Light-responsive functional nanomaterials as pioneering therapeutics: a paradigm shift to combat age-related disorders
Shubham Kumar
Singh†
a,
Shivay
Parihar†
a,
Sanskar
Jain†
a,
Ja-An Annie
Ho
cdef and
Raviraj
Vankayala
 *ab
*ab
aDepartment of Bioscience and Bioengineering, Indian Institute of Technology Jodhpur, Karwar 342030, India. E-mail: rvankayala@iitj.ac.in
bInterdisciplinary Research Platform, Smart Healthcare, Indian Institute of Technology Jodhpur, Karwar 342030, India
cBioanalytical Chemistry and Nanobiomedicine Laboratory, Department of Biochemical Science and Technology, National Taiwan University, Taipei 10617, Taiwan
dDepartment of Chemistry, National Taiwan University, Taipei 10617, Taiwan
eCenter for Emerging Materials and Advanced Devices, National Taiwan University, Taipei 10617, Taiwan
fCenter for Biotechnology, National Taiwan University, Taipei 10617, Taiwan
First published on 17th July 2024
Abstract
Aging, marked by dysregulated cellular systems, gives rise to a spectrum of age-related disorders, including neurodegeneration, atherosclerosis, immunosenescence, and musculoskeletal issues. These conditions contribute significantly to the global disease burden, posing challenges to health span and economic resources. Current therapeutic approaches, although diverse in mechanism, often fall short in targeting the underlying cellular pathologies. They fail to address the issues compounded by altered pharmacokinetics in the elderly. Nanotechnology emerges as a transformative solution, offering tissue-specific targeted therapies through nanoparticles. Functional nanomaterials (FNMs) respond to internal or external stimuli, with light-responsive nanomaterials gaining prominence. Harnessing the benefits of deep tissue penetration and ease of manipulation particularly in the near-infrared spectrum, light-responsive FNMs present innovative strategies for age-related comorbidities. This review comprehensively summarizes the potential of light-responsive FNM-based approaches for targeting cellular environments in age-related disorders, and also emphasizes the advantages over traditional treatment modalities. Specifically, it focuses on the development of various classes of light-responsive functional nanomaterials including plasmonic nanomaterials, nanomaterials as carriers, upconversion nanomaterials, 2D nanomaterials, transition metal oxide and dichalcogenide nanomaterials and carbon-based nanomaterials against age related diseases. We foresee that such advanced developments in the field of nanotechnology could provide a new hope for clinical diagnosis and treatment of age-related disorders.
1. Introduction
Aging is an inherent and inevitable aspect of life, marking the progressive changes that occur in living organisms over time. Estimates have shown that an increment in life expectancy by just one year can lead to economic benefits of US$38 trillion, and by 10 years, about US$367 trillion.1 Aging comprises of various physiological, cellular, and molecular changes that cumulatively contribute to the aging phenotype. Arising from a plethora of dysregulated cellular systems,2 aging manifests as a myriad of age-related disorders, like neurodegeneration, atherosclerosis, immunosenescence, bone and muscle loss, etc.3,4 These medical conditions account for more than 50% of all the ailments, which impose a significant burden on the health span of individuals.5 The various age-related problems manifest themselves in different systems causing a plethora of different diseases. Despite the broad diversity in the diseases and the systems they affect, their underlying mechanisms share common characteristics across all age-related disorders. Mainly, these disorders arise due to an interplay of dysfunctional regulatory systems as the body ages. These failures can result in aggregation of cellular materials such as proteins (such as in the case of neurodegenerative disorders) and lipids (such as in the case of atherosclerosis). Both cases show a sharp correlation between their increased incidence and the seniority of the individual.6 These aggregates induce and worsen another hallmark of aging which is the accumulation of senescent cells. Senescent cells are characterized by loss of cell division, inhibition of apoptosis, and the senescence-associated secretory phenotype (SASP) that promotes a pro-inflammatory environment responsible for pathologies related to aging.2,7 This can affect the regular function of the system they inhabit as well as induce senescence in their neighbouring cells. The resultant inflammatory environment gives rise to a condition called inflammaging. As the age progresses, this mild inflammatory condition persists in the body which is present to combat infections by having a pro-inflammatory environment.8 Despite its protective function, inflammation can also be extremely detrimental for the aged individuals as inflammation is one of the major causes of systemic organ disorders in the elderly. Many age-related disorders have an inflammatory pathophysiology.9 With the gradual increase in age, the immune system of our body becomes fragile. This condition is called immunosenescence.10 As thymus deteriorates it affects T cell production and B cell function, and subsequently, antibody production. Consequently, the aging body becomes more sensitive to infections.11 Tissue repair is another facet dependent on the immune responses as the repair of tissues usually begins once the inflammation has reduced, followed by migration of immune cells, and cellular proliferation.12,13 All of these mechanisms are affected in the aging population.14Despite sharing common pathways,4,15 the currently prescribed therapies for the age-related disorders differ in terms of their mechanism of action, with most therapies failing to target the underlying cellular pathologies. The altered pharmacokinetic profiles of elderly patients further challenge the efficacy of conventional therapies.16 Additionally, most of the disorders are complicated due to weakened tissue homeostasis of patients caused by reduced regenerative capacities with age. The co-existence of many of these pathologies, unrelated co-morbidities and subsequent polypharmacy creates hotspots for adverse drug reactions and drug–drug interactions making the choice, dose, and duration of the prescribed drugs limited.17 Such a scenario highly demands unified therapeutic strategies that can target the common underlying cellular hallmarks of aging and be applied to multiple age-related diseases. Over the years, the field of nanotechnology has set a benchmark for achieving tissue-specific targeted therapies. Specifically, functional nanomaterials (FNMs) have been utilized for targeted drug delivery using the intracellular and extracellular biomarkers of different tissue types. They also ensure improved solubility,18 bioavailability19 and sustained release profiles of conventional medicines20 compared to traditional administration techniques. With extensive potential for surface modifications, FNMs can be tailored to respond to either internal or external stimuli, such as pH, temperature, light, focused ultrasound, electromagnetic fields, etc., to exert therapeutic effects on demand.21 Among these, light provides several important benefits, such as minimal invasiveness, deeper tissue penetration, excellent spatio-temporal resolution, and ease of manipulation. Such remotely controllable FNMs can provide new routes towards the therapeutics of age-related diseases many of which require targeted drug delivery, long treatments, and time-controlled therapy (Fig. 1).
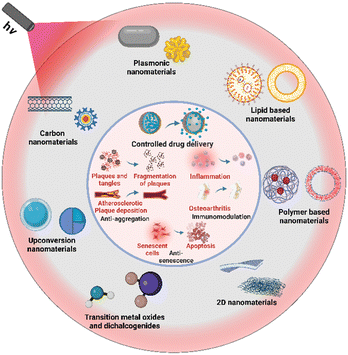 | ||
| Fig. 1 Schematic representation of light responsive FNMs used against age-related disorders. Created from Biorender.com. | ||
The functional requirements for nanomaterials ideal for combating age-related diseases include the ability to precisely target the pathological sites, such as arteries in atherosclerosis, joint cavities in osteoarthritis, and neurons in neurodegenerative disorders. To address these conditions effectively, we demonstrate that these nanomaterials can be conjugated with targeting peptides and chemical moieties, which selectively enhance their uptake at the sites of pathology. Herein, we review the recent developments of different types of light-responsive FNMs to combat age-related disorders. In particular, we focus on the potential advantages that light-responsive nanomaterials can offer over conventional treatment strategies. The different types of nanomaterials reviewed here include plasmonic nanomaterials, nanomaterials as carriers, upconversion nanomaterials, 2D nanomaterials, transition metal oxide and dichalcogenide nanomaterials and carbon-based nanomaterials. These light responsive FNMs can certainly guide the treatment of age-related disorders as well as improve the life expectancy globally.
2. Conventional therapies and advantages of light-responsive FNMs
The rate of aging can be controlled with the assistance of lifestyle modifications, such as caloric restriction, fasting and even exercise.22 However, the treatment of age-related disorders depends on the administration of small molecules, hormonal therapies, stem cell therapy, cytokine therapy, and antioxidants. These conventional approaches have been limited by several shortcomings. For example, small molecule-based drugs have low bioavailability and poor specificity towards the diseased site.23 This certainly requires higher dosages of drugs for it to be available in sufficient quantities at the target site for the therapeutic effect to be observed. This is especially true for age-related neurodegenerative disorders, as small molecules are unable to cross the blood–brain barrier (BBB).24 Yet, small molecules are still employed for managing neurodegenerative disorders, particularly Alzheimer's disease (AD) and Parkinson's disease (PD). Despite approval by the Food and Drug Administration (FDA) and widespread use, their efficacies are limited to symptomatic relief, with no substantial impact on disease progression. In fact, many of these therapeutics can have long-term adverse effects, including motor fluctuations and dyskinesias.25,26 This is not limited to therapies for neurodegenerative treatments, as many of the current therapeutics for age-associated problems suffer from severe side effects. For example, treatments for osteoarthritis (OA) and osteoporosis are known to cause side effects in the gastrointestinal tract and complications in the liver.27,28 Some conventional drugs for osteoporosis such as bisphosphonates trigger side effects such as atypical fractures and osteonecrosis of the jaw.29 Apart from the side effects, high percentage of the elderly are on multiple prescription drugs, leading to drug–drug interactions which are further exaggerated by disturbed pharmacokinetics and pharmacodynamic parameters.17,30 Absorption of drugs is also another factor which gets affected due to various age-associated factors such as decreased blood flow, reduced active transporters across the intestinal membrane31 and age-related changes in gastric pH.32 Furthermore, serum concentrations of drugs and their eventual distribution are affected by the altered glomerular filtration rates and fat percentages in the elderly.31 Similar problems arise with the cellular uptake and metabolism of the drugs as well.33 Some of these drugs remain in the body for longer periods in the elderly which may increase the chances of drug–drug interactions.30 Other limitations of conventional therapies include non-controlled and non-targeted drug release (common in atherosclerotic patients),34 transport across biological barriers (prevalent in small drug molecule therapy for neurodegenerative disorders),26 lack of dosage adjustment and optimization (crucial in hormone therapy) and lack of spatiotemporal control of drug activity (as seen in the delivery of antibiotics and anticancer drugs). Table 1 shows the disadvantages of conventional therapies for age-related diseases in different organ systems.| System | Disease | Conventional therapy | Limitations | Ref. |
|---|---|---|---|---|
| Central nervous system | Alzheimer's disease | Small molecule therapies such as anticholinesterase inhibitors and anti-glutaminergic agents | Peripheral degradation, transport across the BBB limited, high dosage due to low bioavailability and side effects | 35–37 |
| Parkinson's disease | Small molecule therapies such as levodopa, dopamine agonists, COMT inhibitors | Transport across the BBB, uncontrolled on/off cycle, reduced effect with disease progression, side effects such as motor dyskinesis | 26 | |
| Cardiovascular system | Atherosclerosis | Small molecule therapy such as statin to reduce cholesterol deposition and anti-platelet therapy to reduce platelet accumulation | Low efficiency, side effects, and bleeding risks for anti-platelet therapy | 38 |
| Surgical interventions such as coronary artery bypass grafting and percutaneous coronary intervention | Invasive procedure, restenosis, longer recovery time, patient mortality | 38 | ||
| Immune system | Weakened immune response | Antibiotic therapy and vaccination for fighting infections | High dosage, inefficient killing, reduced immunization in elderly | 39 |
| Chemotherapy, radiotherapy, surgical removal of tumor for cancer | Lateral damage, organ failure, high morbidity, patient mortality | 40 | ||
| Anti-inflammatory agents and antioxidants for chronic inflammation | Low efficiency, non-specific action, high dosage requirements | 41 and 42 | ||
| Musculo-skeletal system | Osteoporosis | Small molecule therapy such as anti-resorptive therapy | Atypical femur fracture, multiple side effects, jaw-bone osteonecrosis | 29 |
| Osteoarthritis | Small molecule therapy and stem cell therapy | Low efficiency, short life, and accumulation at the joints | 43 |
With the advent of nanotechnology, many of these challenges have been overcome. Functional nanomaterials (FNMs) can act as efficient transport systems to carry small drug molecules across membranes and deliver the therapeutics specifically to the target site while avoiding the off target unwanted effects.44 The FNMs offer several advantages, including minimal invasiveness, a reduction in the number of side effects, effective biological barrier crossing, spatio-temporal control over the delivery site, monitoring of disease progression as well as therapeutic effects, and reduced adverse drug reactions caused by polypharmacy in the elderly. One of the key advantages provided by FNMs is that they can be modified to become stimuli-responsive. Stimuli-responsive drug delivery systems are designed to release therapeutic agents in response to specific external or internal stimuli. In addition, light-responsive FNMs offer the advantage of being tailored to specific wavelengths, allowing customization for different applications. This tunability increases the diversity of these nanoparticles in various fields, from bioimaging to phototherapy. However, one of the barriers that hamper the effectiveness of phototherapy is the depth of penetration of light into biological tissues. The depth of penetration in biological tissues depends on several factors, one of the most important being tissue heterogeneity. The tissue's uneven refractive index results in the scattering of incident light upon entry, causing deviation from its initial trajectory and attenuation.45 It has been observed that 408 nm visible light can only penetrate 1 mm into the skin, and 633 nm light has a penetration depth of only 6.3 mm, making it evident that the skin significantly restricts the entry of visible light into the depths of the body.46
However, light scattering coefficients of skin, subcutaneous tissue, and muscle show a significant decrease in the wavelength range of 400–1700 nm, which is especially pronounced in the 400–700 nm range.47 When the wavelength is greater than 700 nm, the light scattering coefficients of these tissues tend to stabilize, which suggests that using light with wavelengths greater than 700 nm can facilitate the penetration of soft tissue to reach deep-seated regions for phototherapy.48 Bodily fluids like water and blood are thought to dominate light absorption in tissues, while some other components, such as melanin and fat, also absorb light that penetrates tissues. However, each of these tissue components has a unique spectral distribution; for example, blood primarily absorbs light at wavelengths less than 600 nm. Additionally, water exhibits local absorption peaks at around 970 nm, 1200 nm, and 1450 nm, while showing a local minimum at 808 nm.49,50 Due to these special tissue optical properties, absorption and scattering of light can be minimized within some specific windows.51 Currently, there are confirmed biological transparency windows, including the first window from 700 nm to 950 nm (NIR-I), the second window covering the region from 1000 to 1350 nm (NIR-II), and the third window from 1550 to 1870 nm (NIR-III).52 Within these windows, light can penetrate several centimetres of tissue, which is beneficial for achieving phototherapy for bone repair by allowing light to penetrate soft tissue.53 NIR responsive nanoparticles promote higher tissue penetrance54 which means they can be used to target organ systems protected by superficial tissue layers. Upon photoexcitation, these light-responsive functional nanomaterials are capable of producing photothermal heat via non-radiative decay and/or reactive oxygen species (ROS) via radiative decay pathways.55 Here the evolved heat can be used for precise tumor ablation, pathogen elimination, and even targeted immune modulation. With respect to temperature, there are several modes of hyperthermia including long-term low-temperature hyperthermia (40–41 °C for 6–72 h), moderate-temperature hyperthermia (42–45 °C for 15–60 min), and thermal ablation or high-temperature hyperthermia (>50 °C for >4–6 min).56 There is a plethora of parameters that can be manipulated to control the generation of high temperatures including the irradiation conditions (wavelength of irradiated light used, duration of light exposure, power density of irradiation), concentration of nanomaterials, specific absorbance coefficient and choice of nanomaterials.57,58 As far as the temperature requirement for hyperthermia is concerned, a temperature of 42 °C to 45 °C for periods of 30 to 60 min is required to cause irreversible cellular damage. As the tissue temperature reaches 50 °C, denaturation of protein begins, and the time required to achieve irreversible cellular damage decreases exponentially leading to cell death. Alternatively, the photodynamic ROS generation can be used to kill cells and potentially tackle age-related chronic inflammation (inflammaging).59 Functional nanomaterials can also be equipped with nanoreactors that can be used to generate other radicals such as nitric oxide (NO),60 and peroxynitrite (ONOO−),61 as well as carbon monoxide (CO),62 which are known to be utilized for their anti-inflammatory activity. This also addresses the problem of inflammaging that is prevalent in the older population.63 All the aforementioned strategies can be used to impart therapeutic potential to nanomaterials against various diseases.64 In the subsequent sections, deeper insights are provided on how light-responsive FNMs are used in different ways to treat age-related disorders. The typically employed wavelengths of near-infrared (NIR) light can stimulate multiple chromophore centres.65 NIR light has been extensively used to activate FNMs to mediate photothermal heat generation,66 ROS generation,59 photoacoustic imaging,67 and fluorescence imaging modalities.68 Among the vast ocean of available nanomaterials, one of the frontrunners includes organic nanomaterials which can be derived from organic sources. They can offer a biocompatible approach for treating age-related diseases. These include carbon-based nanomaterials, which range from fullerenes to nanotubes and provide tuneable surfaces. These materials can show several benefits in drug delivery. Another class of nanomaterials in organic nanomaterials include polymeric nanomaterials which are used as versatile carriers, carrying therapeutics directly to diseased cells and with the potential to exhibit stimuli-responsive release. Apart from this, inorganic nanomaterials have also emerged as a major class of FNMs. These inorganic nanomaterials exhibit inherent stability, stimuli responsiveness, as well as several other properties such as upconversion, ion chelation, cell stimulation, etc.
These nanomaterials include plasmonic nanomaterials, which have been used in the therapy of age-related diseases by harnessing the unique interaction between light and their structure. Another class of inorganic nanomaterials include 2D nanomaterials which have a large surface area and possess unique chemical tunability for precisely designed interfaces, enhancing their interaction with targets in age-related diseases. Upconversion nanoparticles follow this trend by converting low-energy light to higher energy light, offering unique tools for therapy in age-related diseases. Transition metal oxides and dichalcogenides are next in line for treating age-related diseases as the metals and chalcogens can be manipulated according to use and they also offer a stimuli-responsive effect that can be beneficial against age-related diseases. Fig. 2 presents an overview of different types of organic and inorganic nanomaterials used against age-related diseases.
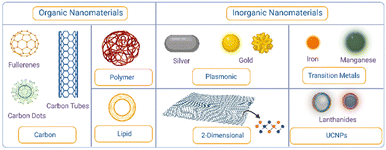 | ||
| Fig. 2 Overview of organic and inorganic nanomaterials used against age-related diseases. Created from Biorender.com. | ||
3. Light responsive functional nanomaterials against age-related diseases
3.1. Organic nanomaterials
Organic nanomaterials are a class of materials with size on the nanoscale (1–100 nm) which can be formed from the compounds containing carbon. These include materials ranging from graphene containing majorly carbon atoms to some of the complex biomolecules like carbohydrates, proteins, and lipids. This organic origin makes them inherently biocompatible, decreasing the toxicity in the body compared to some inorganic nanomaterials. One of the most fascinating properties of organic nanomaterials is their ability to self-assemble. These materials can be used as targeted drug delivery vehicles or as biosensors for disease detection. Organic nanomaterials offer a versatile and promising platform for advancements in nanotechnology. In this review, this class of nanomaterials have been divided into 3 different subclasses: lipid-based nanomaterials, carbon-based nanomaterials and polymeric nanomaterials. Let us discuss these mentioned classes of nanomaterials and how they are aiding in tackling the age-related diseases in detail.In the war with age-related disorders, liposomal formulations have been used against neurodegenerative diseases. For instance, brain targeted liposomes (BTLs) were demonstrated by decorating with transferrin which helped to improve targeting through overexpressed transferrin receptors on the BBB in Parkinson's disease.72 These BTLs were loaded with a monoclonal antibody (mAb) SynO4 that inhibits α-synuclein (AS) aggregation. BTLs below 100 nm were able to cross the BBB and were taken up by primary neurons where SynO4 was released from nanoparticles. This release led to the binding of SynO4 to the target receptors resulting in decreased protein aggregation and enhanced neuronal viability. In vivo results showed improved behavioral motor function and learning abilities in mice, offering a great nano-platform for drug delivery against Parkinson's disease. In another example, complex liposomes modified with leptin to cross the BBB were prepared to rescue the degenerated dopaminergic neurons.73 These liposomes were used as a drug carrier for resveratrol (RES) and epigallocatechin gallate (EGCG) against Parkinson's disease. The activity of drugs against neurotoxicity was investigated in vitro in MPTP treated SH-SY5Y cells. The modified liposomes showed an enhanced ability to cross the BBB and enhanced cell viability and target efficiency. Further, these results showed that the conjugation of leptin with liposomes enabled the docking of human brain microvascular endothelial cells (HBMECs) and SH-SY5Y cells via Lep receptors and enhanced their ability to permeate the BBB and cellular uptake. It was also revealed that RES and EGCG encapsulated into liposomes could be a neural defensive strategy by reducing the pro-apoptotic proteins and α-synuclein, and enhancing the anti-apoptotic proteins and the dopamine transporter.74 Another study involved the use of liposomes for delivery of Aphanamixis polystachya leaf extract against neurodegenerative diseases. GC-MS data showed that the major constituents of Aphanamixis polystachya leaf extract are 2-pentanone, different acids (octadec-9-enoic acid, 5-hydroxypipeloic acid, etc.), beta-elemene, etc. In vivo results showed a significant improvement in the memory function, locomotor activity and ambulatory performance of dementia induced mice. In addition, a lipid nanocarrier modified with folic acid (FA) moieties was also developed to target activated macrophages for treating rheumatoid arthritis (an inflammatory disorder) with plasmonic gold nanorods (AuNRs), dexamethasone (DEX) and a decoy oligodeoxynucleotide (ODN) to inhibit inflammation. They encapsulated Au NRs which could absorb near infrared light, DEX (common drug for RA) and ODN (to interfere with the pro-inflammatory cascade activated in macrophages by NF-κB) inside the lipid carriers. Along with photothermal therapy (PTT) generated by Au NRs, these therapeutics controlled the inflammation and reduced the swelling and redness in mouse models of RA. The group treated with the entire system and then irradiated with NIR light showed the lowest arthritis scores compared to other groups that were treated with individual drugs themselves.75 These liposomal nanoparticles can also be synthesized to show a thermosensitive nature, releasing their payload on temperature rise. Thrombosis caused by the rupture of atherosclerotic plaques presents an example of employing such nanoparticles that can release fibrinolytic plasminogen activators (Pas) like urokinase (uPA) spatiotemporally using light. This has been achieved by a liposomal platform synthesized as a hybrid formulation of Au NRs along with thermosensitive phospholipid dipalmitoyl phosphatidylcholine (DPPC) and a non-ionic surfactant (Brij 58) as shown in Fig. 3.76
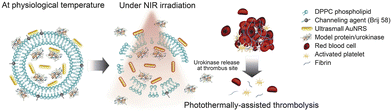 | ||
| Fig. 3 Graphical abstract showing the combined thrombolytic impact caused by the liposomal carrier loaded with gold nanorods and urokinase.76 Copyright 2021 Elsevier. | ||
The liposomal carrier allows the loading of both Au NRs as the light-responsive agent and urokinase protein as the thrombolytic agent. The Au NRs show plasmonic photothermal response upon NIR irradiation while Brij58 acts as a channeling agent to create nanopores upon heating (melting point = 38–39 °C), thus leading to the release of urokinase in a stimuli-responsive manner at the clot site. This liposomal hybrid achieved thrombolysis in vitro in a halo shaped clot model derived from freshly collected blood from healthy volunteers. Although not demonstrated yet, such nanoplatforms once conjugated with targeting ligands can be used for site-specific controlled thrombolysis preventing off-site bleeding which remains a major concern and adverse effect of fibrinolytic therapies in atherosclerosis management. The excellent biocompatibility of liposomes makes their use ideal for deep-seated issues such as atherosclerotic plaques that rupture and lead to thrombosis. However, it is well known that the majority of liposomal formulations are composed of synthetic lipids and they tend to disintegrate in the bloodstream owing to their poor stability. To overcome these limitations, biological materials, such as erythrocytes, have been given significant attention. To this end, a theranostic nanoconstruct system derived from erythrocytes doped with the FDA-approved NIR imaging agent, indocyanine green (ICG), and surface-functionalized with tPA was developed. Using an in vitro clot model, the dual functionality of these nanoconstructs was tested for NIR fluorescence imaging and clot lysis. These biomimetic theranostic nanoconstructs may provide an ultimate hope for the clinical translation, and can be effectively used in imaging and treatment of blood clots involved in ischemic stroke.77
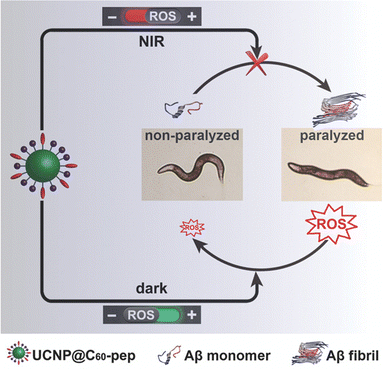 | ||
| Fig. 4 UCNP@C60-pep inhibited Aβ aggregation in vivo and attenuated the oxidative stress to prolong the lifespan of the CL2006 strain. UCNP@C60-pep produced ROS under NIR to disturb Aβ aggregation and scavenged the overproduced ROS in the dark. As a result, UCNP@C60-pep could ameliorate Aβ-triggered paralysis in CL2006 worms. Reproduced and adapted with permission.86 Copyright 2018 Wiley. | ||
These carbon-based materials hence present exciting avenues to age-related disorder treatments with their tunable light-responsive properties, ability to act as carriers, the dual modalities of ROS generation, and scavenging along with excellent biocompatibility profiles. While carbon-based nanomaterials have unmatched impacts on the therapy of age-related diseases, concerns regarding their biocompatibility, specifically in the case of carbon nanotubes, limit their therapeutic applications.
To this end, several strategies have been devised to bypass the BBB and deliver the nanoparticles/drug molecules for maximum utilization of therapeutic modalities including the use of targeting peptides against biomarkers. For example, a versatile polymer-dispersed liquid crystal (PDLC) nanoparticle functionalized with the LPFFD (leucine–proline–phenylalanine–phenylalanine–aspartic acid) peptide is designed with high Aβ-binding affinity, NIR-responsive drug release, and photothermal degradation properties for efficient disassembly of Aβ (Fig. 5).89 A photothermal hydrophobic core made of conjugated PDPP3T-O14 polymer was used which shows strong NIR absorption. The 5-mer β sheet breaker peptide LPFFD was conjugated to specifically target Aβ. Using curcumin as an additional inhibitor of Aβ aggregation, this nanoparticle system succeeded in dramatically lowering the Aβ aggregation induced cytotoxicity in PC12 neuronal cells. Such targeting strategies that address the blood–brain barrier's selectivity are of huge importance, as they can enhance therapeutic effects on the brain, potentially decreasing the advancement of age-related neurodegenerative diseases and enhancing overall treatment outcomes. With the advancing age, just like Alzheimer's disease the aggregation and accumulation of misfolded proteins (α-synuclein) results in the pathogenesis of Parkinson's disease. Towards this end, polydopamine-based NIR photothermal-assisted magnesium oxide-based nanoparticles (MgOp@PPLP) were used as neuroprotectants, gene transfection agents, and photothermal agents. An SH-SY5Y in vitro model induced by rotenone was used to show the suppression of α-synuclein levels and the reduction of inflammation. The nanoparticles were injected into MPTP-induced Parkinson's mouse models leading to restoration of Nissl granules, reduction of brain inflammation, and decrease in TH+ neuronal loss.90 Synergistic approaches using different treatment modalities in a single platform have been used extensively to tackle neurodegenerative diseases.
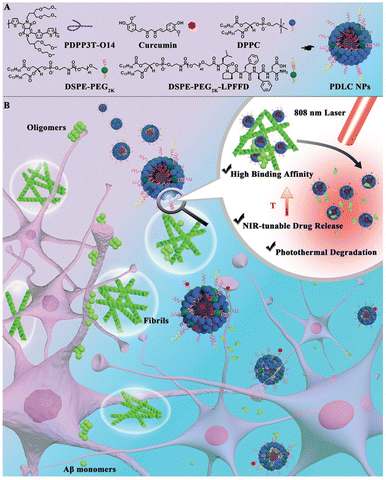 | ||
| Fig. 5 Schematic illustration of PDLC NPs for chemo-photothermal treatment for Aβ aggregation. (A) Chemical structures and the synthesis of PDLC NPs. (B) Schematic illustration of PDLC NP-mediated chemo-photothermal treatment by combining NIR light-tunable drug release and photodegradation of amyloid β. Reproduced with permission.89 Copyright 2021 Wiley-VCH. | ||
For instance, lipid-coated polydopamine nanoparticles (L-PDNPs) have been proposed as antioxidants, neuroprotective agents, and photothermal agents against Parkinson's disease.91 The L-PDNPs were designed to act as a photothermal conversion platform to increase the intracellular temperature to produce a Ca2+ influx in SH-SH5Y models. Upon NIR stimulation of treated cells, a constant increment in temperature (up to 34 °C) and Ca2+ ions was observed along with a subsequent decrease in ROS levels. With the irradiation of an 808 nm laser, L-PDNPs showed a concentration dependent increase in temperature generation with a positive correlation. The temperature increase showed 15 °C at 0.1 mg mL−1, 20 °C at 0.25 mg mL−1 and 35 °C at 5 mg mL−1. The L-PDNPs also showed an increment in output temperature with the increase in the power density of the 808 nm laser with the maximum reaching up to 8 °C. In another example, PEGylated polydopamine coated gold nanoparticles with specific binding to the thermosensitive ion channel were developed as nano-transducers for NIR-II window photo-stimulation of neurons in rats.92 The nanoparticles were functionalized with an anti-TRPV1 antibody for targeting and exhibiting photothermal performance to activate the Ca2+ influx of neurons in vitro. The use of these nanoparticles helps in the NIR-II window in achieving a non-invasive photothermal stimulation of neurons in living rats, inducing excitation of neurons as deep as 5 mm beneath the cortex. As the immune system deteriorates, infections like UTI (urinary tract infection) also become more frequent with age, especially in women. Towards this end, a biomimetic core–shell low temperature photothermal antibiotic nanoplatform against UPEC (uropathogenic Escherichia coli) was developed.93 The polydopamine core imparts photothermal property while the biphenyl mannoside shell shows multivalent high-affinity to UPEC leading to an ultra-strong binding of nanoparticles towards UPEC. Under 808 nm laser irradiation, the nanosystem showed an increase in temperature with the increase in power density with a temperature rise of around 30 °C at 1.5 W cm−2. Similarly, a controlled increase in temperature was observed with the increase in the concentration of nanoparticles. The nanoparticles showed a low temperature photothermal bactericidal effect (∼48 °C) and eliminated almost 100% bacteria in a mouse-based UTI model. Consequently, the high photothermal energy of the nanoparticles appears to be destructive in the affected bacterial area, while the overall environment remains at a low temperature. In addition to eradicating infectious agents, polymer-based NPs have also been used to activate the immune system. This sort of immunomodulation has been used to stimulate the immune cells against cancer. An approach for immunomodulation is shown by semiconducting polymer nano-enzyme (SPNK) particles made of the semiconducting polymer poly(cyclopentadithiophene-alt-benzothiadiazole) (PCB), and linked with an immune activating enzyme KYNase through a singlet oxygen responsive linker.94 These SPNKs accumulate at tumor sites and upon 808 nm NIR excitation generate singlet oxygen radicals to release KYNase. The KYNase enzyme reverses Kyn induced T cell suppression that mediates increased proliferation and infiltration of effector T cells at tumor sites.
Inflammation is also modulated using polymer based FNMs. Since cytokines are the major mediators of cellular communication and play a pivotal role in inflammation regulation (especially in the elderly), anti-cytokine therapy against pro-inflammatory cytokines is one of the preferred choices of treatment that can be utilized against inflammation. For instance, multifunctional alveolar macrophage like nanoparticles with photothermal inactivation property have been used against SARS-COV-2 infection.95 The nanoparticles were designed to be administered through atomization inhalation followed by NIR irradiation of the respiratory tract. Poly(lactic-co-glycolic acid) (PLGA) cores were coated with alveolar membranes and doped with an NIR activable photothermal agent 2PTE-2NDTA. The nanoparticle system was able to absorb a variety of proinflammatory cytokines through the various cytokine receptors (IL-6 and IFN-γ) present on the coated nanoparticles and upon NIR light irradiation, photothermal based antiviral activity of the nanoparticles was switched on. Another important disorder with an inflammation-based pathology is osteoarthritis. It is one of the most prevalent inflammatory diseases arising from the risk factors of older age, characterized by articular degeneration.96,97 Several risk factors lead to age-related changes in the joints such as chondrocyte senescence, increased oxidative damage, and reduced matrix synthesis and repair. These changes arise due to increased expression of cytokines and matrix degradation enzymes. The current pharmacological therapies target matrix degradation, inflammatory processes, pain, or bone turnover.98 These therapies are constrained by limited accumulation and short life at the affected joint sites, multiple dosing, and numerous unwanted side effects. NIR-responsive nanomaterials have been used for deeper tissue penetration and site-specific controlled anti-inflammatory therapies for osteoarthritis (OA). One recent study utilized the anti-inflammatory effect of nitric oxide (NO) by pairing haemoglobin (Hb) bound NO as a NIR-responsive nanogenerator.99 The authors also paired a Notch-1 siRNA to the Hb for RNA interference of Notch-1 signalling necessary for immune cell proliferation and used PLGA–PEG nanoparticles to carry the cargo. In this nano-construct, Hb acted as a photothermal agent creating local hyperthermia enough to break S–NO bonds and release nitric oxide upon 650 nm laser exposure. The siRNA was electrostatically bound to Hb and an amphiphilic coating with a poly[lactic-co-glycolic acid] (PLGA) core and a polyethylene glycol (PEG) shell increased cellular uptake and RNAi efficiency. The photothermal heat showed a synergistic effect with the nanoparticles with a reduction in the levels of cytokines like TNF-α, IL-1β, IL-6, COX-2, and IL-8 in vitro. Additionally, there was an increased accumulation of these NPs at the joints in papain-induced OA mouse models owing to Notch-1-siRNA specificity to OA. In vivo, the OA progression was slowed down due to a decrease in synovial inflammation and cartilage erosion. Another study used ROS scavenging along with a drug delivery system by coating melanin loaded into the Pluronic F127 micellar core with polydopamine (PDA) to form DHMP/M nanoparticles.100 These nanoparticles were responsive to both ROS and NIR (808 nm) due to the presence of melanin and PDA shell respectively. The study demonstrated that in a ROS-abundant environment of osteoarthritic knees, the PDA shell is slowly oxidized and broken enabling a controlled long-term release of loaded drugs, while exogenous NIR laser irradiation can cause a short burst and a faster release of the loaded cargo, making the construct ideal for personalized therapy post-intra-articular administration. Additionally, the release of melanin from the nanoparticles caused ROS scavenging both in vitro in chondrocyte cultures and in vivo in an OA mouse model. The PTT effect was synergistic with ROS scavenging of melanin to reduce OA progression via an effect on the toll-like receptor/protein kinase-B (TLR-2/Akt) axis. All these examples highlight how several strategies involving the pairing of light with nanomaterials can allow modulation of different aspects of the immune system to counter many age-related problems like infections, wound healing and osteoarthritis. While organic nanomaterials offer biocompatibility and self-assembly advantages, the diverse world of inorganic nanomaterials presents a broader spectrum of properties for age-related disease management. Inorganic materials, like plasmonic nanomaterials and upconversion nanomaterials, exhibit additional properties like tuneability in the therapeutic properties including plasmonic effects, the upconversion phenomenon and material associated photothermal and photodynamic therapeutic effects.
3.2. Inorganic nanomaterials
Inorganic nanomaterials are structured with inorganic metals such as transition metals, plasmonic metals, lanthanides, etc. Unlike organic nanomaterials, these materials are robust and can withstand harsh environments and maintain their precise structure. Some of these nanomaterials show plasmonic effects, interacting with light in unique ways to enhance the killing of diseased cells. Others possess the remarkable ability to convert low-energy light into higher-energy light, enabling deeper tissue penetration for diagnostics and therapy. Still others boast a dynamic combination of properties, acting as magnetic hyperthermia mediators or efficient conductors. This unique blend of functionalities allows scientists to tailor these nanomaterials for various applications. They can be designed to target specific molecules within cells, act as heat generators for precise cell destruction, or even illuminate biomarkers for early disease detection. In essence, inorganic nanomaterials offer a versatile and powerful platform for advancements in healthcare, particularly in the fight against age-related diseases.Traditional therapy of atherosclerosis focuses on reduction of cholesterol deposits and platelet accumulation and on lumen dilation using angioplasty or coronary artery bypass grafting. Cholesterol-lowering statin therapies are challenged by limited reductions in plaque deposits and a wide array of side effects. The invasive procedures for revascularization like percutaneous coronary intervention (PCI) are challenged by restenosis, and preventative dual anti-platelet therapy increases bleeding risks. Meanwhile, surgical options like coronary artery bypass grafting face concerns of longer recovery times, and patient mortality and morbidity risks.102 The use of photothermal treatments for the ablation of plaques and fibrin deposits presents an excellent non-invasive option to overcome many of the traditional treatment limitations. For example, through plasmonic photothermal therapy (PPTT) using gold coated sub-micrometer sized silica particles, significant atheroma volume reductions post laser irradiation have been achieved for over a period of 6 months (ΔV = −7.54% at baseline and/−22.92% after 6 months) in the Yucatan miniature swine.103 This was followed up as a clinical trial of plasmonic nano-photothermal therapy of atherosclerosis that delivered the same particles using two cardiac catheterization strategies: (1) nanoparticles on a bioengineered “on-artery” patch and (2) nanoparticles delivered through targeted stem cells or microbubbles, and compared their pathological markers with stenting controls.104 At 12 months of follow-up, the total atheroma volume and the total plaque burden reduced more as compared to stenting controls while the arterial remodelling/restenosis levels remained lower, proving PPTT to be an excellent alternative to stenting using minimally invasive catheterization. Although this modality proves to be promising, the plaques that get ruptured become new hotspots for thrombus formation composed of insoluble fibrils and the traditional fibrinolytic therapy utilizes the natural anti-clotting mechanisms of the body. This increases the risks of haemorrhage-based complications that can be fatal and require highly precise dosing. To overcome this, certain strategies using PTT have been developed for fibrinolysis. One of the studies in the concerned area showed gold nanorods (GNRs) conjugated with anti-fibrin antibodies that could photothermally ablate blood clots in vitro and in vivo in the blood vessels of mice. These GNRs showed a temperature increase of 10 °C when the concentration of nanorods was increased twice. They also showed that GNRs conjugated to streptokinase, a traditional therapeutic drug, could reduce the required dose significantly below therapeutic doses leading to risk aversion.105
Another clinical concern which arises due to accumulation of biomolecules is the age-related neurodegenerative diseases. Conventional therapies for age-related neurodegenerative diseases primarily focus on symptom management and slowing down the disease progression. These include small drug molecules, monoclonal antibodies, gene therapies, and stem cell therapies which have limitations like long-term side effects, immunogenicity, and low precision in targeting. Nanoparticle-based therapy holds promising potential for addressing age-related neurodegenerative diseases offering unique advantages in this context.
For instance, researchers have explored the use of diverse nanomaterials for drug delivery to the brain in neurodegenerative diseases.106 Among these, the plasmonic nanomaterials have been used up to their extreme potential. Several studies have demonstrated the effectiveness of plasmonic nanoparticle-based photothermal therapy in preclinical models of neurodegenerative diseases. With the aid of optimized heat generation, these not only protect neurons from degeneration but also hold the promise of reducing the senescence-associated factors, assisting in a multidimensional approach for dealing with age-related neurodegenerative challenges. For instance, researchers have developed spherical gold nanoparticles functionalized with specific ligands for beta-amyloid, enabling targeted delivery to amyloid plaques in an Alzheimer's disease model.107 Upon 532 nm laser irradiation, these nanoparticles induced plasmon-dependent localized hyperthermia, promoting the disaggregation of beta-amyloid and reducing plaque burden. Studies in this field have further demonstrated that NIR light-responsive nanoparticles hold significant promise in addressing these diseases. In one of the studies, pramipexole (a non-ergot dopamine agonist) loaded hollow gold nanospheres were used for the treatment of Parkinson's disease where the nanoparticles were irradiated with an 808 nm laser to perform photothermal therapy attaining a temperature of 50 °C and a higher drug release rate from the nanoparticles. This also led to improved pharmacokinetics, pharmacodynamics, and early recovery in mice.108 In another study, the authors have reported the use of targeted gold nanoparticle composites against Parkinson's disease where they have investigated the effect of size of nanoparticles for successful therapeutic effects. These nanoparticles have been synthesized via a combination of electrostatic adsorption and photochemical immobilization, and they can carry plasmid DNA against α-synuclein (SNCA gene) and were targeted against the nerve growth factor (NGF) receptor. In vitro results showed that the nanoparticles were internalized by PC12 cells and the pDNA escaped from the lysosome and expressed shRNA which then got associated with the RNA-induced silencing complex (RISC) to suppress SNCA expression via mRNA degradation. In the in vivo studies, the nanoparticles showed potential to cross the blood–brain barrier and inhibited the apoptosis of substantia nigra striatum dopaminergic neurons.109 Similarly, silver nanoparticles have also been used against neurodegenerative diseases. However, there are controversial data regarding the use of silver nanoparticles due to their ability to ionize after entry into cells, causing the release of silver ions inside the cytoplasm. This leads to increased ROS formation and mitochondrial toxicity leading to apoptosis or necrosis.110 Hence, in order to use these nanoparticles against neurodegenerative diseases, surface functionalization becomes the best probable solution. Silver nanoparticles synthesized via green synthesis methods have shown less toxicity and can be used against neurodegenerative diseases.111 One of such studies shows a green synthesis method used for silver nanoparticle preparation and investigation of their preventive effects on brain deficits in a sporadic Alzheimer's rat model. The aqueous extract of N. khasiana leaves was used as a reducing and stabilizing agent in silver nanoparticle preparation. The authors confirmed the coating of leaf extract polyphenols onto the surface of silver nanoparticles with FTIR. Streptozotocin was injected intracerebroventrically to induce a sporadic AD effect in rat models. Finally, with the help of the Barnes maze test and object recognition tests, the authors confirmed the ability of silver nanoparticles to prevent the damage caused due to Alzheimer's disease.112 Similarly, the leaf extract of Mucuna pruriens has also been utilized to synthesize silver nanoparticles. The extract of this plant is known to contain high concentrations for L-dopa which can be a source for the formation of dopamine in patients with Parkinson's disease. In this case, the authors investigated the effect of these nanoparticles on one of the typical conditions of Parkinson's disease, which is the loss of reaction to external stimuli called catalepsy. The results showed that the silver nanoparticles showed potential to counteract catalepsy in 3 month old mice in a dose dependent way.113 Platinum, another plasmonic metal, has also been explored in the field of neuroprotection. However, the studies have not been conducted against age-related neurodegeneration and also do not take light into consideration. Also, studies show that the size of platinum nanoparticles plays a major role in cytocompatibility, where large size particles cause cell damage in neural cell lines.114 However, studies have also shown that plasmonic nanoparticles act as strong ROS scavengers. A study involving platinum nanoparticles used against ischemic stroke and tissue plasminogen activator related brain damage showed a strong neuroprotective effect in mice. The strong ROS reduction capacity of the platinum nanoparticles has been shown to be accountable for the neuroprotective and neurological effects.115 Another study involving platinum nanoparticles demonstrated the superoxide anion reducing effect leading to improved neuroprotective effects in the mouse ischemic brain.116 Thus, further studies are required to confirm the biocompatibility and usability of platinum nanoparticles against age-related neurodegenerative diseases.
As discussed above, immunosenescence, the gradual deterioration of the immune system with age, poses another critical challenge in combating age-related disorders. Addressing this decline in immune function along with increased inflammation is paramount for developing effective interventions as these changes become characteristic pathologies for various age-related disorders. Since infections are the 3rd biggest cause of death in populations above 65 years,117 it is imperative to look for strategies to defend the elderly against these infections. To this end, plasmonic nano-therapies have been utilized as they harbour the potential to disrupt the cellular components of pathogens causing their death. For instance, surface adaptive gold nanoparticles were functionalized with the integration of both weak and strong electrolyte layers, forming a pH responsive nanoplatform.118 Under physiological conditions (pH 7.4), these nanoparticles dispersed well in healthy tissues, while under negatively charged bacterial surfaces of the MRSA (methicillin resistant Staphylococcus aureus) biofilm (pH 5.5), they showed strong adherence. Also, under pH 5.5, the nanoparticles showed an aggregation dependent shift in the absorption peak and an enhanced absorption in the NIR window. When irradiated with an 808 nm laser in a rabbit model, the gold nanoparticles showed a photothermal rise leading to the elimination of biofilms observed by the decrease in colony forming units. These reports make this strategy a suitable candidate for tackling other pathogens that are prevalent in older populations. Killing of macrophages through PTT has been utilized to drive down inflammation in age-associated diseases like atherosclerosis using nanomaterials like gold. For instance, under 808 nm laser irradiation, the PTT effects of gold nanorods were investigated in macrophage cell line Ana-1 and ApoE knockout mice. Gold nanorods exhibited a significant cell-killing efficacy of macrophages and inflammatory macrophages in femoral artery restenosis at very low concentrations as well as under low NIR powers.119 Wound healing hampered by infections can also cause severe tissue damage leading to potentially fatal complications. One of the studies showed hastened wound healing in cutaneous wounds by photo-biomodulation therapy using 808 nm laser irradiated gold nanoparticles. In another example, gold nanoparticles under 904 nm laser irradiation when paired with hyaluronic acid acted as stimulants for collagen production which helped in wound closure in excisional wound in Wistar rats.120 The mechanisms for the acceleration of wound closure involved a reduction in pro-inflammatory cytokines (IFNϒ, IL-1β, TNFα, and IL-6) and oxidative damage and an increment in the levels of the antioxidant glutathione (GSH).
Plasmonic nanoparticles have also been used to target and clear senescent cells where conventional approaches have failed. When paired with conventional medicine, these FNMs can show a synergistic effect. Currently, many anti-senescence drugs have entered the discovery chain, which can either eliminate senescent cells (senolytic) or alter the secretory phenotype (SASP) of senescent cells (senomorphic).121 Recently, a study showed the clearance of senescent microglia by surface-targeted chiral gold NPs which significantly improved the symptoms of Parkinson's disease in mice as shown in Fig. 6.122
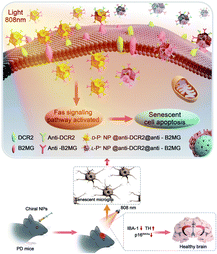 | ||
| Fig. 6 Illustration of the apoptosis pathways of senescent microglia cells induced by chiral NPs under the irradiation of an 808 nm laser in the brain of PD mice. Reproduced with permission.104 Copyright 2015 Royal Society of Chemistry. | ||
Upon 808 nm irradiation, these particles were able to activate apoptosis pathways in senescent microglia without any photothermal damage to the healthy cells. However, further studies on use of light mediated plasmonic nanomaterials are needed to confirm the effects on clearance of senescent cells. Plasmonic nanomaterials have been used as invaluable tools for managing age-related diseases. However, their therapeutic applications are limited due to the dependence on low-energy light radiation to keep the FNM therapy safe for the body. Upconversion nanomaterials offer a game-changing approach. These materials possess the remarkable ability to convert low-energy light to higher-energy light which can be used to activate therapeutic agents or directly generate heat within diseased cells. This deeper tissue penetration and targeted therapy holds immense promise for treating age-related diseases.
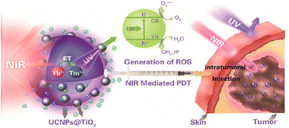 | ||
| Fig. 7 UCNPs absorbing NIR and emitting UV, which gets absorbed by TiO2 for PTT and PDT. Reproduced with permission.126 Copyright 2015 American Chemical Society. | ||
Since cancer becomes more prevalent with age due to a systemic decline in the immune system, UCNPs that can treat as well as help understand the development of disease become an important combat strategy. UCNPs also offer a novel strategy to manage inflammation directly (by reducing infection load) and indirectly (by interrupting pro-inflammatory signals and stimulating anti-inflammatory signals). UCNPs-Ce6–Mn (CO)5Br@Silane is an interesting system developed by Liu and team that could convert NIR to red light, which was absorbed by Ce6 to generate ROS that would break the Mn–CO bond liberating CO and Mn2+. Mn2+ can degrade H2O2 to generate O2, regenerating the oxygen supply for PDT while the CO molecules manage inflammation due to their noted anti-inflammatory effect. This system was tested against E. coli and S. aureus and it showed a remarkable 80% reduction in bacterial load.127 Apart from bacterial infections, yeast infections are also more likely to be observed with progressing age, as more than 50% of incidences of candidiasis are reported in people above 65,128 and hence many photo-responsive nanoformulations have been developed to combat it. For instance, a UCNP based nano-system was utilized as a functional nanocarrier to load protoporphyrin IX (PpIX) for NIR mediated PDT.129 The PpIX loaded UCNPs were modified with oligo-chitosan to increase the affinity of nanoparticles towards the yeast and hyphal cells. Upon irradiation, the nanoparticles absorb NIR light (808 nm) and emit light in the visible range (540 nm) which in turn excites the PpIX molecules leading to the generation of ROS inhibiting the Candida albicans biofilm formation and destroying the already existing biofilms. Similarly, neuroinflammation was treated by Hu and team, who employed the release of NO using an upconverted UV signal by externally irradiating the selective region with NIR. UCNP was co-loaded with SNAP (a NO donor which releases NO under UV radiation) and Pae (an anti-oxidant traditionally used for its neuroprotective effect) for a synergistic treatment.130 Wound healing has also been greatly worked on using UCNPs and has been reviewed by another group, which becomes relevant in the context of battling aging and its related disorders.131 Upconversion NPs have also been used for anti-aggregation therapies under external light irradiation. A group achieved cholesterol efflux from macrophage foam cells using UCNPs coated with a silica shell and conjugated with Ce6 to form UCNPs@SiO2-Ce6.132 Upon 980 nm laser irradiation, the UCNPs@SiO2-Ce6 nanosystem produces upconversion emission that further activates the Ce6 photosensitizer (660 nm) to generate ROS for photodynamic therapy. This PDT effect leads to the activation of autophagic pathways resulting in reduced lipid accumulation in the macrophages and eventually reduces the progression of atherosclerosis. Upconverting nanosystems have also been designed for the controlled release of nitric oxide for stimulating osteogenesis.133 This approach exploits the high photoconversion efficiencies of lanthanide UCNPs with a core of NaYF4:Yb/Tm and two outer shell layers of NaYF4:Nd/Yb and NaYF4 respectively to excite the NO donor BNN contained within mesoporous silica NPs functionalized with alendronate for bone targeting. Upon 808 nm irradiation, UCNPs get excited to generate UV/blue light which when absorbed by BNN triggers NO release within a controllable therapeutic window, overcoming the challenge of conventionally short-lived NO delivery in tissues. In OVX mice, the UCNPs-BNN showed much higher bone mass, bone volume, and bone thickness retention as compared to controls. Apart from inducing cell division to combat ageing, UCNPs have also been shown to activate existing cells for treating age-related neurodegeneration. A group utilized UCNPs for cell activation by linking the release of bacterial lipopolysaccharide (LPS) with the upconverted signal. The LPS could activate microglia in the brain under the exact spatial control of NIR radiation. For multiple therapies that require precise control of their duration and therapeutic windows, photosensitized nanomaterials can help overcome short-term failures and longer-term treatment side effects. Upconversion nanomaterials have been worked upon against therapy for age-related diseases by enabling targeted activation of drugs deep within tissues. However, the field of nanomedicine is constantly evolving. 2D nanomaterials present a unique platform for therapeutic interventions. Their atomically thin structure and exceptional surface area allow for efficient drug loading and precise interaction with diseased cells. This opens doors for exploring novel therapeutic strategies, such as targeted drug delivery with controlled release with improved cellular uptake.
Working as an essential component for cellular energetics, mitochondria are susceptible to gradual and cumulative damage over time including oxidative stress, impaired mitochondrial dynamics, and compromised bioenergetics. One such example has been demonstrated by using a zeolitic imidazolate framework 8-coated Prussian blue nanocomposite which was loaded with quercetin (ZIF-8@PB@QCT). The nanoparticle system holds the ability to penetrate through the BBB to the site of mitochondrial damage and under NIR irradiation imparts its photothermal effect and quercetin-mediated anti-inflammatory action.136 A 2D graphdiyne-based smart dual functional nanoplatform (GDY) endowed with both chemotherapy and photothermal therapy was used against PD. The nanosheets were loaded with minocycline as a neuroprotective agent by π–π stacking. Upon irradiation with a NIR laser, GDY showed a photothermal conversion efficiency of 32% and a release of more than 30% loaded drugs. Upon NIR irradiation, the nanosystem showed an increase of 20 °C as the nanoparticle concentration was doubled.137 Another interesting 2D nanoplatform shuttled by black phosphorus was targeted to the brain with the help of lactoferrin and loaded with paeoniflorin as a neuroprotective agent. The nanoplatform showed high biocompatibility, and upon NIR irradiation, a photothermal efficiency of 37% and a payload release of up to 94.9%. Upon 808 nm laser irradiation, the nanoplatform showed a regular increase of temperature with a corresponding increase in the concentration of nanoparticles in vitro. Similarly, with an increase in power density, a regular increment in temperature generation was observed. The nanoplatform showed a dose-dependent increase in cell viability and an improved ability to cross the blood–brain barrier with NIR irradiation both in vitro and in vivo.138 Furthermore, WS2 nanosheets present another class of 2D nanomaterials that have been investigated for their anti-inflammatory and photothermal properties. To this end, a strategy involving chemically exfoliated WS2 nanosheets helped in efficiently inhibiting the amyloid-β peptide aggregation and enabled NIR irradiation-dependent photothermal treatment of Alzheimer's disease. The amyloid monomers get adsorbed over the surface of nanosheets, which inhibits their aggregation. Additionally, irradiation with a NIR laser (808 nm) helps to dissolve the amyloid aggregates which was further confirmed by circular dichroism of amyloid fibrils after irradiation leading to a 30% decrease in beta fibril secondary structures.139 Overall, the synergistic approaches of nanoparticle-based therapy represent a promising frontier in the battle against neurodegenerative diseases. By combining various modalities, each designed to address specific facets of neurodegeneration, researchers have aimed to create synergies that enhance therapeutic efficacy. Whether it is crossing the blood–brain barrier, targeting pathological proteins, or mitigating oxidative stress, the collaborative potential of therapeutic modalities demonstrates a comprehensive approach. In one study, a semi-oxidized tin sulphide nanosheet based CO nanogenerator was utilized to reduce inflammation.140 Upon irradiation with an 808 nm laser, the tin sulphide nanosheets acted as photothermal agents while reduced levels of pro-inflammatory markers such as TNF-α, IL-6, and IL-1β were observed when irradiated with a 561 nm laser (Fig. 8).
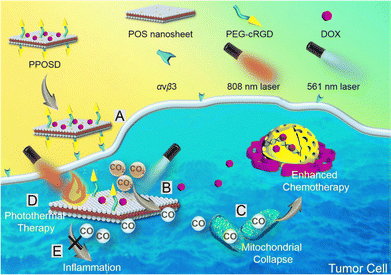 | ||
| Fig. 8 CO generation via CO2 photoreduction by the POS (partially oxidised disulphide) moiety in the FNM upon 561 nm laser irradiation to reduce inflammation and generate photothermal heat for tumor ablation. Reproduced with permission.140 Copyright 2019, American Chemical Society. | ||
Interestingly, it is also possible to activate both innate and adaptive immune systems using light-responsive FNMs. This was demonstrated in a nanoplatform containing Ti3C2 MXene paired with ovalbumin (OVA) and Mn2+ targeting osteosarcoma.141
The irradiation of this nanoplatform through PTT induced the release of mitochondrial DNA (mt-DNA) along with the release of OVA and Mn2+ at the tumor sites. The released mt-DNA and Mn2+ induce the activation of the innate immune responses through the cGAS–STING signalling pathway. The cGAS–STING pathway is a component of the innate immune system that functions to detect the presence of cytosolic DNA and, in response, triggers the expression of inflammatory genes that can lead to senescence or to the activation of defense mechanisms. On the other hand, the released OVA along with other tumor antigens enhances the adaptive T lymphocyte activity presentation, thus causing both facets of the immune system to target osteosarcoma tumors. Another study used chitosan-modified molybdenum disulfide (MoS2) nanosheets loaded with dexamethasone (Dex) as a NIR-responsive nanocarrier (MCD).142 The positive anti-inflammatory profile of Dex is hampered by quick clearance, which was overcome by loading Dex onto MoS2 nanosheets. Upon 808 nm laser irradiation, photothermal heat generation occurred leading to controlled drug release making MCD an on-demand remotely operable drug release system. The NIR-irradiated MCD nanoparticles reduced the survival of activated macrophages, which play a role in the progression of OA, while also reducing the excessive secretion of TNF-α, IL-1β, and IL-8. This was also demonstrated in papain-induced OA mice, where MCD injected into the joint cavity led to drug release causing a reduction in inflammatory cytokines, and the increased retention of MCD in the cavities enabled PA imaging. MCD-treated mice had reduced cartilage erosion and improved mobility without any signs of potential toxicity. The remarkable surface area of 2D nanomaterials makes them utilizable for targeted drug delivery in age-related diseases. However, their therapeutic effects might be limited by the type of drug they can carry. Transition metal oxides and dichalcogenide nanomaterials offer a broader therapeutic spectrum. These materials, with their unique combination of catalytic activity, magnetism, and conductivity, can go beyond simple drug delivery. They can be engineered to directly kill diseased cells through heat generation (photothermal therapy) or act as catalysts for targeted drug activation within the diseased cells, providing more potent and versatile therapeutic options for age-related diseases.
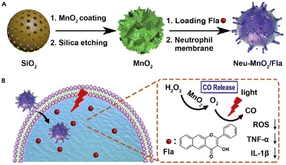 | ||
| Fig. 9 Schematic illustration of (A) the preparation of Neu-MnO2/Fla and (B) neutrophil membrane-targeted in situ CO release for synergistic anti-inflammatory effects promoted by MnO2 nanozymes. Reproduced with permission.147 Copyright 2019, Cell Press. | ||
4. Biodistribution, toxicity and clinical status of functional nanomaterials
The effectiveness of drugs significantly depends on their biodistribution. Similarly, biodistribution for nanoparticles decides whether they deliver therapeutic drugs on-site or offsite. Unless they can reach the target tissue site in sufficient concentrations, their efficacy will not be optimal. There are many factors which affect the biodistribution of a nanoparticle such as size, surface charge, type of material, surface functionalization, etc.153 Although the mode of administration of nanoparticles is the primary factor which affects the biodistribution, most therapeutic applications would require intravenous injection of nanoparticles to reach the target site. Pulmonary and dermal endothelia allow only very small (<3 nm in size) hydrodynamic diameter (HD) to pass through capillaries.154,155 Bigger sized nanoparticles can pass through discontinuous and fenestrated endothelia present in capillaries and vessels associated with the kidney and liver. Hence nanoparticles aggregate in these sites. Size is one of the most important factors that alter the retention time of these particles inside the body. Particles of size below 6 nm will be excreted too fast, not allowing for proper therapeutic application. The half-life of particles below 25 nm is less than 4 hours.156 But the half-life of nanoparticles increases exponentially with the increase in size. Yet, increased size also correlates with a higher clearance due to the reticuloendothelial system. The optimal size lies between 100 nm and 200 nm with optimal retention time and lower ERS clearance.157 However, some nanomaterials may be smaller to fit their intended purpose such as preventing toxicity due to long-term exposure. Surface charge also changes how particles are cleared and which tissues they selectively aggregate in. For example, if the nanoparticles are negatively charged, it will be difficult for them to pass through the negatively charged glomerular capillaries.158 Other tissue membranes and barriers such as the BBB also have charge preferences, which makes it important to consider the charge of nanoparticles based on the target tissue site. Along with these factors, the functional groups that are present on the surface of the nanoparticles can change the proteins that adhere to it. Some nanoformulations have pre-conjugated proteins while others do not. However, both the protein conjugated as well as non-protein conjugated nanoformulations will be exposed to proteins in the bloodstream that will adhere to the surface of the nanoparticles based on their functionalization, charge and hydrophobicity. If opsonizing proteins get adhered to the particle, they can trigger inflammation and phagocytosis by leukocytes and hence will start accumulating in lymph organs and hepatic cells for clearance.159When we also consider the altered pharmacokinetics of drugs in elderly due to the physiological changes in the ageing body, the situation becomes more complicated. The renal function is usually impaired which enhances excretion, also affecting liver metabolism of drugs including nanodrugs. The serum albumin concentration is also lower which affects the adherence of proteins to the nanoparticle. Chronic inflammation also makes the body more sensitive to therapeutics which can trigger an immune cascade. Overall, all these changes make the absorption of the nanodrug lower in elderly compared to younger individuals.160 Given the sensitivity of the aging process to small factors and the multifaceted pathologies of many age-related disorders, an evaluation of the toxicity of such nanomaterials is necessary to achieve maximum therapeutic benefit. The toxicity depends on many factors like size, surface functionality and charge, and composition of nanoparticles, all of which should be taken into account while choosing the right nanomaterial class for light-responsive therapeutic applications for age-related disorders. Among lipid-based nanoparticles, liposomes represent a class with the most examples of clinically approved anticancer drug formulations161 Even with chronic dosage studies, liposomes have demonstrated insignificant toxicity in different organs and systemic parameters.162 However, despite their high biocompatibility profiles, liposomes can still induce immunological activation in some cases depending on their size, charge, and percentage of cholesterol and lipid concentrations.163 Ideal formulations are suggested to have a neutral charge and low mol% of cholesterol and lower lipid concentrations.163,164
The carbon-based materials like carbon dots are highly biocompatible which show low levels of bioaccumulation and rare induction of inflammatory responses. One of the factors affecting the toxicity of carbon dots includes a size above 6 nm (hydrodynamic diameter). Aside from the size, the nature and density of surface charge also play a role in biological interactions with cationic charge, and higher charge density results in oxidative stress, mitochondrial dysfunction, and inflammatory responses.165 Similar to carbon dots fullerenes also have been reported to induce minimal cytotoxic and immunogenic responses. The fullerene (nC60) nanoparticles show no significant toxicity in C. elegans while its derivatives amino fullerene and UV irradiated nC60 show limited toxicity.166
Polymeric nanoparticles are among another class of nanoparticles known for their safety profiles. Often used for improving the bioavailability and biocompatibility of encapsulated drugs, many polymeric nanoparticles like poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and poly(lactic-co-glycolide) (PLGA) have been approved by the US FDA and European Medicine agency.161,167 For plasmonic nanoparticles derived from gold, the toxicity data are two-sided, with many in vitro and in vivo studies reporting little toxicity while others report toxicity based on size, surface charge, and shape, with higher size, higher surface charge, and spherical size being some contributors to toxicity.168 Unlike relatively non-toxic gold nanoparticles, silver nanoparticles have been shown to exhibit significant levels of toxicity in C. elegans models owing to their metallic nature and dissolution in an aqueous phase.166 The release of Ag+ ions can enhance the toxicity of Ag nanoparticles which can drive toxicity in the skin, eye, respiratory system, hepatobiliary system, kidney, and reproductive system.169 The potential mechanisms to reduce the toxicity of Ag-nanoparticles include biological methods over physical and chemical ones for synthesis and angstrom-scale threshold for the size of silver nanoparticles to induce minimal toxicity.169
Upconversion nanoparticles like lanthanide-doped nanoparticles have shown little toxicity in many studies.170 However, these nanoparticles are still not assessed for long-term evaluation of health risks, secondary toxicity due to decomposition, and detailed effects of accumulation in the body. Reduction in any cytotoxicity can be achieved by the use of various coatings like ethylenediamine tetra(methylene phosphonate) (EDTMP) and poly(maleic anhydride-alt-1-octadecene) (PMAO), as well as thick silica shell coatings that provide higher cell viability than bare UCNPs because of the release of fluoride ions from bare UCNPs.171 Upon toxicity assessment of 2D nanomaterials, it is seen that toxicity is limited in both in vitro mammalian cell lines and in vivo studies for materials. A low and descending order of cytotoxicity has been observed for graphene oxide nanomaterials, layered black phosphorus, and transition metal dichalcogenides (TMDs) like MoS2 and WS2. The reduction of toxicity of graphene oxide NPs can be achieved through PEGylation and dispersal in nanocomposite matrices, while for TMDs the type of exfoliation can be a major factor in determining toxicity. These reports of various toxicity assessments highlight that many of the developed nano-formulations available for light-responsive biomedical applications against age-related disorders show limited cytotoxicity and good biocompatibility; however, a low likelihood of cellular damage and immunological activation can be achieved through different physical and morphological modifications to nanoparticles based on their size, surface charge, and surface functionalization.
One of the limitations that specifically needs to be discussed about is the limited penetration of light. Light doesn’t penetrate deep into living tissue for long, but there's debate on what distance to consider as average. The energy from light gets absorbed quickly, with around 95% of light being absorbed within 10–15 mm into tissues (the exact depth varies with different light types). Despite efforts to use different near-infrared light windows, limited penetration remains an unresolved challenge. Several alternative non-invasive therapies have been utilized to overcome this limitation, some of which include electrotherapy or ultrasound therapy. Unlike phototherapy, which relies on light wavelengths for therapeutic effects, electrotherapy involves the use of localized lower voltage currents across the tissues to produce the desired therapeutic effects. Electrotherapy has been shown to reach penetration depths up to 8 mm depending upon the mode of electrotherapy chosen.172 In one of the modes of electrotherapies, i.e., interference current therapy, a resultant wave is generated which can penetrate deeper into tissues. Ultrasound therapy uses low or high frequency sound waves that penetrate the body tissues and result in the generation of heat within tissues. Ultrasound waves have the potential to penetrate deeper into the body allowing the waves to target body tissues. Ultrasound at a frequency of 1 MHz is absorbed primarily by tissues at a depth of 3–5 cm and is thus recommended for deeper injuries. A frequency of 3 MHz is recommended for more superficial lesions at depths of 1–2 cm.173 While looking closely through the alternative strategies for their tissue penetration ability, it is of vital importance to consider the role of light in therapeutics.
Clinical trials have long been a subject of debate when it comes to nanomedicine as the technology can involve materials with established toxicity in higher concentrations. However, this notion is slowly but surely changing as more clinical trials have highlighted the safety and efficacy of various nanoparticle-based treatments across multiple therapeutic areas. For instance, liposomal formulations, such as liposomal doxorubicin, have demonstrated significant efficacy in oncology, particularly in treating ovarian cancer and Kaposi's sarcoma, by improving drug stability and reducing systemic toxicity.174 The U.S. Food and Drug Administration (FDA) has approved several nanomedicines, marking significant milestones in the journey towards more effective and targeted medical treatments. Doxil, a liposomal formulation of the chemotherapy drug doxorubicin, was approved in 1995. It encapsulates doxorubicin within a liposome, a spherical vesicle with a lipid bilayer. This encapsulation allows the drug to be delivered directly to cancer cells, minimizing exposure to healthy tissues and reducing cardiotoxicity. Another notable example is abraxane, approved in 2005, which is an albumin-bound form of paclitaxel, a chemotherapy drug. Abraxane enhances the solubility and delivery of paclitaxel, allowing for higher doses to be administered with fewer side effects compared to the traditional formulation. This innovation has proven particularly effective in treating breast cancer, non-small cell lung cancer, and pancreatic cancer. Additionally, in the realm of neurodegenerative diseases, nanomedicine strategies have shown promise in enhancing drug delivery across the blood–brain barrier (BBB). Liposomal and polymeric nanoparticles have been utilized to improve the biodistribution and pharmacokinetics of therapeutic agents for Alzheimer's disease, demonstrating positive outcomes in early-phase clinical trials.175 Furthermore, the successful deployment of mRNA vaccines for COVID-19, using lipid nanoparticles (LNPs) to deliver mRNA, has established a new paradigm in vaccine development, with extensive clinical data supporting their safety and efficacy. These advancements underscore the potential of nanoparticle-based therapies in addressing complex medical challenges, paving the way for future innovations in nanomedicine.
5. Summary and future perspectives
The global increase in lifespan is followed by increments in the incidence of age-related disorders. The process of aging affects all tissues and organs through many common pathways that present as distinct symptoms and pathologies. Coupled with markedly different treatment options, age-related diseases are complicated by the fact that they co-occur with each other and are tricky to treat given the altered pharmacokinetics and pharmacodynamics in the elderly populations. This review highlights a unifying approach to overcome the shortcomings of these conventional therapies using light-activated nanomaterials. In addition to the classical advantages of nanotherapeutics such as increased bioavailability, reduced off-target effects, and longer retention times, the light responsive FNMs enable excellent spatiotemporal control over therapy. Advancements in the field of nanotechnology have enabled the development of nanomaterials that can be activated by light to produce heat, ROS and ultrasound. These capabilities can then be exploited to realize different therapeutic outcomes at the molecular, cellular, and tissue levels. We have focused on different types of organic and inorganic nanosystems activated by light that are commonly effective against distinct age-related diseases. The ability of photothermal treatment to ablate aggregates of proteins has led to excellent clearance of plaques in the case of neurodegenerative diseases like Alzheimer's and Parkinson's, while the same heat generation has enabled clearance of atheroma formation in vasculature leading to atherosclerosis resolution. This has been successful due to the deep penetration capabilities of near-infrared light wavelengths leading to non-invasive treatment options for deep-seated vulnerable tissues like the brain and cardiac vasculature. Following in this direction, it would be interesting for future studies to explore targeting other kinds of age-related aggregation in different organs like fibrosis in the lungs, fatty deposits in the liver, etc. These photoresponsive nanosystems can also help offset the changes occurring in the immune system, which is a key player in many age-related pathologies as well as the entire process of aging. Not only can the heat and ROS generation from these photoresponsive systems help overcome the suppressed state of the immunity in the case of infections and cancers due to immunosenescence, but they can also help limit the effects of immune overactivation in the case of chronic inflammatory conditions like osteoarthritis, by causing the apoptosis of macrophages or helping deliver anti-inflammatory molecules in a controlled manner. Furthermore, these systems can help reduce inflammation to hasten wound healing and tissue repair, another afflicted physiological process in the elderly. Much of the work against aging has been done through nanomaterials either supplementing the immune system or dampening the effect of excessive immune stimulation. It could be greatly beneficial if light-responsive systems that address the reduction in immune function or immune cells can be designed to combat the immunosenescence that occurs with aging. Another important ability of these nanomaterials includes the dissociation or release of drugs in a precise manner. The advantage of this strategy towards age-related disease therapy lies in the prevention of risky side effects in the case of many therapies, as well as the ability to deliver therapeutics in a cyclic or pulsatile manner. This is especially true for hormonal treatments or replacements, which often affect the elderly, and the long and unobserved use of which can lead to serious complications. While these hormonal treatments are being explored in diseases like osteoporosis in great detail, it remains to be seen if the advantage of such controlled therapy can benefit other kinds of hormonal replacement/supplementation therapies such as in the case of estrogen, testosterone, growth hormone, etc. The review finally also talks about senescence which is one of the most targeted hallmarks of aging and one of the most ubiquitous in terms of age-related pathologies. Recent years have seen an upward trend of light-responsive nanomaterials that can not only detect senescent cells using fluorescence but also eliminate them using photodynamic therapy. The heterogeneity of senescence in different tissues, its positive and negative implications for normal physiology, and the absence of distinct localization render senescence an excellent target for the spatial control offered by light-activated systems. Not only can the senescence removal be tissue-specific, but also restricted to ROS-mediated apoptosis in single cells preventing hyperthermia-induced damage to healthy cells. Although light-responsive nanomaterials have also been targeted to senescent cells as a part of age-related disorders, the literature still lacks in this aspect, with most studies focusing on senescence in fibroblasts. In context of using light-responsive nanomaterials against senescence, another unaddressed factor is their ability to prevent the pro-inflammatory and secretory phenotype of senescent cells without killing them. Acheiving the same could potentially prevent several unwanted effects of senolysis such as cancers and defects in wound healing.The expanding field of nanomedicine holds immense promise for revolutionizing the treatment of various diseases beyond traditional drug delivery, particularly in addressing age-related disorders such as atherosclerosis, neurodegeneration, and osteoarthritis. Despite significant preclinical advancements, there is a critical need for more clinical trials to thoroughly evaluate the safety, efficacy, and therapeutic potential of nanomaterials in these settings. It has been shown that nanomaterials can target and treat more efficiently than conventional treatments. In atherosclerosis, nanomaterials offer novel mechanisms to prevent or reverse plaque formation. In neurodegenerative diseases, they possess the potential to cross the blood–brain barrier and deliver neuroprotective agents directly to affected neurons. For osteoarthritis, nanomaterials can facilitate targeted delivery of anti-inflammatory drugs or support tissue regeneration. Expanding clinical trials in these areas is essential to translate promising laboratory results into tangible clinical outcomes, ultimately improving the quality of life for the ageing population.
With the discoveries of new and promising anti-aging therapies like epigenetic rejuvenation through reprogramming, mitochondrial rehabilitation, and gene therapies to control signalling and prevent DNA damage, also arise the potential risks of uncontrolled treatments that could lead to life-threatening complications like tumors and cancers. Light-controlled nanosystems could provide a pioneering platform for these upcoming therapies, especially in terms of controlling their safety and specificity through real-time monitoring. Although the benefits of such light-controlled nanomaterials are extremely promising and exciting, caution is always warranted because of the rather less investigated toxicity and safety profiles of many nanomaterials for long-term effects, especially in the case of aging where the objective is to minimize damage across tissues. With appropriate research into the safety of these systems, light-responsive functional nanomaterials showcase the ideal capabilities required for tackling age-related diseases which are currently challenged by several deficiencies in therapy and could one day be the mainstay of therapy for the aging populations by significantly enhancing health span and reducing the burden of adverse reactions, drug interactions and polypharmacy.
Author contributions
S. K. S., S. P. and S. J. have contributed equally to this work. J. A. A. H. has provided critical insights into this work. R. V. has conceived the idea and supervised the manuscript. All authors have read and approved the article.Data availability
This is to indicate that no primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
R. V. acknowledges the funding support provided by the Department of Biotechnology (DBT), Government of India, for the Ramalingaswami Biomedical Fellowship (BT/RLF/Re-entry/17/2018), the seed grant (I/SEED/RRV/20200076) funded by Indian Institute of Technology, International Research Mobility Grant (I/IMRG/RRV/20210021) and the Core Research Grant (CRG/2023/001189), Science and Engineering Research Board (SERB), Government of India.References
- A. J. Scott, M. Ellison and D. A. Sinclair, Nat. Aging, 2021, 1, 616–623 CrossRef PubMed.
- C. López-Otín, M. A. Blasco, L. Partridge, M. Serrano and G. Kroemer, Cell, 2023, 186, 243–278 CrossRef PubMed.
- Z. Li, Z. Zhang, Y. Ren, Y. Wang, J. Fang, H. Yue, S. Ma and F. Guan, Biogerontology, 2021, 22, 165–187 CrossRef PubMed.
- J. Guo, X. Huang, L. Dou, M. Yan, T. Shen, W. Tang and J. Li, Signal Transduct. Target Ther., 2022, 7, 391 CrossRef CAS PubMed.
- Y. Chang Scd, N. J. Kassebaum, J. L. Dieleman, A. Y. Chang, V. F. Skirbekk, S. Tyrovolas, N. J. Kassebaum and J. L. Dieleman, A Measuring population ageing: an analysis of the Global Burden of Disease Study 2017, Lancet, 2019, 4, e159–e167 Search PubMed.
- D. J. Tyrrell and D. R. Goldstein, Nat. Rev. Cardiol., 2021, 18, 58–68 CrossRef CAS PubMed.
- B. G. Childs, M. Durik, D. J. Baker and J. M. Van Deursen, Nat. Med., 2015, 21, 1424–1435 CrossRef CAS PubMed.
- A. Larbi, C. Franceschi, D. Mazzatti, R. Solana, A. Wikby and G. Pawelec, Physiology, 2008, 23, 64–74 CrossRef CAS PubMed.
- B. Li, W. Wang, W. Song, Z. Zhao, Q. Tan, Z. Zhao, L. Tang, T. Zhu, J. Yin, J. Bai, X. Dong, S. Tan, Q. Hu, B. Z. Tang and X. Huang, Adv. Sci., 2021, 8, 03556 Search PubMed.
- Z. Liu, Q. Liang, Y. Ren, C. Guo, X. Ge, L. Wang, Q. Cheng, P. Luo, Y. Zhang and X. Han, Signal Transduct. Target. Ther., 2023, 8, 200 CrossRef PubMed.
- J. R. Glynn and P. A. H. Moss, Sci. Data, 2020, 7, 239 CrossRef PubMed.
- H. N. Wilkinson and M. J. Hardman, Exp. Dermatol., 2021, 30, 68–73 CrossRef PubMed.
- K. Meyer, B. Hodwin, D. Ramanujam, S. Engelhardt and A. Sarikas, Essential Role for Premature Senescence of Myofibroblasts in Myocardial Fibrosis, J. Am. Coll. Cardiol., 2016, 67, 2016–2018 CrossRef PubMed.
- J. A. Coffman, S. Rieger, A. N. Rogers, D. L. Updike and V. P. Yin, NPJ Regen. Med., 2016, 3, 16003 CrossRef.
- C. Franceschi, P. Garagnani, C. Morsiani, M. Conte, A. Santoro, A. Grignolio, D. Monti, M. Capri and S. Salvioli, Front. Med., 2018, 5, 61 CrossRef PubMed.
- L. C. Sera and M. L. McPherson, Clin. Geriatr. Med., 2012, 28, 273–286 CrossRef PubMed.
- J. W. Wastesson, L. Morin, E. C. K. Tan and K. Johnell, Expert Opin. Drug Saf., 2018, 17, 1185–1196 CrossRef PubMed.
- S. Song, Y. Wang, J. Xie, B. Sun, N. Zhou, H. Shen and J. Shen, ACS Appl. Mater. Interfaces, 2019, 11, 34258–34267 CrossRef CAS PubMed.
- M. Yao, D. J. McClements and H. Xiao, Curr. Opin. Food Sci., 2015, 2, 14–19 CrossRef.
- C. Shao, H. Zhao and P. Wang, Nano Convergence, 2022, 9, 11 CrossRef CAS PubMed.
- K. T. Nguyen and Y. Zhao, Acc. Chem. Res., 2015, 48, 3016–3025 CrossRef CAS PubMed.
- L. Vitetta and B. Anton, Lifestyle and nutrition, caloric restriction, mitochondrial health and hormones: Scientific interventions for anti-aging, Clin. Interventions Aging, 2007, 2, 537–543 Search PubMed.
- M. Keeney, X. Y. Jiang, M. Yamane, M. Lee, S. Goodman and F. Yang, J. Mater. Chem. B, 2015, 3, 8757–8770 RSC.
- S. Yu, X. Xu, J. Feng, M. Liu and K. Hu, Int. J. Pharm., 2019, 560, 282–293 CrossRef CAS PubMed.
- B. Schmitt, T. Bernhardt, H.-J. Moeller, I. Heuser and L. Frölich, Combination Therapy in Alzheimer’s Disease A Review of Current Evidence, CNS Drugs, 2004, 18, 827–844 CrossRef CAS PubMed.
- J. Jankovic and G. Aguilar, Current approaches to the treatment of Parkinson’s disease, Neuropsychiatr. Dis. Treat., 2008, 4, 743–757 CrossRef CAS PubMed.
- W. Zhang, W. B. Robertson, J. Zhao, W. Chen and J. Xu, Front. Endocrinol., 2019, 10, 431 CrossRef PubMed.
- K. N. Tu, J. D. Lie, V. Wan, M. Cameron, A. G. Austel, J. K. Nguyen, K. Van and D. Hyun, Osteoporosis: A Review of Treatment Options, P T, 2018, 43, 92–104 Search PubMed.
- M. K. Skjødt, M. Frost and B. Abrahamsen, Br. J. Clin. Pharmacol., 2019, 85, 1063–1071 CrossRef PubMed.
- R. J. Cadieux, Postgrad. Med., 1989, 86, 179–186 CrossRef CAS PubMed.
- S. N. Hilmer, Expert Opin. Drug Metab. Toxicol., 2008, 4, 1321–1331 CrossRef CAS PubMed.
- R. An, E. Wilms, A. A. M. Masclee, H. Smidt, E. G. Zoetendal and D. Jonkers, Gut, 2018, 67, 2213–2222 CrossRef CAS PubMed.
- N. J. Hunt, P. A. G. McCourt, Z. Kuncic, D. G. Le Couteur and V. C. Cogger, Front. Nanotechnol., 2022, 4, 832524 CrossRef.
- I. Hetherington and H. Totary-Jain, Mol. Ther., 2022, 30, 3106–3117 CrossRef CAS PubMed.
- E. Passeri, K. Elkhoury, M. Morsink, K. Broersen, M. Linder, A. Tamayol, C. Malaplate, F. T. Yen and E. Arab-Tehrany, Int. J. Mol. Sci., 2022, 23, 13954 CrossRef CAS PubMed.
- P. V. Fish, D. Steadman, E. D. Bayle and P. Whiting, Bioorg. Med. Chem. Lett., 2019, 29, 125–133 CrossRef CAS PubMed.
- E. Zenaro, G. Piacentino and G. Constantin, Neurobiol. Dis., 2017, 107, 41–56 CrossRef CAS PubMed.
- C. Weber and H. Noels, Nat. Med., 2011, 17, 1410–1422 CrossRef CAS PubMed.
- M. Falcone, M. Paul, D. Yahav, G. Orlando, G. Tiseo, V. Prendki, R. Güerri-Fernández, G. Gavazzi, N. T. Mutters, B. Cookson and M. Tinelli Marco, Clin. Microbiol. Infect., 2019, 25, 562–569 CrossRef CAS PubMed.
- U. Anand, A. Dey, A. K. S. Chandel, R. Sanyal, A. Mishra, D. K. Pandey, V. De Falco, A. Upadhyay, R. Kandimalla, A. Chaudhary, J. K. Dhanjal, S. Dewanjee, J. Vallamkondu and J. M. Pérez de la Lastra, Genes Dis., 2023, 10, 1367–1401 CrossRef CAS PubMed.
- X. Li, C. Li, W. Zhang, Y. Wang, P. Qian and H. Huang, Signal Transduct. Target. Ther., 2023, 8, 239 CrossRef PubMed.
- D. Placha and J. Jampilek, Pharmaceutics, 2021, 13, 1–27 CrossRef PubMed.
- S. Grässel and D. Muschter, F1000Res, 2020, 9 Search PubMed.
- A. Z. Wilczewska, K. Niemirowicz, K. H. Markiewicz and H. Car, Pharmacol. Rep., 2012, 64, 1020–1037 CrossRef CAS PubMed.
- Z. H. Hsieh, C. H. Fan, Y. J. Ho, M. L. Li and C. K. Yeh, Sci. Rep., 2020, 10, 17406 CrossRef CAS PubMed.
- P. Juzenas, A. Juzeniene, O. Kaalhus, V. Iani and J. Moan, Photochem. Photobiol. Sci., 2002, 1, 745–748 CrossRef CAS PubMed.
- G. Hong, A. L. Antaris and H. Dai, Nat. Biomed. Eng., 2017, 1, 0010 CrossRef CAS.
- M. Inyushin, D. Meshalkina, L. Zueva and A. Zayas-Santiago, Molecules, 2019, 24, 2388 CrossRef CAS PubMed.
- J. A. Carr, M. Aellen, D. Franke, P. T. C. So, O. T. Bruns and M. G. Bawendi, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9080–9085 CrossRef CAS PubMed.
- L. Tian, Z. Xu, S. Zhao, Y. Cui, Z. Liang, J. Zhang and X. Xu, Materials, 2014, 7, 7289–7303 CrossRef PubMed.
- H. Lin, S. Gao, C. Dai, Y. Chen and J. Shi, J. Am. Chem. Soc., 2017, 139, 16235–16247 CrossRef CAS PubMed.
- E. Hemmer, A. Benayas, F. Légaré and F. Vetrone, Nanoscale Horiz., 2016, 1, 168–184 RSC.
- Z. Zhang, R. Wang, H. Xue, S. Knoedler, Y. Geng, Y. Liao, M. Alfertshofer, A. C. Panayi, J. Ming, B. Mi and G. Liu, Biomater. Res., 2023, 27, 123 CrossRef CAS PubMed.
- C. Ash, M. Dubec, K. Donne and T. Bashford, Lasers Med. Sci., 2017, 32, 1909–1918 CrossRef PubMed.
- M. Kim, J. H. Lee and J. M. Nam, Adv. Sci., 2019, 6, 1900471 CrossRef PubMed.
- R. W. Y. Habash, R. Bansal, D. Krewski, H. T. Alhafid and R. W. Y. Habash, Thermal Therapy, Part 1: An Introduction to Thermal Therapy, Crit. Rev. Biomed. Eng., 2006, 34, 459–489 CrossRef PubMed.
- M. W. Dewhirst, B. L. Viglianti, M. Lora-Michiels, P. J. Hoopes and M. Hanson, Thermal Dose Requirement for Tissue Effect: Experimental and Clinical Findings, Proc. SPIE-Int. Soc. Opt. Eng., 2003, 4954, 37–57 CrossRef PubMed.
- P. S. Yarmolenko, E. J. Moon, C. Landon, A. Manzoor, D. W. Hochman, B. L. Viglianti and M. W. Dewhirst, Int. J. Hyperthermia, 2011, 27, 320–343 CrossRef PubMed.
- D. K. Chatterjee, L. S. Fong and Y. Zhang, Adv. Drug Delivery Rev., 2008, 60, 1627–1637 CrossRef CAS PubMed.
- S. S. Lucky, K. C. Soo and Y. Zhang, Chem. Rev., 2015, 115, 1990–2042 CrossRef CAS PubMed.
- D. Zhang, D. Liu, C. Wang, Y. Su and X. Zhang, Adv. Colloid Interface Sci., 2023, 322, 103037 CrossRef CAS PubMed.
- S. B. Wang, C. Zhang, J. J. Ye, M. Z. Zou, C. J. Liu and X. Z. Zhang, ACS Cent. Sci., 2020, 6, 555–565 CrossRef CAS PubMed.
- C. Franceschi, Nutr. Rev., 2008, 65, 173–176 CrossRef PubMed.
- A. Jiménez-Balsa, S. Pinto, E. Quartin, M. M. Lino, V. Francisco and L. Ferreira, Bioconjugate Chem., 2018, 29, 1485–1489 CrossRef PubMed.
- K. G. Chernov, T. A. Redchuk, E. S. Omelina and V. V. Verkhusha, Chem. Rev., 2017, 117, 6423–6446 CrossRef CAS PubMed.
- F. Gao, C. Xue, T. Zhang, L. Zhang, G. Y. Zhu, C. Ou, Y. Z. Zhang and X. Dong, Adv. Mater., 2023, 35, 2302559 CrossRef CAS PubMed.
- X. Yang, Y. H. Chen, F. Xia and M. Sawan, Photoacoustics, 2021, 23, 100287 CrossRef PubMed.
- W. Li, G. Zhang and L. Liu, Front. Bioeng. Biotechnol., 2021, 9, 786927 Search PubMed.
- S. Mura, J. Nicolas and P. Couvreur, Nat. Mater., 2013, 12, 991–1003 CrossRef CAS PubMed.
- V. V. Ranade, J. Clin. Pharmacol., 1989, 29, 685–694 CrossRef CAS PubMed.
- S. Das, R. A. Lazenby, Z. Yuan, R. J. White and Y. C. Park, Langmuir, 2020, 36, 3573–3582 CrossRef CAS PubMed.
- M. Sela, M. Poley, P. Mora-Raimundo, S. Kagan, A. Avital, M. Kaduri, G. Chen, O. Adir, A. Rozencweig, Y. Weiss, O. Sade, Y. Leichtmann-Bardoogo, L. Simchi, S. Aga-Mizrachi, B. Bell, Y. Yeretz-Peretz, A. Z. Or, A. Choudhary, I. Rosh, D. Cordeiro, S. Cohen-Adiv, Y. Berdichevsky, A. Odeh, J. Shklover, J. Shainsky-Roitman, J. E. Schroeder, D. Hershkovitz, P. Hasson, A. Ashkenazi, S. Stern, T. Laviv, A. Ben-Zvi, A. Avital, U. Ashery, B. M. Maoz and A. Schroeder, Adv. Mater., 2023, 35, 2304654 CrossRef CAS PubMed.
- Y. C. Kuo, I. H. Wang and R. Rajesh, Acta Biomater., 2021, 119, 360–374 CrossRef CAS PubMed.
- M. H. Shariare, M. Rahman, S. R. Lubna, R. S. Roy, J. Abedin, A. L. Marzan, M. A. Altamimi, S. R. Ahamad, A. Ahmad, F. K. Alanazi and M. Kazi, Sci. Rep., 2020, 10, 6938 CrossRef CAS PubMed.
- L. Xue, D. Wang, X. Zhang, S. Xu and N. Zhang, Int. J. Pharm., 2020, 584, 119642 CrossRef PubMed.
- A. Refaat, B. del Rosal, J. Palasubramaniam, G. Pietersz, X. Wang, S. E. Moulton and K. Peter, J. Controlled Release, 2021, 337, 212–223 CrossRef CAS PubMed.
- R. Vankayala, S. R. Corber, J. T. Mac, M. P. Rao, M. Shafie and B. Anvari, Macromol. Biosci., 2018, 18, 1870009 CrossRef.
- K. D. Patel, R. K. Singh and H. W. Kim, Mater. Horiz., 2019, 6, 434–469 RSC.
- N. Rao, R. Singh and L. Bashambu, Mater. Today: Proc., 2021, 44, 608–614 CAS.
- Y. J. Chung, C. H. Lee, J. Lim, J. Jang, H. Kang and C. B. Park, ACS Nano, 2020, 14, 16973–16983 CrossRef CAS PubMed.
- Y. J. Chung, K. Kim, B. Il Lee and C. B. Park, Small, 2017, 13, 00983 CrossRef PubMed.
- Y. J. Chung, B. Il Lee and C. B. Park, Nanoscale, 2019, 11, 6297–6306 RSC.
- P. Ye, L. Li, X. Qi, M. Chi, J. Liu and M. Xie, J. Colloid Interface Sci., 2023, 650, 1749–1761 CrossRef CAS PubMed.
- Q. Chen, X. Duan, Y. Yu, R. Ni, G. Song, X. Yang, L. Zhu, Y. Zhong, K. Zhang, K. Qu, X. Qin and W. Wu, Adv. Sci., 2023, 11, 07441 Search PubMed.
- Z. Du, N. Gao, X. Wang, J. Ren and X. Qu, Small, 2018, 14, 01852 Search PubMed.
- J. Liu, B. Zhou, Y. Guo, A. Zhang, K. Yang, Y. He, J. Wang, Y. Cheng and D. Cui, ACS Appl. Mater. Interfaces, 2021, 13, 29349–29362 CrossRef CAS PubMed.
- B. Begines, T. Ortiz, M. Pérez-Aranda, G. Martínez, M. Merinero, F. Argüelles-Arias and A. Alcudia, Nanomaterials, 2020, 10, 1–41 CrossRef PubMed.
- N. Baig, I. Kammakakam, W. Falath and I. Kammakakam, Mater. Adv., 2021, 2, 1821–1871 RSC.
- Y. Lai, Y. Zhu, Z. Xu, X. Hu, M. Saeed, H. Yu, X. Chen, J. Liu and W. Zhang, Adv. Funct. Mater., 2019, 11, 08473 Search PubMed.
- Y. Gao, Y. Cheng, J. Chen, D. Lin, C. Liu, L. K. Zhang, L. Yin, R. Yang and Y. Q. Guan, Adv. Healthcare Mater., 2022, 11, 01655 Search PubMed.
- M. Battaglini, A. Marino, A. Carmignani, C. Tapeinos, V. Cauda, A. Ancona, N. Garino, V. Vighetto, G. La Rosa, E. Sinibaldi and G. Ciofani, ACS Appl. Mater. Interfaces, 2020, 12, 35782–35798 CrossRef CAS PubMed.
- J. Liu, J. Li, S. Zhang, M. Ding, N. Yu, J. Li, X. Wang and Z. Li, Nano Convergence, 2022, 9, 13 CrossRef CAS PubMed.
- S. Wenjie, A. Jinxia, T. He, J. Mengran and G. Hui, Adv. Healthcare Mater., 2021, 11, 06133 Search PubMed.
- Z. Zeng, C. Zhang, J. Li, D. Cui, Y. Jiang and K. Pu, Adv. Mater., 2020, 33, 07247 Search PubMed.
- B. Li, W. Wang, W. Song, Z. Zhao, Q. Tan, Z. Zhao, L. Tang, T. Zhu, J. Yin, J. Bai, X. Dong, S. Tan, Q. Hu, B. Z. Tang and X. Huang, Adv. Sci., 2020, 8, 03556 Search PubMed.
- B. Xia, D. Chen, J. Zhang, S. Hu, H. Jin and P. Tong, Calcif. Tissue Int., 2014, 95, 495–505 CrossRef CAS PubMed.
- A. Shane Anderson and R. F. Loeser, Best Pract. Res. Clin. Rheumatol., 2010, 24, 15–26 CrossRef CAS PubMed.
- M. Maqbool, G. Fekadu, X. Jiang, F. Bekele, T. Tolossa, E. Turi, G. Fetensa and K. Fanta, Ann. Med. Surg., 2021, 72, 103077 CrossRef PubMed.
- X. Chen, Y. Liu, Y. Wen, Q. Yu, J. Liu, Y. Zhao, J. Liu and G. Ye, Nanoscale, 2019, 11, 6693–6709 RSC.
- J. Ruan, Q. Yu, H. Cui, X. Qin, L. Qin, S. Chen, D. Niu and C. Fan, Appl. Mater. Today, 2021, 25, 101216 CrossRef.
- S. K. Singh, S. Mazumder, A. Vincy, N. Hiremath, R. Kumar, I. Banerjee and R. Vankayala, ACS Appl. Nano Mater., 2023, 6, 1508–1521 CrossRef CAS.
- I. Hetherington and H. Totary-Jain, Mol. Ther., 2022, 30, 3106–3117 CrossRef CAS PubMed.
- A. N. Kharlamov and J. L. Gabinsky, Rejuvenation Res., 2012, 15, 222–230 CrossRef CAS PubMed.
- A. N. Kharlamov, A. E. Tyurnina, V. S. Veselova, O. P. Kovtun, V. Y. Shur and J. L. Gabinsky, Nanoscale, 2015, 7, 8003–8015 RSC.
- N. Singh, A. Varma, A. Verma, B. N. Maurya and D. Dash, Nano Res., 2016, 9, 2327–2337 CrossRef CAS.
- B. A. Witika, M. S. Poka, P. H. Demana, S. K. Matafwali, S. Melamane, S. M. M. Khamanga and P. A. Makoni, Pharmaceutics, 2022, 14, 836 CrossRef CAS PubMed.
- R. C. Triulzi, Q. Dai, J. Zou, R. M. Leblanc, Q. Gu, J. Orbulescu and Q. Huo, Colloids Surf., B, 2008, 63, 200–208 CrossRef CAS PubMed.
- S. Li, J. Liu, G. Li, X. Zhang, F. Xu, Z. Fu, L. Teng, Y. Li and F. Sun, Eur. J. Pharm. Biopharm., 2019, 141, 1–11 CrossRef CAS PubMed.
- K. Hu, X. Chen, W. Chen, L. Zhang, J. Li, J. Ye, Y. Zhang, L. Zhang, C. H. Li, L. Yin and Y. Q. Guan, Nanomedicine, 2018, 14, 1123–1136 CrossRef CAS PubMed.
- M. M. Rohde, C. M. Snyder, J. Sloop, S. R. Solst, G. L. Donati, D. R. Spitz, C. M. Furdui and R. Singh, Part. Fibre Toxicol., 2021, 18, 37 CrossRef CAS PubMed.
- V. De Matteis, L. Rizzello, M. Cascione, E. Liatsi-Douvitsa, A. Apriceno and R. Rinaldi, Nanomaterials, 2020, 10, 1083 CrossRef CAS PubMed.
- X. Zhang, Y. Li and Y. Hu, Colloids Surf., A, 2020, 605, 125288 CrossRef CAS.
- R. E. Sardjono, F. Khoerunnisa, I. Musthopa, N. S. M. M. Akasum and R. Rachmawati, J. Phys.: Conf. Ser., 2018, 1013 Search PubMed.
- M. Manikandan, N. Hasan and H. F. Wu, Biomaterials, 2013, 34, 5833–5842 CrossRef CAS PubMed.
- M. Takamiya, Y. Miyamoto, T. Yamashita, K. Deguchi, Y. Ohta and K. Abe, Neuroscience, 2012, 221, 47–55 CrossRef CAS PubMed.
- M. Takamiya, Y. Miyamoto, T. Yamashita, K. Deguchi, Y. Ohta, Y. Ikeda, T. Matsuura and K. Abe, J. Neurosci. Res., 2011, 89, 1125–1133 CrossRef CAS PubMed.
- K. A. Kline and D. M. E. Bowdish, Curr. Opin. Microbiol., 2016, 29, 63–67 CrossRef PubMed.
- D. Hu, H. Li, B. Wang, Z. Ye, W. Lei, F. Jia, Q. Jin, K. F. Ren and J. Ji, ACS Nano, 2017, 11, 9330–9339 CrossRef CAS PubMed.
- J. Qin, Z. Peng, B. Li, K. Ye, Y. Zhang, F. Yuan, X. Yang, L. Huang, J. Hu and X. Lu, Nanoscale, 2015, 7, 13991–14001 RSC.
- C. Mendes, A. Thirupathi, R. P. Zaccaron, M. E. A. B. Corrêa, J. V. S. Bittencourt, L. de, R. Casagrande, A. C. S. de Lima, L. L. de Oliveira, T. A. M. de Andrade, Y. Gu, P. E. Feuser, R. A. Machado-de-Ávila and P. C. L. Silveira, Antioxidants, 2022, 11, 2257 CrossRef CAS PubMed.
- L. Zhang, L. E. Pitcher, V. Prahalad, L. J. Niedernhofer and P. D. Robbins, FEBS J., 2023, 290, 1362–1383 CrossRef CAS PubMed.
- Z. Xu, A. Qu, H. Zhang, W. Wang, C. Hao, M. Lu, B. Shi, L. Xu, M. Sun, C. Xu and H. Kuang, Chem. Sci., 2022, 13, 6642–6654 RSC.
- S. Borse, R. Rafique, Z. V. P. Murthy, T. J. Park and S. K. Kailasa, Analyst, 2022, 147, 3155–3179 RSC.
- M. Wang, G. Abbineni, A. Clevenger, C. Mao and S. Xu, Nanomedicine, 2011, 7, 710–729 CrossRef CAS PubMed.
- Y. Liu, J. Zhang, C. Zuo, Z. Zhang, D. Ni, C. Zhang, J. Wang, H. Zhang, Z. Yao and W. Bu, Nano Res., 2016, 9, 3257–3266 CrossRef CAS.
- Z. Hou, Y. Zhang, K. Deng, Y. Chen, X. Li, X. Deng, Z. Cheng, H. Lian, C. Li and J. Lin, ACS Nano, 2015, 9, 2584–2599 CrossRef CAS PubMed.
- W. Liu, Y. Sun, B. Zhou, Y. Chen, M. Liu, L. Wang, M. Qi, B. Liu and B. Dong, J. Colloid Interface Sci., 2024, 663, 834–846 CrossRef CAS PubMed.
- B. G. J. Dekkers, A. Veringa, D. J. E. Marriott, J. M. Boonstra, K. C. M. van der Elst, F. F. Doukas, A. J. McLachlan and J. W. C. Alffenaar, Drugs Aging, 2018, 35, 781–789 CrossRef CAS PubMed.
- P. Janda, R. Sroka, B. Mundweil, C. S. Betz, R. Baumgartner and A. Leunig, Lasers Surg. Med., 2003, 33, 93–101 CrossRef PubMed.
- B. Hu, H. Fang, Z. Huang, W. Huang, L. Huang, H. Liu, F. Lv, W. Huang and X. Wang, Chem. Eng. J., 2023, 465, 142959 CrossRef CAS.
- H. E. Gültekin, G. Yaşayan, A. Bal-Öztürk, A. Bigham, A. (Arash) Simchi, A. Zarepour, S. Iravani and A. Zarrabi, Mater. Horiz., 2023, 11, 363–387 RSC.
- X. B. Han, H. X. Li, Y. Q. Jiang, H. Wang, X. S. Li, J. Y. Kou, Y. H. Zheng, Z. N. Liu, H. Li, J. Li, D. Dou, Y. Wang, Y. Tian and L. M. Yang, Cell Death Dis., 2017, 242, e2864 CrossRef PubMed.
- J. Ye, J. Jiang, Z. Zhou, Z. Weng, Y. Xu, L. Liu, W. Zhang, Y. Yang, J. Luo and X. Wang, ACS Nano, 2021, 15, 13692–13702 CrossRef CAS PubMed.
- N. Baig, Composites, Part A, 2023, 165, 107362 CrossRef CAS.
- N. Hiremath, R. Kumar, K. C. Hwang, I. Banerjee, S. Thangudu and R. Vankayala, ACS Appl. Nano Mater., 2022, 5, 1719–1733 CrossRef CAS.
- Y. Liu, H. Hong, J. Xue, J. Luo, Q. Liu, X. Chen, Y. Pan, J. Zhou, Z. Liu and T. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 37746–37760 CrossRef CAS PubMed.
- T. Li, Y. Liu, W. Bao, J. Luo, L. Gao, X. Chen, S. Wang, J. Yu, Y. Ge, B. Zhang, N. Xie, Z. Xie, T. Chen and H. Zhang, Adv. Ther., 2021, 4, 00082 Search PubMed.
- S. Xiong, Z. Li, Y. Liu, Q. Wang, J. Luo, X. Chen, Z. Xie, Y. Zhang, H. Zhang and T. Chen, Biomaterials, 2020, 260, 120339 CrossRef CAS PubMed.
- M. Li, A. Zhao, K. Dong, W. Li, J. Ren and X. Qu, Nano Res., 2015, 8, 3216–3227 CrossRef CAS.
- S. B. Wang, C. Zhang, Z. X. Chen, J. J. Ye, S. Y. Peng, L. Rong, C. J. Liu and X. Z. Zhang, ACS Nano, 2019, 13, 5523–5532 CrossRef CAS PubMed.
- S. Zhang, J. Wang, Z. Kong, X. Sun, Z. He, B. Sun, C. Luo and J. Sun, Biomaterials, 2022, 282, 121433 CrossRef CAS PubMed.
- Y. Zhao, C. Wei, X. Chen, J. Liu, Q. Yu, Y. Liu and J. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 11587–11601 CrossRef CAS PubMed.
- M. Ahmadi, O. Zabihi, S. Jeon, M. Yoonessi, A. Dasari, S. Ramakrishna and M. Naebe, J. Mater. Chem. A, 2020, 8, 845–883 RSC.
- S. A. Han, R. Bhatia and S. W. Kim, Nano Convergence, 2015, 2, 17 CrossRef.
- Q. Peng, Z. Qian, H. Gao and K. Zhang, Front. Chem., 2022, 10, 899231 Search PubMed.
- C. J. Jeong, S. M. Sharker, I. In and S. Y. Park, ACS Appl. Mater. Interfaces, 2015, 7, 9469–9478 CrossRef CAS PubMed.
- C. Liu, Z. Du, M. Ma, Y. Sun, J. Ren and X. Qu, iScience, 2020, 23, 101483 CrossRef CAS PubMed.
- L. Wu, X. Cai, H. Zhu, J. Li, D. Shi, D. Su, D. Yue and Z. Gu, Adv. Funct. Mater., 2018, 28, 04324 Search PubMed.
- M. Popova, L. S. Lazarus, S. Ayad, A. D. Benninghoff and L. M. Berreau, J. Am. Chem. Soc., 2018, 140, 9721–9729 CrossRef CAS PubMed.
- M. C. White, D. M. Holman, J. E. Boehm, L. A. Peipins, M. Grossman and S. Jane Henley, Am. J. Prev. Med., 2013, 46, S7–S15 CrossRef PubMed.
- P. Zhang, Q. Zhao, M. Shi, C. Yin, Z. Zhao, K. Shen, Y. Qiu, Y. Xiao, Y. Zhao, X. Yang and Y. Zhang, Nano Lett., 2020, 20, 261–271 CrossRef CAS PubMed.
- W. Kang, Y. Liu and W. Wang, Acta Pharm. Sin. B, 2023, 13, 2346–2368 CrossRef CAS PubMed.
- J. P. M. Almeida, A. L. Chen, A. Foster and R. Drezek, Nanomedicine, 2011, 6, 815–835 CrossRef CAS PubMed.
- M. Li, K. T. Al-Jamal, K. Kostarelos and J. Reineke, ACS Nano, 2010, 4, 6303–6317 CrossRef CAS PubMed.
- H. S. Choi and J. V. Frangioni, Mol. Imaging, 2010, 9, 291–310 CAS.
- S. D. Perrault, C. Walkey, T. Jennings, H. C. Fischer and W. C. W. Chan, Nano Lett., 2009, 9, 1909–1915 CrossRef CAS PubMed.
- Y. Wei, L. Quan, C. Zhou and Q. Zhan, Nanomedicine, 2018, 13, 1495–1512 CrossRef CAS PubMed.
- M. Longmire, P. L. Choyke and H. Kobayashi, Nanomedicine, 2008, 3, 703–717 CrossRef CAS PubMed.
- A. J. Aaron, A. Bumb and M. W. Brechbiel, Chem. Rev., 2010, 110, 2921–2959 CrossRef PubMed.
- S. R. R. dos Reis, S. R. Pinto, F. D. de Menezes, R. Martinez-Manez, E. Ricci-Junior, L. M. R. Alencar, E. Helal-Neto, A. O. da Silva de Barros, P. C. Lisboa and R. Santos-Oliveira, Pharm. Res., 2020, 37, 40 CrossRef PubMed.
- D. Lombardo, M. A. Kiselev and M. T. Caccamo, J. Nanomater., 2019, 3702518 CAS.
- J. Fonseca-Gomes, J. A. Loureiro, S. R. Tanqueiro, F. M. Mouro, P. Ruivo, T. Carvalho, A. M. Sebastião, M. J. Diógenes and M. C. Pereira, Int. J. Nanomed., 2020, 15, 8609–8621 CrossRef CAS PubMed.
- C. T. Inglut, A. J. Sorrin, T. Kuruppu, S. Vig, J. Cicalo, H. Ahmad and H. C. Huang, Nanomaterials, 2020, 10, 190 CrossRef CAS PubMed.
- J. B. Strachan, B. P. Dyett, Z. Nasa, C. Valery and C. E. Conn, J. Colloid Interface Sci., 2020, 576, 241–251 CrossRef CAS PubMed.
- A. Truskewycz, H. Yin, N. Halberg, D. T. H. Lai, A. S. Ball, V. K. Truong, A. M. Rybicka and I. Cole, Small, 2022, 18, e2106342 CrossRef PubMed.
- S. K. Jung, X. Qu, B. Aleman-Meza, T. Wang, C. Riepe, Z. Liu, Q. Li and W. Zhong, Environ. Sci. Technol., 2015, 49, 2477–2485 CrossRef CAS PubMed.
- A. Zielinska, F. Carreiró, A. M. Oliveira, A. Neves, B. Pires, D. Nagasamy Venkatesh, A. Durazzo, M. Lucarini, P. Eder, A. M. Silva, A. Santini and E. B. Souto, Molecules, 2020, 25, 3731 CrossRef CAS PubMed.
- A. Sani, C. Cao and D. Cui, Biochem. Biophys. Rep., 2021, 26, 100991 CAS.
- L. Xu, Y. Y. Wang, J. Huang, C. Y. Chen, Z. X. Wang and H. Xie, Theranostics, 2020, 10, 8996–9031 CrossRef CAS PubMed.
- A. Gnach, T. Lipinski, A. Bednarkiewicz, J. Rybka and J. A. Capobianco, Chem. Soc. Rev., 2015, 44, 1561–1584 RSC.
- V. Bastos, P. Oskoei, E. Andresen, M. I. Saleh, B. Rühle, U. Resch-Genger and H. Oliveira, Sci. Rep., 2022, 12, 3770 CrossRef CAS PubMed.
- K. Yorimoto, Y. Ariake, J. Ogawa, T. Saotome and Y. Kobayashi, Physiotherapy, 2015, 101, e82–e83 CrossRef.
- C. A. Speed, Therapeutic ultrasound in soft tissue lesions, Rheumatology, 2001, 40, 1331–1336 CrossRef CAS PubMed.
- E. Beltrán-Gracia, A. López-Camacho, I. Higuera-Ciapara, J. B. Velázquez-Fernández and A. A. Vallejo-Cardona, Cancer Nanotechnol., 2019, 10, 11 CrossRef.
- L. Hu, Y. Tao, Y. Jiang and F. Qin, Front. Cell Dev. Biol., 2023, 11, 1228679 CrossRef PubMed.
Footnote |
| † Equally contributed. |
| This journal is © The Royal Society of Chemistry 2024 |