Electrospun organic/inorganic hybrid nanofibers for accelerating wound healing: a review
Sai
Yan
 a,
Yuqi
Qian
a,
Marjan
Haghayegh
a,
Yuqi
Qian
a,
Marjan
Haghayegh
 a,
Yuhan
Xia
a,
Shengyuan
Yang
a,
Yuhan
Xia
a,
Shengyuan
Yang
 a,
Ran
Cao
a,
Ran
Cao
 *ab and
Meifang
Zhu
*ab and
Meifang
Zhu
 a
a
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, P. R. China. E-mail: rancao@dhu.edu.cn; caoranaw@sina.com
bShanghai Engineering Research Center of Nano-Biomaterials and Regenerative Medicine, Donghua University, Shanghai 201620, P. R. China
First published on 5th March 2024
Abstract
Electrospun nanofiber membranes hold great promise as scaffolds for tissue reconstruction, mirroring the natural extracellular matrix (ECM) in their structure. However, their limited bioactive functions have hindered their effectiveness in fostering wound healing. Inorganic nanoparticles possess commendable biocompatibility, which can expedite wound healing; nevertheless, deploying them in the particle form presents challenges associated with removal or collection. To capitalize on the strengths of both components, electrospun organic/inorganic hybrid nanofibers (HNFs) have emerged as a groundbreaking solution for accelerating wound healing and maintaining stability throughout the healing process. In this review, we provide an overview of recent advancements in the utilization of HNFs for wound treatment. The review begins by elucidating various fabrication methods for hybrid nanofibers, encompassing direct electrospinning, coaxial electrospinning, and electrospinning with subsequent loading. These techniques facilitate the construction of micro–nano structures and the controlled release of inorganic ions. Subsequently, we delve into the manifold applications of HNFs in promoting the wound regeneration process. These applications encompass hemostasis, antibacterial properties, anti-inflammatory effects, stimulation of cell proliferation, and facilitation of angiogenesis. Finally, we offer insights into the prospective trends in the utilization of hybrid nanofiber-based wound dressings, charting the path forward in this dynamic field of research.
1. Introduction
The skin, being the largest organ of the human body, serves as a crucial barrier protecting us from external threats. Beyond thwarting the invasion of bacteria, viruses, and other microorganisms, the skin plays a pivotal role in the regulation of the endocrine system.1–3 Therefore, prompt treatment of skin damage is essential to prevent potential risks. Unfortunately, chronic wounds, such as diabetic foot ulcers and severe burns, often necessitate prolonged and advanced medical interventions, imposing substantial financial burdens on patients.4,5 Conventional wound treatment strategies include autografts, allografts, and xenografts.6 However, these methods suffer from limited availability, donor shortage, and the risk of immune rejections.7–9 Consequently, advanced wound dressings that can replace partial functions of skin grafts and promote wound healing are highly desirable.Wound dressings can be categorized into traditional options such as gauze, and newer alternatives including hydrogels, foams, and electrospun nanofibrous membranes.10,11 Among them, electrospun nanofibrous membranes have gained considerable attention due to their high porosity, large specific surface area, and suitable mechanical strength.12–14 Moreover, the nano-network structure of electrospun nanofibrous membranes closely resembles the natural extracellular matrix (ECM) of skin, making them promising candidates for wound treatment.15–17 Additionally, electrospinning has evolved from single-fluid18,19 to side-by-side20,21 or coaxial,22,23 triaxial24,25 or even other multi-fluid derived structures including core–shell,22 porous,26 Janus27,28 and even more complex and fine structures.29,30 This offers a straightforward and practical method for utilizing a wide range of raw or modified materials, providing control over the nanofiber morphology and structure.31–33 Furthermore, nanofiber membranes can function as carriers for the controlled delivery and release of biomolecules and drugs, expanding their application in the field of wound healing. In fact, some electrostatically spun wound dressings have already been commercially applied, such as FibriSEAL™ from St Teresa Medical, USA, PHOENIX™ from Nanofiber SOLUTIONS, USA, and Spincare® System from Nanomedic, Israel.
Skin primarily consists of water and an organic matrix, yet it also contains various inorganic elements, including calcium, iron, silicon, and zinc.34,35 By incorporating these inorganic components with the organic matrix, the bioactivity of the hybrid nanofibrous membranes can be greatly enhanced.36–39 As such, the use of electrospinning to create organic/inorganic hybrid nanofibers as novel wound dressings has seen rapid evolvement in recent years.
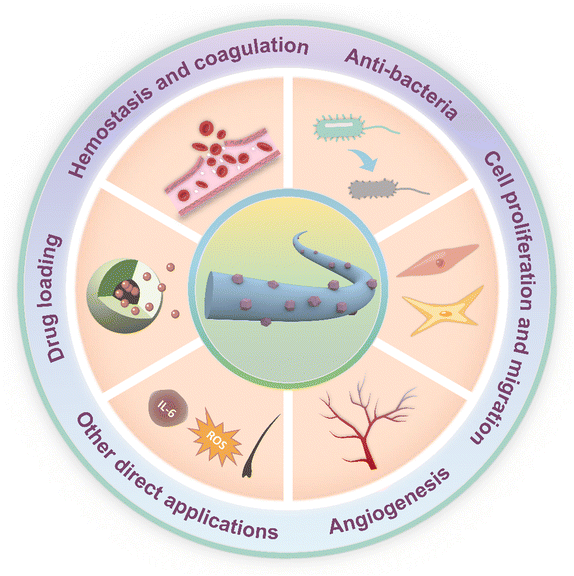
While many excellent review articles have explored the potential of hybrid materials for wound healing, they tend to focus on specific aspects, such as bioactive properties,6 or the synthesis pathways of natural hybrids.36 However, despite electrospinning being one of the most prevalent methods for preparing wound dressings, there is a notable absence of reviews summarizing recent progress in electrospun hybrid materials. Therefore, this review aims to bridge the gap by summarizing the fabrication and specific applications of hybrid nanofibers as wound dressings. We commence by discussing three typical preparation methods for electrospun organic/inorganic hybrid nanofibers, including direct electrospinning, coaxial electrospinning, and electrospinning with subsequent loading. The applications of electrospun organic/inorganic hybrid nanofibers are systematically summarized thereafter, including hemostasis, anti-bacteria, promoting cell proliferation and migration, accelerating angiogenesis and other direct (anti-inflammatory, antioxidant, and follicle regeneration) and indirect applications (drug loading) (Fig. 1). Moreover, potential mechanisms of the HNFs in promoting wound healing were discussed. Finally, we provide a comprehensive summary of the current state of research and offer insights into future development trends.
 | ||
| Fig. 1 Schematic diagram of electrospun organic/inorganic hybrid nanofibers (HNFs) for wound healing. | ||
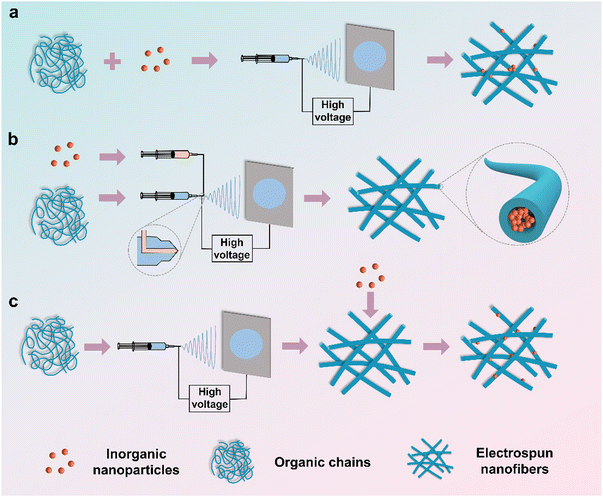
2. Preparation methods of HNFs
Electrospun organic/inorganic hybrid nanofibers (HNFs), combining the polymer matrixes with inorganic nanomaterials, possess a unique blend of superior properties from both components, rendering them attractive in various fields.40 Furthermore, the electrospinning technique holds great promise for the cost-effective manufacture of continuous nanofibers. In essence, the HNFs can be categories into direct electrospinning, coaxial electrospinning, and electrospinning with subsequent loading (Fig. 2).2.1. Direct electrospinning
Premixing organic matrixes and inorganic nanoparticles and then directly subjecting them to electrospinning (Fig. 2a) is the simplest and most efficient method.39,41 However, this method suffers from the inferior distribution of various components due to the fact that the high viscosity of polymer solutions retards the dispersion of inorganic nanoparticles. As a result, the inorganic nanomaterials are either dispersed on the nanofiber surface or encapsulated within the polymer nanofibers. For example, Chen et al.42 physically mixed melatonin and Fe3O4 magnetic nanoparticles (Fe3O4-MNPs) with polycaprolactone (PCL) pellets in a solvent mixture of N,N-dimethylformamide (DMF) and dichloromethane (DCM). The obtained hybrid nanofibers acted as artificial nerve catheters for repairing nerve injuries.2.2. Coaxial electrospinning
Coaxial electrospinning involves constructing core–shell nanofibers by using a multi-channel needle, where the core solution typically contains inorganic nanoparticles dissolved in a solvent or polymer solution, while the shell solution is another polymer solution (Fig. 2b).32,43,44 This method allows the simultaneous electrospinning of core and shell solutions using a coaxial needle. Mahdieh et al.45 fabricated antibacterial sheath–core fibers through coaxial electrospinning, using citrate-coated silver nanoparticles (AgNPs) dispersed in sodium citrate aqueous solution as the core solution and the shell solution comprising ZnO particles, polycaprolactone (PCL), and polyethylene glycol (PEG) in a chloroform/DMF mixture. The superiority of this method lies in the controllable release of the inorganic components or drugs in the core layer.46 Nevertheless, the coaxial electrospinning process is complicated, and the compatibility of the core–shell solution limits the choice of materials.46,472.3. Electrospinning with subsequent loading
In this method, a pre-electrospun nanofibrous membrane is immersed in inorganic materials where the latter forms nanoparticles on the surface of the former (Fig. 2c). Rivero et al.48 combined poly(acrylic acid) (PAA) with β-cyclodextrin (β-CD) to create nanofibrous membranes via electrospinning. The nanofibrous membranes were subsequently immersed in a silver nitrate solution, resulting in Ag+ bonding to the carboxyl group of PAA. Via reducing Ag+ with dimethylamine borane (DMAB) solution, Ag nanoparticles were successfully decorated on the nanofibrous membrane. The post-loading method ensures the even distribution of inorganic nanomaterials only on the surface of nanofibers.49 However, this method requires suitable synthesis conditions for both organic and inorganic materials, and achieving precise control of the particle size and loading rate of inorganic materials is a challenge.3. Mechanisms of the HNFs in accelerating wound healing
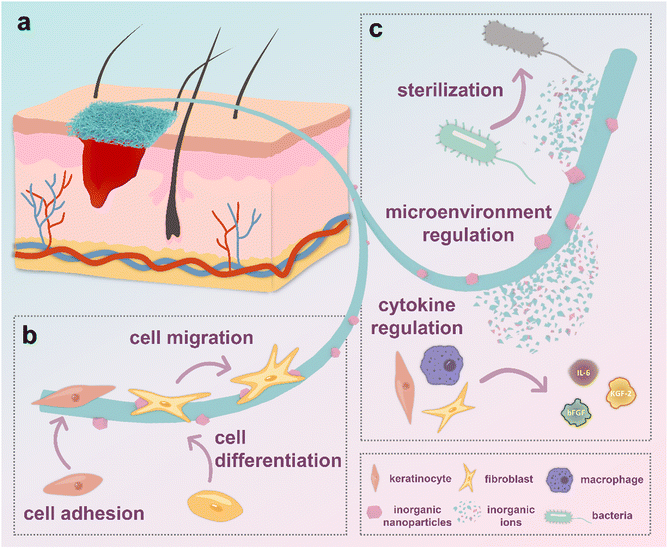
The efficacy of HNFs in expediting wound healing can be categorically delineated into two primary mechanisms. Firstly, the hierarchical nanostructures arising from the interplay of organic fibers and inorganic nanoparticles serve to intricately guide cellular behavior. Secondly, the controlled release of bioactive substances such as ions from the HNFs actively modulates the microenvironment surrounding the wound site, thereby orchestrating a concerted influence on cellular behavior (Fig. 3).3.1. Hierarchical nano-structures
It is a notion long acknowledged in scientific literature that the microstructure exerts a profound influence on cells and tissues.50,51 The introduction of nanoparticles allows the electrospun nanofiber network to form a hierarchical nano-structure, which leads to increased surface roughness, and provision of better sites for cell adhesion.52,53 Consequently, cell shapes and morphologies can be tuned according to the distribution of nanoparticles (Fig. 3b). Moreover, mechanical stimulation caused by the nanoparticles allows cells to enhance phagocytosis, accompanied by improved cellular viability. To some extent, this simulation provides an optimistic impact on the regulation of cell differentiation.54,55 Besides, it is believed that the engineered uneven roughness surface can accelerate the spatial and temporal alternation between the extension of the pseudopods in the head of the cell, and the contraction of the tail of the cell body. As a result, cells tend to migrate faster, thus accelerating wound closure.54,56 Wu and colleagues57 prepared hierarchical nanofiber scaffolds with controllable micropattern substrates (squared-shaped, hole shaped, strip-shaped and hexagon shaped) and bioglass nanoparticles. Consequently, a 2-dimensional patterned structure with 300–400 μm variation, 1-dimensional fibers with the diameter in the range of 500–1000 nm, and the surface of individual nanofibers was composed of 0-dimensional bioglass nanoparticles (approximately 30 nm) were prepared. They observed that the layered micro-nanostructures and nano bioglass in the scaffold could work together to improve the efficiency of wound healing and re-epithelialization. Their research shows that electrospun nanofibers with square-shaped structures showed better healing efficiency due to its high porosity. In addition, bioglass nanoparticles smaller coated on the nanofibers are believed to promote the preferential adsorption molecules such as hyaline, which plays a crucial role in stimulating cell adhesion. Similarly, Lin et al.54 prepared a hierarchical micro/nanofibrous scaffold incorporated with curcumin and zinc ion eutectic metal organic frameworks (ZIF-8) by using an electrospinning and crystal engineering method. Compared with the control group, these microscopic/nanofibrous scaffolds increase the number of antennae and diffusion area of the cells, significantly affecting cell adhesion and migration. As can be seen from the above examples, hierarchical nano-structures constructed by nanofibers and nanoparticles have important implications for cell behavior and even wound healing.3.2. Active substance release
In addition to their role in fostering hierarchical nanostructures, HNFs contribute to the promotion of wound healing through the release of active substances into the humoral environment (Fig. 3c). Those active substances such as ions exhibit versatility, with some inducing cells to generate cytokines, thereby expediting the wound healing process.55,58,59 Besides, certain active substances contribute to the production of nutrients or modify the microenvironment through chemical reactions. For example, the generation of oxygen by nanoparticles can accelerate angiogenesis.60,61 Furthermore, inorganic nanoparticles possessing charges or specific electron-pairing active sites on their surfaces can selectively bind to cells or other substances.62,65,66 It should be noted that some inorganic nanoparticles also shows antimicrobial activity by releasing ions or having chemical reactions.67,68 A number of specific experimental reports support these views. Augustine et al.69 reported that the prolonged effect of endothelial cell proliferation and cell density increase is attributed to the slow and sustained release of europium hydroxide from the hybrid nanofiber scaffold. Ning and colleagues70 demonstrated that their Ag-MOF hybrid electrospun scaffolds showed excellent antibacterial activity due to the slow release of Ag+ ions. The previously mentioned hierarchical micro/nanofibrous scaffold incorporated with ZIF-8 prepared by Lin et al. also showed a sustained release of curcumin and Zn2+, contributing to the scaffold's increased cell proliferation, anti-inflammatory performance and antioxidant capacity.544. Applications of HNFs in wound healing
The wound healing process can be divided into four essential stages: hemostasis, inflammation, cell proliferation and migration, and maturation.71–73 Different hybrid nanofibers have been developed to address specific requirements during each stage of wound healing. For example, the initial stage necessitates hybrid nanofibers with hemostatic and antibacterial properties.4.1. Hemostasis and coagulation
Hemostasis is a prerequisite for the wound healing process. Inorganic materials such as montmorillonite, kaolinite, halloysite, and palygorskite have demonstrated the ability to accelerate hemostasis by actively stimulating the body's endogenous coagulation factors and facilitating the aggregation of blood cells.74,75 However, a drawback of using the powdered form of the inorganic materials is their challenging aggregation and removal, which could potentially hinder the subsequent stages of wound healing.75–78 Therefore, the combination of electrospun nanofibers and inorganic hemostatic powder is a promising method for improved hemostatic performance.62 Moreover, nanofiber membranes, owing to their high flexibility, skin-friendly properties, and finely tuned pore size distribution, are proven to be effective in staunching blood exudation.79–81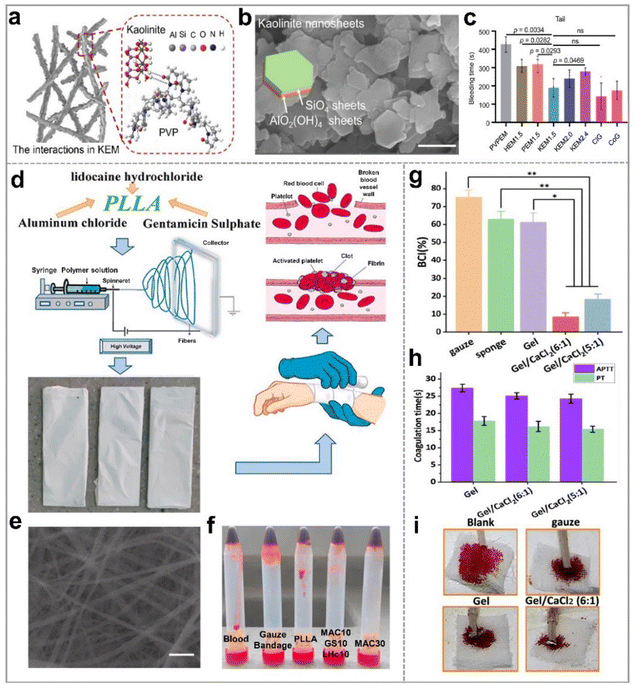 | ||
Fig. 4 Nanoclay-based electrospun membranes for hemostasis of wounds; (a) illustration of kaolinite/PVP nanofibers. (b) Scanning electron microscope (SEM) image of kaolinite (scale bar, 500 nm); (c) the bleeding time of the wounds treated with NEMs in rat-tail amputation hemostasis model; reproduced with permission,62 CC BY 4.0. (d) Schematic illustration of the electrospun PLLA/AlCl3 for hemostatic application; (e) SEM image of PLLA nanofibrous membrane loaded with 30% w/w AlCl3 (scale bar, 2 μm); (f) optical photo of the blood clotting effect of five samples; reproduced with permission.63 Copyright 2021, Elsevier. (g) BCI of gauze, gelation sponge, gelatin (Gel), and gelatin/calcium chloride electrospun nanofibers; (h) APTT and PT of Gel, Gel/CaCl2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and Gel/CaCl2 (5 1), and Gel/CaCl2 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanofibrous membranes; (i) tail vein hemostasis of mice treated with different samples; reproduced with permission.64 Copyright 2023, Royal Society of Chemistry. 1) nanofibrous membranes; (i) tail vein hemostasis of mice treated with different samples; reproduced with permission.64 Copyright 2023, Royal Society of Chemistry. | ||
Additionally, calcium, an essential element in hemostasis, functions as coagulation factor IV, accelerating thrombosis formation by increasing the polymerization rate of the fibrin monomer, and vascular contraction.64 Moreover, calcium accumulating in the vascular smooth muscle cells can trigger vascular contraction, facilitating fast hemostasis. Yu et al.64 employed gelatin and calcium chloride as raw materials to prepare a hemostatic wound dressing (Gel/CaCl2) through electrospinning. Hemostatic properties of calcium-doped nanofiber membranes are evident in the blood-clotting index (BCI) (Fig. 4g), activated partial thromboplastin time (APTT) and prothrombin time (PT) values of Gel/CaCl2 and other samples (Fig. 4h). Furthermore, images of tail vein hemostasis in mice (Fig. 4i) underscore the effectiveness of these membranes. Notably, the Gel/CaCl2 membranes with varying contents of gelatin and calcium chloride displayed BCI values of 8.57% and 18.4%, respectively, significantly lower than those of the gelatin nanofiber (Gel) membranes. Besides, the APTT value of Gel/CaCl2 decreased by about 28 s compared with that of Gel, indicating that the addition of calcium ions improved the hemostatic ability of nanofiber membranes in an exogenous pathway. With a mean hemostatic time of 289 seconds and minimal blood loss (0.06 g), the Gel/CaCl2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) membrane outperformed commercially available gauze (698 s, 0.31 g), blank control (846 s, 0.63 g), and Gel membranes (304s, 0.36g). In addition, silver,85 zinc,86 and other nanoparticle-based hybrid electrospun nanofibers have also exhibited exceptional hemostatic and coagulation properties.
1) membrane outperformed commercially available gauze (698 s, 0.31 g), blank control (846 s, 0.63 g), and Gel membranes (304s, 0.36g). In addition, silver,85 zinc,86 and other nanoparticle-based hybrid electrospun nanofibers have also exhibited exceptional hemostatic and coagulation properties.
4.2. Anti-bacterials
Wound healing is a protracted process during which bacteria can infiltrate the wound site, leading to infections – a significant impediment to the healing process. Repeat infections not only retard the wound recovery but also pose considerable risks to individuals. Therefore, preventing bacterial intrusion into wounds is of paramount importance. Previous studies have demonstrated that certain inorganic nanoparticles possess broad-spectrum antibacterial properties and could circumvent bacterial drug resistance. Incorporating these anti-bacterial inorganic nanoparticles into polymer fibers proves to be an effective strategy for avoiding infections during the wound healing process.87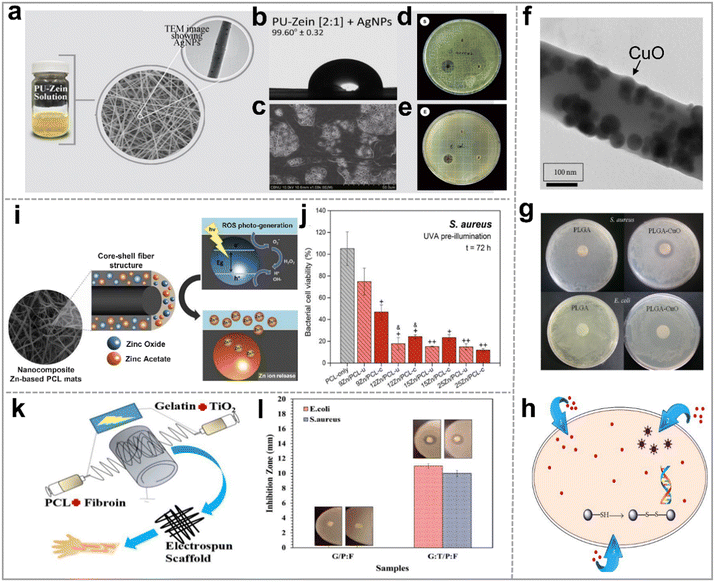 | ||
| Fig. 5 Metal and metal oxide nanoparticle based hybrid nanofibers for anti-bacterial application. (a) Silver-doped polyurethane zein hybrid nanofibrous scaffold (AgNPs/PU-zein); (b) the water contact angle of AgNPs/PU-zein; (c) SEM image of the cell-cultured AgNPs/PU-zein scaffold for 7 days; the antibacterial activity of samples in against (d) S. aureus and (e) E. coli, respectively; reproduced with permission.91 Copyright 2016, Elsevier. (f) Transmission electron microscope (TEM) image of PLGA/CuO hybrid nanofiber; (g) image of the inhibition zones of different nanofiber scaffolds against E. coli and S. aureus; (h) schematic diagram of the antibacterial mechanism of nano CuO/Cu2+ ions; reproduced with permission,92 CC BY 4.0. (i) Schematic diagram of the antibacterial mechanism of electrospun PCL/ZnONPs; (j) the bacterial cell viability (%) of S. aureus in the different mats; reproduced with permission.93 Copyright 2018, Elsevier. (k) Co-electrospun gelatin:TiO2/PCL:silk fibroin scaffolds (G:T/P:F) for antisepsis; (l) the antibacterial activity of G/P:F and G:T/P:F scaffolds against S. aureus and E. coli. Reproduced with permission.94 Copyright 2022, Wiley Periodicals LLC. | ||
These nanofibers facilitated the controlled release of Zn2+ and displayed enhanced antibacterial properties under UVA light irradiation (Fig. 5i), significantly reducing bacterial activity against E. coli and S. aureus (Fig. 5j) to about 20% after 72 hours when compared to pure PCL. Titanium dioxide nanoparticles (TiO2NPs) can trigger antibacterial activity under ultraviolet irradiation. This effect is adjustable by manipulating parameters such as wavelength, light intensity, pH value, and temperature.100,101 Consequently, TiO2NPs find extensive application in wound treatment.94,102–106 Recently, Golipour et al.94 developed wound healing dressings by co-electrospinning of gelatin:TiO2 and polycaprolactone:silk fibroin (G:T/P:F) (Fig. 5k). These scaffolds exhibited pronounced antibacterial activity against E. coli and S. aureus (Fig. 5l). Furthermore, the possible bacterial inhibitory mechanisms of TiO2NPs were suggested: (i) TiO2 leads to the peroxidation of polyunsaturated phospholipids in cell membranes and the loss of bacterial respiratory activity; (ii) the electrostatic interaction between TiO2 and cell wall causes damage to the latter; (iii) TiO2 generates ROS in body fluid environment, resulting in bacterial oxidative stress, thus destroying bacterial cells.
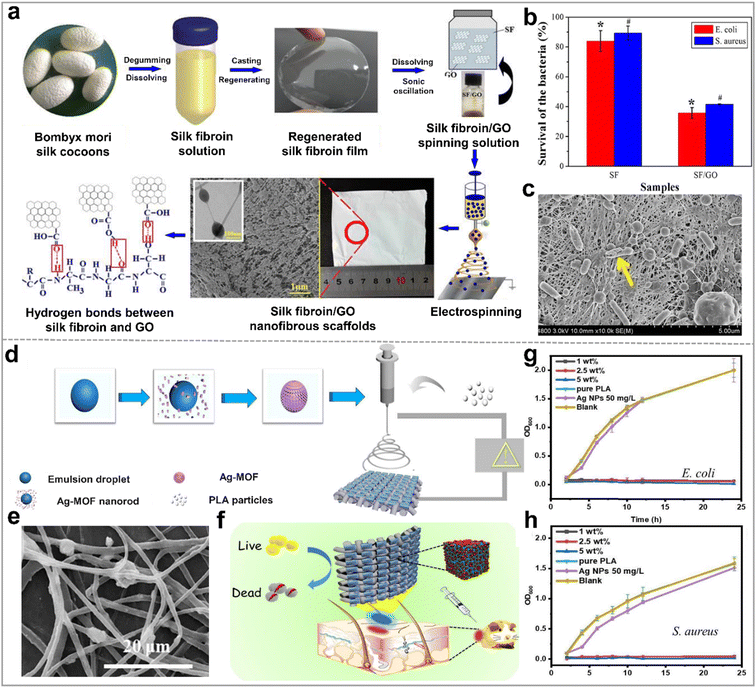 | ||
| Fig. 6 Anti-bacterial ability of inorganic nonmetallic nanoparticle based hybrid nanofibers. (a) Schematic illustration of the preparation process of silk fibroin/GO nanofibrous scaffolds(SF/GO); (b) survival rates of bacteria (%) on the nanofibers (c) SEM image of E. coli on the surface of SF/GO-blended nanofibers; reproduced with permission,99 CC BY 4.0. (d) Schematic diagram of the preparation process of the electrospun fibrous mat based on silver(I)-MOF/poly(lactic acid) (Ag-MOF/PLA) for bacterial killing; (e) SEM image of Ag-MOF/PLA; (f) schematic diagram of the antibacterial applications of Ag-MOF/PLA; growth activity of (g) E. coli and (h) S. aureus on Ag-MOF/PLA, pure PLA, and commercial AgNPs; reproduced with permission.70 Copyright 2020, Elsevier. | ||
Metal–organic frameworks (MOFs) have also attracted extensive attention as antibacterial materials in recent years because of their designability of molecular structure and controllability of releasing metal ions. The fine-tuning of antibacterial performance at the molecular level is achievable by regulating the metal ions and organic ligands within MOFs.70,108–110 Most of the antimicrobial strategies of MOFs are mainly derived from two aspects: MOFs are reservoirs and sustained-release agents of antimicrobial metal ions or MOFs as porous materials are carriers of antimicrobial drugs. For example, as shown in Fig. 6d, Ning and colleagues70 synthesized a silver(I) based metal organic framework (Ag2[HBTC][IM], abbreviated as Ag-MOF). In their work, this Ag-MOF achieved high antimicrobial efficiency by slowly releasing silver ions. The Ag-MOF was uniformly mixed with PLA/DCM solution and subsequently electrospinning to form a Ag-MOF/PLA hybrid nanofiber membrane (Fig. 6e). Based on their results, Ag-MOF/PLA hybrid nanofibers presented potent inhibitory effects against E. coli, P. aeruginosa, S. aureus, and M. smegmatis (Fig. 6f). In contrast, both pure PLA and blank control group displayed negligible antibacterial activity, as is evident in Fig. 6g and h. However, the inhibition rates against E. coli and S. aureus of Ag-MOF/PLA with a concentration of 2.5 wt% Ag-MOF reached 97.2%, and 97.5%, respectively.
We have summarized the HNFs with antibacterial activity and other functions in Table 1.
| Inorganic components | Polymers | Methods of incorporation | Functions | Ref. |
|---|---|---|---|---|
| AgNPs | Polyurethane | Electrospinning with subsequent loading | Antibacterial activity against E. coli and S. aureus, and promotion of cell proliferation | 91 |
| Polyvinyl alcohol/chitosan | Direct electrospinning | Antibacterial activity against E. coli and S. aureus | 114 | |
| Polycaprolactone | Direct electrospinning | Antibacterial activity against E. coli and S. aureus, and promoting cell attachment, proliferation and spreading | 89 | |
| Thermoplastic polyurethane, polyvinyl alcohol | Electrospinning with subsequent loading | Antibacterial activity against E. coli, S. aureus, MRSA, Acinetobacter, and Klebsiella-pneumoniae | 88 | |
| Polycaprolactone/polyvinyl pyrrolidone | Direct electrospinning | Antibacterial activity against E. coli and S. aureus | 115 | |
| Silk fibroin | Direct electrospinning | Antibacterial activity against S. aureus, S. epidermidis, and P. aeruginosa | 116 | |
| Polyethylene oxide/carboxymethyl chitosan | Direct electrospinning | Antibacterial activity against P. aeruginosa, E. coli, and S. aureus | 117 | |
| Polyvinyl alcohol/chitosan | Electrospinning with subsequent loading | Antibacterial activity against E. coli | 118 | |
| Cu, CuONPs | Poly(lactide-co-glycolide) | Direct electrospinning | Antibacterial activity against E. coli and S. aureus, and promotion of cell adhesion, proliferation and viability | 92 |
| Polyvinyl pyrrolidone | Direct electrospinning | Antibacterial activity against E. coli, P. aeruginosa, S. aureus and Bacillus cereus | 95 | |
| Polycaprolactone/gelatin | Direct electrospinning | Antibacterial activity against E. coli and S. aureus | 96 | |
| ZnONPs | Polyacrylic acid/polyallylamine hydrochloride | Electrospinning with subsequent loading | Antibacterial activity against E. coli and S. aureus | 97 |
| Polycaprolactone | Coaxial electrospinning | Antibacterial activity against E. coli and S. aureus | 93 | |
| Chitosan/polycaprolactone | Direct electrospinning | Antibacterial activity against S. aureus and B. subtilis, antioxidant activity and ability to accelerate wound healing | 98 | |
| Vinylidene fluoride-tetrafluoroethylene copolymer/polyvinyl pyrrolidone | Direct electrospinning | Antibacterial activity against S. aureus, and positive contribution to purulent wound healing | 119 | |
| TiO2NPs | Polycaprolactone | Direct electrospinning | Antibacterial activity against E. coli and S. aureus, and promotion of cell adhesion and viability | 100 |
| Polyurethane | Electrospinning with subsequent loading | Antibacterial activity against S. aureus and P. aeruginosa, and good cell adhesion | 105 | |
| Chitosan/pectin | Direct electrospinning | Antibacterial activity against E. coli, S. aureus, P. aeruginosa, absorption of wound exudate and acceleration of wound closure | 102 | |
| Silk fibroin | Direct electrospinning | Antibacterial activity against E. coli, and promotion of cell adhesion and growth | 104 | |
| Chitosan/polyvinyl pyrrolidone | Direct electrospinning | Antibacterial activity against E. coli, S. aureus, P. aeruginosa, and B. subtilis, and B. subtilis, and promotion of wound closure | 106 | |
| Gelatin/polycaprolactone/silk fibroin | Direct electrospinning | Antibacterial activity against E. coli and S. aureus, and acceleration of cell proliferation and migration | 94 | |
| Polylactic acid | Direct electrospinning | Antibacterial activity against S. aureus | 101 | |
| Chitosan | Direct electrospinning | Antibacterial activity against S. aureus | 120 | |
| Iodine | Polyvinyl pyrrolidone/polyvinyl butyral | Direct electrospinning | Antibacterial activity against E. coli and S. aureus | 112 |
| GO | Silk fibroin | Direct electrospinning | Antibacterial activity against E. coli and S. aureus, and acceleration of cell proliferation and migration | 99 |
| MOFs | Polylactic acid | Direct electrospinning | Antibacterial activity against E. coli, P. aeruginosa, S. aureus, and M. smegmatis, and acceleration of wound healing | 70 |
| Chitosan/polyvinyl alcohol | Direct electrospinning | Antibacterial activity against E. coli and S. aureus | 108 | |
| CeO2/bioglass | Chitosan/polyethylene oxide | Direct electrospinning | Antibacterial activity against E. coli and S. aureus | 111 |
| SeNPs | Polycaprolactone/gelatin | Direct electrospinning | Antibacterial activity against S. aureus and P. aeruginosa, and promotion of wound closure | 121 |
4.3. Cell proliferation and migration
Proliferation and migration of the fibroblasts, endothelial cells, and keratinocytes allow the establishment of a new extracellular matrix and granulation tissue, which are essential for wound closure.122 Inorganic nanoparticles, including metals (Ag123) and metal oxides (ZnO,124 and Fe2O3125) as well as inorganic nonmetallic materials (Si,56,126 GO,99 and MOF109) have demonstrated the ability to promote cell proliferation and migration. In addition to the above, some materials have been considered for their versatility to promote cell proliferation and migration in wound therapy, such as cuprous sulfide (CuS), cerium oxide (CeO2) and biological glass (BG).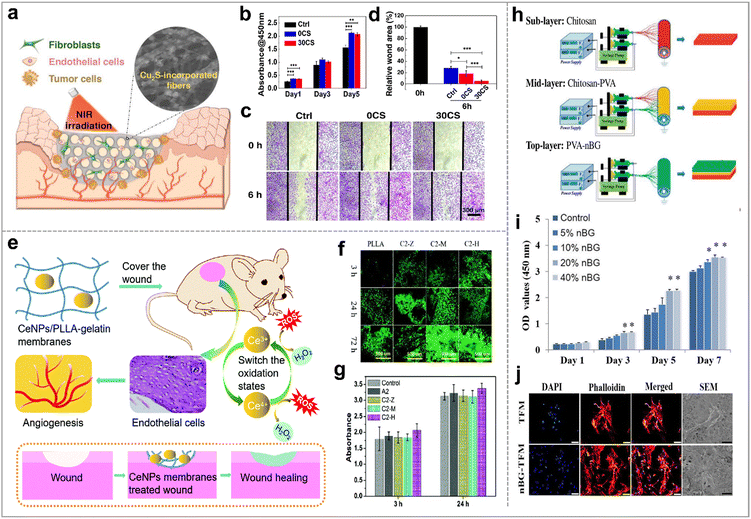 | ||
| Fig. 7 Bioglass-based hybrid nanofibers for promoting cell proliferation and migration. (a) Electrospun nanocomposites incorporated with Cu2S nanoflowers for skin tumor therapy and wound healing; (b) the cell proliferation of HDFs cultured on the different membranes for 1, 3, 5 days; (c) in vitro scratch assay of HUVECs on the various membranes. (d) The relative wound area; reproduced with permission.127 Copyright 2017, American Chemical Society. (e) Schematic illustration of the mechanism of CeNPs/PLLA–gelatin for wound healing via generation of ROS, proliferation and migration of the endothelial cells, and angiogenesis; (f) distribution and density of NIH 3T3 cells (AO/EB staining), and (g) cell activity of L929 cells (CCK-8 assay) on the various nanofiber membranes; reproduced with permission.128 Copyright 2022, Royal Society of Chemistry. (h) Schematic diagram of the preparation of the nano bioglass/three-layer composite nanofibrous membrane (nBG–TFM); (i) proliferation of L929 cells on nBG–TFM; (j) morphology of HDF cells on the nBG–TFM and non-nBG (TFM); reproduced with permission.129 Copyright 2019, Elsevier. | ||
4.4. Angiogenesis
Blood vessels play a crucial role in facilitating the exchange of substances between the blood and tissue, providing essential nutrients for cell growth. Therefore, angiogenesis, the formation of new blood vessels, is a pivotal step in wound healing. Growth factors such as the vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), stromal cell-derived factor (SDF) have been proven effective in accelerating wound revascularization. However, medical products based on growth factor face limitations such as short release time and high cost.58,131,133 Thus, researchers are searching satisfactory organic/inorganic hybrid materials to accelerate angiogenesis.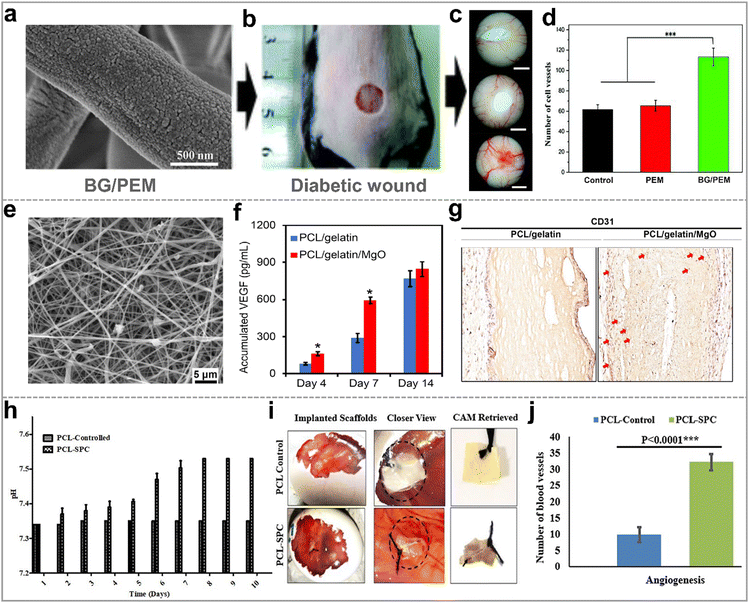 | ||
| Fig. 8 Hybrid nanofibers for promoting blood vessel generation. (a) SEM image of BG/PEM; (b) BG/PEM used for the diabetic wound treatment and (c) digital image of new blood vessels for 15 days; (d) statistical analyses of the number of new blood vessels; reproduced with permission.131 Copyright 2017, Royal Society of Chemistry. (e) SEM image of PCL/Gelatin/MgO; (f) PCL/Gelatin/MgO produced more VEGF than PCL/Gelatin; (g) immunohistochemical staining of subcutaneously implanted electrospun membranes for 14 days; reproduced with permission.132 Copyright 2021, Elsevier. (h) Evaluation of the oxygen release of the electrospun PCL–SPC nanofibers over 10 days; (i) digital images of the new blood vessels in chorioallantoic membrance (CAM) assay; (j) quantification of the blood vessels using different scaffolds; originally published by and used with permission from Dove Medical Press Ltd.61 | ||
4.5. Other direct applications
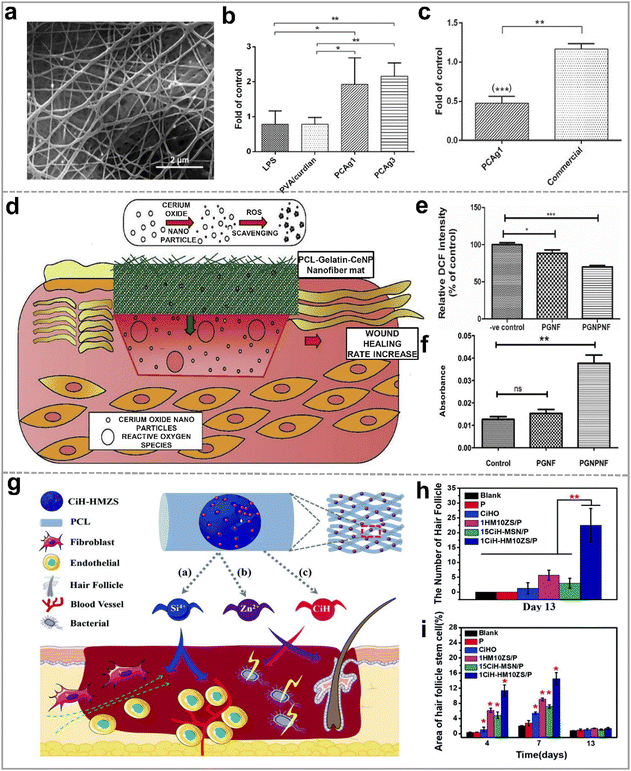 | ||
| Fig. 9 (a) SEM image of PVA/curdlan nanofibers with 1% AgNO3 (PCAg1); (b) in vitro mRNA expression levels of TGFβ1 inflammatory cytokines in the mouse macrophages with the different nanofibrous mats; (c) effect of PCAg1 on mRNA expression levels of IL1β on day 14; reproduced with permission.141 Copyright 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (d) Schematic illustration of the antioxidant mechanism of CeNPs functionalized PCL-Gelatin nanofibers; (e) ROS level measurement in 3T3-L1 cells on various nanofibers by the DCF fluorescence intensity; (f) the cell viability of 3T3 L1 on different nanofibers against the ROS measurement by the alamar blue assay; reproduced with permission,142 CC BY-NC-ND 4.0. (g) Schematic diagram of zinc-loaded hollow mesoporous silica/PCL electrospun nanofibers promoting hair follicle regeneration; (h) quantification of the new hair follicles for 13 days; (i) quantification of the area of hair follicle stem cell on day 4, 7, and 13; reproduced with permission.124 Copyright 2019, Royal Society of Chemistry. | ||
4.6. Drug loading
While the premixed drug/polymer followed by electrospinning method is simple and convenient, it falls short in achieving controlled and sustained drug release, limiting its potential in accelerating wound healing. This can be suitably resolved by using integrated nanofiber membrane/porous inorganic as drug carriers. Consequently, a wide array of drugs, including hemostatic agents, antibacterial agents, antioxidants, and many other drugs are encapsulated in these materials for wound treatment.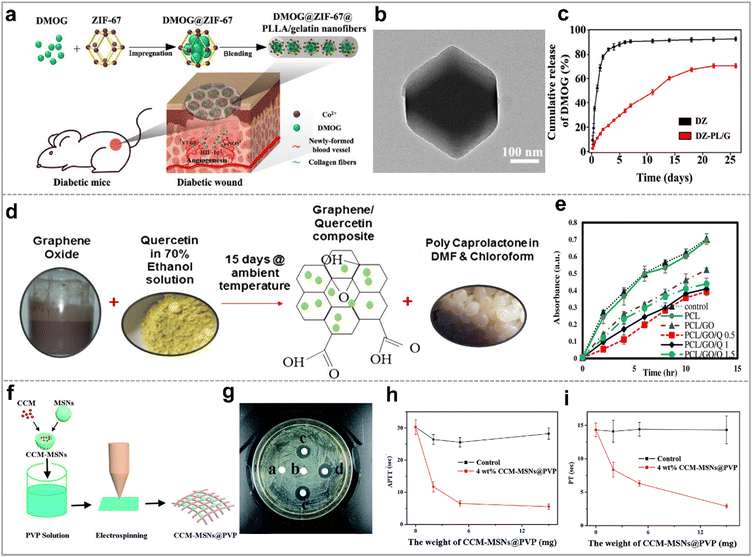 | ||
| Fig. 10 Hybrid nanofibers as drug carriers for promoting wound healing. (a) Schematic illustration of PCL/gelatin nanofibers with ZIF-67 that loading DMOG for wound healing; (b) TEM image of ZIF-67; (c) the release profiles of DMOG from (ZIF-67)DZ and (ZIF-67-PLLA/gelatin)DZ-PL/G; reproduced with permission.148 Copyright 2020, Tsinghua University Press and Springer-Verlag GmbH Germany, part of Springer Nature. (d) Schematic diagram of electrospun PCL/GO/quercetin nanofibrous scaffolds; (e) antibacterial activities of PCL nanofibrous scaffolds with the varying contents of GO and quercetin against S. aureus; reproduced with permission.149 Copyright 2020, Elsevier. (f) Schematic illustration of the preparation process of CCM-MSNs@PVP nanofiber mats; (g) image of the inhibition zones of the different mats against MRSA strain for 48 h, (a)–(e) represent pure PVP, 4 wt% MSNs@PVP nanofiber mats, 2 wt% MSNs@PVP nanofiber mats, 4 wt% CCM-MSNs@PVP nanofiber mats, and 8 wt% CCM-MSNs@PVP nanofiber mats, respectively; results of (h) APTT and (i) PT tests of CCM-MSNs@PVP nanofiber mats; reproduced with permission.150 Copyright 2017, Royal Society of Chemistry. | ||
We have investigated and summarized some cases of electrospun nanofibers loaded with wound-healing drugs using MOF, GO or MSN in Table 2.
| Inorganic carriers | Organic polymers | Drugs | Application | Ref. |
|---|---|---|---|---|
| ZIF-67 | Polylactic acid | Dimethyloxalylgl-ycine (DMOG) | Enhances angiogenesis, promotes collagen deposition, and eliminates inflammation | 148 |
| ZIF-8 | Chitosan/polyvinyl alcohol | Tannic acid (TA) | Improves antibacterial activity and accelerates the aggregation of the coagulation factors and platelets | 153 |
| Polycaprolactone | Rose Bengal (RB) | Improves antibacterial activity and accelerates wound healing | 161 | |
| Polylactic acid | Curcumin (CCM) | Inhibits inflammatory response and promotes collagen deposition, angiogenesis, and re-epithelialization | 54 | |
| HKUST-1 | Citrus pectin | Folic acid | Improves the mechanical strength and antibacterial activity, induces the angiogenesis, and promotes fibroblast migration and proliferation | 162 |
| Polycaprolactone | Nitric oxide (NO) | Promotes endothelial cell growth and improves angiogenesis, collagen deposition, and anti-inflammatory property | 163 | |
| GO | Polycaprolactone | Quercetin | Improves antibacterial activity | 149 |
| Chitosan/polyvinyl alcohol | Ciprofloxacin | Improves antibacterial activity | 154 | |
| Polyethylene oxide | CeO2 and peppermint oil | Improves antibacterial activity and accelerates re-epithelialization and collagen deposition | 39 | |
| Polylactic acid | Se/clarithromycin | Improves antibacterial activity | 113 | |
| MSNs | Polyvinyl pyrrolidone | Curcumin (CCM) | Improves antibacterial activity and activates the clotting system to stop wound bleeding | 150 |
| Poly(lactide-co-glycolide) | Andrographolide | Promotes epidermal cell adhesion and reduces inflammation process | 155 | |
| Polylactic acid | Levofloxacin (lev) and Ag | Inhibites bacterial growth and infection | 156 | |
| Polycaprolactone | Curcumin (CCM) | Improves antibacterial activity | 157 | |
| Polycaprolactone | Levofloxacin | Improves antibacterial activity | 158 | |
| Polycaprolactone | Methylene blue | Improves antibacterial activity | 159 | |
5. Conclusions and future perspectives
Electrospun organic/inorganic hybrid nanofibers are highly promising candidates for promoting wound healing due to the synergistic effect of electrospun nanofibers' ECM-mimicking architecture of and the biological activities in inorganic nanomaterials. This paper has delineated the several methods for crafting these hybrid nanofibers. Notably, premixing inorganic and organic constituents before electrospinning stands as the simplest and most prevalent technique, offering precise control over component ratios. While coaxial electrospinning introduces complexity, its paramount advantage lies in controlled release of inorganic ions or therapeutic agents. The approach of electrospinning with subsequent loading adeptly secures inorganic nanoparticles onto nanofiber surfaces, affording tailored morphologies conducive to regulating cell responses. However, unlocking the full potential of uniform nanoparticle distribution within polymer nanofibers mandates advanced electrospinning and synthetic methodologies. Furthermore, comprehensively investigating the bonding mechanisms underpinning interactions between inorganic nanoparticles and organic nanofibers is imperative, as it will further illuminate the design principles governing hybrid nanofiber fabrication.The applications of electrospun organic/inorganic hybrid nanofibers in accelerating the various stages of wound healing, encompassing hemostasis, antibacterial, cell proliferation and migration, angiogenesis, etc. have been summarized. Briefly, clay mineral nanoparticle-based hybrid nanofibers emerge as preferable choices for hemostasis. Beyond frequently used metal-based nanomaterials (Ag, ZnO, CuO, TiO2, etc), inorganic non-metallic nanomaterials (GO, MOF, etc) have demonstrated remarkable efficacy in thwarting bacterial infections. Bioglass has proven to be effective in promoting cell proliferation and migration, and even angiogenesis. Among the array of inorganic nanoparticles, ZnO stands as the most favored due to the antibacterial activity of Zn2+ ions and their capacity to stimulate blood vessel regeneration. Polymers like PLLA and PLGA are also commonly employed, owing to their biocompatibility and facile electrospinning characteristics. However, the biotoxicity of these inorganic nanoparticles and their circulation/expulsion pathways in vivo remain to be fully investigated. In other words, the concentration of each component in the hybrid nanofibers and controllable release of ions need to be delicately regulated to avoid potential side effects.
Looking ahead, a pivotal shift in the landscape of wound dressings foresees the emergence of bioabsorbable materials to alleviate the challenges associated with dressing changes. Consequently, there is an escalating demand for multifunctional organic/inorganic hybrid nanofibers engineered to accelerate all four stages of the wound healing process within a single dressing. Besides, multiple fluid electrospinning and the resultant multiple-chamber nanostructures that can tailor the ingredients and hierarchical structures of the hybrid nanofibers is a new direction worth developing. The biological mechanisms governing the efficacy of inorganic materials and the in vivo degradation products of biopolymers in promoting wound healing require further exploration. Innovative methodologies like high-throughput screening and organs-on-chips hold immense potential for expediting the development of novel hybrid nanofibers and reducing reliance on animal experimentation. Through collaborative endeavors among researchers from diverse domains including materials science, bioengineering, and clinical practice, electrospun organic/inorganic hybrid nanofibers are poised for a promising and commercially prosperous future.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFA1201304/2021YFA1201300), the National Natural Science Foundation of China (52103298 and 52350410453), the Fundamental Research Funds for the Central Universities (2232022D-01), the Science and Technology Commission of Shanghai Municipality (20DZ2254900), and the Young Elite Scientists Sponsorship Program by CAST (YESS20220259). We thank Dr Xuechen Wu from Shanghai Starriver Bilingual School for language editing.Notes and references
- S. Homaeigohar and A. R. Boccaccini, Acta Biomater., 2020, 107, 25–49 CrossRef CAS PubMed.
- K. Chen, H. Hu, Y. Zeng, H. Pan, S. Wang, Y. Zhang, L. Shi, G. X. Tan, W. S. Pan and H. Liu, Eur. Polym. J., 2022, 178, 111490 CrossRef CAS.
- G. Rivero, M. Meuter, A. Pepe, M. G. Guevara, A. R. Boccaccini and G. A. Abraham, Colloids Surf., A, 2020, 587, 124313 CrossRef CAS.
- E. J. Jang, R. Patel and M. Patel, Pharmaceutics, 2023, 15, 1144 CrossRef CAS PubMed.
- L. Yang, D. Zhang, W. Li, H. Lin, C. Ding, Q. Liu, L. Wang, Z. Li, L. Mei, H. Chen, Y. Zhao and X. Zeng, Nat. Commun., 2023, 14, 7658 CrossRef CAS PubMed.
- X. Wang, J. Chang and C. Wu, Appl. Mater. Today, 2018, 11, 308–319 CrossRef.
- A. G. Kurian, R. K. Singh, V. Sagar, J.-H. Lee and H.-W. Kim, Nano-Micro Lett., 2024, 16, 110 CrossRef CAS.
- B. Magne, A. Demers, É. Savard, M. Lemire-Rondeau, N. Veillette, V. Pruneau, R. Guignard, A. Morissette, D. Larouche, F. A. Auger and L. Germain, Acta Biomater., 2023, 167, 249–259 CrossRef CAS PubMed.
- K. Kang, S. Ye, C. Jeong, J. Jeong, Y.-S. Ye, J.-Y. Jeong, Y.-J. Kim, S. Lim, T. H. Kim, K. Y. Kim, J. U. Kim, G. I. Kim, D. H. Chun, K. Kim, J. Park, J.-H. Hong, B. Park, K. Kim, S. Jung, K. Baek, D. Cho, J. Yoo, K. Lee, H. Cheng, B.-W. Min, H. J. Kim, H. Jeon, H. Yi, T.-I. Kim, K. J. Yu and Y. Jung, Nat. Commun., 2024, 15, 10 CrossRef CAS.
- C. Gao, L. Zhang, J. Wang, M. Jin, Q. Tang, Z. Chen, Y. Cheng, R. Yang and G. Zhao, J. Mater. Chem. B, 2021, 9, 3106–3130 RSC.
- P. I. Campa-Siqueiros, T. J. Madera-Santana, M. M. Castillo-Ortega, J. Lopez-Cervantes, J. F. Ayala-Zavala and E. L. Ortiz-Vazquez, RSC Adv., 2021, 11, 15340–15350 RSC.
- J. Xue, T. Wu, Y. Dai and Y. Xia, Chem. Rev., 2019, 119, 5298–5415 CrossRef CAS PubMed.
- K. R. S. Dinuwan Gunawardhana, R. B. V. B. Simorangkir, G. B. McGuinness, M. S. Rasel, L. A. Magre Colorado, S. S. Baberwal, T. E. Ward, B. O’Flynn and S. M. Coyle, ACS Nano, 2024, 18, 2649–2684 CrossRef CAS PubMed.
- Z. Dong, Q. Liu, X. Han, X. Zhang, X. Wang, C. Hu, X. Li, J. Liang, Y. Chen and Y. Fan, J. Mater. Chem. B, 2023, 11, 6346–6360 RSC.
- K. A. Rieger, N. P. Birch and J. D. Schiffman, J. Mater. Chem. B, 2013, 1, 4531–4541 RSC.
- A. Keirouz, M. Chung, J. Kwon, G. Fortunato and N. Radacsi, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2020, 12, e1626 Search PubMed.
- Z. Huang, H. An, H. Guo, S. Ji, Q. Gu, Z. Gu and Y. Wen, Adv. Fiber Mater., 2024 DOI:10.1007/s42765-023-00364-7.
- X. Han, L. Wang, Y. Shang, X. Liu, J. Yuan and J. Shen, J. Mater. Chem. B, 2022, 10, 8672–8683 RSC.
- E. Hosseini-Alvand and M.-T. Khorasani, J. Mater. Chem. B, 2023, 11, 890–904 RSC.
- T. L. Braga, A. R. de Souza Rossin, J. A. Fernandes, P. de Souza Bonfim de Mendonça, L. V. de Castro-Hoshino, M. L. Baesso, C. F. de Freitas, E. Radovanovic and W. Caetano, Appl. Mater. Today, 2024, 36, 102073 CrossRef.
- Y. Hou, X. Xu, Y. Zhou, Q. Li, L. Zhu, C. Liu, S. Chen and J. Pang, ACS Appl. Mater. Interfaces, 2024, 16, 8228–8237 CrossRef CAS.
- X. Li, L. Pattelli, Z. Ding, M. Chen, T. Zhao, Y. Li, H. Xu, L. Pan and J. Zhao, Adv. Funct. Mater., 2024, 2315315 CrossRef.
- W. He, C. Li, S. Zhao, Z. Li, J. Wu, J. Li, H. Zhou, Y. Yang, Y. Xu and H. Xia, Bioact. Mater., 2024, 34, 338–353 CAS.
- D.-G. Yu, X.-Y. Li, X. Wang, J.-H. Yang, S. W. A. Bligh and G. R. Williams, ACS Appl. Mater. Interfaces, 2015, 7, 18891–18897 CrossRef CAS PubMed.
- T. Zhao, Y. Zheng, X. Zhang, D. Teng, Y. Xu and Y. Zeng, Mater. Des., 2021, 205, 109705 CrossRef CAS.
- J. Yin, L. Xu and A. Ahmed, Adv. Fiber Mater., 2022, 4, 832–844 CrossRef CAS.
- J. Zhou, T. Yi, Z. Zhang, D.-G. Yu, P. Liu, L. Wang and Y. Zhu, Adv. Compos. Hybrid Mater., 2023, 6, 189 CrossRef CAS.
- Z. Xu, J. Fan, W. Tian, X. Ji, Y. Cui, Q. Nan, F. Sun and J. Zhang, Adv. Funct. Mater., 2023, 34, 2307449 CrossRef.
- J. Li, Q. Du, J. Wan, D.-G. Yu, F. Tan and X. Yang, Mater. Des., 2024, 238, 112657 CrossRef CAS.
- L. Liu, H. Sun, J. Zhang, B. Xu, Y. Gao, D. Qi, Z. Mao and J. Wu, Adv. Fiber Mater., 2022, 5, 574–587 CrossRef.
- E. Mele, J. Mater. Chem. B, 2016, 4, 4801–4812 RSC.
- D. Ji, Y. Lin, X. Guo, B. Ramasubramanian, R. Wang, N. Radacsi, R. Jose, X. Qin and S. Ramakrishna, Nat. Rev. Methods Primers, 2024, 4, 1 CrossRef CAS.
- Y. Si, S. Shi and J. Hu, Nano Today, 2023, 48, 101723 CrossRef.
- M. Tarnowska, S. Briancon, J. Resende de Azevedo, Y. Chevalier and M. A. Bolzinger, Int. J. Pharm., 2020, 591, 119991 CrossRef CAS.
- M. A. Zoroddu, J. Aaseth, G. Crisponi, S. Medici, M. Peana and V. M. Nurchi, J. Inorg. Biochem., 2019, 195, 120–129 CrossRef CAS PubMed.
- N. Ninan, M. Muthiah, I.-K. Park, T. W. Wong, S. Thomas and Y. Grohens, Polym. Rev., 2015, 55, 453–490 CrossRef CAS.
- X. Yang, J. Yang, L. Wang, B. Ran, Y. Jia, L. Zhang, G. Yang, H. Shao and X. Jiang, ACS Nano, 2017, 11, 5737–5745 CrossRef CAS PubMed.
- J. Yang, K. Wang, D. G. Yu, Y. Yang, S. W. A. Bligh and G. R. Williams, Mater. Sci. Eng., C, 2020, 111, 110805 CrossRef CAS PubMed.
- B. Suganya Bharathi and T. Stalin, Mater. Today Commun., 2019, 21, 100664 CrossRef CAS.
- S. Pina, J. M. Oliveira and R. L. Reis, Adv. Mater., 2015, 27, 1143–1169 CrossRef CAS PubMed.
- K. E. Mosaad, K. R. Shoueir and M. M. Dewidar, J. Cluster. Sci., 2021, 1–12 Search PubMed.
- X. Chen, X. Ge, Y. Qian, H. Tang, J. Song, X. Qu, B. Yue and W. E. Yuan, Adv. Funct. Mater., 2020, 30, 2004537 CrossRef CAS.
- L. Dong, M. Ren, Y. Wang, G. Wang, S. Zhang, X. Wei, J. He, B. Cui, Y. Zhao, P. Xu, X. Wang, J. Di and Q. Li, Sci. Adv., 2022, 8, eabq7703 CrossRef CAS PubMed.
- H. Jiang, L. Wang and K. Zhu, J. Controlled Release, 2014, 193, 296–303 CrossRef CAS PubMed.
- Z. Mahdieh, S. Mitra and A. Holian, ACS Appl. Polym. Mater., 2020, 2, 4004–4015 CrossRef CAS.
- Y. Sun, S. Cheng, W. Lu, Y. Wang, P. Zhang and Q. Yao, RSC Adv., 2019, 9, 25712–25729 RSC.
- J. Yoon, H. S. Yang, B. S. Lee and W. R. Yu, Adv. Mater., 2018, 30, e1704765 CrossRef PubMed.
- P. J. Rivero, A. Urrutia, J. Goicoechea, Y. Rodríguez, J. M. Corres, F. J. Arregui and I. R. Matías, J. Appl. Polym. Sci., 2012, 126, 1228–1235 CrossRef CAS.
- Q. Li, Y. Yin, D. Cao, Y. Wang, P. Luan, X. Sun, W. Liang and H. Zhu, ACS Nano, 2021, 15, 11992–12005 CrossRef CAS PubMed.
- C. S. Chen, M. Mrksich, S. Huang, G. M. Whitesides and D. E. Ingber, Science, 1997, 276, 1425–1428 CrossRef CAS PubMed.
- J. M. Gao, X. Y. Yu, X. L. Wang, Y. N. He and J. D. Ding, Engineering, 2022, 13, 31–45 CrossRef CAS.
- R. Augustine, Y. B. Dalvi, V. K. Y. Nath, R. Varghese, V. Raghuveeran, A. Hasan, S. Thomas and N. Sandhyarani, Mater. Sci. Eng., C, 2019, 103, 109801 CrossRef CAS PubMed.
- S. Wang, J. Li, Z. Zhou, S. Zhou and Z. Hu, Molecules, 2018, 24, 75 CrossRef PubMed.
- Y. T. Wang, T. Ying, J. X. Li, Y. F. Xu, R. Q. Wang, Q. F. Ke, S. G. F. Shen, H. Xu and K. L. Lin, Chem. Eng. J., 2020, 402, 126273 CrossRef CAS.
- P. J. Zhang, Y. Q. Jiang, D. Liu, Y. Liu, Q. F. Ke and H. Xu, Nanomedicine, 2020, 15, 2241–2254 CrossRef CAS.
- Q. Yu, Y. Han, X. Wang, C. Qin, D. Zhai, Z. Yi, J. Chang, Y. Xiao and C. Wu, ACS Nano, 2018, 12, 2695–2707 CrossRef CAS PubMed.
- H. Xu, F. Lv, Y. Zhang, Z. Yi, Q. Ke, C. Wu, M. Liu and J. Chang, Nanoscale, 2015, 7, 18446–18452 RSC.
- F. Lv, J. Wang, P. Xu, Y. Han, H. Ma, H. Xu, S. Chen, J. Chang, Q. Ke, M. Liu, Z. Yi and C. Wu, Acta Biomater., 2017, 60, 128–143 CrossRef CAS PubMed.
- Z. Zhang, W. Li, Y. Liu, Z. Yang, L. Ma, H. Zhuang, E. Wang, C. Wu, Z. Huan, F. Guo and J. Chang, Bioact. Mater., 2021, 6, 1910–1920 CAS.
- A. Ullah, A. A. Mamun, M. B. Zaidi, T. Roome and A. Hasan, Biomed. Pharmacother., 2023, 165, 115156 CrossRef CAS PubMed.
- M. Zehra, W. Zubairi, A. Hasan, H. Butt, A. Ramzan, M. Azam, A. Mehmood, M. Falahati, A. A. Chaudhry, I. U. Rehman and M. Yar, Int. J. Nanomed., 2020, 15, 3511–3522 CrossRef CAS PubMed.
- Y. Cui, Z. Huang, L. Lei, Q. Li, J. Jiang, Q. Zeng, A. Tang, H. Yang and Y. Zhang, Nat. Commun., 2021, 12, 5922 CrossRef CAS.
- S. Nasser, M. Ibrahim and Y. Atassi, Mater. Chem. Phys., 2021, 267, 124686 CrossRef CAS.
- X. R. Yu, Z. C. Gao, J. X. Mu, H. Lian and Z. X. Meng, Biomater. Sci., 2023, 11, 2158–2166 RSC.
- M. Delyanee, A. Solouk, S. Akbari and M. J. Daliri, Macromol. Biosci., 2022, 22, e2100313 CrossRef PubMed.
- R. Augustine, A. Hasan, N. K. Patan, Y. B. Dalvi, R. Varghese, A. Antony, R. N. Unni, N. Sandhyarani and A.-E. A. Moustafa, ACS Biomater. Sci. Eng., 2019, 6, 58–70 CrossRef PubMed.
- F. Zhou, C. J. Cui, S. B. Sun, S. H. Wu, S. J. Chen, J. W. Ma and C. M. Li, Carbohydr. Polym., 2022, 282, 119131 CrossRef CAS PubMed.
- L. Yin, Q. Tang, Q. Ke, X. Zhang, J. Su, H. Zhong and L. Fang, ACS Appl. Mater. Interfaces, 2023, 15, 48903–48912 CrossRef CAS.
- R. Augustine, S. K. Nethi, N. Kalarikkal, S. Thomas and C. R. Patra, J. Mater. Chem. B, 2017, 5, 4660–4672 RSC.
- S. Zhang, J. Ye, Y. Sun, J. Kang, J. Liu, Y. Wang, Y. Li, L. Zhang and G. Ning, Chem. Eng. J., 2020, 390, 124523 CrossRef CAS.
- S. Enoch and D. J. Leaper, Surgery, 2008, 26, 31–37 Search PubMed.
- J. R. Dias, P. L. Granja and P. J. Bártolo, Prog. Mater. Sci., 2016, 84, 314–334 CrossRef.
- B. Guo, R. Dong, Y. Liang and M. Li, Nat. Rev. Chem., 2021, 5, 773–791 CrossRef CAS PubMed.
- Y. Yang, Y. Du, J. Zhang, H. Zhang and B. Guo, Adv. Fiber Mater., 2022, 4, 1027–1057 CrossRef CAS.
- Y. Zheng, W. Ma, Z. Yang, H. Zhang, J. Ma, T. Li, H. Niu, Y. Zhou, Q. Yao, J. Chang, Y. Zhu and C. Wu, Chem. Eng. J., 2022, 430, 132912 CrossRef CAS.
- Y. Feng, Y. He, X. Lin, M. Xie, M. Liu and Y. Lvov, Adv. Healthcare Mater., 2022, 12, 2202265 CrossRef PubMed.
- Y. Feng, X. Luo, F. Wu, H. Z. Liu, E. Y. Liang, R. R. He and M. X. Liu, Chem. Eng. J., 2022, 428, 132049 CrossRef CAS.
- X. Sun, Z. Tang, M. Pan, Z. Wang, H. Yang and H. Liu, Carbohydr. Polym., 2017, 177, 135–143 CrossRef CAS.
- X. X. Wang, Q. Liu, J. X. Sui, S. Ramakrishna, M. Yu, Y. Zhou, X. Y. Jiang and Y. Z. Long, Adv. Healthcare Mater., 2019, 8, e1900823 CrossRef PubMed.
- S. Y. Ong, J. Wu, S. M. Moochhala, M. H. Tan and J. Lu, Biomaterials, 2008, 29, 4323–4332 CrossRef CAS PubMed.
- K. H. Nam, E. Yeom, H. Ha and S. J. Lee, Ultrasound Med. Biol., 2012, 38, 468–475 CrossRef PubMed.
- M. Delyanee, A. Solouk, S. Akbari and M. Daliri Joupari, Polym. Adv. Technol., 2021, 32, 3934–3947 CrossRef CAS.
- R. N. Udangawa, P. E. Mikael, C. Mancinelli, C. Chapman, C. F. Willard, T. J. Simmons and R. J. Linhardt, ACS Appl. Mater. Interfaces, 2019, 11, 15447–15456 CrossRef CAS PubMed.
- A. J. Tompeck, A. U. R. Gajdhar, M. Dowling, S. B. Johnson, P. S. Barie, R. J. Winchell, D. King, T. M. Scalea, L. D. Britt and M. Narayan, J. Trauma Acute Care Surg., 2020, 88, e1–e21 CrossRef CAS.
- X. Liang, C. Jintao and W. Jiana, Guangdong Chem. Ind., 2018, 45, 67–69 Search PubMed.
- S. S. Yuan, X. X. Sun, Y. Shen and Z. B. Li, Macromol. Biosci., 2022, 22, e2100524 CrossRef PubMed.
- K. Madhumathi, P. T. Sudheesh Kumar, S. Abhilash, V. Sreeja, H. Tamura, K. Manzoor, S. V. Nair and R. Jayakumar, J. Mater. Sci.: Mater. Med., 2010, 21, 807–813 CrossRef CAS PubMed.
- L. A. Alshabanah, M. Hagar, L. A. Al-Mutabagani, G. M. Abozaid, S. M. Abdallah, N. Shehata, H. Ahmed and A. H. Hassanin, Polymers, 2021, 13, 1776 CrossRef CAS.
- A. A. Hassan, H. A. Radwan, S. A. Abdelaal, N. S. Al-Radadi, M. K. Ahmed, K. R. Shoueir and M. A. Hady, Int. J. Pharm., 2021, 593, 120143 CrossRef CAS.
- Z. J. Wang, W. K. Hu, W. Wang, Y. Xiao, Y. Chen and X. H. Wang, Adv. Fiber Mater., 2023, 5, 107–129 CrossRef CAS.
- B. Maharjan, M. K. Joshi, A. P. Tiwari, C. H. Park and C. S. Kim, J. Mech. Behav. Biomed. Mater., 2017, 65, 66–76 CrossRef CAS.
- A. Haider, S. Kwak, K. C. Gupta and I.-K. Kang, J. Nanomater., 2015, 2015, 1–10 CrossRef.
- G. Prado-Prone, P. Silva-Bermudez, A. Almaguer-Flores, J. A. Garcia-Macedo, V. I. Garcia, S. E. Rodil, C. Ibarra and C. Velasquillo, Nanomedicine, 2018, 14, 1695–1706 CrossRef CAS PubMed.
- H. Golipour, E. Ezzatzadeh and A. Sadeghianmaryan, J. Appl. Polym. Sci., 2022, 139, e52505 CrossRef CAS.
- E. Ghasemian Lemraski, H. Jahangirian, M. Dashti, E. Khajehali, S. Sharafinia, R. Rafiee-Moghaddam and T. J. Webster, Int. J. Nanomed., 2021, 16, 223–235 CrossRef PubMed.
- H. Chen, J. Zhang, H. Wu, Y. Li, X. Li, J. Zhang, L. Huang, S. Deng, S. Tan and X. Cai, ACS Appl. Bio Mater., 2021, 4, 6137–6147 CrossRef CAS PubMed.
- M. Bandeira, B. S. Chee, R. Frassini, M. Nugent, M. Giovanela, M. Roesch-Ely, J. D. S. Crespo and D. M. Devine, Materials, 2021, 14, 2889 CrossRef CAS.
- R. Ahmed, M. Tariq, I. Ali, R. Asghar, P. Noorunnisa Khanam, R. Augustine and A. Hasan, Int. J. Biol. Macromol., 2018, 120, 385–393 CrossRef CAS PubMed.
- S. D. Wang, Q. Ma, K. Wang and H. W. Chen, ACS Omega, 2018, 3, 406–413 CrossRef CAS.
- K. Ghosal, C. Agatemor, Z. Špitálsky, S. Thomas and E. Kny, Chem. Eng. J., 2019, 358, 1262–1278 CrossRef CAS.
- T. V. Toniatto, B. V. M. Rodrigues, T. C. O. Marsi, R. Ricci, F. R. Marciano, T. J. Webster and A. O. Lobo, Mater. Sci. Eng., C, 2017, 71, 381–385 CrossRef CAS PubMed.
- D. Archana, J. Dutta and P. K. Dutta, Int. J. Biol. Macromol., 2013, 57, 193–203 CrossRef CAS.
- K. Ghosal, A. Manakhov, L. Zajickova and S. Thomas, AAPS PharmSciTech, 2017, 18, 72–81 CrossRef CAS PubMed.
- W.-C. Jao, M.-C. Yang, C.-H. Lin and C.-C. Hsu, Polym. Adv. Technol., 2012, 23, 1066–1076 CrossRef CAS.
- L. Yan, S. Si, Y. Chen, T. Yuan, H. Fan, Y. Yao and Q. Zhang, Fibers Polym., 2011, 12, 207–213 CrossRef CAS.
- D. Archana, B. K. Singh, J. Dutta and P. K. Dutta, Carbohydr. Polym., 2013, 95, 530–539 CrossRef CAS PubMed.
- M. S. Al Mogbel, M. T. Elabbasy, R. S. Mohamed, A. E. Ghoniem, M. F. H. A. El-Kader and A. A. Menazea, J. Polym. Res., 2021, 28, 1–8 CrossRef.
- H. Zhang, Z. Xu, Y. Mao, Y. Zhang, Y. Li, J. Lao and L. Wang, Polymers, 2021, 13, 3942 CrossRef CAS PubMed.
- S. Wang, F. Yan, P. Ren, Y. Li, Q. Wu, X. Fang, F. Chen and C. Wang, Int. J. Biol. Macromol., 2020, 158, 9–17 CrossRef CAS.
- C. Pettinari, R. Pettinari, C. Di Nicola, A. Tombesi, S. Scuri and F. Marchetti, Coord. Chem. Rev., 2021, 446, 214121 CrossRef CAS.
- A. Saatchi, A. R. Arani, A. Moghanian and M. Mozafari, Ceram. Int., 2021, 47, 9447–9461 CrossRef CAS.
- G. S. Liu, X. Yan, F. F. Yan, F. X. Chen, L. Y. Hao, S. J. Chen, T. Lou, X. Ning and Y. Z. Long, Nanoscale Res. Lett., 2018, 13, 309 CrossRef PubMed.
- F. Ciftci, S. Ayan, N. Duygulu, Y. Yilmazer, Z. Karavelioglu, M. Vehapi, R. Cakır Koc, M. Sengor, H. Yılmazer, D. Ozcimen, O. Gunduz and C. B. Ustundag, Int. J. Polym. Mater. Polym. Biomater., 2021, 71, 1–12 Search PubMed.
- K. Santiago-Castillo, A. M. Torres-Huerta, D. Del Angel-Lopez, M. A. Dominguez-Crespo, H. Dorantes-Rosales, D. Palma-Ramirez and H. Willcock, Polymers, 2022, 14, 674 CrossRef CAS PubMed.
- R. Li, Z. Cheng, X. Yu, S. Wang, Z. Han and L. Kang, Mater. Lett., 2019, 254, 206–209 CrossRef CAS.
- S. Calamak, E. A. Aksoy, N. Ertas, C. Erdogdu, M. Sagıroglu and K. Ulubayram, Eur. Polym. J., 2015, 67, 99–112 CrossRef CAS.
- M. M. Fouda, M. R. El-Aassar and S. S. Al-Deyab, Carbohydr. Polym., 2013, 92, 1012–1017 CrossRef CAS PubMed.
- A. M. Abdelgawad, S. M. Hudson and O. J. Rojas, Carbohydr. Polym., 2014, 100, 166–178 CrossRef CAS PubMed.
- I. V. Lukiev, L. S. Antipina, S. I. Goreninskii, T. S. Tverdokhlebova, D. V. Vasilchenko, A. L. Nemoykina, D. A. Goncharova, V. A. Svetlichnyi, G. T. Dambaev, V. M. Bouznik and E. N. Bolbasov, Membranes, 2021, 11, 986 CrossRef CAS PubMed.
- G. Q. Blantocas, A. S. Alaboodi and A.-b H. Mekky, Arabian J. Sci. Eng., 2017, 43, 389–398 CrossRef.
- M. Salehi, K. Shahporzadeh, A. Ehterami, H. Yeganehfard, H. Ziaei, M. M. Azizi, S. Farzamfar, A. Tahersoltani, A. Goodarzi, J. Ai and A. Ahmadi, Fibers Polym., 2020, 21, 1713–1721 CrossRef CAS.
- B. Dalisson and J. Barralet, Adv. Healthcare Mater., 2019, 8, e1900764 CrossRef PubMed.
- U. Dashdorj, M. K. Reyes, A. R. Unnithan, A. P. Tiwari, B. Tumurbaatar, C. H. Park and C. S. Kim, Int. J. Biol. Macromol., 2015, 80, 1–7 CrossRef CAS PubMed.
- Y. Zhang, M. Chang, F. Bao, M. Xing, E. Wang, Q. Xu, Z. Huan, F. Guo and J. Chang, Nanoscale, 2019, 11, 6315–6333 RSC.
- E. Fallahiarezoudar, M. Ahmadipourroudposht, A. Idris, N. M. Yusof, M. Marvibaigi and M. Irfan, J. Mater. Sci., 2016, 51, 8361–8381 CrossRef CAS.
- S. Chen, Z. Huan, L. Zhang and J. Chang, Int. J. Surg., 2018, 52, 229–232 CrossRef.
- X. Wang, F. Lv, T. Li, Y. Han, Z. Yi, M. Liu, J. Chang and C. Wu, ACS Nano, 2017, 11, 11337–11349 CrossRef CAS PubMed.
- Y. Lv, Y. Xu, X. Sang, C. Li, Y. Liu, Q. Guo, S. Ramakrishna, C. Wang, P. Hu and H. S. Nanda, J. Mater. Chem. B, 2022, 10, 1116–1127 RSC.
- Q. Chen, J. Wu, Y. Liu, Y. Li, C. Zhang, W. Qi, K. W. K. Yeung, T. M. Wong, X. Zhao and H. Pan, Mater. Sci. Eng., C, 2019, 105, 110083 CrossRef CAS PubMed.
- X. Zhang, Y. Li, Z. Ma, D. He and H. Li, Bioact. Mater., 2021, 6, 3692–3704 CAS.
- J. Li, F. Lv, H. Xu, Y. Zhang, J. Wang, Z. Yi, J. Yin, J. Chang and C. Wu, J. Mater. Chem. B, 2017, 5, 1926–1934 RSC.
- M. Liu, R. Wang, J. Liu, W. Zhang, Z. Liu, X. Lou, H. Nie, H. Wang, X. Mo, A. I. Abd-Elhamid, R. Zheng and J. Wu, Biomater. Adv., 2022, 133, 112609 CrossRef PubMed.
- A. P. Veith, K. Henderson, A. Spencer, A. D. Sligar and A. B. Baker, Adv. Drug Delivery Rev., 2019, 146, 97–125 CrossRef CAS PubMed.
- P. Gao, B. Fan, X. Yu, W. Liu, J. Wu, L. Shi, D. Yang, L. Tan, P. Wan, Y. Hao, S. Li, W. Hou, K. Yang, X. Li and Z. Guo, Bioact. Mater., 2020, 5, 680–693 Search PubMed.
- C. Zhu, R. Cao, Y. Zhang and R. Chen, Front. Cell Dev. Biol., 2021, 9, 660571 CrossRef PubMed.
- P. K. Chandra, C. L. Ross, L. C. Smith, S. S. Jeong, J. Kim, J. J. Yoo and B. S. Harrison, Wound Repair Regen., 2015, 23, 830–841 CrossRef PubMed.
- Y. Hua and G. Bergers, Front. Immunol., 2019, 10, 2178 CrossRef CAS PubMed.
- L. W. Fu, Q. Feng, Y. J. Chen, J. Z. Fu, X. J. Zhou and C. L. He, Adv. Fiber Mater., 2022, 4, 1334–1356 CrossRef CAS.
- Z. Tu, M. Chen, M. Wang, Z. Shao, X. Jiang, K. Wang, Z. Yao, S. Yang, X. Zhang, W. Gao, C. Lin, B. Lei and C. Mao, Adv. Funct. Mater., 2021, 31, 2100924 CrossRef CAS.
- J. Hu, T. Wei, H. Zhao, M. Chen, Y. Tan, Z. Ji, Q. Jin, J. Shen, Y. Han, N. Yang, L. Chen, Z. Xiao, H. Zhang, Z. Liu and Q. Chen, Matter, 2021, 4, 2985–3000 CrossRef CAS.
- R. Yunus Basha, T. S. Sampath Kumar, R. Selvaraj and M. Doble, Macromol. Mater. Eng., 2018, 303, 1800234 CrossRef.
- H. A. Rather, R. Thakore, R. Singh, D. Jhala, S. Singh and R. Vasita, Bioact. Mater., 2018, 3, 201–211 Search PubMed.
- J. Sun, W. Jia, H. Qi, J. Huo, X. Liao, Y. Xu, J. Wang, Z. Sun, Y. Liu, J. Liu, M. Zhen, C. Wang and C. Bai, Adv. Mater., 2024, 2312440 CrossRef PubMed.
- R. Goldman, Adv. Skin Wound Care, 2004, 17, 24–35 CrossRef PubMed.
- C. K. Sen, S. Khanna, B. M. Babior, T. K. Hunt, E. C. Ellison and S. Roy, J. Biol. Chem., 2002, 277, 33284–33290 CrossRef CAS PubMed.
- H. Wu, F. Li, S. Wang, J. Lu, J. Li, Y. Du, X. Sun, X. Chen, J. Gao and D. Ling, Biomaterials, 2018, 151, 66–77 CrossRef CAS.
- A. Pellicoro, P. Ramachandran, J. P. Iredale and J. A. Fallowfield, Nat. Rev. Immunol., 2014, 14, 181–194 CrossRef CAS.
- J. Li, F. Lv, J. Li, Y. Li, J. Gao, J. Luo, F. Xue, Q. Ke and H. Xu, Nano Res., 2020, 13, 2268–2279 CrossRef CAS.
- S. Faraji, N. Nowroozi, A. Nouralishahi and J. Shabani Shayeh, Life Sci., 2020, 257, 118062 CrossRef CAS.
- D. Li, W. Nie, L. Chen, Y. Miao, X. Zhang, F. Chen, B. Yu, R. Ao, B. Yu and C. He, RSC Adv., 2017, 7, 7973–7982 RSC.
- A. Arno, A. H. Smith, P. H. Blit, M. A. Shehab, G. G. Gauglitz and M. G. Jeschke, Pharmaceuticals, 2011, 4, 1355–1380 CrossRef CAS.
- S. He, L. Wu, X. Li, H. Sun, T. Xiong, J. Liu, C. Huang, H. Xu, H. Sun, W. Chen, R. Gref and J. Zhang, Acta Pharm. Sin. B, 2021, 11, 2362–2395 CrossRef CAS.
- E. Lamei and M. Hasanzadeh, Int. J. Biol. Macromol., 2022, 208, 409–420 CrossRef CAS PubMed.
- S. Yang, X. Zhang and D. Zhang, Int. J. Mol. Sci., 2019, 20, 4395 CrossRef CAS PubMed.
- Y. Jia, H. Zhang, S. Yang, Z. Xi, T. Tang, R. Yin and W. Zhang, Nanomedicine, 2018, 13, 2881–2899 CrossRef CAS PubMed.
- Z. Song, Y. Ma, G. Xia, Y. Wang, W. Kapadia, Z. Sun, W. Wu, H. Gu, W. Cui and X. Huang, J. Mater. Chem. B, 2017, 5, 7632–7643 RSC.
- S. Rathinavel, P. S. Korrapati, P. Kalaiselvi and S. Dharmalingam, Eur. J. Pharm. Sci., 2021, 167, 106021 CrossRef CAS PubMed.
- J. Jalvandi, M. White, Y. B. Truong, Y. Gao, R. Padhye and I. L. Kyratzis, J. Mater. Sci., 2015, 50, 7967–7974 CrossRef CAS.
- J. Sun, Y. Fan, P. Zhang, X. Zhang, Q. Zhou, J. Zhao and L. Ren, J. Colloid Interface Sci., 2020, 559, 197–205 CrossRef CAS PubMed.
- R. H. Dong, Y. X. Jia, C. C. Qin, L. Zhan, X. Yan, L. Cui, Y. Zhou, X. Jiang and Y. Z. Long, Nanoscale, 2016, 8, 3482–3488 RSC.
- S. Qian, L. Song, L. Sun, X. Zhang, Z. Xin, J. Yin and S. Luan, J. Photochem. Photobiol., A, 2020, 400, 112626 CrossRef CAS.
- S. Zirak Hassan Kiadeh, A. Ghaee, M. Farokhi, J. Nourmohammadi, A. Bahi and F. K. Ko, Int. J. Biol. Macromol., 2021, 173, 351–365 CrossRef CAS PubMed.
- P. Zhang, Y. Li, Y. Tang, H. Shen, J. Li, Z. Yi, Q. Ke and H. Xu, ACS Appl. Mater. Interfaces, 2020, 12, 18319–18331 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |