on the mechanical and self-healing properties of the hydrogels was investigated (the PA content was fixed at 40.0 wt%). In Fig. 4(f), the CNCs-g-PPy content has a significant effect on the mechanical properties of the hydrogels. As non-covalent cross-linking points, the mechanical strength of the hydrogels was accelerated effectively by CNCs-g-PPy. When the CNCs-g-PPy contents were 0.0 mg, 4.0 mg and 8.0 mg, respectively, the fracture stress (fracture strain) of the hydrogel increased to 123.3 kPa (783.0%), 144.5 kPa (782.8%) and 164.6 kPa (851.8%). More importantly, the self-healing efficiency increased to 83.3%, 92.9%, and 95.8%, respectively, owing to the hydrogen bonding interaction between CNCs-g-PPy and PA and PVA. We explored the effect of the nanomaterial CNCs-g-PPy on the swelling properties of hydrogels. From Fig. S4 (ESI†), it can be seen that the swelling ability of CNCs-g-PPy hydrogels was lower than that of the original hydrogels, which further indicated that the introduction of nanofillers could effectively improve the crosslinking density of polymer networks. We performed 10 cyclic stretching experiments on the hydrogels (PA 40.0 wt%, CNCs-g-PPy 4.0 mg) healed for 3 h at −15 °C. The chemical structures of PVA/PDDA/PA and PVA/PDDA/PA/CNCs-g-PPy were analyzed by FT-IR and the results are shown in Fig. S5 (ESI†). The peak value of PVA/PDDA/PA around 1640 cm−1 can be attributed to the P-OH telescopic vibration of PA. The characteristic peaks of PVA/PDDA/PA/CNCs-g-PPy at 1050 cm−1 and 1455 cm−1 were attributed to the C–O–C stretching vibration and the C–N stretching vibration of the pyrrole ring, respectively. As shown in Fig. 4(g), the original and healed hydrogels exhibited obvious hysteresis during the first cycle and then remained relatively stable in different cycles, indicating that the hydrogels displayed anti-fatigue properties at low temperatures. Similarly, the hydrogels exhibited excellent mechanical compressibility at different strains. As shown in Fig. S6 (ESI†), the compression curves of the hydrogel in each cycle almost overlapped, proving its excellent compressive elasticity and fatigue resistance. The self-healing process of the hydrogels is demonstrated in Fig. S7(a)–(c) (ESI†). It is more intuitive to place semicircular hydrogels of different colors at −15 °C for self-healing experiments without external stimulation. LED lights brightened when healed hydrogels were placed on the circuit. In addition, the healed hydrogels could withstand stabs and support 200.0 g weight. The self-healing properties of hydrogels were further demonstrated from a microscopic perspective using optical microscopy (Fig. S7(d), ESI†). It was observed that the scars of the hydrogels became shallower over time and definitely vanished after 5 h, illustrating the marked self-healing properties of hydrogels. Finally, the properties of the PA-based nanocomposite hydrogels were compared with those of the previously reported hydrogels. The results of the comparison are shown in Table S1 (ESI†). In conclusion, the hydrogels introduced with PA and CNCs-g-PPy exhibited good self-healing and mechanical properties at low temperatures.
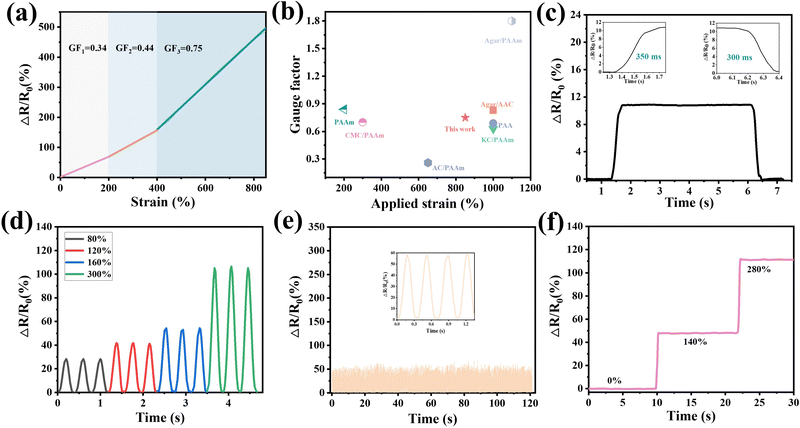
The excellent freezing resistance of hydrogels provides the possibility for hydrogels to exhibit electrical conductivity, flexibility and self-healing in harsh environments, which is beneficial to ensure the normal operation of flexible sensors based on the hydrogels in sub-zero environments. The sensing performance was investigated after the hydrogels were placed at −15 °C for 24 h. The gauge factor (GF) is considered a considerable factor for examining the sensitivity of hydrogels. The GF is obtained by the formula of GF = (ΔR/R0)/ε, where ε is the strain of the hydrogels.47 According to the definition of the GF, the tensile response process could be divided into three stages with strain ranges of 0–200%, 200–400% and 400–800%, respectively. Therefore, the GF value of the hydrogel increased with the increase of strain, and the maximum value could reach 0.75 (Fig. 5(a)). Compared with previous hydrogel-based flexible sensors,48–54 the obtained sensors exhibited good sensitivity and a broad strain detection extent (Fig. 5(b)). In Fig. 5(c), the hydrogels exhibited a short response time (350 ms) and recovery time (300 ms) during stretching cycles. In particular, the hydrogel embodied agreeable sensitivity and stability in both large and small deformations (Fig. 5(d)). Relatively small strain changes (80%) could be detected by the hydrogels, and at the same time the hydrogels were stable in detecting larger deformations (300%). The hydrogels were cyclically stretched 500 times under 140% strain; as depicted in Fig. 5(e), the change of relative resistance was generally stable during the tensile process, which meant that the hydrogels showed excellent sensing stability and fatigue resistance. As shown in Fig. 5(f), the change in relative resistance increases as the strain increases. What was noteworthy was that since the hydrogels maintained a decided deformation, the change value of relative resistance also maintained a relatively stable state.
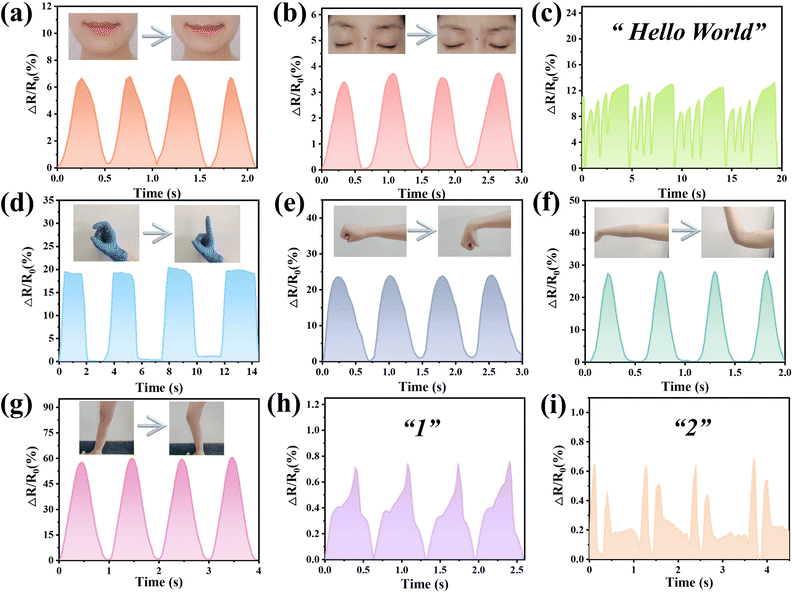
Based on the excellent sensitivity and stability of hydrogels, the hydrogels were assembled into flexible sensors and applied to the detection of human motion signals, which can maintain prominent durability at −15 °C. The biocompatibility of the hydrogel was evaluated before performing the sensing assay. Therefore, the hydrogels were applied to the skin of volunteers. After 24 and 48 h, skin irritation signs such as erythematous, infiltration, edema, and pimples did not appear in the PVA/PDDA/PA/CNCs-g-PPy hydrogel group, indicating that the hydrogel would not cause skin irritation (Fig. S8, ESI†). These results showed that the hydrogel has good biocompatibility. The sensors based on hydrogels were stored at −15 °C for 24 h. In order to verify the sensitivity of the sensors under small deformation, as shown in Fig. 6(a) and (b), the hydrogels were applied to the cheeks and eyebrows, respectively. The sensors not only successfully produced a stable signal for smile and frown behaviors, but also responded sensitively to speech. As shown in Fig. 6(c), the sensors were placed on the throat to detect muscle movements during speech, and volunteers repeated the phonetic alphabet of “Hello World”. Surprisingly, a characteristic curve with four peaks appeared. This shows that the sensors have the ability to recognize different words, and as a word was spoken, similar curves were recorded, further verifying the reproducibility of the sensors. Next, the sensing performance of the sensors for large deformation was investigated to demonstrate the strain detection capability of the sensors. As shown in Fig. 6(d), the sensors were fixed to the index finger joint, and the output signal changed accordingly with the bending of the index finger. When the finger was bent to 90°, the relative resistance value reached the maximum. As the finger returned to the initial state, the relative resistance changes also restored to the original value. In addition, the sensors were installed on the wrist, elbow and knees; when the volunteers repeated bending movements, the stability of the output signals can be seen from Fig. 6(e)–(g); at the same time, the sensors showed different resistance changes in various detection parts due to diverse deformation, revealing the sensitivity and stability of the sensors at −15 °C. When numbers 1 and 2 were written on the sensors, the sensors could even convert that behavior into electrical signals (Fig. 6(h) and (i)). It could be observed that different numbers would correspond to various characteristic curves. The occurrence of single and double peaks were observed in 1 and 2, respectively. The motion sensing properties of the hydrogel strain sensors after long-term cryogenic storage and after self-healing were further explored, including finger bending, wrist bending, elbow bending, knee bending, smile and frown. As shown in Fig. S9 and S10 (ESI†), the relative resistance changes of the treated hydrogel strain sensors were close to those of the original hydrogel strain sensors. The results confirmed that the hydrogel had good low-temperature resistance and self-healing properties, and the service life of the hydrogel strain sensor was significantly extended.
4 Conclusion
In this work, we developed an anti-freezing nanocomposite hydrogel based on PA and CNCs-g-PPy. Based on the non-covalent interactions between PVA, PDDA, CNCs-g-PPy and PA, the hydrogel exhibited excellent mechanical (strain: 851.8%) and self-healing properties (the self-healing efficiency could achieve 92.9% for storage at −15 °C for 3 h). Hydrogels with three-dimensional network structures have been widely used in various fields, but traditional hydrogels face the problem of low-temperature freezing, which makes the hydrogels able to work only in a very narrow temperature range. Therefore the development of hydrogels with anti-freezing properties and low temperature mechanical properties is a promising research direction. It was worth noting that PVA/PDDA/PA/CNCs-g-PPy hydrogels exhibited excellent mechanical flexibility and stability over a wide temperature range. Therefore, this simple and effective low-temperature-resistance strategy will provide new ideas for designing and synthesizing a new generation of flexible sensing devices that can function in harsh environments. Meanwhile, this work will be also well prepared for further development of bio-based highly frost-resistant, self-healing and conductive nanocomposite hydrogels.Author contributions
Dongqi Yue: writing – original draft, writing – review & editing. Shaoning Shi: writing – review & editing, methodology. Hou Chen: funding acquisition, methodology, supervision. Liangjiu Bai: conceptualization, supervision, writing – review & editing. Wenxiang Wang: conceptualization, validation. Huawei Yang: visualization, validation. Lixia Yang: methodology, resources. Donglei Wei: project administration, methodology.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was financially supported by the National Natural Science Foundation of China (No. 51973086), the Natural Science Foundation of Shandong Province (No. ZR2023YQ043), the Project of Shandong Province Higher Educational Science (No. 2019KJA011) and the Natural Science Foundation of Shandong Province (No. ZR2021MB124).Notes and references
- W. J. Ma, W. X. Cao, T. Lu, Z. C. Jiang, R. H. Xiong, S. K. Samal and C. B. Huang, ACS Appl. Mater. Interfaces, 2021, 13, 58048–58058 CrossRef CAS PubMed.
- Z. X. Deng, T. L. Hu, Q. Lei, J. K. He, P. X. Ma and B. L. Guo, ACS Appl. Mater. Interfaces, 2019, 11, 6796–6808 CrossRef CAS PubMed.
- B. Song, X. Fan, J. L. Shen and H. B. Gu, Chem. Eng. J., 2023, 474, 145780 CrossRef CAS.
- J. C. Liu, X. Fan, D. Astruc and H. B. Gu, Collagen Leather, 2023, 5, 17 CrossRef CAS.
- X. Fan, T. Ke and H. B. Gu, Adv. Funct. Mater., 2023, 33, 2304015 CrossRef CAS.
- H. W. Zhou, Z. Y. Jin, Y. Yuan, G. Zhang, W. F. Zhao, X. L. Jin, A. J. Ma, H. B. Liu and W. X. Chen, Colloids Surf., A, 2020, 592, 124587 CrossRef CAS.
- B. Peng, F. N. Zhao, J. F. Ping and Y. B. Ying, Small, 2020, 16, 2002681 CrossRef CAS PubMed.
- N. Wang, X. Yang and X. X. Zhang, Nat. Commun., 2023, 14, 814 CrossRef CAS PubMed.
- Z. X. Deng, H. Wang, P. X. Ma and B. L. Guo, Nanoscale, 2020, 12, 1224–1246 RSC.
- L. Zhao, Q. J. Ling, X. Fan and H. B. Gu, ACS Appl. Mater. Interfaces, 2023, 15, 40975–40990 CrossRef CAS PubMed.
- B. Song, X. Fan and H. B. Gu, ACS Appl. Mater. Interfaces, 2023, 15, 2147–2162 CrossRef CAS PubMed.
- X. Fan, L. Zhao, Q. J. Ling, J. C. Liu and H. B. Gu, Polymer, 2022, 256, 125270 CrossRef CAS.
- X. M. Zhang, K. J. Liu, M. Qin, W. W. Lan, L. F. Wang, Z. W. Liang, X. C. Li, Y. Wei, Y. C. Hu, L. Q. Zhao, X. J. Lian and D. Huang, Carbohydr. Polym., 2023, 309, 120702 CrossRef CAS PubMed.
- X. F. Zhang, X. F. Ma, T. Hou, K. C. Guo, J. Y. Yin, Z. G. Wang, L. Shu, M. He and J. F. Yao, Angew. Chem., Int. Ed., 2019, 58, 7366–7370 CrossRef CAS PubMed.
- M. Zhang, Y. X. Wang, K. Liu, Y. Liu, T. Xu, H. S. Du and C. L. Si, Carbohydr. Polym., 2023, 305, 120567 CrossRef CAS PubMed.
- F. C. Lin, Y. J. Qiu, X. X. Zheng, Z. H. Duanmu, Q. L. Lu, B. Huang, L. Tang and B. L. Lu, Chem. Eng. J., 2022, 437, 135286 CrossRef CAS.
- W. H. Zheng, L. J. Xu, Y. Y. Li, Y. D. Huang, B. Li, Z. X. Jiang and G. L. Gao, J. Colloid Interface Sci., 2021, 594, 584–592 CrossRef CAS PubMed.
- L. Shu, X. F. Zhang, Y. F. Wu, Z. G. Wang and J. F. Yao, Int. J. Biol. Macromol., 2023, 240, 124438 CrossRef CAS PubMed.
- X. Y. Qu, S. Y. Wang, Y. Zhao, H. Huang, Q. Wang, J. J. Shao, W. J. Wang and X. C. Dong, Chem. Eng. J., 2021, 425, 131523 CrossRef CAS.
- S. X. Pan, M. Xia, H. H. Li, X. L. Jiang, P. X. He, Z. G. Sun and Y. H. Zhang, J. Mater. Chem. C, 2020, 8, 2827–2837 RSC.
- G. Li, C. L. Li, G. D. Li, D. H. Yu, Z. P. Song, H. L. Wang, X. N. Liu, H. Liu and W. X. Liu, Small, 2022, 18, 2101518 CrossRef CAS PubMed.
- A. Diouf-Lewis, S. Commereuc and V. Verney, Eur. Polym. J., 2017, 96, 190–199 CrossRef CAS.
- L. J. Zeng and G. H. Gao, ACS Appl. Mater. Interfaces, 2023, 15, 28993–29003 CrossRef CAS PubMed.
- W. S. Dai, J. C. Wang, K. L. Xiang, W. Y. Hu, J. B. Sun, H. Zhang and L. M. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 29499–29510 CrossRef CAS PubMed.
- R. J. Liu, Q. Y. Wan, Y. H. Yu, X. Zhang, L. C. Liu, H. Wang and C. T. Yue, J. Water Process Eng., 2023, 53, 103659 CrossRef.
- Q. Zhang, X. Liu, J. W. Zhang, L. J. Duan and G. H. Gao, J. Mater. Chem. A, 2021, 9, 22615–22625 RSC.
- Y. Nie, D. Q. Yue, W. M. Xiao, W. X. Wang, H. Chen, L. J. Bai, L. X. Yang, H. W. Yang and D. L. Wei, Chem. Eng. J., 2022, 436, 135243 CrossRef CAS.
- H. L. Bui and C. J. Huang, Polymers, 2019, 11, 1721 CrossRef CAS PubMed.
- X. K. Li, J. Z. Liu, D. D. Li, S. Q. Huang, K. Huang and X. X. Zhang, Adv. Sci., 2021, 8, 2101295 CrossRef PubMed.
- Q. K. Cui, X. Huang, X. Y. Dong, H. Y. Zhao, X. H. Liu and X. X. Zhang, Chem. Mater., 2022, 34, 10778–10788 CrossRef CAS.
- X. K. Li, J. Z. Liu, Q. Q. Guo, X. X. Zhang and M. Tian, Small, 2022, 18, 2201012 CrossRef CAS PubMed.
- H. S. Du, W. Liu, M. M. Zhang, C. L. Si, X. Y. Zhang and B. Li, Carbohydr. Polym., 2019, 209, 130–144 CrossRef CAS PubMed.
- Z. X. Deng, Y. Guo, X. Zhao, P. X. Ma and B. L. Guo, Chem. Mater., 2018, 30, 1729–1742 CrossRef CAS.
- Q. C. Fan, Y. Nie, Q. Sun, W. X. Wang, L. J. Bai, H. Chen, L. X. Yang, H. W. Yang and D. L. Wei, ACS Appl. Polym. Mater., 2022, 4, 1626–1635 CrossRef CAS.
- B. Vellaichamy, P. Periakaruppan, R. Arumugam, K. Sellamuthu and B. Nagulan, J. Colloid Interface Sci., 2018, 514, 376–385 CrossRef CAS PubMed.
- Y. X. Chen, J. Y. Zhu, H. Y. Yu and Y. Z. Li, Compos. Sci. Technol., 2020, 194, 108165 CrossRef CAS.
- A. H. P. de Oliveira and H. P. de Oliveira, J. Power Sources, 2014, 268, 45–49 CrossRef CAS.
- D. Cheng, Y. B. Wen, L. J. Wang, X. Y. An, X. H. Zhu and Y. H. Ni, Carbohydr. Polym., 2015, 123, 157–163 CrossRef CAS PubMed.
- S. Zhang, Y. H. Zhang, B. Li, P. Zhang, L. Kan, G. J. Wang, H. Wei, X. Y. Zhang and N. Ma, ACS Appl. Mater. Interfaces, 2019, 11, 32441–32448 CrossRef CAS PubMed.
- J. Shojaeiarani, D. S. Bajwa, N. M. Stark, T. M. Bergholz and A. L. Kraft, Sustainable Mater. Technol., 2020, 26, e00212 CrossRef CAS.
- B. Song, Z. J. Ren and H. B. Gu, Mater. Today Commun., 2023, 35, 105919 CrossRef CAS.
- Z. He, J. C. Liu, X. Fan, B. Song and H. B. Gu, Ind. Eng. Chem. Res., 2022, 61, 17915–17929 CrossRef CAS.
- Z. X. Zhou, C. H. Qian and W. Z. Yuan, Compos. Sci. Technol., 2021, 203, 108608 CrossRef CAS.
- Q. J. Ling, X. Fan, M. J. Ling, J. C. Liu, L. Zhao and H. B. Gu, ACS Appl. Mater. Interfaces, 2023, 15, 12350–12362 CrossRef CAS.
- Z. J. Ren, T. Ke, Q. J. Ling, L. Zhao and H. B. Gu, Carbohydr. Polym., 2021, 273, 118533 CrossRef CAS.
- W. Liu, L. H. Geng, J. M. Wu, A. Huang and X. F. Peng, Compos. Sci. Technol., 2022, 225, 109494 CrossRef.
- Z. X. Pei, Z. W. Yu, M. N. Li, L. J. Bai, W. X. Wang, H. Chen, H. W. Yang, D. L. Wei and L. X. Yang, Int. J. Biol. Macromol., 2021, 179, 324–332 CrossRef CAS PubMed.
- S. J. Liu and L. Li, ACS Appl. Mater. Interfaces, 2017, 9, 26429–26437 CrossRef CAS PubMed.
- Z. J. Gao, L. S. Kong, R. N. Jin, X. Liu, W. Hu and G. H. Gao, J. Mater. Chem. C, 2020, 8, 11119–11127 RSC.
- W. L. Zhang, Y. W. Zhang, Y. Dai, F. Xia and X. J. Zhang, J. Mater. Chem. B, 2022, 10, 757–764 RSC.
- R. X. Tang, Q. Y. Meng, Z. S. Wang, C. J. Lu, M. H. Zhang, C. C. Li, Y. Y. Li, X. P. Shen and Q. F. Sun, ACS Appl. Mater. Interfaces, 2021, 13, 57725–57734 CrossRef CAS PubMed.
- B. W. Yang and W. Z. Yuan, ACS Appl. Mater. Interfaces, 2019, 11, 16765–16775 CrossRef CAS PubMed.
- J. L. Lai, H. W. Zhou, Z. Y. Jin, S. L. Li, H. B. Liu, X. L. Jin, C. Y. Luo, A. J. Ma and W. X. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 26412–26420 CrossRef CAS PubMed.
- H. Li, H. Zheng, Y. J. Tan, S. B. Tor and K. Zhou, ACS Appl. Mater. Interfaces, 2021, 13, 12814–12823 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3tb02482b |
| This journal is © The Royal Society of Chemistry 2024 |