Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Breakthrough in cancer therapy: lutetium texaphyrin–celecoxib conjugate for immune and photodynamic treatment
Qihang
Ding†
a,
Yue
Wang†
b,
Pengfei
Zhang
 *b and
Ling
Mei
*c
*b and
Ling
Mei
*c
aDepartment of Chemistry, Korea University, Seoul 02841, Korea
bGuangdong Key Laboratory of Nanomedicine, CAS-HK Joint Lab of Biomaterials, CAS Key Labora-tory of Biomedical Imaging Science and System, Institute of Biomedicine and Biotechnology, Shenzhen Institute of Advanced Technology (SIAT), Chinese Academy of Sciences, Shenzhen 518055, China. E-mail: pf.zhang@siat.ac.cn
cEngineering Research Center for Pharmaceuticals and Equipments of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu 610106, China. E-mail: meiling@cdu.edu.cn
First published on 7th October 2024
Abstract
Immuno-photodynamic therapy (IPDT) has become a promising approach for cancer treatment. Innovative photosensitizers are essential to fully realize the potential of IPDT, specifically the complete elimination of tumors without recurrence. In this context, Jong Seung Kim et al. introduce a small molecule photosensitizer conjugate, LuCXB. This IPDT agent combines a celecoxib (cyclooxygenase-2 inhibitor) moiety with a near-infrared absorbing lutetium texaphyrin photocatalytic core. In aqueous solutions, the two components of LuCXB self-associate through inferred donor–acceptor interactions. As a result of this intramolecular association, LuCXB generates superoxide radicals (O2−˙) via a type I photodynamic pathway upon irradiation with 730 nm light. This serves as a primary defense against the tumor and enhances the IPDT effect. For in vivo applications, they developed a CD133-targeting, aptamer-functionalized exosome-based nanophotosensitizer (Ex-apt@LuCXB) aimed at targeting cancer stem cells. Ex-apt@LuCXB demonstrated excellent photosensitivity, satisfactory biocompatibility, and strong tumor-targeting capabilities. Under photoirradiation, Ex-apt@LuCXB amplifies IPDT and produces significant antitumor effects in liver and breast cancer mouse models. The therapeutic outcomes are attributed to a synergistic mechanism that combines antiangiogenesis with photoinduced cancer immunotherapy.
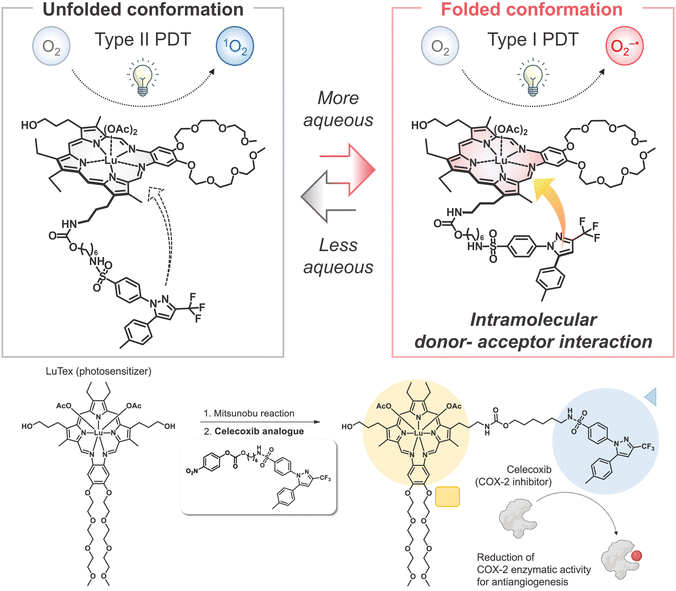
Cancer remains a significant global health issue, with current treatment methods often necessitating a delicate balance between efficacy and side effects.1 Recently, immuno-photodynamic therapy (IPDT) has emerged as a promising novel treatment modality. In this study, Jong Seung Kim's team reported the potential application of an innovative lutetium texaphyrin–celecoxib conjugate (LuCXB) for IPDT.2 This groundbreaking photosensitizer combines celecoxib, a cyclooxygenase-2 inhibitor, with a near-infrared absorbing lutetium texaphyrin photocatalytic core to enhance the effectiveness of photodynamic therapy and overcome challenges posed by hypoxic tumor microenvironments.3,4
The conjugation of LuCXB results in the formation of a distinctive photosensitizer by combining the cyclooxygenase-2 inhibitor (celecoxib) with a lutetium texaphyrin photocatalytic core. This unique combination not only facilitates the generation of superoxide radicals (O2−˙) through photocatalysis but also enables excitation using near-infrared light at 730 nm, allowing it to effectively operate within deep tissues. Traditional photodynamic therapy often relies on oxygen for producing reactive oxygen species (ROS), which limits its efficacy in hypoxic environments.5,6 In contrast, LuCXB generates superoxide radicals through a type I photodynamic pathway, ensuring its effectiveness even under hypoxic conditions. Moreover, the study utilizes an Ex-apt@LuCXB, a CD133-targeted and aptamer-functionalized exosome-based nanoparticle photosensitizer. This innovative approach precisely targets and attacks cancer stem cells, thereby enhancing treatment precision while minimizing potential side effects. In vivo experiments conducted on liver and breast cancer mouse models have demonstrated significant antitumor effects, highlighting the practical applications of this targeted strategy and its biocompatibility.7,8
The mechanism of LuCXB primarily relies on the type I photodynamic pathway, which generates superoxide radicals upon near-infrared light irradiation. These radicals possess high cytotoxicity within the tumor microenvironment. Additionally, LuCXB exhibits a dual mechanism of action: it inhibits tumor growth through anti-angiogenesis and enhances antitumor immunity via photoinduced immune responses. This synergistic mechanism not only directly attacks tumor cells but also activates the immune system to further eliminate residual cancer cells, thereby reducing the likelihood of tumor recurrence.
Despite the promising potential demonstrated by LuCXB in preliminary studies, further research and optimization are required in several areas. Firstly, it is essential to enhance the photostability of LuCXB to ensure its stability and effectiveness under physiological conditions and during light exposure. Additionally, optimizing the design of the conjugate is crucial for maximizing ROS generation in hypoxic tumor environments.9 Detailed mechanistic studies are also necessary to elucidate the molecular pathways through which LuCXB induces tumor cell death and its role in immune responses. Understanding how LuCXB adapts and functions in hypoxic environments will further refine its application in solid tumors. On the other hand, biocompatibility and safety are crucial for clinical applications, necessitating comprehensive long-term safety studies to evaluate the potential adverse effects of LuCXB and its derivatives in vivo. Investigating the impact of repeated treatments on the immune system is also imperative to ensure absence of any unfavourable immune reactions. In terms of clinical translation, developing cost-effective and scalable synthesis methods for LuCXB and related nanoparticle photosensitizers is vital to meet the demands of large-scale production. Navigating the intricate regulatory landscape to ensure compliance with safety and efficacy standards for new therapies poses another significant challenge. Lastly, conducting comparative studies to benchmark the efficacy and safety of LuCXB against existing photodynamic therapy agents and other cancer treatments is essential in establishing its advantages and clinical potential.10,11
In summary, the LuCXB represents a significant innovation and potential in the field of immuno-photodynamic therapy. Its efficacy in hypoxic tumor environments and enhanced antitumor effects through dual mechanisms make it a breakthrough in cancer treatment. However, addressing challenges related to conjugate design, mechanistic studies, biocompatibility, safety, clinical translation, and comparative research is crucial for its successful development. By resolving these issues comprehensively, LuCXB and its derivatives have the potential to offer a novel and effective treatment option for cancer patients, instilling new hope into cancer therapy (Fig. 1).
Data availability
This study was carried out using publicly available data from Lutetium Texaphyrin–Celecoxib Conjugate as a Potential Immuno-Photodynamic Therapy Agent Journal of the American Chemical Society at https://pubs.acs.org/doi/10.1021/jacs.4c05978.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from National Key R&D Programs (China) (2021YFA0910001), Guangdong Provincial Key Area R&D Program (2020B1111540001), the Shenzhen Science and Technology Program (KQTD20210811090115019), Shenzhen Basic Research (key project) (China) (JCYJ20210324120011030, JCYJ20210324115804013), the Major Instrumentation Development Program of the Chinese Academy of Sciences (Project Number: ZDKYYQ20220008).Notes and references
- Q. Ding, Y. Luo, J. Hu, S. Zhang, W. Zhang, Y. Feng, K. Qian, X. Li, Z. Cheng and M. Gu, Chem. Eng. J., 2024, 495, 153395 CrossRef CAS.
- J. An, K. Lv, C. V. Chau, J. H. Lim, R. Parida, X. Huang, S. Debnath, Y. Xu, S. Zheng, A. C. Sedgwick, J. Y. Lee, D. Luo, Q. Liu, J. L. Sessler and J. S. Kim, J. Am. Chem. Soc., 2024, 146, 19434–19448 CrossRef CAS PubMed.
- Y. Liu, M. Gu, Q. Ding, Z. Zhang, W. Gong, Y. Yuan, X. Miao, H. Ma, X. Hong, W. Hu and Y. Xiao, Angew. Chem., Int. Ed., 2023, 62, e202214875 CrossRef CAS PubMed.
- Q. Ding, X. Xu, Y. Li, B. Li, Q. Saiding, M. Gu, W. Tao, B. Z. Tang and J. S. Kim, Chem, 2024, 10, 2031–2073 Search PubMed.
- S. Li, Q. Ding, L. Zhang, F. Shi, C. Liu, T. Li, Y. Shi, M. Qi, L. Wang, B. Dong, S. Song, J. Sun, J. S. Kim and C. Li, J. Controlled Release, 2024, 370, 600–613 CrossRef CAS PubMed.
- L. Tu, C. Li, Q. Ding, A. Sharma, M. Li, J. Li, J. S. Kim and Y. Sun, J. Am. Chem. Soc., 2024, 146, 8991–9003 CrossRef CAS PubMed.
- P. Devant and J. C. Kagan, Nat. Immunol., 2023, 24, 1064–1075 CrossRef CAS PubMed.
- Q. Ding, X. Wang, Y. Luo, X. Leng, X. Li, M. Gu and J. S. Kim, Coord. Chem. Rev., 2024, 508, 215772 CrossRef CAS.
- Q. Ding, M. He and M. Gu, Sci. China: Chem., 2024, 67, 1–2, DOI:10.1007/s11426-024-2224-9.
- Q. Yang, X. Sun, Q. Ding, M. Qi, C. Liu, T. Li, F. Shi, L. Wang, C. Li and J. S. Kim, Natl. Sci. Rev., 2024, 11, nwae225 CrossRef PubMed.
- Q. Ding, L. Mei, Y. Liu, S. Zhou, L. Chen, Y. Liang, M. Li, H. Zhou, C. Yin and J. S. Kim, Chem. Commun., 2023, 59, 8127–8130 RSC.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |