Clearly fluorescent delineating ER+ breast tumor incisal edge and identifying tiny metastatic tumor foci at high resolution†
Changle
Li
a,
Changyu
Zhang
*ab,
Wenkai
Liu
a,
Jia
Liu
b,
Wanying
Ma
b,
Chengyuan
Lv
a,
Zhuoran
Xia
a,
Yingchao
Chen
a,
Hua
Gu
ab,
Wen
Sun
 ab,
Jianjun
Du
ab,
Jianjun
Du
 ab,
Jiangli
Fan
ab,
Jiangli
Fan
 *ab and
Xiaojun
Peng
*ab and
Xiaojun
Peng
 a
a
aState Key Laboratory of Fine Chemicals, Frontiers Science Center for Smart Materials Oriented Chemical Engineering, Dalian University of Technology, Dalian, 116024, China. E-mail: fanjl@dlut.edu.cn
bNingbo Institute of Dalian University of Technology, Ningbo, 315016, China. E-mail: zhangcy_nbi@dlut.edu.cn
First published on 20th June 2024
Abstract
Fluorescence-image guided surgery (FGS) can intraoperatively provide real-time visualization of a tumor incisal edge and high-resolution identification of tumor foci to improve treatment outcomes. In this contribution, we report a fluorescent probe NB-TAM based on intramolecularly folded photoinduced electron transfer (PET), which displayed a prominent turn-on response in the near-infrared (NIR) window upon specific interaction with the estrogen receptor (ER). Significantly, NB-TAM could delineate a clear tumor incisal edge (tumor-to-normal tissue ratio > 5) in a 70-min time window, and was successfully used to guide the facile and precise resection of ER+ breast tumors in mice. To our surprise, NB-TAM was found to be capable of identifying very tiny lung metastatic ER+ breast tumor foci (0.4 × 0.3 mm), and this ultrahigh resolution was essential to effectively promote tumor resection precision and early diagnosis of tiny tumors. These results clearly elucidate the promising application of NB-TAM as a diagnostic agent for intraoperative fluorescence imaging of ER+ breast cancer.
Introduction
Clinically, surgery continues to be the first-line treatment of cancers.1 Complete resection of tumor tissue, including the primary tumor and metastases, without the removal of too much peritumoral normal tissue, is the aim of surgery therapy, which relies on the clear identification of the incisal edge and tiny tumor foci.2 Currently, surgeons usually depend on preoperative imaging systems (e.g., magnetic resonance imaging, ultrasonography, and X-ray computed tomography) as well as intraoperative experience, visual and tactile information obtained during the operation, which is always challenging and can leave residual lesions resulting in local recurrence.3 Alternatively, fluorescence-image guided surgery (FGS) allows for intraoperative real-time delineation of the tumor incisal edge and high-resolution identification of tumor foci, thereby improving operation precision and treatment outcomes.4 An intraoperative fluorescence imaging probe is one of the keys to FGS, as it emits signals to assist surgeons. Indocyanine green (ICG), methylene blue (MB) and cytalux are the only three near-infrared (NIR) probes approved by the United States Food and Drug Administration as diagnostic agents in FGS.5–7 But these probes are always on and are still limited by low tumor-to-normal tissue (T/N) ratios.8 Moreover, only cytalux is tumor-targeted, and is dedicated to imaging folate receptor-overexpressed ovarian cancer.7 As such, fluorescence imaging probes featuring tumor-targeting properties, a high T/N ratio and high resolution are still an urgent need.Estrogen receptors (ERs), a member of the nuclear receptor superfamily, are ligand-dependent transcriptional regulators,9 which are located at the cell membrane, cytoplasm as well as nucleus, and play key roles in the development and progression of cancers, including breast cancer, ovarian cancer, prostate cancer, acute myeloid leukemia, etc.10 ERs are regarded as main targets for cancer therapy,11 and therefore should also be good targets for designing tumor-targeted fluorescent probes. Of note, breast cancer has been the primary female malignancy worldwide11 and ER+ breast cancer, characterized by an upregulation of ERs, accounts for about 80% of this malignancy.12 Additionally, ER+ breast cancer tends to metastasize to bone and lungs, and these metastases foci result in the vast majority of deaths in patients with ER+ breast tumor,13 as they are too tiny to be identified with preoperative imaging systems or naked eyes. Thus, it is significant to develop ER-targeted fluorescence imaging probes as contrast agents for delineating ER+ breast tumor and identifying metastatic foci during surgery. To date, although a few fluorescent probes for ER+ breast cancer detection have been reported,14–23 most of them are restricted by a short emission wavelength or a low T/N ratio.
To address the aforementioned limitations, we herein report an ER+ breast tumor-targeted NIR fluorescence probe NB-TAM based on intramolecularly folded photoinduced electron transfer (PET).24–27 This probe was in a folded conformation (fluorescence was quenched) in its free state, and could release remarkable fluorescence in the NIR window upon specific interaction with ERs. This turn-on response provided NB-TAM with high T/N ratios to selectively illuminate ER+ breast cancer cells and identify human ER+ breast tumor tissue from peritumoral tissue. Further studies showed that NB-TAM was able to clearly (T/N ratio > 5) delineate the incisal edge of an ER+ xenograft breast tumor for 70 min, which assisted us in resecting the tumor in the mice precisely. To our surprise, NB-TAM was found to be capable of identifying very tiny lung metastatic ER+ breast tumor foci (only 0.3 mm in length), indicating its ultrahigh resolution. These findings indicate the superior properties of NB-TAM as a fluorescent diagnostic agent for image-guided resection of ER+ breast tumors.
Results and discussion
Design and synthesis of NB-TAM
PET is a well-established strategy used to design off–on fluorescence probes.28 As illustrated in Scheme 1, NB-TAM consists of Nile Blue (NB) dye, tamoxifen (TAM) and a flexible alkane linker. NB was selected as the fluorophore due to its large absorption coefficient and excellent photostability as well as NIR excitation and emission wavelength,29 and TAM, the estrogen agonist and antagonist, was used as the ligand.30 These two moieties were linked through a flexible hexyl straight-chain alkyl, which allowed formation of the folded conformation in its free state (quenching the fluorescence) and effective binding of the active site of the ER (releasing the fluorescence). Fluorophore and ligand moieties were prepared by referring to reported methods,31,32 then NB-TAM was synthesized through a facile amidation reaction (Fig. S1, ESI†) and all compounds were well-characterized using 1H NMR and 13C NMR spectra, and high-resolution mass spectrometry (Fig. S6–S17, ESI†).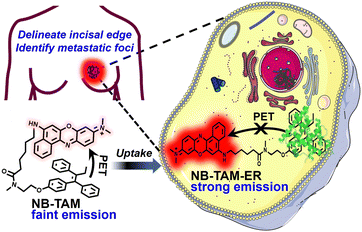 | ||
| Scheme 1 Illustration of the recognition mechanism of NB-TAM towards ERs for delineating the ER+ breast tumor incisal edge and identifying tiny metastatic tumor foci at high resolution. | ||
Off–on mechanism and the spectroscopic properties of NB-TAM
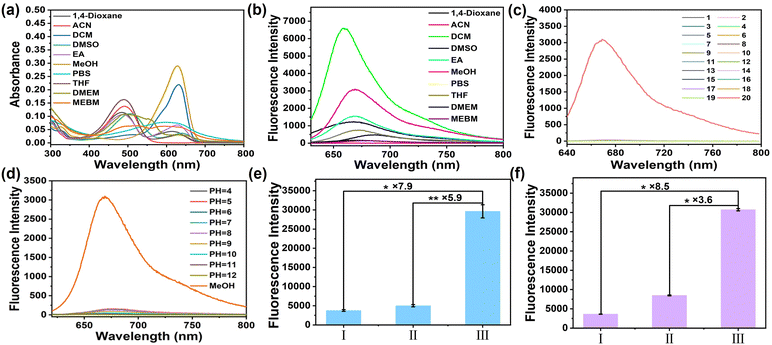
To understand the off–on mechanism of NB-TAM toward ERs, the molecular conformation of NB-TAM was optimized using density functional theory simulation. As shown in Fig. 1a, in aqueous medium, NB-TAM presents as a folded conformation as the total energy is much lower in a folded structure (−2303.48044 Ha) than that in an unfolded state (−2303.45270 Ha). Distances between NB and TAM were determined to be 0.5 nm (folded) and 1.5 nm (unfolded), respectively. Optimized geometries and frontier molecular orbitals were further simulated based on time-dependent density functional theory (B3LYP/TZVP). The results in Fig. 1b show that the HOMO molecular orbital was located in TAM while the HOMO−1 and LUMO molecular orbitals were located in NB. The oscillator strength of the HOMO to LUMO was only 0.052, implying that the electron transition should be prohibited. Moreover, energy levels of the HOMO lay between the HOMO−1 and LUMO. Therefore, upon irradiation in an aqueous solvent, for the folded NB-TAM, the electron should transit from the HOMO−1 to LUMO, then the electron in the HOMO would transit to the HOMO−1 due to the short distance between TAM and NB, so as to quench the fluorescence via a PET process. However, for the unfolded NB-TAM, after binding ERs, the PET process was prohibited due to the too long distance between NB and TAM, thus NB-TAM could emit strong fluorescence (Fig. 1c). Meanwhile, the absorbance and fluorescence properties of NB-TAM in aqueous medium and organic solvents were studied (Fig. 2a and b and Fig. S2, ESI†). A well-fitted linear relationship was plotted between the absorbance maximum and NB-TAM concentration over the range from 0 to 20 μM, implying a good water solubility of NB-TAM (Fig. S2, ESI†). Of note, in aqueous medium, e.g., phosphate buffered saline (PBS) and cell culture media, NB-TAM displayed very faint fluorescence in the NIR window (Fig. 2b). The absolute fluorescence quantum yield of NB-TAM in PBS was determined to be only 0.024, which was in accord with the molecular simulation. Moreover, common analytes (including ions, reactive oxygen species, reactive sulfur species and amino acids) showed no interference with the fluorescence of NB-TAM (Fig. 2c). Additionally, NB-TAM was found to show very weak emission in PBS over a broad pH value of 4–12 (Fig. 2d). However, after incubation with human recombinant protein ERs for 10 min, NB-TAM showed remarkable fluorescence enhancements (7.9-fold for ERα and 8.5-fold for ERβ), and when ERs were pretreated with excess TAM, the fluorescence increase was significantly suppressed (Fig. 2e and f and Fig. S3, ESI†), indicating that NB-TAM could specifically bind with ERs.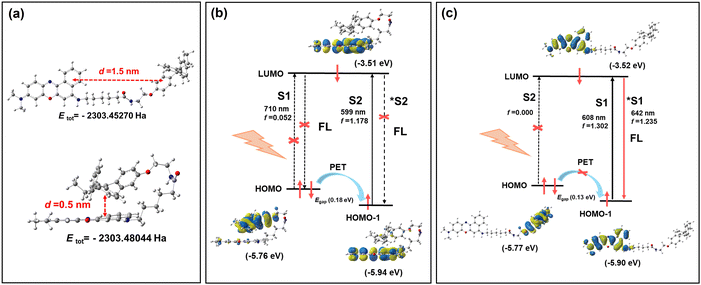 | ||
| Fig. 1 (a) Optimized molecular conformation of NB-TAM in aqueous medium. (b) Optimized geometries and frontier molecular orbitals of the folded (b) and unfolded (c) NB-TAM. | ||
Specific imaging of ER+ breast cancer cells
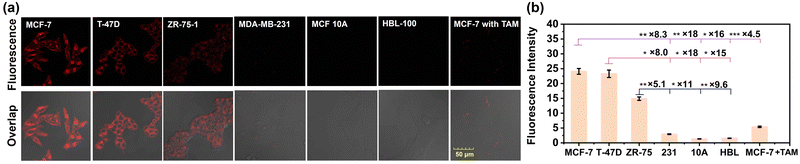
Prior to imaging ER+ breast cancer cells, the cytotoxicity of NB-TAM was first evaluated using an MTT assay with the ER+ breast cancer cell line MCF-7,33 triple-negative breast cancer cell line MDA-MB-23134 and normal mammary epithelial cell line MCF 10A.35 The results showed that after incubation with 15 μM of NB-TAM for 24 h, the viabilities of both cancerous breast cells and noncancerous breast cells were over 90% (Fig. S4, ESI†), indicating a negligible cytotoxicity of NB-TAM. To evaluate whether NB-TAM could specifically illuminate ER+ cancer cells, ER+ breast cancer cells (MCF-7, T-47D, and ZR-75-1),36 ER− breast cancer cells MDA-MB-231 and mammary epithelial cells (MCF 10A and HBL-100) were stained with 0.25 μM of NB-TAM separately for 30 min, and MCF-7 cells preincubated with 5 μM of TAM were used as the control group. As shown in Fig. 3, NB-TAM-stained ER+ cancer cells showed much stronger fluorescence intensities than those in MDA-MB-231, MCF 10A and HBL-100 cells, and fluorescence in the control group was suppressed due to pretreatment of TAM. These data demonstrated that NB-TAM was able to selectively identify ER+ breast cancer cells via specific binding with ERs.Identification of human ER+ breast cancer
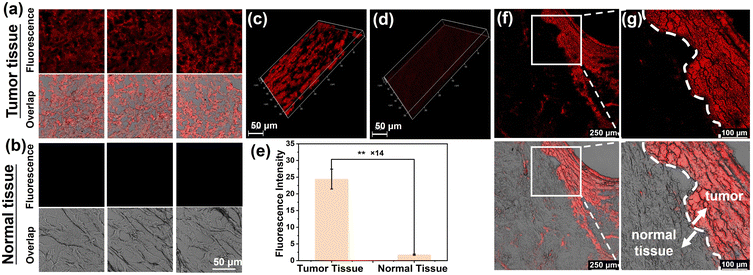
Classification at the molecular level is significant in grading breast cancer and implementing the intervention. In view of this, we investigated the clinical application potential of NB-TAM in molecular classification of human ER+ breast cancer. Isolated fresh tumor and peritumoral tissue were from an ER+ breast cancer patient, which was confirmed by immunohistochemistry. These fresh tissues were frozen, sliced, stained with NB-TAM for 30 min and washed with PBS buffer prior to imaging. As shown in Fig. 4a and b, tumor tissue slices exhibited obvious strong fluorescence signals while these peritumoral tissue slices showed very faint fluorescence signals. Deep scan imaging was further performed and subsequent reconstructed 3D images indicated that tumor tissue indeed displayed much stronger fluorescence (Fig. 4c and d). The relative fluorescence intensity between tumor slices and peritumoral tissue slices was calculated to be as high as 14 (Fig. 4e). Moreover, as shown in Fig. 4f and g, a clear edge between the tumor and the peritumoral normal tissue was obtained in a single image (the white dashed line), which further demonstrated the attractive potential of NB-TAM to delineate the ER+ breast tumor incisal edge. To sum up, NB-TAM was capable of quickly and facilely classifying human ER+ breast cancer, which could shorten the detection time and lower labor costs compared with current immunohistochemistry techniques.Fluorescence image-guided resection of ER+ breast tumor in mice
NB-TAM showed a turn-on response towards ER, which should provide this probe with high T/N ratios in imaging. Prior to exploring the feasibility of NB-TAM in FGS, we first studied its ability to illuminate ER+ breast tumors in mice. The ER+ breast cancer mouse model was established by subcutaneous injection of MCF-7 cells into the right armpit, and NB-TAM was administrated by subcutaneous peritumoral injection. Mice pretreated with TAM via peritumoral injection were used as the control group and normal mice not bearing a tumor were used as the blank group. As shown in Fig. 5a, after administration of NB-TAM (10 nmol), a clear incisal edge (T/N ratio > 5) was recorded in the xenograft tumor over a time window from 30 to 100 min, and reached the maximal T/N ratio of 6.6 at 60 min. In contrast, the fluorescence intensity of the xenograft tumor in the control group was significantly suppressed (Fig. 5b). And after subcutaneous injection of NB-TAM for 60 min, only very faint fluorescence was observed in the normal mice (Fig. S5, ESI†). These results indicated that NB-TAM could specifically illuminate ER+ breast tumor with a high T/N ratio and a relatively long imaging time window. We next performed the image-guided resection of the tumor by in situ spraying NB-TAM. As shown in Fig. 5c and d, the tumor was exposed by removing the surrounding skin, and a very clear tumor incisal edge was delineated after spraying NB-TAM for 15 min, implying a well and fast dispersion of this probe. The fluorescence intensity of the tumor hit a plateau around a timepoint of 30 min and remained steady for another 30 min, which guided us to resect the tumor with ease. The residual peritumoral tissue displayed no fluorescence, which indicated that the tumor lesion was absolutely removed. Taken together, NB-TAM could be a promising contrast agent for FGS to guide the resection of ER+ breast tumor by providing a clear incisal edge in a 70-min time window.Ultrahigh-resolution imaging of metastatic ER+ breast cancer foci in vitro
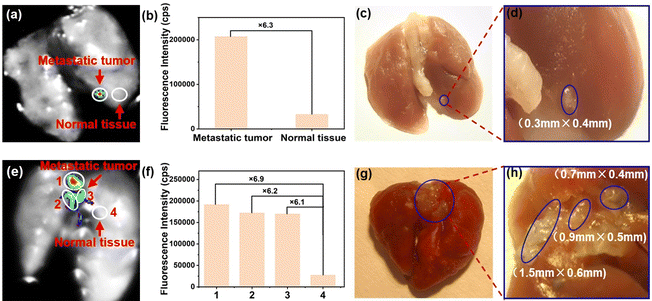
Metastatic tumor foci are hard to identify by the naked eye during surgery or by preoperative imaging systems37 due to limited resolution. Omission of foci is the key cause of poor overall survival in patients with malignant tumors.8 In view of this, to further investigate the potential clinical application of NB-TAM in identifying metastatic ER+ breast cancer foci in vitro, MCF-7 cells were injected in situ into the mammary (method I), or administrated through the tail vein (method II) to establish a lung metastatic tumor model in BABL/c nude mice. After sacrifice, the lungs were removed, washed three times with cold PBS buffer, and sprayed with NB-TAM. 30 min later, for the lung from method I, as shown in Fig. 6a–d, a very tiny metastatic tumor was identified based on the fluorescence signal with a high T/N ratio of 6.3, and the tumor size was further measured to be as small as 0.4 × 0.3 mm. This ultrahigh resolution was far ahead of current clinical approaches. For the lung from method II, we found three main metastatic foci, and their T/N ratios were determined to be as high as 6.9, 6.2 and 6.1, respectively (Fig. 6e and f). Two of these metastatic lesions were less than 1 mm in length (Fig. 6g and h). Taken together, upon spraying after 30 min, NB-TAM was capable of identifying very small ER+ metastatic lesions in vitro with ultrahigh resolutions and high T/N ratios, which would inevitably improve tumor resection precision.Conclusions
In summary, we reported a turn-on NIR fluorescent probe NB-TAM for clearly delineating ER+ breast tumor incisal margin and identifying very tiny metastatic tumor foci at high resolution. NB-TAM was prepared by linking the NIR dye NB and the ligand TAM via a flexible linker, which was folded in aqueous medium so as to quench the fluorescence through a PET process. After binding with the ER, the fluorescence of NB-TAM was recovered as the electron transfer from the TAM to NB was restricted. Due to this sort of enhanced fluorescence signal, NB-TAM was able to illuminate ER+ breast cancer cells, human ER+ breast tumor tissues and the xenograft ER+ breast tumor with high T/N ratios. Of note, NB-TAM could delineate a clear incisal edge for 70 min and was used to successfully guide the resection of tumors in mice precisely. In addition, NB-TAM was further found to be capable of clearly identifying very small lung metastatic foci (0.4 × 0.3 mm) of ER+ breast tumor in vitro, and this ultrahigh resolution will improve tumor resection precision and promote the diagnosis of tiny tumors. These results sufficiently demonstrate the excellent properties of NB-TAM and its promising application as a new fluorescent agent for intraoperative imaging of ER+ breast tumors.Methods
Synthesis of probe NB-TAM
As shown in Fig. S1 (ESI†), 2-(7-azabenzotriazolyl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (0.66 mmol, 0.25 g) and compound 2 (0.49 mmol, 0.20 g) were added to a solution of 3 mL N,N′-dimethylformamide (DMF), then 0.32 mL of N,N′-diisopropylethylamine was added. After 20 min, compound 3 (0.59 mmol, 0.21 g) in 2 mL of DMF was added. After 3 hours, the solvent was removed under vacuum and the residue was purified by silica gel column chromatography (DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 7
MeOH = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain NB-TAM as a dark blue powder, (0.34 g, 92%). 1H NMR (400 MHz, DMSO-d6) δ 10.53 (s, 1H), 8.79–8.67 (m, 2H), 7.95 (dd, J = 13.9, 7.0 Hz, 1H), 7.87–7.78 (m, 2H), 7.40–7.31 (m, 2H), 7.27 (t, J = 7.2 Hz, 1H), 7.22–6.98 (m, 9H), 6.80 (s, 1H), 6.70 (d, J = 8.6 Hz, 1H), 6.65 (d, J = 8.6 Hz, 1H), 6.57 (t, J = 7.7 Hz, 2H), 3.96 (t, J = 5.0 Hz, 1H), 3.90 (t, J = 5.6 Hz, 1H), 3.72 (s, 2H), 3.61 (t, J = 4.8 Hz, 1H), 3.54 (t, J = 5.6 Hz, 1H), 3.25 (s, 6H), 2.98 (s, 1.5H), 2.81 (s, 1.5H), 2.40–2.26 (m, 4H), 1.81–1.72 (m, 2H), 1.62–1.52 (m, 2H), 1.48–1.37 (m, 2H), 0.83 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 173.28, 157.86, 156.61, 156.34, 155.55, 151.77, 147.71, 143.51, 143.42, 142.11, 142.01, 141.31, 141.26, 138.18, 138.11, 135.76, 135.55, 132.52, 131.67, 130.94, 130.22, 129.70, 129.66, 129.22, 129.18, 128.71, 128.68, 128.31, 128.25, 127.13, 126.61, 124.42, 123.60, 113.77, 113.64, 96.21, 93.98, 65.54, 48.64, 44.70, 40.95, 36.83, 33.43, 32.11, 28.33, 26.38, 24.78, 13.71. HRMS: m/z calcd for [M]+: 743.3956, found 743.4314.
1) to obtain NB-TAM as a dark blue powder, (0.34 g, 92%). 1H NMR (400 MHz, DMSO-d6) δ 10.53 (s, 1H), 8.79–8.67 (m, 2H), 7.95 (dd, J = 13.9, 7.0 Hz, 1H), 7.87–7.78 (m, 2H), 7.40–7.31 (m, 2H), 7.27 (t, J = 7.2 Hz, 1H), 7.22–6.98 (m, 9H), 6.80 (s, 1H), 6.70 (d, J = 8.6 Hz, 1H), 6.65 (d, J = 8.6 Hz, 1H), 6.57 (t, J = 7.7 Hz, 2H), 3.96 (t, J = 5.0 Hz, 1H), 3.90 (t, J = 5.6 Hz, 1H), 3.72 (s, 2H), 3.61 (t, J = 4.8 Hz, 1H), 3.54 (t, J = 5.6 Hz, 1H), 3.25 (s, 6H), 2.98 (s, 1.5H), 2.81 (s, 1.5H), 2.40–2.26 (m, 4H), 1.81–1.72 (m, 2H), 1.62–1.52 (m, 2H), 1.48–1.37 (m, 2H), 0.83 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 173.28, 157.86, 156.61, 156.34, 155.55, 151.77, 147.71, 143.51, 143.42, 142.11, 142.01, 141.31, 141.26, 138.18, 138.11, 135.76, 135.55, 132.52, 131.67, 130.94, 130.22, 129.70, 129.66, 129.22, 129.18, 128.71, 128.68, 128.31, 128.25, 127.13, 126.61, 124.42, 123.60, 113.77, 113.64, 96.21, 93.98, 65.54, 48.64, 44.70, 40.95, 36.83, 33.43, 32.11, 28.33, 26.38, 24.78, 13.71. HRMS: m/z calcd for [M]+: 743.3956, found 743.4314.
Live cell imaging
MCF-7, T-47D, ZR-75-1, MDA-MB-231, MCF 10A and HBL-100 cells were seeded in confocal dishes and allowed to grow to 70% confluency before usage. Next, cells were incubated with 0.25 μM of NB-TAM separately for 30 min, and washed with cold PBS buffer 3 times prior to capturing images. As a control, MCF-7 cells were pretreated with 5 μM of TAM for 6 hours before incubation with NB-TAM. Fluorescence images were obtained using an Olympus FV3000 with excitation at 638 nm and emission at 650–750 nm.Imaging and resection of ER+ breast tumors
The bare Balb/c mice were purchased from Liaoning Changsheng Biological. All experiments were approved by the Animal Care and Use Committee of Dalian University of Technology (DUTNI220308_02). Fluorescence images were captured on a NightOWL II LB983 small animal in vivo imaging system with a 630 nm excitation and a 700 nm (fwhm 20 nm) emission filter.Imaging of xenograft ER+ breast tumor. MCF-7 cells were first injected subcutaneously into the right armpit of mice to establish a xenograft ER+ breast tumor model. The volumes of the tumors were determined to be 0.5 × L × W2 (L = longest axis and W = axis perpendicular to L in millimeters). Once the tumor volumes reached about 200–300 mm3, NB-TAM (100 μM, 100 μL) was administrated by peritumorally subcutaneous injection. In the control group, mice were peritumorally injected with 50 nmol of TAM 1.5 hours ahead of administration of NB-TAM. Normal mice not bearing tumor were also used to study the background fluorescence of NB-TAM. The whole-body images of mice were recorded at different time intervals. The experiment was repeated three times.
Resection of xenograft ER+ breast tumors. The tumors were exposed by removing the surrounding skin, and were sprayed with NB-TAM (100 μM, 100 μL). The whole-body images were recorded at different time intervals. At a timepoint of 60 min, the tumors were resected with the help of fluorescence images. After surgery, no fluorescence was recorded in the peritumoral tissue, indicating that the tumor was clearly removed. The experiment was repeated three times.
Imaging of lung metastasis ER+ breast tumor foci
MCF-7 cells were injected in situ into the mammary (method I), or injected through the tail vein (method II) to establish the lung metastatic tumor model in BABL/c nude mice. After sacrifice, the lungs were removed, stained with NB-TAM (100 μM) for 30 min and washed 3 times with cold PBS buffer before capturing fluorescent images.Identification of human ER+ breast tumor tissue
Isolated fresh tumor and peritumoral tissue were from an ER+ breast cancer patient of Ningbo Medical Center Lihuili Hospital and this experiment was approved by the Animal Care and Use Committee of Ningbo University (12446). These fresh tissues were frozen, sliced (20 μm in thickness), stained with NB-TAM (5 μM) for 30 min and washed with PBS buffer 3 times prior to fluorescence imaging.Data availability
The ESI† includes materials and instrumentation, experimental methods, synthesis, optical spectra, cytotoxicity and characterization data.Author contributions
Conceptualization: J. Fan, C. Zhang, C. Li; methodology: C. Li and C. Zhang; formal analysis: C. Li, C. Zhang, W. Liu, W. Ma and J. Liu; investigation: C. Li and C. Zhang; resources: J. Fan and C. Zhang; supervision: J. Fan and C. Zhang; project administration, J. Fan and C. Zhang; funding acquisition, J. Fan, C. Zhang; writing – original draft: C. Li and C. Zhang; writing – review & editing: J. Fan, W. Sun, J. Du and X. Peng.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21925802, 22338005, 22208045), Liaoning Binhai Laboratory (LBLB-2023-03), the Fundamental Research Funds for the Central Universities (DUT22LAB60), the Zhejiang Provincial Natural Science Foundation (TGY24B060002), and the Ningbo Natural Science Foundation (2022J012, 2023S115).References
- Nat. Cancer, 2021, 2, 245–246 Search PubMed.
- L. Wyld, R. A. Audisio and G. J. Poston, Nat. Rev. Clin. Oncol., 2014, 12, 115–124 CrossRef.
- L. J. Lauwerends, P. B. A. A. van Driel, R. J. Baatenburg de Jong, J. A. U. Hardillo, S. Koljenovic, G. Puppels, L. Mezzanotte, C. W. G. M. Löwik, E. L. Rosenthal, A. L. Vahrmeijer and S. Keereweer, Lancet Oncol., 2021, 22, 186–195 CrossRef.
- K. Wang, Y. Du, Z. Zhang, K. He, Z. Cheng, L. Yin, D. Dong, C. Li, W. Li, Z. Hu, C. Zhang, H. Hui, C. Chi and J. Tian, Nat. Rev. Bioeng., 2023, 1, 161–179 CrossRef.
- C. Tomasz, W. Polom, L. Marano, G. Roviello, A. D’Angelo, N. Cwalina, M. Matuszewski, F. Roviello, J. Jaskiewicz and K. Polom, J. Clin. Med., 2020, 9, 3538 CrossRef.
- G. M. Son, H.-M. Ahn, I. Y. Lee and G. W. Ha, Ann. Coloproctol., 2021, 37, 133–140 CrossRef PubMed.
- S. M. Mahalingam, S. A. Kularatne, C. H. Myers, P. Gagare, M. Norshi, X. Liu, S. Singhal and P. S. Low, J. Med. Chem., 2018, 61, 9637–9646 CrossRef CAS PubMed.
- H. Li, Q. Yao, W. Sun, K. Shao, Y. Lu, J. Chung, D. Y. Kim, J. Fan, S. Long, J. Du, Y. Li, J. Wang, J. Yoon and X. Peng, J. Am. Chem. Soc., 2020, 142, 6381–6389 CrossRef CAS PubMed.
- X. Zhan, X. Wang and J. Zhang, Prog. Vet. Med., 2005, 26, 35–39 Search PubMed.
- P. Miziak, M. Baran, E. Błaszczak, A. Przybyszewska-Podstawka, J. Kałafut, J. Smok-Kalwat, M. Dmoszyńska-Graniczka, M. Kiełbus and A. Stepulak, Cancers, 2023, 15, 4689 CrossRef CAS.
- N. Harbeck, F. Penault-Llorca, J. Cortes, M. Gnant, N. Houssami, P. Poortmans, K. Ruddy, J. Tsang and F. Cardoso, Nat. Rev. Dis. Primer, 2019, 5, 66 CrossRef PubMed.
- C. N. Haines, S. E. Wardell and D. P. McDonnell, Essays Biochem., 2021, 65, 985–1001 CrossRef CAS PubMed.
- L. Jin, B. Han, E. Siegel, Y. Cui, A. Giuliano and X. Cui, Cancer Biol. Ther., 2018, 19, 858–868 CrossRef CAS PubMed.
- Z. Hu, L. Yang, W. Ning, C. Tang, Q. Meng, J. Zheng, C. Dong and H. Zhou, Chem. Commun., 2018, 54, 3887–3890 RSC.
- F. J. Céspedes-Guirao, A. B. Ropero, E. Font-Sanchis, Á. Nadal, F. Fernández-Lázaro and Á. Sastre-Santos, Chem. Commun., 2011, 47, 8307–8309 RSC.
- S. H. Kim, J. R. Gunther and J. A. Katzenellenbogen, J. Am. Chem. Soc., 2010, 132, 4685–4692 CrossRef CAS.
- E. L. Rickert, S. Oriana, C. Hartman-Frey, X. Long, T. T. Webb, K. P. Nephew and R. V. Weatherman, Bioconjugate Chem., 2010, 21, 903–910 CrossRef CAS.
- F. Abendroth, M. Solleder, D. Mangoldt, P. Welker, K. Licha, M. Weber and O. Seitz, Eur. J. Org. Chem., 2015, 2157–2166 CrossRef CAS.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899–4031 CrossRef PubMed.
- L. Yang, Q. Meng, Z. Hu, W. Ning, J. Zheng, C. Dong and H. Zhou, Sens. Actuators, B, 2018, 272, 589–597 CrossRef CAS.
- Q. Meng, B. Xie, X. Ma, Z. Hu, F. Zhou, H. Zhou and C. Dong, Chem. Commun., 2020, 56, 10493–10496 RSC.
- C. Ding and T. Ren, Coord. Chem. Rev., 2023, 482, 215080 CrossRef CAS.
- C. Tang, Y. Du, Q. Liang, Z. Cheng and J. Tian, Mol. Pharmaceutics, 2018, 15, 4702–4709 CrossRef CAS PubMed.
- H. Zhang, J. Fan, J. Wang, S. Zhang, B. Dou and X. Peng, J. Am. Chem. Soc., 2013, 135, 11663–11669 CrossRef CAS PubMed.
- J. Fan, S. Guo, S. Wang, Y. Kang, Q. Yao, J. Wang, X. Gao, H. Wang, J. Du and X. Peng, Chem. Commun., 2017, 53, 4857–4860 RSC.
- S. Guo, J. Fan, B. Wang, M. Xiao, Y. Li, J. Du and X. Peng, ACS Appl. Mater. Interfaces, 2018, 10, 1499–1507 CrossRef CAS PubMed.
- M. Xiao, W. Sun, J. Fan, J. Cao, Y. Li, K. Shao, M. Li, X. Li, Y. Kang, W. Zhang, S. Long, J. Du and X. Peng, Adv. Funct. Mater., 2018, 28, 1805128 CrossRef.
- H. Niu, J. Liu, H. O’connor, T. Gunnlaugsson, T. James and H. Zhang, Chem. Soc. Rev., 2023, 52, 2322–2357 RSC.
- V. Martinez and M. Henary, Chem. – Eur. J., 2016, 22, 13764–13782 CrossRef CAS.
- A. Jameera Begam, S. Jubie and M. J. Nanjan, Bioorg. Chem., 2017, 71, 257–274 CrossRef CAS PubMed.
- B. Wang, J. Fan, X. Wang, H. Zhu, J. Wang, H. Mu and X. Peng, Chem. Commun., 2015, 51, 792–795 RSC.
- B. E. Gryder, M. K. Rood, K. A. Johnson, V. Patil, E. D. Raftery, L.-P. D. Yao, M. Rice, B. Azizi, D. F. Doyle and A. K. Oyelere, J. Med. Chem., 2013, 56, 5782–5796 CrossRef CAS PubMed.
- A. R. Michmerhuizen, L. M. Lerner, A. M. Pesch, C. Ward, R. Schwartz, K. Wilder-Romans, M. Liu, C. Nino, K. Jungles, R. Azaria, A. Jelley, N. Zambrana Garcia, A. Harold, A. Zhang, B. Wharram, D. F. Hayes, J. M. Rae, L. J. Pierce and C. W. Speers, npj Breast Cancer, 2022, 8, 31 CrossRef CAS PubMed.
- K. J. Chavez, S. V. Garimella and S. Lipkowitz, Breast Dis., 2011, 32, 35–48 Search PubMed.
- Y. Qu, B. Han, Y. Yu, W. Yao, S. Bose, B. Y. Karlan, A. E. Giuliano and X. Cui, PLoS One, 2015, 10, 7 Search PubMed.
- D. Tao, M. Jiang, J. Wu, Y. Feng and J. Gong, Chin. J. Cancer., 2004, 23, 339–341 CAS.
- M. E. Menezes, S. K. Das, I. Minn, L. Emdad, X. Wang, D. Sarkar, M. G. Pomper and P. B. Fisher, Adv. Cancer Res., 2016, 132, 1–44 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb00558a |
| This journal is © The Royal Society of Chemistry 2024 |