Simple synthesis of carbon dots/organosilicon composites with tunable solid-state emission and size for accurate latent fingerprint identification†
Xiyue
Cao
a,
Jiashi
Chen
a,
Yue
Chen
a,
Xuanfeng
Jiang
 a,
Wen
Fan
a,
Huijuan
Ma
b,
Zhengguang
Sun
*a and
Yuan
Zhan
a,
Wen
Fan
a,
Huijuan
Ma
b,
Zhengguang
Sun
*a and
Yuan
Zhan
 *a
*a
aKey Laboratory for the Green Preparation and Application of Functional Materials, Ministry of Education, Hubei Key Laboratory of Polymer Materials, School of Materials Science and Engineering, Hubei University, Wuhan 430062, China. E-mail: sunshine@hubu.edu.cn; zy@hubu.edu.cn
bHubei Three Gorges Laboratory, Yichang 443000, China
First published on 17th November 2023
Abstract
Carbon dots (CDs) have desirable prospects for replacing conventional fluorescent materials in the field of fingerprint powder due to their attractive fluorescence, environmental friendliness, and stability. Designing solid-state fluorescent CD-based composites with suitable sizes for accurate latent fingerprint identification is still a huge challenge. Herein, CDs embedded in organosilicon (CDs–OSi) composites were synthesized by utilizing the reaction between 3-ureidopropyl triethoxy silane and L-(−)-malic acid. The obtained CDs–OSi composites display tunable fluorescence from blue to green just by controlling the volume ratio of H2O and ethanol in a solvothermal process. In particular, CDs–OSi composites simultaneously exhibit a controllable and uniform size from 0.12 μm to 5 μm. The CDs–OSi composites with different emissions and sizes were further applied to detect latent fingerprints (LFPs). The results show that CDs–OSi composites can be easily adsorbed onto the grease of the fingerprint, and level 2 and level 3 characteristics can be clearly identified by a simple powder dusting method. Furthermore, CDs–OSi composites are validated for the collection and identification of LFPs on different substrates. The results prove that CDs–OSi composites have great development prospects as developers in LFP visualization for forensic investigations.
1. Introduction
Fingerprints show one of the highest levels of reliability and are used extensively in various security applications due to their characteristics of being unique to an individual and remaining unchanged throughout a person's life.1,2 Latent fingerprints (LFPs) are the universal type of vital evidence for personal identification in criminal investigations of crime scenes.3 The details of fingerprints can be classified into three levels. Level 1 characteristics are global features of the fingerprint, including arches, loops, whorls, mixed patterns, and creases. Level 2 characteristics refer to the macroscopic and detailed local features of handprinted lines, such as bifurcation, combination, starting points, end points, small points, small sticks, small hooks, small eyes, and small bridges. Level 3 characteristics are microscopic details of fingerprints, mainly including ridge edge shape, ridge width, scars, and pores.4–6 For identity authentication through fingerprints, it is necessary to achieve the resolution of level 2 and level 3 characteristics.7 LFPs are generally invisible, and various methods, such as physical imaging techniques, chemical methods and fluorescent sensor imaging, have been explored for their collection and visualization.8–10 However, traditional physical methods usually have problems of low sensitivity and development contrast, while chemical techniques can be harmful and damaging by using iodine, ninhydrin or silver nitrate.11,12 Fluorescent sensors and imaging are an effective strategy to develop LFPs, in which fluorescent materials dusted on LFPs can effectively enhance the developing signal with a light source.13,14At present, various fluorescent materials have been developed for LFP recording and analysis, including organic fluorophores, aggregation-induced emission (AIE) materials, organometallics, and semiconductor quantum dots.15,16 But these materials also exhibit some inherent shortcomings. For example, organic fluorophores, like rhodamine composites, easily stain the surface of substrates, so that the lines of a fingerprint cannot be drawn clearly due to background interference.17 AIE materials usually involve the use of environmentally unfriendly organic solvents with long incubation time, which can easily cause damage to fingerprints.18 Organometallics and semiconductor quantum dots typically use expensive metal or toxic heavy metal ions.19 Therefore, it is highly desirable to design safe and excellent novel fluorescent materials to enhance LFP development.
Carbon dots (CDs) might be promising candidates for fingerprint analysis owing to their simple preparation, controlled fluorescence, low cost, environmental friendliness and easy functionalization.20–23 CDs have an abundance of surface functional groups, which can combine with fingermark residues by covalent coupling or electrostatic adsorption to develop the characteristics of fingermarks.24 Bandosz reported N-doped CDs for developing the recognition of LFPs.25 However, CDs frequently undergo fluorescence quenching in an agglomerated state. A spraying method is usually selected to capture a fingerprint. As a CD dispersal medium, H2O is usually difficult to adhere stably on smooth surfaces.26 But an organic solvent would be corrosive to most objects, such as rubber and paint layers.27 Subsequently, Xiong reported red-emissive CDs mixed with starch to form R-CDs/starch phosphors, and the bright red fluorescence of R-CDs/starch phosphors lit up LFPs, showing very clear ridge patterns with a typical powder dusting method.28 Very recently, Lu utilized molecular self-assembly to prepare four chlorosalicylaldehyde-functionalized CDs with tunable emission ranging from blue to yellow, as well as changing morphologies from three-dimensional to one-dimensional.29 The efficiency of developing latent fingerprints with fluorescent materials is determined by several main factors, including the emissive color and intensity, morphology, size, and powder dispersion of the developer.30 However, designing solid-state fluorescent CD-based composites with multicolored solid-state emission, uniform size and high dispersion for accurate latent fingerprint identification remains a great challenge.
In this paper, we provide a simple strategy to synthesize CDs embedded in organosilicon (CDs–OSi) composites with controllable fluorescence from blue to green, accompanied by increasing size from 0.12 μm to 5 μm by adjusting the volume ratio of H2O and ethanol in a solvothermal process. The CDs–OSi composites are used as developers to identify LFPs on several substrates by a powder-dusting method. It is found that CDs–OSi composites with different luminescent colors, and appropriate sizes can be selected for LFP display on different substrates, which can provide details of the fingermarks with effective definition and contrast. This work not only provides an efficient strategy for the preparation of CD-based materials with tunable solid-state fluorescence and size, but also provides a versatile, adaptable, nontoxic, and cheap solution suitable for fingermark development at crime scenes.
2. Experimental section
2.1 Materials
L-(−)-malic acid (99%) was obtained from Energy Chemical. 3-Ureidopropyl triethoxy silane (UPTES, 40.0–50.0% in methanol) was purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. Bromobenzene (AR) was produced by Damao Chemical Reagent Factory. Ethanol (EtOH, ≥99.7%), acetone (AR), trichloromethane (TM, AR), carbon tetrachloride (CTC, CP), methanol (CP), dimethyl sulfoxide (DMSO, CP), potassium chloride (KCl, AR) and sodium hydroxide (NaOH, AR) were purchased from Sinopharm Group Chemical Reagent Co. Ltd. Hydrochloric acid (HCl, AR) was purchased from Xinyang Chemical Reagent Factory.2.2 Preparation of CDs–OSi composites
For the preparation of the CDs–OSi composites, 0.134 g (1 mmol) of L-(−)-malic acid was dissolved in a mixed solvent consisting of distilled H2O and EtOH (total volume: 18 mL, volume ratios of distilled H2O and EtOH of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 12
3, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 9
6, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 6
9, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 and 3
12 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, respectively). Then 0.87 g (3 mmol) of UPTES was added into the solution and it was kept under stirring for 5 min. The above mixed solution was transferred to a Teflon-lined stainless-steel autoclave and reacted for 12 h in a constant-temperature air blast drying oven at 180 °C. After cooling naturally to room temperature, the obtained mixtures were first centrifuged using distilled H2O three times at 8000 rpm for 5 min; then the above process was repeated using EtOH to remove organic moieties. The CDs–OSi composites were then obtained by drying in an oven at 55 °C.
15, respectively). Then 0.87 g (3 mmol) of UPTES was added into the solution and it was kept under stirring for 5 min. The above mixed solution was transferred to a Teflon-lined stainless-steel autoclave and reacted for 12 h in a constant-temperature air blast drying oven at 180 °C. After cooling naturally to room temperature, the obtained mixtures were first centrifuged using distilled H2O three times at 8000 rpm for 5 min; then the above process was repeated using EtOH to remove organic moieties. The CDs–OSi composites were then obtained by drying in an oven at 55 °C.
2.3 Fingerprint imaging
Fingerprints were collected from the index finger of one individual volunteer. First, the volunteer's index finger was washed with EtOH. After drying, the forefinger was wiped on the nose or forehead three times to stain it with sweat and grease. Then LFPs were left on several surfaces of different materials, including aluminum foil, glass, plastic bags, and transparent plastic sheets, and the CDs–OSi composite was applied to the LFP surface using a feather brush until a fingerprint appeared. Finally, the fingerprint images were obtained using a stereoscopic microscope (connected to a computer) and iPhone 13 separately under 365 nm ultraviolet (UV) light or LED white light.2.4 Characterization
The morphology of the CDs–OSi composites was observed using field-emission scanning electron microscopy (FESEM) under an accelerating voltage of 5 kV. High-resolution transmission electron microscopy (HRTEM) images were obtained with a JEOL JEM 2100F electron microscope. Fourier-transform infrared spectroscopy (FT-IR) spectra were collected on a Thermo Fisher Nicolet iS50 Spectrometer with KBr pellets. An Escalab 250Xi X-ray photoelectron spectrometer was used to obtain X-ray photoelectron spectroscopy (XPS, Thermo Fisher) measurements to analyze the surface composition and chemical state of the product with mono X-ray source Al Kα excitation (1486.6 eV). The binding energy was calibrated with a C1s peak at 284.8 eV. Avantage software was used to fit the XPS spectra. A conjunct Gaussian–Lorentzian function with Shirley background subtraction was applied in the XPS data fitting. The main peak at 284.8 eV attributed to hydrocarbon contamination was used as an internal reference to calibrate the spectra. Crystallographic information was obtained using powder X-ray diffraction (P-XRD) equipped with a D8 Advance X-ray diffractometer (Bruker, Cu KR radiation (λ = 1.5406 Å)). A fluorescence spectrophotometer (PicoQuant FluoTime 300) was used to measure the fluorescence spectra. The photoluminescence quantum yield (PLQY) and fluorescence lifetime curves (420 nm laser) were obtained with the fluorescence spectrophotometer with an integrating sphere. A UV-3600 spectrophotometer (Shimadzu Corp, Tokyo, Japan) was used to record the UV-visible absorption spectra.3. Results and discussion
CDs–OSi composites were directly synthesized by solvothermal reaction of H2O/EtOH medium at 180 °C for 12 h (Fig. 1a). L-(−)-malic acid, like citric acid, possesses a few oxygen-containing functional groups, which can easily carbonize to CDs in the solvothermal process. Furthermore, there are abundant active groups (like hydroxyl and carboxyl) on the surface of these CDs, which can react with the amino groups of UPTES to form nitrogen-related species under solvothermal conditions.31,32 Through further hydrolysis and condensation of UPTES, solid-state composites of CDs–OSi can be prepared. It should be noted that the resulting CDs–OSi composites are insoluble in the H2O/EtOH reaction medium, and they could be easily collected as a powder after centrifugation and drying. These CDs–OSi powders gradually become darker brown in the daylight (Fig. 1b, top). More interestingly, the emission color of the CDs–OSi composites changes from blue to green under 365 nm UV light (Fig. 1b, bottom) just by tuning the volume ratio of H2O/EtOH.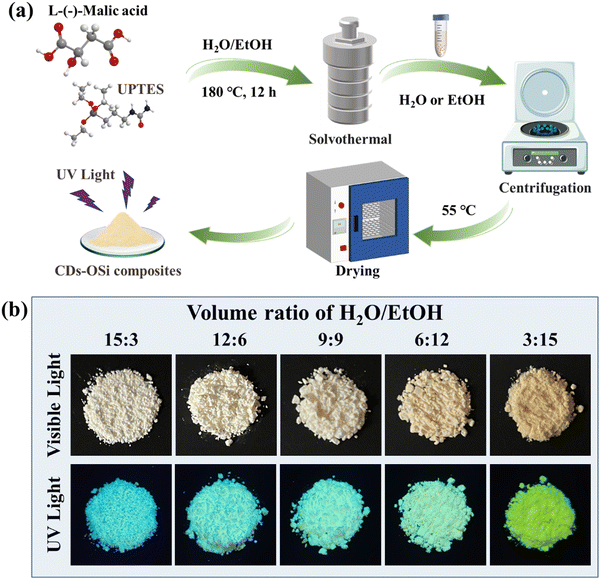 | ||
| Fig. 1 (a) Synthesis route of the CDs–OSi composites. (b) Optical images of CDs–OSi composite synthesis at different volume ratios of H2O/EtOH. | ||
The chemical structure and composition of the CDs–OSi composites were first investigated using FT-IR and XPS spectra. The FT-IR spectra of the five CDs–OSi composites are similar (Fig. S1, ESI†), indicating their similar chemical compositions. Several sharp bands at 1563 cm−1, 1652 cm−1, and 1709 cm−1 should be noted, which can be attributed to the stretching band of the –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, and sharp bands at 3011–2824 cm−1, which can be assigned to stretching vibrations of the –NHCH2CH2– group.33 In addition, there is a peak at 1770 cm−1, which can be assigned to the C
O group, and sharp bands at 3011–2824 cm−1, which can be assigned to stretching vibrations of the –NHCH2CH2– group.33 In addition, there is a peak at 1770 cm−1, which can be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of imide.34 These characteristic bands confirm that the reaction successfully took place between the –NH2 of the UPTES and the –COOH of L-(−)-malic acid during the synthesis process. The presence of obvious Si–O–Si and Si–O–C bond stretching vibrations is found at 1129 and 1027 cm−1, respectively. This proves that hydrolytic polycondensation of UPTES forms an “Si–O–Si” network structure to coat the CDs.35 The XPS spectrum of the CDs–OSi composites demonstrates the presence of carbon (C), silicon (Si), oxygen (O), and nitrogen (N) elements (Fig. S2a, ESI†). As shown as Fig. S2b (ESI†), the content of C, N, O, and Si elements approaches that of CDs–OSi composites prepared with different H2O/EtOH volume ratios, so it can be concluded that the content of doped element is not a factor causing the redshift of the emission wavelength of CDs–OSi composites. Analysis of the high-resolution scans (Fig. S2c–e, ESI†) reveals that the C 1s band can be divided into four peaks at around 283.88, 284.80, 285.85, and 287.50 eV, belonging to C–Si, C–C, C–N, and C
O stretching vibration of imide.34 These characteristic bands confirm that the reaction successfully took place between the –NH2 of the UPTES and the –COOH of L-(−)-malic acid during the synthesis process. The presence of obvious Si–O–Si and Si–O–C bond stretching vibrations is found at 1129 and 1027 cm−1, respectively. This proves that hydrolytic polycondensation of UPTES forms an “Si–O–Si” network structure to coat the CDs.35 The XPS spectrum of the CDs–OSi composites demonstrates the presence of carbon (C), silicon (Si), oxygen (O), and nitrogen (N) elements (Fig. S2a, ESI†). As shown as Fig. S2b (ESI†), the content of C, N, O, and Si elements approaches that of CDs–OSi composites prepared with different H2O/EtOH volume ratios, so it can be concluded that the content of doped element is not a factor causing the redshift of the emission wavelength of CDs–OSi composites. Analysis of the high-resolution scans (Fig. S2c–e, ESI†) reveals that the C 1s band can be divided into four peaks at around 283.88, 284.80, 285.85, and 287.50 eV, belonging to C–Si, C–C, C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.36,37 The N 1s spectrum manifests peaks at around 399.05 and 400.35 eV that are attributed to N–H and N–C, respectively (Fig. S2f–h, ESI†).38 The Si 2p spectrum is fitted with two peaks at around 101.73 and 102.24 eV for Si–C and Si–O, respectively (Fig. S2i–k, ESI†).39 All the XPS spectra show that the five CDs–OSi composites possess the same chemical bonds, in agreement with the FT-IR analysis. According to the above results, we can see that the tunable H2O/EtOH volume ratio has a slight influence on the chemical structure and composition of CDs–OSi composites, with regard to their emission.
O, respectively.36,37 The N 1s spectrum manifests peaks at around 399.05 and 400.35 eV that are attributed to N–H and N–C, respectively (Fig. S2f–h, ESI†).38 The Si 2p spectrum is fitted with two peaks at around 101.73 and 102.24 eV for Si–C and Si–O, respectively (Fig. S2i–k, ESI†).39 All the XPS spectra show that the five CDs–OSi composites possess the same chemical bonds, in agreement with the FT-IR analysis. According to the above results, we can see that the tunable H2O/EtOH volume ratio has a slight influence on the chemical structure and composition of CDs–OSi composites, with regard to their emission.
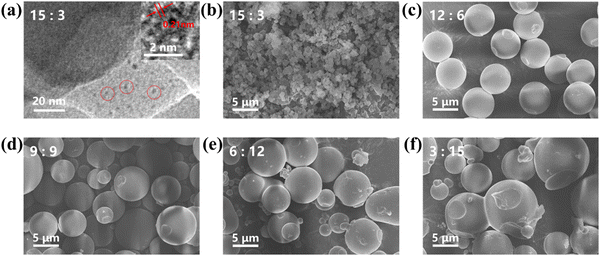
The morphology of the CDs–OSi composites was further observed using HRTEM and FESEM. It has been reported in the literature that the volume ratio of H2O/EtOH has a negligible effect on the size of CDs in a one-step solvothermal method to prepare CDs/SiO2 composites.40,41 CDs–OSi prepared with an H2O/EtOH volume ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was selected to explore the structure of the CDs. The HRTEM image of 15
3 was selected to explore the structure of the CDs. The HRTEM image of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 CDs–OSi reveals that spherical CDs (average particle size around 2.36 nm) can be observed with visible crystalline lattice fringes (Fig. 2a). The lattice spacings of the CDs are about 0.21 nm, indicating the (1 0 0) facet of graphite.42 The XRD pattern presents a broad peak centered at 2θ = 21.27°, indicating that the CDs–OSi composites are amorphous (Fig. S3, ESI†).43Fig. 2b–f show that the morphology of the composites is spherical, and the radius of the particles increases from 0.12 μm to 5 μm as the H2O/EtOH volume ratio decreases from 15
3 CDs–OSi reveals that spherical CDs (average particle size around 2.36 nm) can be observed with visible crystalline lattice fringes (Fig. 2a). The lattice spacings of the CDs are about 0.21 nm, indicating the (1 0 0) facet of graphite.42 The XRD pattern presents a broad peak centered at 2θ = 21.27°, indicating that the CDs–OSi composites are amorphous (Fig. S3, ESI†).43Fig. 2b–f show that the morphology of the composites is spherical, and the radius of the particles increases from 0.12 μm to 5 μm as the H2O/EtOH volume ratio decreases from 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 3
3 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15. It has been reported that the proportion of H2O in the reaction mixed solvent plays a crucial role in the degree of hydrolysis and the subsequent condensation reaction of UPTES. The hydrolysis and polycondensation of UPTES compete in their reaction with CDs.44 Thus, the reduction of H2O content in the reaction may be profitable for inhibiting the hydrolysis of UPTES and advancing the reaction between UPTES and CDs, making the CDs–OSi more regular and larger in size due to the higher degree of crosslinking between the CDs and organosilicon.45 The increased degree of crosslinking indicates that the spacing between the CDs decreases, which will tend to induce a resonance energy transfer (RET) effect.46 CDs–OSi composites with tunable emission and size will have important application prospects in fingerprint analysis for different substrates.
15. It has been reported that the proportion of H2O in the reaction mixed solvent plays a crucial role in the degree of hydrolysis and the subsequent condensation reaction of UPTES. The hydrolysis and polycondensation of UPTES compete in their reaction with CDs.44 Thus, the reduction of H2O content in the reaction may be profitable for inhibiting the hydrolysis of UPTES and advancing the reaction between UPTES and CDs, making the CDs–OSi more regular and larger in size due to the higher degree of crosslinking between the CDs and organosilicon.45 The increased degree of crosslinking indicates that the spacing between the CDs decreases, which will tend to induce a resonance energy transfer (RET) effect.46 CDs–OSi composites with tunable emission and size will have important application prospects in fingerprint analysis for different substrates.
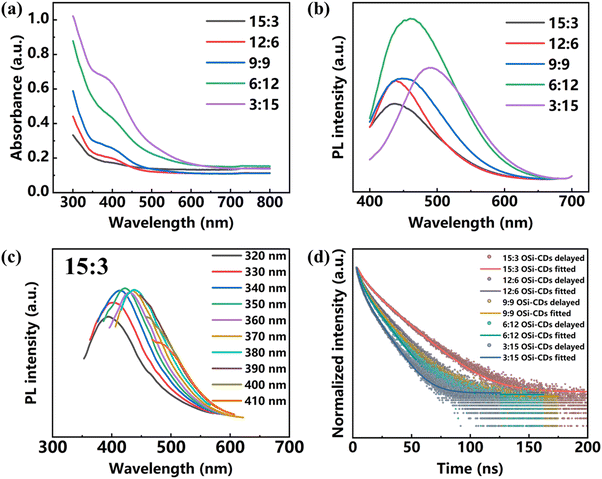
The optical properties of the CDs–OSi composites are characterized in Fig. 3. The absorption spectra of the CDs–OSi composites (Fig. 3a) show that they exhibit a strong absorption band located at λmax ≈ 400 nm, which can be attributed to n–π* transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O caused by the extended conjugation in their structure.47 The photoluminescent (PL) emission spectra are redshifted from 440 to 500 nm when the volume ratio of H2O/EtOH changes from 15
O caused by the extended conjugation in their structure.47 The photoluminescent (PL) emission spectra are redshifted from 440 to 500 nm when the volume ratio of H2O/EtOH changes from 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 3
3 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, consistent with the fluorescent photographs shown in Fig. 1b. In addition, the fluorescence intensity first increases from 15
15, consistent with the fluorescent photographs shown in Fig. 1b. In addition, the fluorescence intensity first increases from 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 6
3 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, and then decreases to 3
12, and then decreases to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (Fig. 3b). To explore the reason for the fluorescence redshift, control experiments treating L-(−)-malic acid or UPTES in ratios of H2O/EtOH of 15
15 (Fig. 3b). To explore the reason for the fluorescence redshift, control experiments treating L-(−)-malic acid or UPTES in ratios of H2O/EtOH of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 or 3
3 or 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 were conducted under the same reaction conditions. As shown in Fig. S4 (ESI†), the CDs–OSi composites exhibit more pronounced redshift emission (about 50 nm) than that of a solution of CDs prepared with L-(−)-malic acid (about 14 nm) and fluorescent powder prepared from UPTES (about 0 nm), which proves that the redshifted emission of the CDs–OSi composites is caused by joint action between CDs and organosilicon rather than by the CDs. Therefore, the redshifted emission of CDs–OSi may be attributed to the following reason: the UPTES have a decreasing hydrolysis and polycondensation rate, and an increasing reaction rate with CDs as the volume ratio of H2O/EtOH changes from 15
15 were conducted under the same reaction conditions. As shown in Fig. S4 (ESI†), the CDs–OSi composites exhibit more pronounced redshift emission (about 50 nm) than that of a solution of CDs prepared with L-(−)-malic acid (about 14 nm) and fluorescent powder prepared from UPTES (about 0 nm), which proves that the redshifted emission of the CDs–OSi composites is caused by joint action between CDs and organosilicon rather than by the CDs. Therefore, the redshifted emission of CDs–OSi may be attributed to the following reason: the UPTES have a decreasing hydrolysis and polycondensation rate, and an increasing reaction rate with CDs as the volume ratio of H2O/EtOH changes from 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 3
3 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, which lead to the CDs–OSi composites having a more compact structure, bigger size and decreasing distance between CDs due to the higher crosslinking degree between CDs with organosilicon.48 It has been reported that the CDs would undergo an RET effect as the distance between CDs decreases to less than the Förster distance (R0), which will lead to their emission redshift.49,50 As Fig. 3c and Fig. S5 (ESI†) show, all the CDs–OSi composites exhibit similar excitation wavelength dependence behavior: that is, the emission peaks appear redshifted as the excitation wavelength increases. This indicates that the properties of CDs embedded in CDs–OSi composites are similar. As shown in Fig. 3d and Table S1 (ESI†), the fluorescence decay curve of the CDs–OSi composites are fitted with bi-exponential functions with average lifetimes of 17.90, 13.42, 12.94, 11.21 and 10.23 ns, respectively, indicating the singlet-state nature of the emission of the CDs–OSi composites.51 The gradually shortened average lifetimes of the CDs–OSi composites can also demonstrate the generation of RET, because the RET competes with radiative transition and thus shortens the fluorescence lifetime.52 The above results can prove that the redshifted emission of the CDs–OSi composites is caused by their different size due to the occurrence of RET. The PLQY of the five CDs–OSi composites is determined to be 18.07%, 18.8%, 12.6%, 10.8% and 6.47%, respectively. The bright solid-state emission intensity would meet the requirements for fingerprint recognition.
15, which lead to the CDs–OSi composites having a more compact structure, bigger size and decreasing distance between CDs due to the higher crosslinking degree between CDs with organosilicon.48 It has been reported that the CDs would undergo an RET effect as the distance between CDs decreases to less than the Förster distance (R0), which will lead to their emission redshift.49,50 As Fig. 3c and Fig. S5 (ESI†) show, all the CDs–OSi composites exhibit similar excitation wavelength dependence behavior: that is, the emission peaks appear redshifted as the excitation wavelength increases. This indicates that the properties of CDs embedded in CDs–OSi composites are similar. As shown in Fig. 3d and Table S1 (ESI†), the fluorescence decay curve of the CDs–OSi composites are fitted with bi-exponential functions with average lifetimes of 17.90, 13.42, 12.94, 11.21 and 10.23 ns, respectively, indicating the singlet-state nature of the emission of the CDs–OSi composites.51 The gradually shortened average lifetimes of the CDs–OSi composites can also demonstrate the generation of RET, because the RET competes with radiative transition and thus shortens the fluorescence lifetime.52 The above results can prove that the redshifted emission of the CDs–OSi composites is caused by their different size due to the occurrence of RET. The PLQY of the five CDs–OSi composites is determined to be 18.07%, 18.8%, 12.6%, 10.8% and 6.47%, respectively. The bright solid-state emission intensity would meet the requirements for fingerprint recognition.
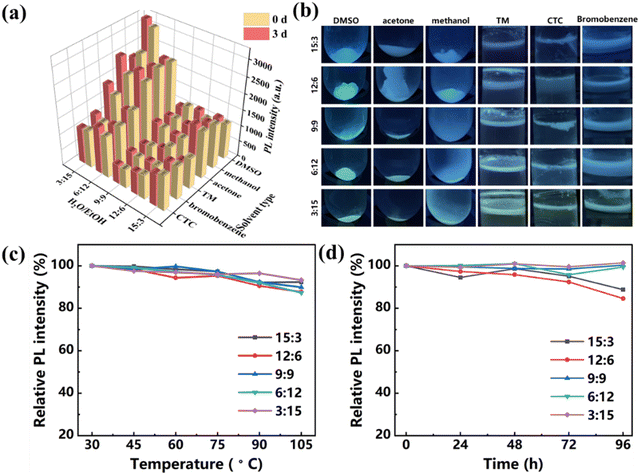
In criminal investigation at an actual crime scene, latent fingerprints sometimes exist in a matrix contaminated by impurities.53,54 Therefore, the corrosion resistance of developers under different complex conditions is very important. The CDs–OSi composites were soaked for three days in organic solvents of different polarities, such as bromobenzene, acetone, TM, CTC, methanol, and DMSO. As shown in Fig. 4a and b and Fig. S6 (ESI†), before and after soaking, none of the CDs–OSi composites dissolved, and the emission peak and PL intensity were basically unchanged, indicating that all of the CDs–OSi composites show good organic corrosion resistance. It is necessary to explore the stability of the CDs–OSi composites in terms of temperature and UV radiation, which are closely related to the effective preservation and actual practical operation for LFP development. Fig. 4c shows the trend of the relative PL intensities of CDs–OSi composites treated at different temperatures. Compared with their initial value at room temperature of 30 °C, the PL intensity of all the CDs–OSi remains as high as about 90% as the temperature rises from 30 to 105 °C. The five CDs–OSi composites were also exposed under 365 nm UV light for 24 h, 48 h, 72 h, and 96 h. The relative PL intensity of the CDs–OSi can also remain as high as about 90% (Fig. 4d). As shown in Fig. S7 (ESI†), the CDs–OSi composites were also treated with different pH, and the fluorescence emission peak and intensity are basically unchanged in the pH range 1–11 for 24 h. This is because the “Si–O–Si” network structure wraps the CDs, avoiding fluorescence quenching caused by the generation of hydrogen bonds in a strongly acidic environment and deprotonation in a strongly alkaline environment with the rich hydroxyl, carboxyl and other functional groups on the surface of the CDs.55 The fluorescence emission peak and intensity of CDs–OSi composites were also hardly changed in 1 mol⋅ L−1 or 3 mol ⋅L−1 of KCl solutions for 24 h (Fig. S8, ESI†), which proves that the surface functional groups of the CDs are not ionized. The above results indicate that CDs–OSi composites show excellent photobleaching resistance, organic solvent resistance, high temperature resistance, acid and alkali resistance and salt tolerance.
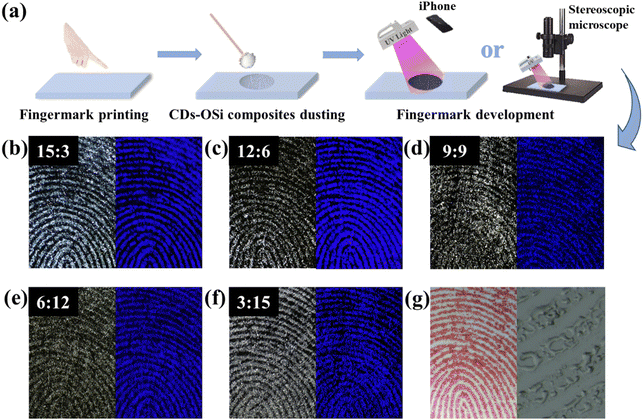
Thanks to their tunable emission and size, and good emission stability, the CDs–OSi composites were further used for fingerprint identification. As shown in Fig. 5a, five kinds of samples were coated on latent fingerprints with goose feather sticks. The fingerprint images were obtained by irradiating them with an LED lamp (Fig. 5b–f, left) and an ultraviolet lamp (Fig. 5b–f, right). Compared with the fingerprints obtained from a traditional ink pad (Fig. 5g, left) and non-fluorescent patterns (Fig. 5g, right), the fluorescent images from CDs–OSi composites under UV light are clearer, which indicates that the use of CDs–OSi composites is beneficial for displaying fingerprint images. It can be observed that the LFPs are formed by residual liquid from the fingers (Fig. 5g, right). To test whether the CDs–OSi composites can be used for LFP identification under higher temperature conditions, the glass plates with the LFPs were placed in a 40 °C oven for 5 days, and then coated with CDs–OSi composites (Fig. S9, ESI†). For observation of the appearance of latent fingerprints, the effect is similar to that at room temperature.
For identity authentication through fingerprints, it is necessary to achieve LFPs with level 2 and 3 characteristics.56–58 The details of LFPs can be successfully detected on glass with the five CDs–OSi composites. We take LFP detection using CDs–OSi composites at an H2O/EtOH volume ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 on glass as an example. As shown in Fig. 6, level 1 characteristics can be clearly identified, including loops, whorls, mixed patterns, and creases. As we all know, each fingerprint has different details, which makes it unique and provides important identification information for identity verification.59 The following featured points can be clearly observed and marked as level 2 characteristics: islands, hooks, bridges, short ridges, bifurcation, crossover, termination and deltas. More interestingly, CDs–OSi composites can even clearly develop level 3 characteristics with sweat holes, ridge edge shape, and scars. Both level 2 and level 3 characteristics can be clearly observed, indicating that LFPs are readily collected and identified by CDs–OSi composites. It is worth mentioning that the adsorption ability between the CDs–OSi composites and the LFP is also very important. Therefore, EtOH in a washing bottle was sprayed on an LFP dusted with CDs–OSi composites. Then the glass plate was dried with a hair dryer to observe the developing effect of the fingerprint. As shown in Fig. S10a (ESI†), CDs–OSi composites can remain present in the LFP (Fig. S10, left, ESI†) and maintain a fluorescence effect (Fig. S10a, right, ESI†). Fig. S10b and S10c (ESI†) show that the level 1, level 2 and level 3 characteristics are still clearly visible, which indicates that there is a strong adsorption ability between CDs–OSi composites and the LFP. These outcomes further extend the reliability and veracity of the new fluorescent powder for discriminating latent fingerprints in forensic analysis.
3 on glass as an example. As shown in Fig. 6, level 1 characteristics can be clearly identified, including loops, whorls, mixed patterns, and creases. As we all know, each fingerprint has different details, which makes it unique and provides important identification information for identity verification.59 The following featured points can be clearly observed and marked as level 2 characteristics: islands, hooks, bridges, short ridges, bifurcation, crossover, termination and deltas. More interestingly, CDs–OSi composites can even clearly develop level 3 characteristics with sweat holes, ridge edge shape, and scars. Both level 2 and level 3 characteristics can be clearly observed, indicating that LFPs are readily collected and identified by CDs–OSi composites. It is worth mentioning that the adsorption ability between the CDs–OSi composites and the LFP is also very important. Therefore, EtOH in a washing bottle was sprayed on an LFP dusted with CDs–OSi composites. Then the glass plate was dried with a hair dryer to observe the developing effect of the fingerprint. As shown in Fig. S10a (ESI†), CDs–OSi composites can remain present in the LFP (Fig. S10, left, ESI†) and maintain a fluorescence effect (Fig. S10a, right, ESI†). Fig. S10b and S10c (ESI†) show that the level 1, level 2 and level 3 characteristics are still clearly visible, which indicates that there is a strong adsorption ability between CDs–OSi composites and the LFP. These outcomes further extend the reliability and veracity of the new fluorescent powder for discriminating latent fingerprints in forensic analysis.
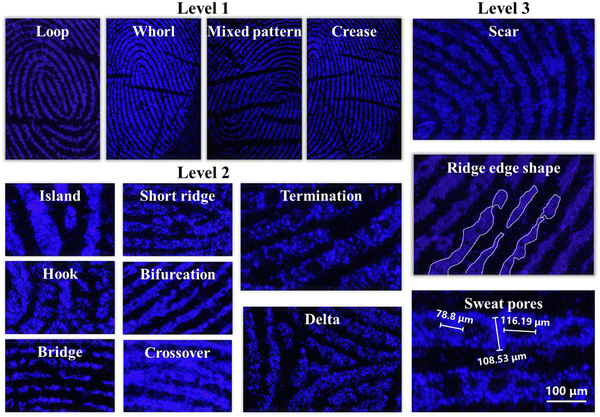 | ||
Fig. 6 The level 1, level 2 and level 3 characteristics developed by CDs–OSi composites at an H2O/EtOH volume ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 on glass using a stereoscopic microscope. 3 on glass using a stereoscopic microscope. | ||
Aged LFPs are vitally important in criminal investigations.60 Latent fingerprints from the same finger were left on many of the glass plates, and the glass plates were then exposed to room-temperature air for 2 h, 2 d, 5 d and 10 d under ventilation to obtain aged LFPs. Then the five CDs–OSi composites were individually coated on the series of aged LFPs. Finally, the characteristics were observed with a stereoscopic microscope under irradiation by an ultraviolet lamp (Fig. S11, ESI†). By comparing the detailed features of sweat holes, islands, and bifurcations at the same location and the clarity of the main structure, it can be found that the resolution decreased with an increase in preservation time, but the details on 10 days can be still recognized.
As is well known, crime scene environments are often complicated, and LFPs may be left on different surfaces and colors of various substrates.61 The same finger was selected to leave LFPs on the surface of different substrates, such as glass, aluminum foil, soft plastic bags, and hard plastic sheets (Fig. 7). The five kinds of CDs–OSi composites were painted on the different substrates to observe, capture and collect the LFPs by using a mobile phone under irradiation by an ultraviolet lamp (wavelength 365 nm). It can be seen that all five kinds of CDs–OSi composite could successfully obtain fingerprint patterns on different substrates. Importantly, the level 2 (crossover) and level 3 (sweat holes) characteristics also can be clearly observed. In addition, the five CDs–OSi composites show different detection effects on substrates with different roughness, colorfulness, and background fluorescence interference. For example, the fingerprint produced by the CDs–OSi composites using an H2O/EtOH volume ratio of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 shows higher contrast on glass, and the fingerprints produced by CDs–OSi composites using H2O/EtOH volume ratios of 12
6 shows higher contrast on glass, and the fingerprints produced by CDs–OSi composites using H2O/EtOH volume ratios of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 9
6 and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 show higher contrast on aluminum. It can also be observed that the contrast of green fluorescence on plastic products is more pronounced than that of blue. Therefore, the appropriate emission and size of CDs–OSi composites can be selected for developers based on the characteristics of the substrates, which can effectively improve the efficiency of fingerprint detection. Therefore, the CDs–OSi composites prepared here exhibit some advantages compared to other fingerprint identification methods (Table S2, ESI†), like excellent fingerprint detection, simple preparation method, and environmental friendliness, making them an extremely likely substitute for traditional fluorescent powders.
9 show higher contrast on aluminum. It can also be observed that the contrast of green fluorescence on plastic products is more pronounced than that of blue. Therefore, the appropriate emission and size of CDs–OSi composites can be selected for developers based on the characteristics of the substrates, which can effectively improve the efficiency of fingerprint detection. Therefore, the CDs–OSi composites prepared here exhibit some advantages compared to other fingerprint identification methods (Table S2, ESI†), like excellent fingerprint detection, simple preparation method, and environmental friendliness, making them an extremely likely substitute for traditional fluorescent powders.
4. Conclusions
In summary, we have successfully synthesized CDs–OSi composites with multicolored emission from blue to green just by controlling the ratio of H2O/EtOH to adjust the size of CDs–OSi. The as-prepared CDs–OSi show good dispersibility, stability, and bright solid-state emission, and they were further used as developers to detect LFPs by a simple powder-dusting method. Thanks to their tunable emission and size, the CDs–OSi composites exhibit excellent performance in detecting LPFs with high contrast and resolution for multifarious substrates regardless of roughness, colorfulness, or background fluorescence interference. Therefore, a series of superior and universal fluorescent developers are provided here, which can be applied for observing hyperfine LFP details for individualization, thus showing great potential in forensic investigation.Author contributions
Xiyue Cao: methodology, investigation, data curation, visualization, and writing–original draft. Jiashi Chen: formal analysis, investigation. Yue Chen: software, supervision. Xuanfeng Jiang: formal analysis. Wen Fan: software. Huijuan Ma: formal analysis, funding acquisition. Zhengguang Sun: formal analysis, supervision. Yuan Zhan: funding acquisition, supervision, writing–review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Our work is financially supported by the Foundation of Three Gorges Laboratory (SK232014).References
- S. Bell, Annu. Rev. Anal. Chem., 2009, 2, 297 CrossRef CAS.
- H. S. Jung, K. J. Cho, S. J. Ryu, Y. Takagi, P. A. Roche and K. C. Neuman, ACS Appl. Mater. Interfaces, 2020, 12, 6641 CrossRef CAS PubMed.
- R. M. Caplan, J. Am. Acad. Dermatol., 1990, 23, 109 CrossRef CAS PubMed.
- K. Scotcher and R. Bradshaw, Sci. Rep., 2018, 8, 8765 CrossRef CAS PubMed.
- L. Yang, Q. Zhang, Y. Han, H. Li, S. Sun and Y. Xu, Nanoscale, 2021, 13, 13057 RSC.
- H. Chen, R. Ma and M. Zhang, ChemistryOpen, 2022, 11, e202200091 CrossRef CAS.
- A. A. Ansari, K. M. Aldajani, A. N. AlHazaa and H. A. Albrithen, Coord. Chem. Rev., 2022, 462, 214523 CrossRef CAS.
- M. Shi, Q. Wei, L. Tian, X. Du, X. Zhang and M. Zhang, Electrochim. Acta, 2020, 350, 136373 CrossRef CAS.
- S. Saharan, A. K. Yadav and B. Yadav, Egypt. J. Forensic Sci., 2020, 10, 16 CrossRef.
- Y. Hu, Z. Zhou, F. Zhao, X. Liu, Y. Gong, W. Xiong and M. Sillanpää, Sci. Rep., 2018, 8, 10277 CrossRef PubMed.
- A. M. Azman, J. Chem. Educ., 2020, 97, 571 CrossRef CAS.
- V. Prasad, L. Prasad, S. Lukose and P. Agarwal, J. Forensic Sci., 2021, 66, 1065 CrossRef CAS.
- Q. Zhu, W. Wang, W. Kong, X. Chao, Y. Bi and Z. Li, Anal. Chim. Acta, 2022, 1209, 339864 CrossRef CAS PubMed.
- M. Wang, D. Shen, Z. Zhu, J. Ju, J. Wu, Y. Zhu, M. Li, C. Yuan and C. Mao, Mater. Today Adv., 2020, 8, 100113 CrossRef.
- D. Chávez, C. R. Garcia, J. Oliva and L. A. Diaz-Torres, Ceram. Int., 2021, 47, 10 CrossRef.
- M. Wang, M. Li, A. Yu, Y. Zhu, M. Yang and C. Mao, Adv. Funct. Mater., 2017, 27, 1606243 CrossRef.
- C. Yuan, M. Li, M. Wang, X. Zhang, Z. Yin, K. Song and Z. Zhang, Chem. Eng. J., 2020, 383, 123076 CrossRef CAS.
- L. Duan, Q. Zheng and T. Tu, Adv. Mater., 2022, 34, 2202540 CrossRef CAS PubMed.
- Z. L. Chen, H. Y. Yang, Z. H. Kang, M. Driess and P. W. Menezes, Adv. Mater., 2022, 34, 2108432 CrossRef CAS.
- M. M. Hussain, W. U. Khan, F. Ahmed, Y. Wei and H. Xiong, Chem. Eng. J., 2023, 465, 143010 CrossRef CAS.
- B. Wang, G. I. N. Waterhouse and S. Lu, Trends Chem., 2022, 5, 76–87 CrossRef.
- Z. Chen, Y. Liu and Z. Kang, Acc. Chem. Res., 2022, 55, 3110–3124 CrossRef CAS PubMed.
- K. Shao, H. Zhang, Q. Ling, W. Xie, D. Gu, Y. Teng, X. Yuan, S. Ye and Z. Pan, J. Mater. Chem. C, 2023, 11, 10341 RSC.
- E. Prabakaran and K. Pillay, J. Mater. Res. Technol., 2021, 12, 1856 CrossRef CAS.
- I. Milenkovic, M. Algarra, C. Alcoholado, M. Cifuentes, J. M. Lázaro-Martínez, E. Rodríguez-Castellón, D. Mutavdžić, K. Radotić and T. J. Bandosz, Carbon, 2019, 144, 791 CrossRef CAS.
- F. Zu, F. Yan, Z. Bai, J. Xu, Y. Wang, Y. Huang and X. Zhou, Microchim. Acta, 2017, 184, 1899 CrossRef CAS.
- J. Chen, J. S. Wei, P. Zhang, X. Q. Niu, W. Zhao, Z. Y. Zhu, H. Ding and H. M. Xiong, ACS Appl. Mater. Interfaces, 2017, 9, 18429 CrossRef CAS PubMed.
- X. Y. Dong, X. Q. Niu, Z. Y. Zhang, J. S. Wei and H. M. Xiong, ACS Appl. Mater. Interfaces, 2020, 12, 29549 CAS.
- T. Liu, G. Yin, Z. Song, J. Yu, X. Yong, B. Zhang, L. Ai and S. Lu, ACS Mater. Lett., 2023, 5, 846 CrossRef CAS.
- D. Peng, X. Liu, M. Huang, D. Wang and R. Liu, Dalton Trans., 2018, 47, 5823 RSC.
- J. Manioudakis, F. Victoria, C. A. Thompson, L. Brown, M. Movsum, R. Lucifero and R. Naccache, J. Mater. Chem. C, 2019, 7, 853 RSC.
- E. V. Kundelev, N. V. Tepliakov, M. Y. Leonov, V. G. Maslov, A. V. Baranov, A. V. Fedorov, I. D. Rukhlenko and A. L. Rogach, J. Phys. Chem. Lett., 2019, 10, 5111 CrossRef CAS PubMed.
- Z. Liu and H. Mao, J. Lumin., 2017, 190, 179 CrossRef CAS.
- W. Chen, W. Chen, B. Zhang, S. Yang and C. Liu, Polymer, 2017, 109, 205–215 CrossRef CAS.
- Y. Zhang, M. Li and S. Lu, Small, 2023, 19, 2206080 CrossRef CAS.
- X. Geng, Z. Li, Y. Hu, H. Liu, Y. Sun, H. Meng, Y. Wang, L. Qu and Y. Lin, ACS Appl. Mater. Interfaces, 2018, 10, 27979 CrossRef CAS PubMed.
- Y. Han, Y. Chen, J. Feng, J. Liu, S. Ma and X. Chen, Anal. Chem., 2017, 89, 3001 CrossRef CAS PubMed.
- Y. Wu, Y. Zhan, W. Xin, W. Cao, J. Li, M. Chen, X. Jiang, J. Wang and Z. Sun, ACS Appl. Energy Mater., 2022, 5, 1781 CrossRef CAS.
- J. Li, J. Chen, X. Zhao, A. Vomiero and X. Gong, Nano Energy, 2023, 115, 108674 CrossRef CAS.
- Y. Wu, L. Zhao, X. Cao, Y. Zhang, X. Jiang, Z. Sun and Y. Zhan, Carbon, 2023, 207, 77 CrossRef CAS.
- Y. Zhan, B. Shang, M. Chen and L. Wu, Small, 2019, 15, 1901161 CrossRef.
- L. Wang, W. Li, L. Yin, Y. Liu, H. Guo, J. La, Y. Ha, G. Li, M. Li, J. Zhang, R. Vajtai, P. M. Ajayan and M. Wu, Sci. Adv., 2020, 6, eabb6772 CrossRef CAS.
- M. Wang, Y. Han, Z. Guo, Z. Huang and W. Yang, ACS Appl. Nano Mater., 2021, 4, 13625–13632 CrossRef CAS.
- D. Zhou, D. Li, P. Jing, Y. Zhai, D. Shen, S. Qu and A. L. Rogach, Chem. Mater., 2017, 29, 1779 CrossRef CAS.
- Z. Sun, F. Yan, J. Xu, H. Zhang and L. Chen, Nano Res., 2022, 15, 414 CrossRef CAS.
- J. Wang, F. Zhang, Y. Wang, Y. Yang and X. Liu, Carbon, 2018, 126, 426 CrossRef CAS.
- C. L. Shen, J. H. Zang, Q. Lou, L. X. Su, Z. Li, Z. Y. Liu, L. Dong and C. X. Shan, Carbon, 2018, 136, 359 CrossRef CAS.
- B. Wang, Z. Sun, J. Yu, G. I. N. Waterhouse, S. Lu and B. Yang, SmartMat., 2022, 3, 337–348 CrossRef CAS.
- Z. Zhou, P. Tian, X. Liu, S. Mei, D. Zhou, D. Li, P. Jing, W. Zhang, R. Guo, S. Qu and A. L. Rogach, Adv. Sci., 2018, 5, 1800369 CrossRef.
- Y. Chen, M. Zheng, Y. Xiao, H. Dong, H. Zhang, J. Zhuang, H. Hu, B. Lei and Y. Liu, Adv. Mater., 2016, 28, 312 CrossRef CAS.
- P. Yang, Z. Zhu, T. Zhang, W. Zhang, W. Chen, Y. Cao, M. Chen and X. Zhou, Small, 2019, 15, 1902823 CrossRef CAS PubMed.
- R. M. Barry, J. Biomed. Opt., 2008, 13, 029901 CrossRef.
- B. K. Joseph, Ann. Appl. Stat., 2018, 12, 771 Search PubMed.
- Z. Hu, H. Dai, W. Zhou, J. Wei, H. Zhang, Z. Ye, Y. Qiu, Y. Chen, Z. Duan, J. Wang, W. Zhang, F. Xie and R. Guo, J. Alloys Compd., 2021, 889, 161660 CrossRef.
- M. Fang, B. Wang, X. Qu, S. Li, J. Huang, J. Li, S. Lu and N. Zhou, Chin. Chem. Lett., 2024, 35, 108423 CrossRef CAS.
- Y. L. Wang, C. Li, H. Q. Qu, C. Fan, P. J. Zhao, R. Tian and M. Q. Zhu, J. Am. Chem. Soc., 2020, 142, 7497 CrossRef CAS PubMed.
- Y. Chen, A. Li, X. Li, L. Tu, Y. Xie, S. Xu and Z. Li, Adv. Mater., 2023, 35, 2211917 CrossRef CAS PubMed.
- K. Song, P. Huang, C. Yi, B. Ning, S. Hu, L. Nie, X. Chen and Z. Nie, ACS Nano, 2015, 9, 12344 CrossRef CAS PubMed.
- L. Xu, C. Zhang, Y. He and B. Su, Sci. China: Chem., 2015, 58, 1090 CrossRef CAS.
- B. Yu, S. Liu, W. Xie, P. Pan, P. Zhou, Y. Zou, Q. Yue and Y. Deng, InfoMat, 2022, 4, e12289 CrossRef CAS.
- M. Wang, M. Li, A. Yu, J. Wu and C. Mao, ACS Appl. Mater. Interfaces, 2015, 7, 28110 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3tc03570k |
| This journal is © The Royal Society of Chemistry 2024 |