Open Access Article
Open Access Article3D structured capillary cell suspensions aided by aqueous two-phase systems†
Amro K. F.
Dyab
 ab and
Vesselin N.
Paunov
ab and
Vesselin N.
Paunov
 *a
*a
aDepartment of Chemistry, Nazarbayev University, Kabanbay Batyr 53, Astana 010000, Kazakhstan. E-mail: vesselin.paunov@nu.edu.kz
bColloids & Advanced Materials Group, Chemistry Department, Faculty of Science, Minia University, Minia 61519, Egypt
First published on 8th October 2024
Abstract
We report a facile technique for 3D structuring of living cells by forming capillary cell suspensions based on an aqueous two-phase system (ATPS) of polyethylene glycol (PEG) and dextran (DEX) solutions. We demonstrate the formation of water-in-water (DEX-in-PEG) capillary bridges using concentrated suspensions of yeast cells which show enhanced rheological properties and distinctive 3D patterns. Capillary structured cell suspensions can potentially find applications in novel ways of 3D cell culturing, instant tissue engineering and many biomedical investigations.
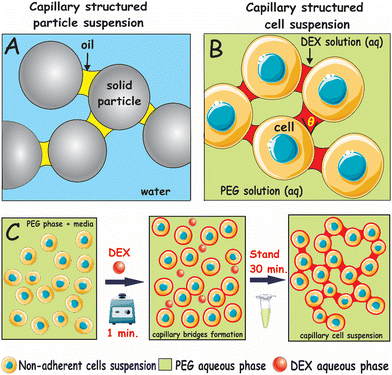
The use of capillary bridge forces to form capillary suspensions of solids with enhanced rheological properties has gained increased attention.1–3 Capillary suspensions are usually concentrated dispersions of solid particles in a continuous liquid phase doped with a very small fraction of a secondary immiscible liquid phase. The addition of the secondary phase can result in a marked increase of the yield stress of the suspension by several orders of magnitude.3–8 The capillary suspensions are formed in two different ways, depending on the wetting behaviour of the particles to the primary and the secondary liquid phases. If the secondary liquid phase wets the particles better than the primary (continuous) phase, it forms capillary bridges in a pendular state, and if the continuous phase wets the solid particles better than the secondary one, this corresponds to liquid bridges in a capillary state.7,9 In both cases, the secondary fluid forms an interconnected network of capillary bridged particles. Depending on the wetting properties of the particles, both oil-based and water-based capillary suspensions can be produced.3–6,8,10 Due to their tuneable rheological behaviour, capillary suspensions have been employed in several applications in food and materials science.7,10–12 A typical capillary suspension formed with solid particles is shown schematically in Fig. 1A where capillary bridges connect particles in clusters.
Aqueous two-phase systems (ATPS) have been used for the separation of substances and purification products based on the incompatibility of two aqueous solutions. ATPS include a polymer/salt system [e.g., polyethylene glycol (PEG) and potassium phosphate], a polymer/polymer system (e.g., PEG/dextran, DEX), an ionic liquid (IL) and a salt system, and a low molecular weight alcohol and a salt system.13 ATPS have recently been employed in biological applications where various scaffold-free techniques based on ATPS have been developed using multicellular spheroids (MCS), the simplest in vitro model of cell clusters, to achieve a larger yield with controlled cell cluster sizes and properties.9,14–26 Despite the growing interest in 3D cell culture based on ATPS based cell clusteroid/spheroid applications,12–17 there is virtually no significant work done so far on capillary suspensions based on ATPS as an immiscible pair of liquids.
This is not surprising, as the interfacial tension between the two aqueous phases (solutions of incompatible polymers) is usually very low, so one would expect the capillary forces resulting from the formation of capillary bridges to be relatively modest.
Here, we report for the first time a simple approach to fabricate a novel type of capillary suspensions of non-adherent living cells based on ATPS by introducing (by vortexing) a small volume fraction of DEX as a secondary aqueous phase, which wets the cells preferentially, into a suspension of cells in PEG as a bulk aqueous phase. Our protocol for cell capillary suspension fabrication is illustrated schematically in Fig. 1B and C for the cells dispersed in the PEG phase where structuring starts within 30 min of the mixing. The formation of these “gentle” capillary forces would be more than sufficient to overwhelm all other types of interactions between suspended cells and to create an extended network bridging the cells. This cost-effective and robust approach allows facile fabrication of structured cell capillary suspensions with a variety of different microstructures, controlled by the volume fraction of the added secondary immiscible liquid phase.
Here we use a biocompatible ATPS composed of aqueous solutions of 10 wt% of DEX (M.W. 40 kDa, Fisher Scientific, UK) and 10 wt% PEG (M.W. 200 kDa, Sigma-Aldrich, UK) where two separate aqueous phases can be obtained above the critical solution point of the liquid pair.9 The same ATPS system (DEX/PEG) forms in pure water as well as in the presence of the culture media (90 vol% DMEM and 10% FBS).27,28 In addition, there are reports published elsewhere where cell spheroids have been produced using the same ATPS from various human cell lines as HaCaT, HEP-G2, ECV304, HEK and HUVEC without a significant loss of cell viability – see ref. 9, 18, 19 and 27–32. Here we just demonstrate a proof of concept of using ATPS in cell capillary suspension as a new direction in this area. The only difference is the extremely small volume fraction of the DEX bridging phase.
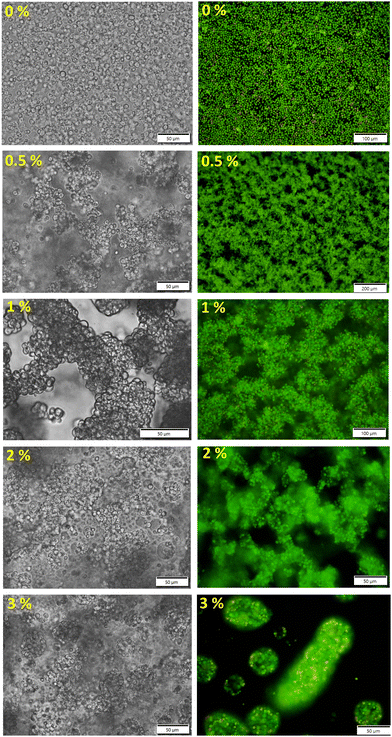
The DEX phase was fluorescently labelled with 1 mg mL−1 of tetramethyl rhodamine isothiocyanate-dextran (DEX-TRITC) for confocal laser scanning microscopy (CLSM). The yeast cells were labelled either with acridine orange solution in DI water (AO), 1 mg mL−1, for fluorescence microscopy or with 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) cell tracker stain for CLSM, labelling at 5 μM. The detailed staining protocols can be found in the ESI.†Fig. 2 shows a series of different microstructures of networked cell clusters obtained by altering the amount of added DEX (from 0 to 3 vol%) to the 10 wt% yeast cell suspension in PEG. Capillary structuring of the cell suspension at 0.5–1 vol% of DEX resulted in the formation of a fine network of interconnected cell clusters. However, the addition of the DEX phase above 1.5 vol% leads to the start of formation of fewer but larger capillary aggregates (engulfment of some cells by DEX) along with large and interconnected clusters of cells. These capillary aggregates transformed into cell clusteroids packed in the DEX drops with DEX-in-PEG emulsion morphology which predominate the microstructure of the cell suspensions at 3–5 vol% of DEX (see Fig. S2 and Video V1, ESI†). It is known that ATPS preserve cell integrity for cell-based applications.18,19,27–32 For cell separation purposes, it is not common to regulate the osmolarity of ATPS when polymers are dissolved in physiological media. Since the DEX phase and the PEG phase are in equilibrium in the ATPS, there is no change in the osmotic pressure around the cells when they transition from one of the aqueous phases to the other, or when a capillary bridge from the DEX phase is formed between them.
There are various human cell lines that have been cultured in DEX/PEG emulsion drops formed from the same ATPS (typically at a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 DEX
90 DEX![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG volume ratio) for up to 7 days without a significant loss of cell viability.18,19,23,33 Since our ATPS system is the same, only differing in the small volume fraction of DEX (typically 1
PEG volume ratio) for up to 7 days without a significant loss of cell viability.18,19,23,33 Since our ATPS system is the same, only differing in the small volume fraction of DEX (typically 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 DEX
99 DEX![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG), there are no changes in the conditions that may impact the cell viability. It has recently been reported that, for capillary suspensions with a ternary system of solid colloid particles and polar–nonpolar primary–secondary liquid pairs, the morphology of the system is dictated by the value of the fraction of the secondary liquid added and the wettability of the particles.7,33 To elucidate this point for the case of cells and the PEG/DEX ATPS, we measured the contact angle (θ) of the DEX and the PEG aqueous solutions on a yeast substrate layer, and both showed θ < 90°.
PEG), there are no changes in the conditions that may impact the cell viability. It has recently been reported that, for capillary suspensions with a ternary system of solid colloid particles and polar–nonpolar primary–secondary liquid pairs, the morphology of the system is dictated by the value of the fraction of the secondary liquid added and the wettability of the particles.7,33 To elucidate this point for the case of cells and the PEG/DEX ATPS, we measured the contact angle (θ) of the DEX and the PEG aqueous solutions on a yeast substrate layer, and both showed θ < 90°.
However, the DEX phase was found to wet the yeast cell layer preferentially when compared to the PEG phase (see Fig. S3 and Table S1, ESI†). In addition, the three-phase DEX/PEG/yeast contact angle was measured to be less than 90° (measured through the DEX phase). This indicates that the pendular/funicular states of the DEX bridges between the cells are preferred.2–6 One can observe both configurations on the CLSM images. We also attempted to form the opposite type of capillary suspension system, where the yeast cells were initially dispersed in DEX aqueous solution and then PEG solution was added in small volume percentage as a secondary liquid. However, no spanning structures were observed indicative of less affinity of the cells to the PEG phase and potential instability of PEG bridges in a capillary state (Fig. S4, ESI†).
To further examine the 2D and 3D morphologies of the capillary bridges that are responsible of connecting cells to form networked clusters, CLSM images were recorded in three channels as shown in Fig. 3A–D. Cell suspension in the PEG phase without DEX as a secondary phase showed random arrangements of cells (Fig. 3A and Video V2, ESI†), whereas predominant pendular state morphology was realized by the addition of 0.5 vol% DEX (Fig. 3B). Upon the addition of 1 vol% DEX, both pendular and capillary states are seen (Fig. 3C and Videos V3 and V4, ESI†). Capillary aggregates started to be the predominant morphology at 2 vol% DEX in the suspension (Fig. 3D and Video V5, ESI†). More CLSM images can be found in Fig. S5–S7 (ESI†) for these capillary structured cell morphologies. The results obtained from the CLSM images confirm the formation of capillary bridges resulting in different structuring morphologies by increasing the volume of the added DEX phase.
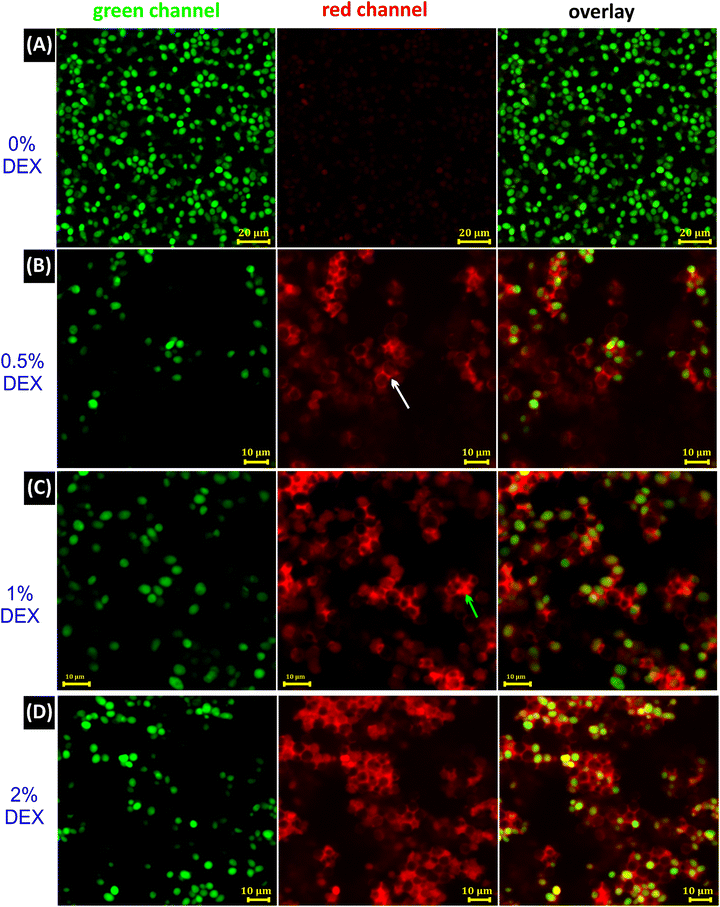 | ||
| Fig. 3 CLSM images of capillary suspensions of yeast cells recorded in XY slices in green, red and overlay channels. (A) Control sample of 10 wt% yeast cell suspension in PEG without the secondary liquid, DEX. (B) Sample with 0.5% (v/v) of the secondary phase (DEX), with the white arrow indicating a DEX bridge in a pendular state. (C) Sample with 1% (v/v) of DEX, with the green arrow indicating a DEX bridge in a capillary state. (D) Sample with 2% (v/v) of DEX, where the capillary aggregates are seen. Yeast cells were stained with CFDA-SE and are shown in green, DEX was stained with DEX-TRITC and is shown in red, and the continuous bulk phase (PEG) was unstained and is shown as a black background. Yeast suspensions in (B)–(D) were diluted in the PEG phase for clearer imaging after preparation. In overlay images, some yeast cells appeared unstained based on their position with respect to the slice and cluster density (see Video S2 in the ESI†). All images were recorded after 24 h incubation at 37 °C. | ||
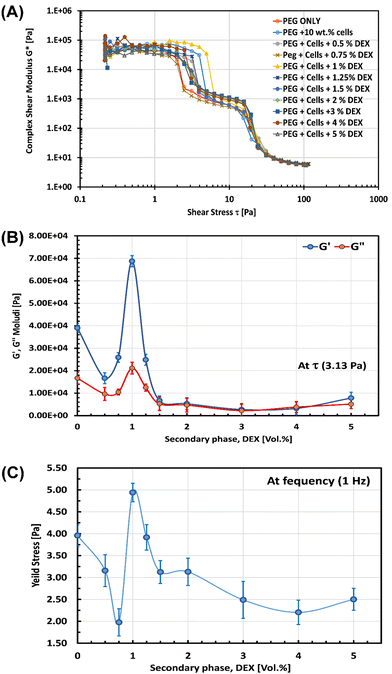
We also examined the rheological behaviour of the obtained capillary structured cell suspension as a function of vol% of the DEX phase. A shear stress amplitude sweep test was carried out using oscillatory rheometry (details can be seen in the ESI†) with oscillating stress ranging from 0.01 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa in the ramp logarithmic profile at a constant frequency of 1 Hz and at 25 °C. We found that, as a function of shear stress, the complex modulus of the 10 wt% yeast suspensions initially decreased upon the addition of 0.5 vol% of DEX phase, reaching the maximum when 1 vol% DEX was added, and declined again with 2–5 vol% DEX as evident from Fig. 4A, indicative of the formation of the most stable network of inter-bridged cells at 1 vol% DEX as a result of optimal formation of DEX-capillary bridges between the cells. In line with that, the storage, G′, and the loss, G′′, moduli were plotted against the vol% of added DEX at 3.13 Pa applied stress where the G′ was higher than that at 0 vol% DEX by about 3 orders of magnitude for the capillary suspension formed with 1 vol% DEX as depicted in Fig. 4B.
000 Pa in the ramp logarithmic profile at a constant frequency of 1 Hz and at 25 °C. We found that, as a function of shear stress, the complex modulus of the 10 wt% yeast suspensions initially decreased upon the addition of 0.5 vol% of DEX phase, reaching the maximum when 1 vol% DEX was added, and declined again with 2–5 vol% DEX as evident from Fig. 4A, indicative of the formation of the most stable network of inter-bridged cells at 1 vol% DEX as a result of optimal formation of DEX-capillary bridges between the cells. In line with that, the storage, G′, and the loss, G′′, moduli were plotted against the vol% of added DEX at 3.13 Pa applied stress where the G′ was higher than that at 0 vol% DEX by about 3 orders of magnitude for the capillary suspension formed with 1 vol% DEX as depicted in Fig. 4B.
A similar trend was observed in the measured yield stress of 10 wt% cell suspensions as a function of added vol% of the DEX phase where a maximum yield stress was reached at 1 vol% of DEX as shown in Fig. 4C. This is in agreement with some typical capillary suspensions with solid particles where a maximum in the yield stress versus the volume fraction of the secondary liquid phase has also been reported.3–5,12 Therefore, cell structured suspensions using an ATPS as primary and secondary liquid phases conforms to similar trends and relationships despite both phases being aqueous solutions. These findings are consistent with the optical, fluorescence and CLSM results shown above where the added secondary liquid plays a crucial rule in the resulting microstructure of the formed systems. It is suggested that the initial decrease in the yield stress upon the addition of 0.5–0.75 vol% DEX (Fig. 4C) might be due to the formation of a thin film of the secondary liquid phase (DEX) on the cell surface that hinders the cell–cell surface interactions. However, upon increasing the added DEX phase to 1 vol%, the number and state morphology of formed DEX bridges significantly increased the complex modulus and yield stress, giving rise to a stable capillary-structured cell suspension. A further increase of the DEX phase above 1 vol% leads to another decrease in the yield stress which could be linked to the start of formation of separate capillary aggregates and cells packed in DEX-in-PEG emulsions as shown in Fig. 2 and Fig. S7 (ESI†), where the cell–cell interactions were minimized.
In summary, we have demonstrated an innovative and robust strategy devised to produce interconnected cell clusters with different microstructures and morphologies from cell capillary suspensions. The latter was achieved by the addition of small fractions of a secondary aqueous liquid to another aqueous immiscible bulk phase, both giving an ATPS. The immiscibility of the two polymeric solutions (PEG and DEX) of the primary and secondary aqueous phases leads to rapid capillary structuring of the cell suspension and the formation of networks. It is worth noting that this approach works even with non-adherent S. cerevisiae (yeast) cells, just using the ATPS of dextran solution as a second phase and polyethylene glycol solution as a bulk phase. Different cell microstructures can be obtained, and the most stable capillary structured system was realized at 1 vol% of the secondary phase that bridged the cells. The results from the oscillatory rheological test are consistent with the different cell microstructures formed. The present capillary structuring strategy of cell suspension generation can be tested in different culture media and applied to other types of adherent cells, e.g. fibroblasts or tumour cells in moulds for scaffold free tissue engineering that would find potential application in 3D cell culturing and biomedical engineering studies which are currently underway.
Author contributions
The manuscript was written through contributions of all authors. V. N. P. gave the idea of the study, conceptualized it as a project, hosted the visiting professor A. K. F. D. and provided the methodology and materials, directed the research and reviewed and edited the draft of the manuscript. A. K. F. D. participated in the conceptualization of the study, performed the experiments, collected experimental data and images, and prepared the figures and the first draft of the manuscript. All authors have given approval to the final version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP23485273). A.K.F.D. thanks Nazarbayev University for supporting his visiting professorship.References
- S. Bindgen, J. Allard and E. Koos, Curr. Opin. Colloid Interface Sci., 2022, 58, 101557 CrossRef.
- T. Domenech and S. S. Velankar, Soft Matter, 2015, 11, 1500–1516 RSC.
- E. Koos and N. Willenbacher, Science, 2011, 331, 897–900 CrossRef PubMed.
- A. A. K. Das, T. S. Dunstan, S. D. Stoyanov, P. Starck and V. N. Paunov, ACS Appl. Mater. Interfaces, 2017, 9, 44152–44160 CrossRef PubMed.
- E. Koos, Curr. Opin. Colloid Interface Sci., 2014, 19, 575–584 CrossRef PubMed.
- T. Domenech and S. Velankar, Rheol. Acta, 2014, 53, 593–605 CrossRef.
- S. Bindgen, J. Allard and E. Koos, Curr. Opin. Colloid Interface Sci., 2022, 58, 101557 CrossRef CAS.
- T. S. Dunstan, A. A. K. Das, P. Starck, S. D. Stoyanov and V. N. Paunov, Langmuir, 2018, 34, 442–452 CrossRef CAS PubMed.
- A. Wang, L. A. Madden and V. N. Paunov, J. Mater. Chem. B, 2020, 8, 10487–10501 RSC.
- S. Roh, D. P. Parekh, B. Bharti, S. D. Stoyanov and O. D. Velev, Adv. Mater., 2017, 29, 1701554 CrossRef.
- S. Hoffmann, E. Koos and N. Willenbacher, Food Hydrocolloids, 2014, 40, 44–52 CrossRef CAS.
- J. Zhang, H. Zhao, W. Li, M. Xu and H. Liu, Sci. Rep., 2015, 5, 16058 CrossRef CAS.
- M. Iqbal, Y. Tao, S. Xie, Y. Zhu, D. Chen, X. Wang, L. Huang, D. Peng, A. Sattar, M. A. B. Shabbir, H. I. Hussain, S. Ahmed and Z. Yuan, Biol. Proced. Online, 2016, 18, 18 CrossRef.
- A. Wang, L. A. Madden and V. N. Paunov, Bioengineering, 2022, 9, 126 CrossRef CAS PubMed.
- V. Mironov, R. P. Visconti, V. Kasyanov, G. Forgacs, C. J. Drake and R. R. Markwald, Biomaterials, 2009, 30, 2164–2174 CrossRef CAS.
- K. E. Kasza, A. C. Rowat, J. Liu, T. E. Angelini, C. P. Brangwynne, G. H. Koenderink and D. A. Weitz, Curr. Opin. Cell Biol., 2007, 19, 101–107 CrossRef CAS PubMed.
- Y. L. Insung Choi and Rawil F. Fakhrullin, Cell Surface Engineering: Fabrication of Functional Nanoshells, 2014.
- A. Wang, L. A. Madden and V. N. Paunov, Mater. Adv., 2020, 1, 3022–3032 RSC.
- A. Wang, L. A. Madden and V. N. Paunov, ACS Appl. Bio Mater., 2022, 5, 1804–1816 CrossRef PubMed.
- T. Sato, R. G. Vries, H. J. Snippert, M. Van De Wetering, N. Barker, D. E. Stange, J. H. Van Es, A. Abo, P. Kujala, P. J. Peters and H. Clevers, Nature, 2009, 459, 262–265 CrossRef PubMed.
- D. Huh, D. C. Leslie, B. D. Matthews, J. P. Fraser, S. Jurek, G. A. Hamilton, K. S. Thorneloe, M. A. McAlexander and D. E. Ingber, Sci. Transl. Med., 2012, 4, 159ra147 Search PubMed.
- J. Friedrich, R. Ebner and L. A. Kunz-Schughart, Int. J. Radiat. Biol., 2007, 83, 849–871 CrossRef.
- D. Kloß, M. Fischer, A. Rothermel, J. C. Simon and A. A. Robitzki, Lab Chip, 2008, 8, 879–884 RSC.
- L. A. Kunz-Schughart, J. P. Freyer, F. Hofstaedter and R. Ebner, J. Biomol. Screening, 2004, 9, 273–285 CrossRef CAS PubMed.
- R. Z. Lin and H. Y. Chang, Biotechnol. J., 2008, 3, 1172–1184 CrossRef CAS PubMed.
- A. G. Teixeira, R. Agarwal, K. R. Ko, J. Grant-Burt, B. M. Leung and J. P. Frampton, Adv. Healthcare Mater., 2018, 7, 1701036 CrossRef.
- A. A. K. Das, B. W. Filby, D. A. Geddes, D. Legrande and V. N. Paunov, Mater. Horiz., 2017, 4, 1196–1200 RSC.
- S. B. Celik, S. R. Dominici, B. W. Filby, A. A. Das, L. A. Madden and V. N. Paunov, Biomimetics, 2019, 4, 50 CrossRef CAS PubMed.
- M. González-González and M. Rito-Palomares, J. Chem. Technol. Biotechnol., 2020, 95, 8–10 CrossRef.
- M. González-González and M. Rito-Palomares, Crit. Rev. Biotechnol., 2014, 34, 318–327 CrossRef.
- H. Tavana, A. Jovic, B. Mosadegh, Q. Y. Lee, X. Liu, K. E. Luker, G. D. Luker, S. J. Weiss and S. Takayama, Nat. Mater., 2009, 8, 736–741 CrossRef CAS.
- K. Chairez-Cantu, M. González-González and M. Rito-Palomares, Food Bioprod. Process., 2023, 139, 157–165 CrossRef CAS.
- S. S. Velankar, Soft Matter, 2015, 11, 8393–8403 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01296h |
| This journal is © The Royal Society of Chemistry 2024 |