Recent advances in enhancing the photodetector performance of 2D materials by combining them with organic thin films
Lei
Guo
 a,
Jiayue
Han
a,
Jiayue
Han
 ab and
Jun
Wang
ab and
Jun
Wang
 *ab
*ab
aSchool of Optoelectronic Science and Engineering, University of Electronic Science and Technology of China, No. 2006, Xiyuan Ave, West Hi-Tech Zone, Chengdu 610054, P. R. China. E-mail: wjun@uestc.edu.cn
bState Key Laboratory of Electronic Thin Films and Integrated Devices, University of Electronic Science and Technology of China, Chengdu 610054, China
First published on 25th December 2023
Abstract
Two dimension (2D) material-based photodetectors usually indicate excellent properties such as ultrafast and broadband response, but the atomic thickness of 2D materials usually leads to low absorption coefficient. Pure 2D material-based photodetectors also suffer from serious drawbacks such as short carrier lifetime, low optical absorption coefficient, and weak built-in field. Organic materials present excellent properties such as flexibility, high absorption coefficient, low cost and large area, which are very suitable for enhancing the detector performance of 2D materials. In this paper, the development of 2D material photodetectors by combining 2D materials with organic thin films since their proposal to date is reviewed. The 2D/organic photodetectors are classified into three categories (graphene-based, MoS2-based and others), and their development history and research status are comprehensively summarized and reviewed. Organic thin films are categorized into small molecule organic thin films, organic polymer thin films, complex organic thin films, and bilayer organic thin films, and the device performance of each type of material and the mechanism behind the high performance are discussed in detail. Finally, the future development of 2D/organic photodetectors is envisaged. The aim of this paper is to provide new research ideas and directions for 2D/organic heterojunction photodetectors with ultra-high performance and promote the application of these combined photodetectors.
1. Introduction
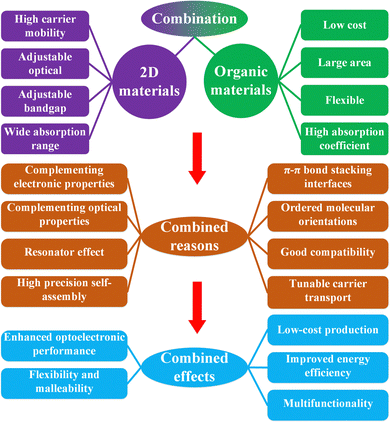
In the field of modern technology, photodetectors play a key role, and their main function is to convert information-carrying optical signals into electronic signals for further processing and application in electronic devices.1–3 This technology is widely used in the fields of image processing,4,5 optical communication,6,7etc., and has a profound impact on our daily life. For example, in smartphones, photodetectors enable automatic screen brightness adjustment; in cameras, charge-coupled devices help capture accurate images; infrared detectors play a key role in night observation and security surveillance;8,9 and photodetectors are also used to acquire high-quality medical images.10–12 Conventional photodetectors mainly utilize semiconductor materials, which can be divided into three according to the stages of development.13 The first stage includes the monolithic semiconductors Si14,15 and Ge;16 the second stage includes the compound semiconductors GaAs,17 InSb,18etc., and the third stage includes wide-bandwidth semiconductors GaN,19 SiC,20 and ZnO.21 For a long time, traditional photodetector materials such as HgCdTe, Si, InGaAs, etc. have been dominating the global commercial photodetector market by virtue of their considerable detection performance as well as mature scale production and integration technologies.22–24 The sensitivity, detection range, and detectivity of traditional material photodetectors can no longer meet the demands of military and civil products. There is an urgent need for novel materials to fabricate high-performance photodetectors.Researchers are actively exploring novel materials, among which graphene25 and other 2D materials have attracted much attention. Due to their excellent electronic properties and flexibility,26 2D materials have great potential for application in the field of improved photodetectors. 2D materials such as graphene have high mechanical strength and high optical transparency,27 with potential use in fabricating transparent electrodes and flexible optoelectronic devices. The number of layers of 2D materials such as MoS2 induces a change in the bandgap and has a low dielectric shielding effect internally, making them promising as stable carriers for electronic device applications.2,28,29 The two-dimensional condition makes it possible to achieve arbitrary stacking between any materials, which is completely unaffected by the lattice mismatch of traditional 3D materials, and exhibits highly compatible integration properties.30 Therefore a large number of 2D materials have been applied in advanced optoelectronic devices,31–33 including photodetectors,1 optical modulators,34 lasers,35 and synaptic photonic chips.36 In 2009, Xia et al. prepared the first graphene photodetector,1 which initiated the large-scale application of graphene in photodetectors. In 2011, A. Kis et al. successfully realized the first single-layer MoS2 field effect transistor,37 with a current switching ratio of 108 in a device with HfO2 as the top gate dielectric layer, and a subthreshold swing of 74 mV dec−1 for the field effect transistor at room temperature. However, 2D materials are usually less resistive, resulting in higher photodetector dark currents. Moreover, pure 2D material photodetectors have the disadvantages of short carrier lifetime, low light absorption coefficient, and weak built-in field. Therefore, further development on the basis of 2D photodetectors is necessary, and combining 2D materials with organic materials brings new development opportunities.38
Organic materials typically have low carrier mobility, high absorption coefficients and broader energy level structures, so photodetectors fabricated using organic materials typically have very low dark current39 and high light sensitivity.40 Combining 2D materials with organic materials results in ultra-high-performance photodetectors that can improve performance by several orders of magnitude.41 Both 2D and organic materials contain a rich library of materials. Common 2D materials include graphene (semimetal),25,26 MoS2 (semiconductor),2 h-BN (insulator),42 FeTe (magnetic material),43etc.; common organic materials include pentacene (semiconductor),44 PVCN (insulator),45 CuPc (polymer),46 C8- BTBT (polymer),47etc. Although 2D materials show great potential in infrared photodetectors, the performance of photodetectors based on pure 2D materials is limited by weak light absorption and short carrier lifetime. 2D materials are capable of forming π–π stacked interfaces with organic materials, and 2D/organic heterojunctions have good compatibility.48,49 In addition, the delocalization of Frenkel excitons in organic materials is beneficial to improve the quantum efficiency and operating wavelength of photodetectors. 2D/organic heterojunction photodetectors have been proved to be an effective alternative to improve the performance of photodetectors. 2D/organic heterojunction photodetectors usually exhibit the advantages of ultra-high responsivity, ultrahigh detectivity, ultra-fast response time, broadband spectral detection, ultra-high gain and ultra-high quantum efficiency. The first applications of 2D/organic heterojunctions are in field effect transistors, with the main objectives of improving dissociation efficiency, increasing responsivity and increasing gain.50,51 For high-efficiency light generation or collection in graphene-based optoelectronic devices, the key challenges are to improve light absorption, construct appropriate band structures and improve the interface quality. Increasing the thickness of 2D material films significantly improves light absorption, but only a small fraction of carriers away from the interface can be collected.52 The integration of 2D materials with other materials to improve light detection is an excellent strategy, and in particular the combination of organic materials with 2D materials is a breakthrough in photodetector performance.48,53 Obtaining high-quality 2D/organic interfaces is also a challenge, which are easily contaminated during the fabrication process.48,53 Heterojunction photovoltaic devices have far surpassed conventional photodetectors in terms of performance, but most of the research is only on individual devices, which have not realized large-scale applications, such as solar cells54–56 and photodetectors, so heterojunction photovoltaic devices still have a sizeable developmental space.
There are five main physical mechanisms of 2D/organic heterojunction photodetectors: surface plasma-wave-assisted effect,57 photovoltaic effect,58 photoconductive effect,59 photothermoelectric effect60 and photobolometric effect.61 A review of some 2D/organic devices, categorized according to the design strategy and physical mechanisms, was conducted by our group in 2019.41 In this review, we present a comprehensive summary of recent advances in enhancing the photodetector performance of 2D materials by combining them with organic thin films from the materials perspective. Given the distinct properties of diverse materials, separate consideration is necessary concerning application areas, manufacturing processes, integration strategies, performance optimization, and research priorities. Categorizing 2D/organic heterojunction photodetectors based on material type aids in better comprehension and utilization of these materials’ unique properties, facilitating the development of targeted application solutions. Initially, this paper classifies 2D/organic heterojunction photodetectors into three categories (graphene-based, MoS2-based, and others) and discusses various photodetector technologies based on the materials’ characteristics and advancements. Further, organic thin films are similarly categorized according to material type, and a comprehensive review of different material types of organic thin films is presented separately, revealing gaps in current research and future directions. This paper aims to systematically examine and discuss the latest advancements in the field of photodetectors, leveraging a material-based classification perspective. It offers an intuitive comparative framework, enabling readers to contrast the performance of detectors made from different materials easily. It is hoped that this review will provide readers with valuable information, which will guide the direction of future research and promote the further development of the field.
2. Two-dimensional (2D) materials and organic materials
Both 2D and organic materials contain a rich library of materials, which ensures the diversity of 2D/organic heterojunction photodetector design. Heterojunctions can also be designed from the structural aspect, such as type-I junction, type-II junction, type-III junction, Z scheme junction, Schottky junction and so on. Achieving effective energy band matching is the focal point of designing high-performance photodetectors, and the type of heterojunction charge transfer is mainly determined by the energy bands of 2D materials and organic materials.622.1. Two-dimensional (2D) materials
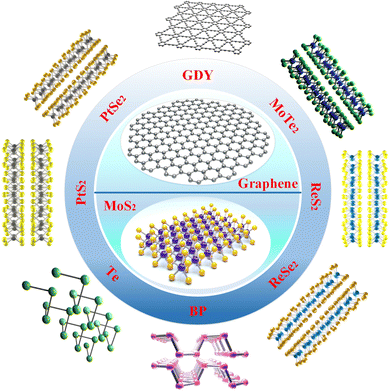
Common materials used for photodetectors can be classified into four types: (1) zero-dimensional materials,63 such as quantum dots, clusters of atoms, etc.; (2) one-dimensional materials,64 such as nanowires, nanorods, nanotubes, etc.; (3) two-dimensional materials,1,2,25,26 such as graphene, MoS2, etc.; and (4) three-dimensional materials, such as Si, Ge, and InSb. 2D materials are those with thickness reduced to the atomic level in one dimension and with larger sizes in the other two dimensions. Common 2D materials include graphene, MoS2, black phosphorus (BP), MoTe2, WSe2, ReS2, Bi2Se3, WS2, etc., and their structures are shown in Fig. 1. As a semimetal, graphene has the advantages of high mobility, broad light absorption spectrum, and ultrafast photogenerated carriers. As for semiconducting TMDs, they have a bandgap that varies with the number of layers (direct bandgap in the case of a single layer and indirect bandgap in the case of multilayers) and unique exciton properties. Graphene and TMDs are most widely used in photodetectors. This paper reports 2D/organic heterojunctions in three categories: graphene-based photodetectors, MoS2-based photodetectors, and photodetectors of other 2D materials.The crystal structure and energy band diagram of the 2D material graphene are given in Fig. 2(a) and (b). Graphene has a linear dispersion relation near the Dirac point, and the theoretical density of states at the Dirac point is zero.65 Therefore, graphene is a semi-metallic material with a band gap of zero. The carriers in graphene can be described as massless Dirac fermions, and this unique property brings about many unique physical phenomena. For example, Dirac fermions can move at 1/300th of the speed of light in graphene. Most importantly, the carrier mobility in graphene can be as high as 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1, much higher than that of commonly used semiconductor materials such as Si. Due to graphene's unique energy band structure, its zero band gap gives it uniform optical absorption over a broad spectrum (Fig. 2c and d), and its optical absorption range can be extended from the UV to the terahertz band.26,66,67 The optical absorption of graphene depends only on the structural parameters, and the intrinsic graphene absorbs only 2.3% of the light in the broad spectrum. Mechanically, Young's modulus of single-layer graphene is greater than 1 TPa. Since the thickness of a single layer is only 0.34 nm, it is very flexible and can be used to fabricate flexible devices, e.g., as a flexible electrode material.
000 cm2 V−1 s−1, much higher than that of commonly used semiconductor materials such as Si. Due to graphene's unique energy band structure, its zero band gap gives it uniform optical absorption over a broad spectrum (Fig. 2c and d), and its optical absorption range can be extended from the UV to the terahertz band.26,66,67 The optical absorption of graphene depends only on the structural parameters, and the intrinsic graphene absorbs only 2.3% of the light in the broad spectrum. Mechanically, Young's modulus of single-layer graphene is greater than 1 TPa. Since the thickness of a single layer is only 0.34 nm, it is very flexible and can be used to fabricate flexible devices, e.g., as a flexible electrode material.
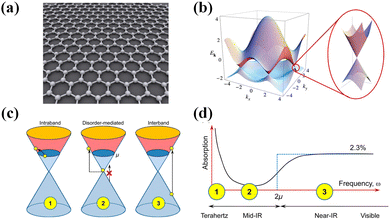 | ||
| Fig. 2 2D material graphene. (a) Crystal structure of graphene.65 Reproduced with permission.65 Copyright 2015, Sciendo. (b) Energy band diagram of graphene.26 Reproduced with permission.26 Copyright 2009, American Physical Society. (c) Illustration of the various optical transition processes.67 (d) Schematic diagram of the absorption spectrum of graphene, with the numbers corresponding to the dominant mechanism in part (c).67 Reproduced with permission.67 Copyright 2014, American Chemical Society. | ||
Unlike graphene, TMDs are a group of 2D materials that can have a band gap. Their common form is MX2, where M is a transition metal (Mo, W, etc.) and X is a sulfur group element (S, Se, Te, etc.). MoS2 is a typical TMD, and its crystal structure, energy band structure, and spin–valley coupling are shown in Fig. 3(a)–(c) respectively. Thick-layer MoS2 is an indirect bandgap semiconductor material, and the bandgap decreases as the layers become thicker. The band gap of thick-layer MoS2 is 1.2 eV, whereas single-layer MoS2 has a direct band gap of 1.9 eV. As shown in Fig. 3(d), which demonstrates the light absorption spectrum of MoS2, the cleavage of the valence bands brings about two unique distinctive excitons, with the higher-energy exciton known as B-exciton and the lower-energy exciton known as A-exciton. In monolayer TMDs, the neutral exciton can also combine with an electron or hole to form a tri-exciton, and the concentration of tri-excitons in TMDs can be regulated by applying a gate voltage.
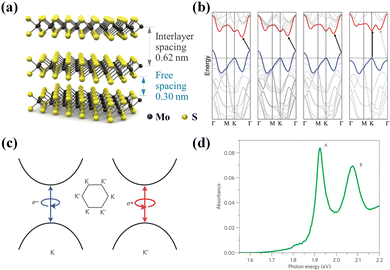 | ||
| Fig. 3 Transition metal dichalcogenide MoS2. (a) Crystal structure of MoS2.68 Reproduced with permission.68 Copyright 2017, American Chemical Society. (b) Energy band diagram of MoS2;69 from left to right, block molybdenum, quadruple molybdenum, double molybdenum, and single molybdenum. Solid arrows indicate the lowest energy conversion. Reproduced with permission.69 Copyright 2010, American Chemical Society. (c) Spin and valley coupling of MoS2.70 Reproduced with permission.70 Copyright 2012, Nature Publishing Group. (d) Light absorption spectra of monolayer MoS2 and A, B excitons.71 Reproduced with permission.71 Copyright 2012, Nature Publishing Group. | ||
Researchers have been exploring the preparation method of high-quality, large-area, expandable, and stable 2D material films for a long time.72 Currently, the main preparation methods include the following four: mechanical exfoliation,73 chemical vapor deposition,74 epitaxial growth75 and reduction of graphene oxide.76 2D materials are unlikely to replace Si but can functionally complement the existing technologies. Microelectronic devices will continue to evolve in the direction of being faster, smaller, cheaper, and more functional. 2D materials are expected to lead the industrial transformation based on material innovation.
2.2. Organic materials
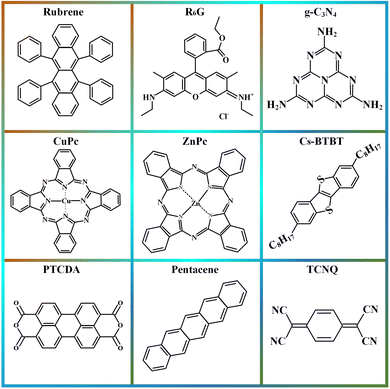
Organic materials mainly include small molecules, polymers, complexes, metal–organic frameworks (MOFs), and covalent organic frameworks (COFs). Organic semiconductor materials are widely used in organic light-emitting diodes (OLEDs) because of their low cost, flexibility, high fluorescence efficiency, and broad spectral coverage.77–79 Organic thin film transistors are widely used in integrated circuits.80–83 Polymer organic semiconductors were the first class of materials to be used in photodetector research, with a long history of research and extensive optimization experience. In general, polymers have good solubility and can be prepared as films by spin-coating, spraying, LB method, and other simple and inexpensive processes. Small molecule materials have inherent advantages over polymers. For example, they have a fixed molecular mass and physical properties, a fixed molecular structure, and a neat and regular crystal arrangement. The acene compounds possess flat and tight conjugated structures and are most frequently used in organic electronics research, such as benzotriazole, tetracene, pentacene, and rubrene, which have excellent properties. Among them, pentacene has become the most shining material in photodetector applications due to its good crystallinity, easy formation of large-size grains, and highly ordered single crystals. The molecular structure of phthalocyanines mainly consists of nitrogen-containing conjugated macrocycles with excellent planarity, and molecules with different properties can be synthesized by coordinating different metal elements. Phthalocyanine materials such as CuPc, CoPc, NiPc, F16CuPc, etc. perform well in photodetectors. In addition to the above materials, many new high-performance organic semiconductor materials have also been developed and applied to photodetectors with good results, such as NTCDA, PTCDI-C8, and C60.COFs are emerging crystalline porous organic polymers with light elements linked by covalent bonds. COFs have controllable properties in the material structure and electronics. COFs are formed by covalent bonding, and the 2D planes are usually stacked face-to-face, with strong π–π interactions between neighboring layers and in the 2D plane, which is very favorable for carrier migration.84–86 2D COFs with characteristic light absorption and exciton generation under light illumination have become a hot research topic. The coupling of optical and electrical properties leads to the application of 2D COFs in photodetectors with their unique advantages, and ultrathin 2D-COF nanosheets or thin films have gained a widespread interest as photodetectors. MOFs are a new class of crystalline nanoporous materials composed of inorganic secondary structural units and organic ligands via coordination bonds.87 The intrinsic energy band structure and electronic properties of 2D MOFs can be systematically tuned by the reaction conditions of the coordination environment, organic ligands, and molecular building blocks during the assembly process.88,89 MOFs have strong light absorption in the SWIR, and their structural and functional diversity and energy band tunability simultaneously provide more opportunities for their integration into photodetectors.
Common organic materials for heterojunction photodetectors are shown in Fig. 4. By choosing different 2D and organic materials, devices with different functions can be realized. The strategy of combining 2D materials with organic materials has excellent application prospects in the field of photodetectors.
2.3. Rational combination of 2D and organic materials
Photodetectors, as critical devices for information perception, have an indispensable position in modern information technology, and their main task is to convert optical signals into electrical signals efficiently. Optoelectronic devices adapted to different wavelength bands can be constructed by selecting semiconductor materials with different bandgaps. In recent years, 2D materials have been widely used in optoelectronic devices due to their wide variety and excellent electrical and optical properties. However, 2D materials suffer from limited light absorption capacity due to their ultrathin property, which limits their application in optoelectronics. Constructing 2D/organic heterojunctions is an advanced material design strategy (Fig. 5) that combines the advantages of 2D and organic materials, solves some of the problems of single-material systems, and significantly improves several material and device properties, especially in photovoltaic conversion and energy storage.In order to construct high-performance heterojunction photodetectors, the energy band structures and properties of different materials need to be fully considered in order to select and match different materials. Fortunately, both 2D and organic materials are equipped with a rich library of materials, so researchers can flexibly select appropriate materials to achieve suitable heterostructures according to specific needs. Graphene has excellent electrical and thermal properties; the downside is that graphene has a zero band gap, which makes it more difficult to suppress its dark current. MoS2 has an adjustable bandgap, which can be realized to suppress the dark current. However, the spectral response of MoS2 is mainly concentrated in the visible range, and the response to bands such as infrared light is relatively poor. Graphene and MoS2 are the most applied 2D materials in photodetectors.
When comparing Wannier excitons in 2D materials and Frenkel excitons in organic materials, Frenkel excitons have higher exciton binding energies, and delocalization of Frenkel excitons is beneficial for improving the quantum efficiency of photodetectors and broadening the operating wavelength range. Combining 2D and organic materials may result in “π–π stacked interfaces” and “ordered molecular orientations”. The 2D materials have low-defect surfaces and show good compatibility with organic materials without the need to consider lattice matching. This provides a solid theoretical basis for the design and application of 2D/organic heterojunctions.48,49
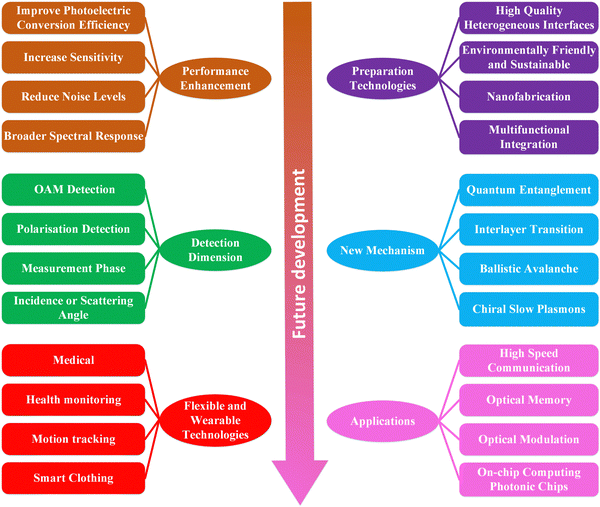
As society enters the high-tech era, there is a growing demand for multifunctional photodetectors, such as in life sciences (X-rays,90 MRIs,91 CT scans,92 and PET scans93), communications and information technology (fiber-optic communications,94 LIDAR,95 photonics computing,96 and optical sensing97), security and military applications (night-vision,98 infrared detection,99 laser ranging,100 and target identification101), industry and manufacturing102 (measuring, detecting and monitoring various physical parameters), etc., which necessitates the development of new photodetectors, especially 2D/organic heterojunction detectors. 2D/organic heterojunctions face challenges in multifunctional, high-performance optoelectronic detection (e.g., interface engineering103 and thin-film preparation techniques104), which can be overcome through materials engineering and technological innovation, enabling them to be useful in a variety of applications.
Combining 2D materials with organic materials offers the following advantages:
(1) Optoelectronic performance enhancement: 2D materials (e.g., graphene, TMDs, etc.) have excellent optoelectronic properties with high carrier mobility and wide spectral response range. Combining 2D materials with organic materials can extend the spectral response range of photodetectors and improve their sensitivity.
(2) Flexibility and malleability: organic materials are often very flexible and can be easily prepared on substrates of different shapes and sizes, thus making photodetectors more flexible. This is important for certain special applications such as wearable technology and flexible electronics.
(3) Low-cost production: organic materials typically have low preparation costs and can be prepared using simple processes such as printing and coating. Combining organic materials with 2D materials allows high performance photodetectors to be produced at low cost, which is very attractive for mass production.
(4) Energy efficiency: 2D materials possess high electron mobility, and thus combining them with organic materials can reduce power consumption and improve the energy efficiency of photodetectors, which is very important in mobile devices and sensor applications.
(5) Multifunctionality: organic materials can be chemically modified and functionalized to achieve a variety of functions, such as enhancing the stability, selectivity, and response speed of photodetectors. Thus, the combination of 2D and organic materials holds great promise for various specific applications.
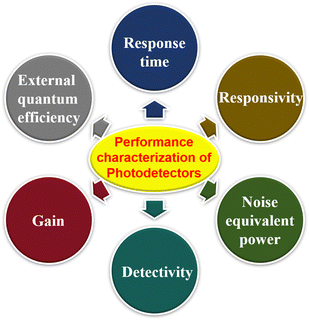
3. Performance characterization of photodetectors
The following parameters are often used to measure the performance of a photodetector (Fig. 6): responsivity, gain, response time, detectivity, noise equivalent power, and so on. These parameters are briefly described below.3.1. Responsivity (R)
Responsivity (R), also known as sensitivity, describes the photoelectric conversion efficiency of a photodetector, which can characterize the magnitude of the photoelectric conversion capability and is an essential parameter for assessing the performance of a photodetector.105 The responsivity refers to the ratio of the photocurrent generated under light conditions to the optical power of the effective area. The defining formula is | (1) |
3.2. Response time (τ)
Response time (τ) refers to the sensitivity of a photovoltaic device to an optical signal. Typically, the response time (τ) is defined as the time for the net photocurrent to rise from 10% to 90% when the light is switched on and off (rise time or response time) or the time for the net photocurrent to fall from 90% to 10% (fall time or recovery time). The rise time is determined by the transport of carriers and the fall time is determined by the collection of carriers by the electrodes.106 The response time is affected by many factors, such as the equivalent intrinsic RC constant of the device and the defect in the 2D material.3.3. Noise equivalent power (NEP)
Noise equivalent power (NEP), also called minimum measurable power, refers to the optical power required to produce a signal with one signal-to-noise ratio.107 The NEP is defined as follows: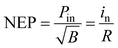 | (2) |
3.4. Detectivity (D*)
Detectivity (D*) represents the ability of the detector to capture weak signals108 and is calculated as follows: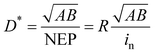 | (3) |
3.5. Gain (G)
Gain (G) can be expressed as the number of regenerated photo-excitons through a multiplication mechanism. For highly responsive photoconductive devices, researchers usually use multiple methods to capture one type of carrier to obtain a higher carrier lifetime, which multiplies the number of other types of carriers within the conducting channel.109 Thus, the gain (G) stems from a longer carrier lifetime and can be defined as follows: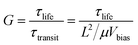 | (4) |
3.6. External quantum efficiency (EQE)
External quantum efficiency (EQE) is one of the main performance indicators of photodetectors and is defined as follows: when photons are irradiated onto the sensing surface of a photodetector, some of the photons will excite the photosensitive material to produce an electron–hole pair to form a photocurrent, and the ratio of the number of collected electrons to the number of all incident photons is called the external quantum efficiency (EQE).110,111 The defining equation is as follows: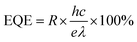 | (5) |
4. Recent progress in 2D/organic heterojunction photodetectors
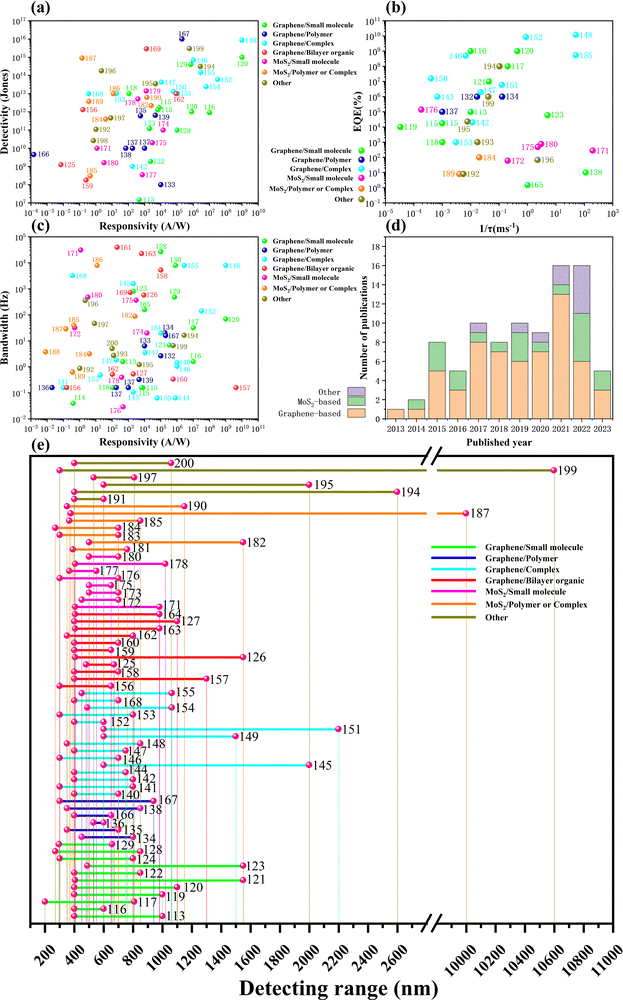
Modern optoelectronic devices, such as detectors, modulators, lasers, LEDs, and solar cells, are widely used daily. Modern optoelectronic devices are essential in imaging, display, optical communications, medicine, security, and energy. Photodetectors are popular optoelectronic devices used to convert optical signals into electrical signals. They play a key role in optics, communications, imaging, sensing and many other applications. The main principle of operation of photodetectors is to use light energy to excite charge carriers (electrons or holes) and convert them into current or voltage signals. Traditional photodetectors are usually manufactured based on traditional semiconductor materials such as Si, Ge, GaAs, etc.13–21 The application fields of photodetectors are becoming increasingly extensive, and the corresponding requirements for photodetectors are also higher. Traditional photodetectors can no longer meet the demand, especially in medicine, quantum communication, and satellite radar, and other more stringent requirements.90–97,99–102 The rise of 2D materials has brought new opportunities for the fabrication of photodetectors, especially since 2004 when Novoselov et al. obtained single-layer graphene by mechanical exfoliation.112 2D materials, including graphene and its derivatives (e.g., graphene oxide, reduced graphene oxide), along with analogous materials (e.g., TMDs, black phosphorus), demonstrate favorable compatibility with organic materials due to their distinct physical and chemical characteristics (low-defect surfaces, tunable surface chemistry, and the absence of a requirement for lattice matching considerations).48,49 Constructing 2D/organic heterojunctions can enhance carrier separation efficiency and mobility, improve interface properties, and increase device flexibility and processability. Consequently, 2D/organic heterojunction photodetectors (Fig. 7) often exhibit ultra-high responsivity, ultra-sensitive optical response, ultra-fast response time, broadband spectral detection, ultra-high gain, and ultra-high quantum efficiency. With society entering the era of big data, there is an increasing demand for multifunctional photodetectors in various fields, including high-speed communication, optical memory, light modulation, and on-chip computational optoelectronics. Therefore, challenges and opportunities persist for 2D/organic heterojunctions in multifunctional and high-performance detection. | ||
| Fig. 7 2D/organic heterojunction photodetectors. Graphene-based photodetectors. Reproduced with permission.126,139,148,152,157,169 Copyright 2018, Wiley-VCH; Copyright 2023, Wiley-VCH; Copyright 2018, Wiley-VCH; Copyright 2019, American Chemical Society; Copyright 2020, Wiley-VCH; Copyright 2017, American Chemical Society; Copyright 2022, American Chemical Society. MoS2-based photodetectors. Reproduced with permission.178,179,182,186,187,189 Copyright 2022, Nature Publishing Group; Copyright 2022, American Chemical Society; Copyright 2015, Wiley-VCH; Copyright 2019, Nature Publishing Group; Copyright 2022, Wiley-VCH; Copyright 2019, Wiley-VCH. Other photodetectors. Reproduced with permission.191,193–198,200 Copyright 2017, American Chemical Society; Copyright 2020, Wiley-VCH; Copyright 2021, Wiley-VCH; Copyright 2021, Nature Publishing Group; Copyright 2022, American Chemical Society; Copyright 2022, Nature Publishing Group; Copyright 2022, Elsevier; Copyright 2022, Wiley-VCH. | ||
4.1. Graphene-based photodetectors
Photodetectors based on the 2D material graphene are a hot research topic in the field of photodetection. However, graphene's thickness of only one atomic layer results in low absorbance (only 2.7%), which limits its responsivity, which is usually on the order of mA W−1. To solve this problem, we can combine graphene's property of high carrier mobility with the excellent light-absorbing properties of organic materials to prepare graphene photodetectors with excellent absorption properties (the summary of graphene/organic devices is shown in Table 1).| 2D materials | Organic thin films | Published year | Active materials | Responsivity [A W−1] | Response time [ms] | Detection range [nm] | Gain/EQE [%] | Detectivity [Jones] | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Graphene | Small molecule organic thin films | 2015 | Graphene/dye R6G | 460 | <100 | 400–1000 | 105% | 1.5 × 107 | 113 |
| 2016 | Nanoribbon graphene/C60 | 0.4 | 4000 | 10 000 | NA | NA | 114 | ||
| 2017 | Graphene/Zn0.9Mg0.1O NPs/BET dye | 8 × 103 | ≈1000 | 530 | 1.87 × 104% | 1.7 × 1012 | 115 | ||
| 2017 | Graphene/Zn0.9Mg0.1O NPs/N4 dye | 6.5 × 103 | ≈1000 | 460, 660 | 1.75 × 104% | 1.3 × 1012 | 115 | ||
| 2017 | Graphene/rubrene | 107 | 100 | 400–600 | 109% | 9 × 1011 | 116 | ||
| 2018 | Graphene/C60 | 107 | <5 | 200–808 | >108% | NA | 117 | ||
| 2018 | Graphene/DPA | 110 | >1000 | 420 | >103% | 1013 | 118 | ||
| 2019 | Graphene/R6G/graphene | 500 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
400–1000 | >104% | NA | 119 | ||
| 2019 | Graphene/C60 | 109 | 2.3 | 400–1100 | 109% | 1015 | 120 | ||
| 2020 | Graphene/C60/graphene | 3.4 × 105 | 23 | 405–1550 | >107% | NA | 121 | ||
| 2020 | Graphene/C12TBP | 2330 | NA | 400–850 | >105% | 1.9 × 109 | 122 | ||
| 2021 | Graphene/HAT-CN/Bi2O2Se | 2000 | 0.197 | 488–1550 | 6 × 104 | 1.2 × 1011 | 123 | ||
| 2021 | IGZO/graphene/CuPc | NA | NA | 300–800 | 150% | NA | 124 | ||
| 2022 | Graphene/pentacene | 105 | 0.006/0.009 | 270–850 | 3.3%/10% | >1011 | 128 | ||
| 2022 | Graphene/BUBD-1 | 7 × 105 | 0.33/0.49 | 295–658 | NA | 4 × 1014 | 129 | ||
| 2023 | Rubrene/graphene | 8 × 105 | 0.02/0.07 | 405 | NA | >1012 | 130 | ||
| 2023 | Pentacene/graphene/Au | >104 | 1 | 658 | 1.51% | NA | 165 | ||
| Polymer organic thin films | 2014 | Graphene/P3HT/PZT | NA | 180 | 325 | 103 | NA | 131 | |
| 2015 | Graphene/P3HT | 105 | 60 | 500 | >106% | NA | 132 | ||
| 2016 | Graphene/C8-BTBT | 104 | 25 | 355 | 1.89 × 108 | 108 | 133 | ||
| 2017 | Graphene/PTB7 | 1.8 × 105 | 7.8 | 450–800 | >106% | NA | 134 | ||
| 2018 | Graphene/PDA | 556 | NA | 350–700 | >105% | 6 × 1011 | 135 | ||
| 2020 | Graphene/J-aggregate TDBC cyanine dye | 0.02 | >1000 | 530–600 | NA | NA | 136 | ||
| 2020 | Graphene/PTCDI-C8/Al | 1.5 × 10−4 | NA | 400–650 | 1.50% | 4.5 × 109 | 166 | ||
| 2021 | Graphene/h-BN/J-aggregate PTCDI-C13 | 103 | >1000 | 550 | >105% | 1010 | 137 | ||
| 2021 | Graphene/h-BN/PTCDI-C13 | 180 | <1000 | 550 | >103% | 1010 | 137 | ||
| 2021 | Graphene/PDVF | 70 | NA | 350–850 | NA | 1010 | 138 | ||
| 2021 | Graphene/PTCDI-C8 dimer | 2 × 105 | 10 | 300–940 | NA | 1016 | 167 | ||
| 2023 | PPy-NGr/SnO2 | 4594.25 | 490/3040 | <240 | NA | 6.47 × 1011 | 139 | ||
| Complex organic thin films | 2013 | Graphene/chlorophyll | 106 | 110 | 400–700 | NA | NA | 140 | |
| 2015 | TiOx/graphene/P3HT:PCBM | ≈0.1 | ≈1000 | 300–800 | NA | NA | 141 | ||
| 2015 | Graphene/CH3NH3PbI3 | 180 | 87 | 400–800 | 2 × 104% | 109 | 142 | ||
| 2016 | Graphene/CH3NH3PbI3/NPs | 2 × 103 | 1500 | 532 | >106% | NA | 143 | ||
| 2017 | Graphene/CH3NH3PbI3:PCBM | 8 × 105 | 2.5 × 103 | 400–750 | NA | NA | 144 | ||
| 2018 | Graphene/TCNQ | 2 × 103 | 0.1 | 600–2000 | NA | NA | 145 | ||
| 2018 | Graphene/MOF | 106 | <150 | 300–700 | 5 × 108% | 6.9 × 1014 | 146 | ||
| 2018 | Graphene/CH3NH3PbI3:MoS2 | 1.08 × 104 | 45 | 400–750 | 2 × 106% | 4.28 × 1013 | 147 | ||
| 2019 | Graphene/CH3NH3PbBr3 QDs/graphene | 109 | 0.02 | 350–850 | 1.2 × 1010% | 8.7 × 1015 | 148 | ||
| 2019 | Graphene/P3HT:F4 TCNQ | 6 × 105 | NA | 600–1500 | >107% | NA | 149 | ||
| 2020 | Graphene/GNSs/MAPbI3 | 5.9 × 104 | 2500 | 460 | 1.59 × 107% | 1.31 × 1013 | 150 | ||
| 2020 | Graphene/TTF:CA | 105 | 8 | 600–2200 | 6 × 106% | 1013 | 151 | ||
| 2020 | Graphene/COF | 3.2 × 107 | 1.14 | 400–600 | 8.5 × 109% | 6 × 1013 | 152 | ||
| 2021 | Graphene/Au array/(BA)2(FA)n−1PbnI3n+1 | 18.71 | 330 | 300–800 | ≈103% | 1013 | 153 | ||
| 2021 | Graphene/ZnO/IEICO-4F:PTB7-Th | 6.1 × 106 | NA | 488–1064 | 8.3 × 108% | 2.43 × 1013 | 154 | ||
| 2021 | Graphene/PBDB-T:PC71BM | 0.36 | 0.049 | 400–700 | 1013 | 1013 | 168 | ||
| 2022 | Graphene/PM6:Y6 | 2.8 × 106 | 0.02/8.4 | 450–1064 | 5.47 × 108% | 1.47 × 1014 | 155 | ||
| Bilayer organic thin films | 2015 | Graphene/PEDOT:PSS/P3HT:PC71BM | 0.16 | ≈1000 | 300–650 | NA | 1.33 × 1012 | 156 | |
| 2017 | Graphene/P3HT/CH3NH3PbI3−xClx | 4.3 × 109 | 1000 | 400–1300 | 8.9 × 109 | NA | 157 | ||
| 2017 | Graphene/PTCDA/pentacene | 105 | 0.03 | 400–700 | 9% | NA | 158 | ||
| 2017 | Graphene/pentacene/PTCDI-C8 | 0.007 | NA | 480–670 | 1.26 × 109 | 1.26 × 109 | 125 | ||
| 2018 | Graphene/C60/pentacene | 9127 | 0.275 | 405–1550 | 5.2 × 105 | NA | 126 | ||
| 2019 | Graphene/P3HT/PEDOT:PSS | 0.25 | NA | 400–650 | NA | 1.8 × 108 | 159 | ||
| 2019 | Graphene/PTCDA/C8-BTBT | 5.76 × 105 | 470 | 400–700 | 1.38 × 109 | NA | 160 | ||
| 2021 | Graphene/ZnPc:C60/ZnPc | 204 | 0.004 | 635 | 3.9 × 104 | NA | 161 | ||
| 2021 | Graphene/PTAA/CH3NH3PbI3−xClx | 105 | 310 | 350–800 | NA | 1013 | 162 | ||
| 2021 | Graphene/C60/ZnPc | 6537 | 0.007 | 405–980 | 1.4 × 105 | NA | 163 | ||
| 2021 | Graphene/C60/pentacene | 2010 | <300 | 400–1100 | NA | NA | 127 | ||
| 2022 | Graphene/MOF/graphene/PVDF | 1.3 × 103 | 0.22 | 530 | 3 × 1010% | 2.9 × 1015 | 169 | ||
| 2022 | Graphene/C60/PbPc | 2.2 × 103 | NA | 405–980 | NA | NA | 164 | ||
| 2022 | Graphene/C60/CuPc | NA | NA | NA | NA | NA | 170 |
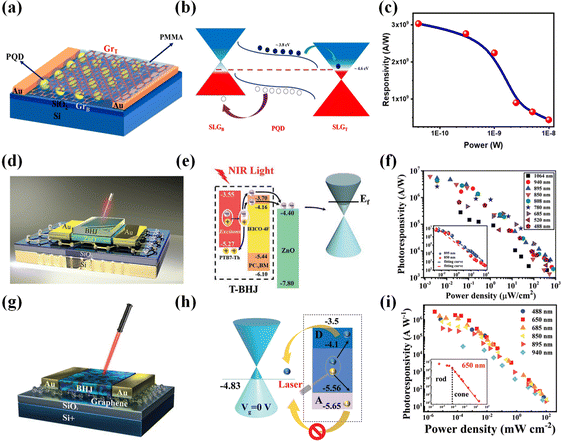
Narrowband photodetection has attracted extensive research, but previous strategies had noticeable drawbacks, such as reduced photoresponsivity, complex configurations, and lack of tunability. 2D/dye molecule heterojunction phototransistors115 provide an ideal solution. The graphene/Zn0.9Mg0.1O NPs/BET dye combination achieves tunable narrowband detection in the range of 470–580 nm, while the graphene/Zn0.9Mg0.1O NPs/N4 dye setup accomplishes narrowband photodetection at 400–500 nm and 610–700 nm. The responsivity reaches levels of 103, EQE reaches levels of 104%, and detectivity reaches levels of 1012. Most importantly, these devices are compatible with flexible configurations. Organic dye molecules typically have distinct isolated energy levels, making them unique, high-absorption, inherently narrowband absorbers. Combining them with 2D materials, where the dye molecules are anchored on graphene, significantly enhances light absorption while mitigating linewidth broadening. Graphene serves as a narrowband hybrid transport layer. In summary, the high performance of these photodetectors results from the controlled adsorption of dye molecules, which suppresses dye–dye coupling and π-resonance, preserving the narrowband detection characteristics of the dye molecules. The rich library of available dye molecule materials enables the tunability of the photodetectors. Gareth et al. were the first to apply single-crystal organic semiconductor materials to 2D/organic heterojunctions, and the stacking within single crystals can promote exciton diffusion over several micrometers, which contributes to improving the phototransistor's EQE. The graphene/rubrene heterojunction phototransistor116 achieves a responsivity of 107 A W−1 and a detectivity of 9 × 1011 Jones at room temperature. This performance is attributed to the long-range order in single-crystal “rubrene”, which helps to efficiently transfer photogenerated charges to graphene. Furthermore, combining single crystals with 2D materials can enhance the response speed of 2D/organic heterojunction phototransistors, holding significant potential applications in optical storage. Qin et al.117 were the first to introduce C60 into 2D/organic heterojunction photodetectors. The authors synthesized an all-carbon hybrid film with solid, tunable ultraviolet spectral absorption. The graphene/C60 heterojunction photodetector exhibits high photocurrent gain, elevated EQE, and exceptional ultraviolet sensitivity (covering 200–400 nm, R > 107 A W−1). This is attributed to the well-organized assembly of C60, which facilitates efficient exciton dissociation at the heterojunction interface. Notably, the device maintains its performance almost unchanged after two months of operation, demonstrating long-term stability and high reliability. The authors simultaneously fabricated a 5 × 5 array of devices, which holds significant potential for integrated optoelectronic applications.
Field-effect transistors have found wide applications due to their excellent optoelectronic properties. However, previously reported 2D/organic heterojunction vertical field-effect transistors did not perform well. DPA is an organic single-crystal material known for its advantages, such as the absence of impurities and defects, uniform molecular orientation, and high mobility. Combining DPA with graphene forms an excellent interface contact that effectively suppresses dark currents, enabling high photo-detection rates and a sizeable on–off ratio. The graphene/DPA heterojunction field-effect transistor,118 by gently transferring the organic single crystal onto the graphene surface using soft contact gold electrodes, achieves a detectivity of 1013 Jones, a responsivity of 110 A W−1, and a switching ratio of 1.5 × 103. This represents a significant enhancement in the performance of 2D/organic field-effect transistors, laying a solid foundation for developing vertical organic optoelectronic devices. Graphene/R6G/graphene (Fig. 8a and b)119 adopts a sandwich structure with a rhodamine 6G (R6G) monolayer as the photoactive layer sandwiched between two graphene films acting as channels. R6G dye molecules possess favorable characteristics such as low cost, high stability, and a high fluorescence yield (0.95). Due to the solid π–π interaction between R6G and graphene interfaces, photo-excited electrons can transfer from graphene to the dye molecules. Compared to R6G/graphene, graphene/R6G/graphene exhibits a 40-fold increase in responsivity (103 A W−1) (Fig. 8c). This enhancement results from the powerful π–π interaction, which reduces the intermolecular distance between the monolayer of R6G and the graphene, allowing photo-excited electrons from the top and bottom graphene layers to more effectively transfer to the encapsulated R6G monolayer. Of greater significance, graphene/R6G/graphene represents a three-layer ultra-thin structure, potentially serving as a critical component in the next generation of highly integrated optoelectronic devices.
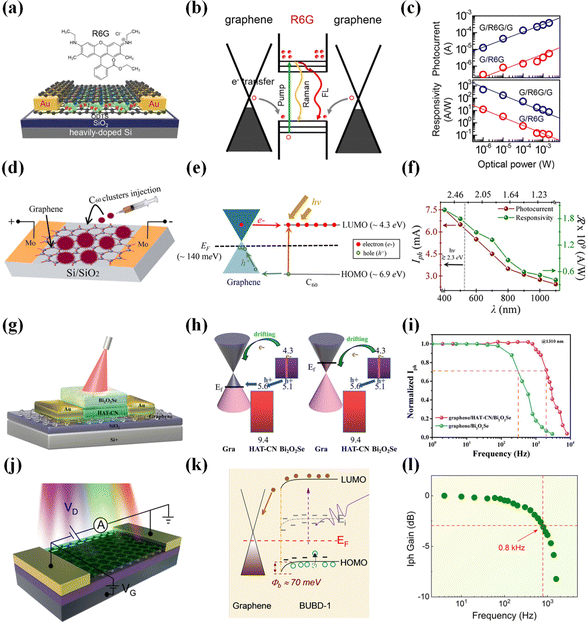 | ||
| Fig. 8 Enhancing the photodetector performance of graphene by combining it with small molecule organic materials. (a) Schematic illustration of graphene/R6G/graphene.119 (b) Schematic band structure.119 (c) Responsivity and photocurrent as a function of incident light power density.119 Reproduced with permission.119 Copyright 2019, American Chemical Society. (d) Schematic representation of graphene/C60.120 (e) Band diagram.120 (f) Iph and responsivity as a function of λ.120 Reproduced with permission.120 Copyright 2019, American Chemical Society. (g) Schematic illustration of graphene/HAT-CN/Bi2O2Se.123 (h) The charge transfer principle of type-III energy band alignment.123 (i) Normalized amplitude as a function of frequency.123 Reproduced with permission.123 Copyright 2021, Royal Society of Chemistry. (j) Schematic of graphene/BUBD-1.129 (k) Energy band diagram.129 (l) Photocurrent gain as a function of frequency.129 Reproduced with permission.129 Copyright 2021, Royal Society of Chemistry. | ||
Electrophoretic deposition technology aids in producing high-quality 2D/organic heterojunctions. Photodetectors fabricated using electrophoretic deposition technology, such as the graphene/C60 heterojunction photodetector (Fig. 8d and e),120 achieve an outstanding detectivity of 1015 Jones, an EQE of 109%, and a remarkably high responsivity of 109 A W−1 (Fig. 8f). The exceptional performance of these photodetectors is primarily attributed to the doping enhancement of graphene upon adsorption of C60, significantly promoting charge transport between the two components. This device capitalizes on C60's strong electron-accepting properties and graphene's high charge carrier mobility, enabling synergistic cooperation for efficient charge collection and, thus, the realization of high-performance photodetectors. Importantly, this device introduces electrophoretic deposition technology into manufacturing 2D/organic heterojunction photodetectors for the first time, extending the detection range to 400–1100 nm. This marks a pivotal advancement for broadband and NIR photodetection. The graphene/C60/graphene heterojunction photodetector121 proposed by our research group has also been utilized to fabricate phototransistor arrays. This approach enables tunability of multiple parameters, such as photocurrent, response intensity, and response speed, making it feasible for wide-band, large-scale array detection of an all-carbon material. Notably, graphene/C60/graphene exhibits bidirectional response photocurrent, with the device displaying a negative response current in the 405–650 nm range and a positive response current in the 808–1550 nm range. The authors employed photolithography and deep reactive ion etching methods to achieve sensitivity (3.4105 A W−1), rapid response (23 ms), and ultra-wideband coverage (405–1550 nm) in the phototransistor's characteristics. The significant contribution of this work lies in enabling the fabrication of large-scale, all-carbon array photodetectors.
The interface layer may have a different arrangement from the bulk material, leading to an unclear understanding of the interface layer's optoelectronic properties and photodetection performance. Shao et al. prepared high-performance graphene/C12TBP heterojunction photodetectors (191 A W−1, 3.9 × 1012 Jones)122 using the in situ retro-Diels–Alder reaction. Notably, the authors investigated the impact of the interface layer stacking on photodetection capabilities and the underlying mechanism. They pointed out that the π–π stacking at the graphene/C12TBP interface edges is critical in achieving ultra-high photodetection efficiency. More accurately, this is due to the adequate overlap of molecular orbitals in adjacent π–π stacked C12TBP molecules, promoting the diffusion, dissociation, and separation of photogenerated charge carriers and charge transfer states. This work provides robust theoretical support for studying interface layer structures and electronic properties. Similar to the challenges in weak light detection in the NIR region, achieving a fast response in the optical communication band (@1310 nm) has been challenging. Our research group has proposed the graphene/HAT-CN/Bi2O2Se heterojunction phototransistor (Fig. 8g and h),123 which demonstrates the capability of ultra-broadband detection (488–1550 nm) (Fig. 8i), covering the optical communication band, and exhibits a rapid response time (101 ms). This is attributed to the presence of HAT-CN, which efficiently transfers photogenerated holes in Bi2O2Se to the graphene layer, resulting in an enhanced responsivity of 2420 A W−1. Additionally, the authors have achieved array detection, which holds significant potential for application in near-infrared focal plane detection and high-speed communication fields. Choi et al. harnessed the anti-ambipolar charge transport behavior of IGZO/graphene/CuPc heterojunction photodetectors124 to achieve high-precision color selectivity. In other words, a monolithic tandem multicolor photodetector has enabled the functionality of an image sensor. This vertically stacked photodetector structure and wavelength-selective photodetection approach have paved the way for the development of image sensors.
Photodetectors based on pentacene and graphene have been manufactured for some time.125–127 However, these devices typically feature relatively complex structures and modulation processes and exhibit limited weak light detection capabilities. The graphene/pentacene heterojunction photodetector proposed by Gan et al.128 offers several advantages, including a simple structure and excellent self-powered weak light detection capabilities. This device exhibits high responsivity (>105 A W−1), high detectivity (>1011 Jones), and ultra-fast response times (0.006/0.009 ms). Its exceptional performance can be attributed to the low charge trap density and short carrier lifetime at the graphene/pentacene interface. Notably, the device achieves high-resolution single-pixel imaging in UV and VIS spectra. Overall, this work provides a design concept for self-powered photodetectors and holds great promise in integrating weak light detection devices and radio equipment. The performance of sensitized devices is highly correlated with the quality of the heterojunction interface, making the simplified production of high-quality heterojunction interfaces a challenging issue. Qin et al. have successfully fabricated a high-quality graphene/BUBD-1 heterojunction interface using a straightforward thermal evaporation technique. The graphene/BUBD-1 heterojunction photodetector (Fig. 8j and k)129 exhibits a high responsivity (7 × 105 A W−1), rapid response times (0.33/0.49 ms), and a high detectivity (4 × 1014 Jones). The cutoff frequency at −3 dB, indicating where the photocurrent signal decreased by 2, was determined to be approximately 0.8 kHz under VG = −60 V (Fig. 8l). Additionally, this device offers the capability for ultraviolet detection and tunable rise/decay times attributed to gate voltage adjustments of the barrier height. This direct growth preparation method holds significant potential for application in large-scale hybrid devices. Organic semiconductors exhibit high optical absorption coefficients, but amorphous and polycrystalline materials often reduce exciton dissociation efficiency due to short exciton diffusion lengths. They also typically struggle to achieve zero-bias photodetection. The use of single-crystal rubrene structures can address these challenges. While rubrene/graphene heterojunction photodetectors have been proposed previously,116 they did not achieve self-powered capabilities. The rubrene/graphene heterojunction photodetector presented by Zhao et al.130 offers self-powered photon detection, high responsivity (8 × 105 A W−1), high detectivity (>1012 Jones), and fast response times (20/70 μs). These attributes are primarily due to the long exciton diffusion length in the rubrene/graphene heterojunction's rubrene single crystal and graphene's high mobility. Furthermore, the asymmetric van der Waals stacking structure of the rubrene/graphene heterojunction forms a high-quality heterojunction interface, which holds significant promise in the field of wireless photon detection.
Poly(3-hexylthiophene) (P3HT) demonstrates a high absorption coefficient, offering a solution to graphene's low absorption limitations. Moreover, P3HT possesses favorable attributes such as ease of manufacturing and cost-effectiveness. In 2014, Tan et al. introduced the graphene/P3HT/PZT heterojunction photodetector (Fig. 9a–c).131 Photogenerated holes transfer from P3HT to graphene, while photogenerated electrons are retained within P3HT. The high hole mobility within graphene allows them to circulate efficiently before recombining with the electrons captured in P3HT. Consequently, photosensitivity is significantly enhanced. Variations in the confinement of the PZT substrate can have a crucial impact on P3HT/graphene photodetectors and substantially improve the light responsivity of the hybrid composite material. This photodetector exhibits an ultra-high gain of 103. Organic polymer thin films have the potential to enhance the performance of 2D phototransistors, but achieving high gain remains a challenging task. Everardus et al. fabricated graphene/P3HT heterojunction phototransistors (Fig. 9d–f),132 obtaining phototransistors with ultra-high gain for the first time. By reducing the channel length and increasing the mobility, the transit time of charge carriers through graphene was minimized, thereby optimizing the optical gain of these devices. Organic semiconductors offer high spectral sensitivity, and graphene provides high charge carrier mobility. However, creating high-quality 2D/organic interfaces remains a challenge. Using van der Waals epitaxy allows for producing single-layer organic semiconductors and high-quality interfaces. Even with just one layer of C8-BTBT, the graphene/C8-BTBT heterojunction phototransistor133 still demonstrates outstanding performance, with a high responsivity (R) of up to 1.57 × 104 A W−1 and an ultra-high gain exceeding 108.
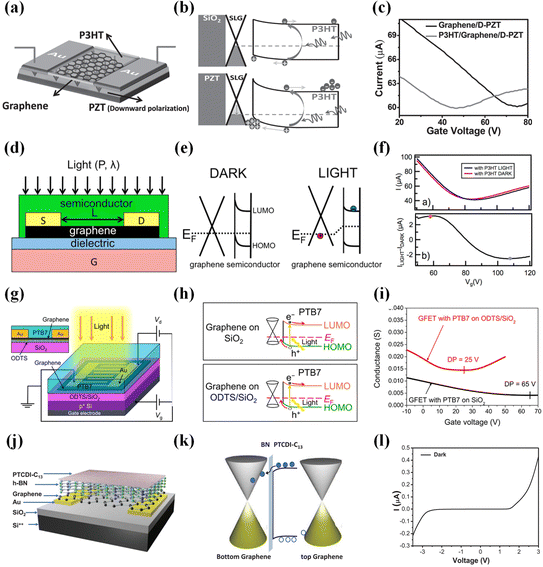 | ||
| Fig. 9 Enhancing the photodetector performance of graphene by combining it with organic polymers. (a) Schematic view of graphene/P3HT/PZT.131 (b) Band diagrams of P3HT/graphene/PZT: the lower Fermi level of graphene produces a higher built-in field by D-PZT than by the SiO2 substrate.131 (c) Drain current as a function of gate voltage.131 Reproduced with permission.131 Copyright 2014, Wiley-VCH. (d) Side view of graphene/P3HT.132 (e) Energy level diagram of graphene/P3HT in the dark and under light.132 (f) Source–drain and light-induced current as a function of gate voltage.132 Reproduced with permission.132 Copyright 2015, American Chemical Society. (g) Schematic illustration of graphene/PTB7.134 (h) Schematic illustration of the photocarrier transfer.134 (i) Channel conductance of PTB7-coated GFETs.134 Reproduced with permission.134 Copyright 2017, American Chemical Society. (j) Schematic of graphene/h-BN/J-aggregate PTCDI-C13.137 (k) Energy-band diagram under illumination at zero bias.137 (l) I–V curve.137 Reproduced with permission.137 Copyright 2021, Wiley-VCH. | ||
Most graphene-based hybrid photodetectors typically exhibit longer response times. However, the “assembled-monolayer (SAM)-functionalized” technique can improve devices’ response time due to the enhanced interface quality. High-sensitivity photodetectors with short response times are of great importance in the field of photodetection. Graphene/PTB7 heterojunction (Fig. 9g–i)134 photodetectors using SAM technology achieve an ultra-high responsivity of 1.8 × 105 A W−1 and a fast response time of 7.8 ms. High-quality interfaces are crucial for photodetector performance and represent an area that needs significant development in the future. Most current research focuses on visible to infrared light, with relatively few studies addressing the ultraviolet part. PDA exhibits extensive strong absorption properties in the VIS and UV regions, offering significant potential in the field of flexible wideband UV-VIS photodetectors. PDA possesses advantages such as low manufacturing costs, flexibility, and ease of production. Therefore, the graphene/PDA heterojunction photodetector135 achieves a responsivity of 556 A W−1 and a detectivity of 6 × 1011 Jones. The graphene/PDA detector maintains reliable sensing capabilities even after undergoing 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bending rounds. This can be attributed to PDA serving as both a transfer support layer and a light-absorbing layer, allowing the production of near-perfect centimeter-scale graphene sheets. The device also possesses eco-friendly characteristics, similar to graphene/MOF. It holds significant potential for application in the field of flexible, portable, and wearable electronic devices. J-aggregates have very narrow absorption bands in the VIS and NIR regions, greatly enhancing the spectral sensitivity of photodetectors due to their rapid and coherent energy transfer capabilities. Therefore, this material holds promise for improving the spectral sensitivity of photodetectors, but effectively integrating it with graphene is a challenge. The graphene/J-aggregate TDBC cyanine dye heterojunction photodetector136 is a VIS light-gated all-carbon phototransistor. While its performance is not outstanding, it offers an innovative thin film fabrication technique. This technique is simple and controllable, allowing the fabrication of thin films of various water-soluble materials on the desired substrate material's surface. It is worth noting that this transistor can reversibly write and erase charge doping in graphene using visible light, paving the way for applying J-aggregates in photodetectors.
000 bending rounds. This can be attributed to PDA serving as both a transfer support layer and a light-absorbing layer, allowing the production of near-perfect centimeter-scale graphene sheets. The device also possesses eco-friendly characteristics, similar to graphene/MOF. It holds significant potential for application in the field of flexible, portable, and wearable electronic devices. J-aggregates have very narrow absorption bands in the VIS and NIR regions, greatly enhancing the spectral sensitivity of photodetectors due to their rapid and coherent energy transfer capabilities. Therefore, this material holds promise for improving the spectral sensitivity of photodetectors, but effectively integrating it with graphene is a challenge. The graphene/J-aggregate TDBC cyanine dye heterojunction photodetector136 is a VIS light-gated all-carbon phototransistor. While its performance is not outstanding, it offers an innovative thin film fabrication technique. This technique is simple and controllable, allowing the fabrication of thin films of various water-soluble materials on the desired substrate material's surface. It is worth noting that this transistor can reversibly write and erase charge doping in graphene using visible light, paving the way for applying J-aggregates in photodetectors.
In the field of biophysics, there is often a need to detect the colors of biomolecules, requiring high-performance narrowband photodetectors. Previous narrowband photodetectors have frequently sacrificed gain and responsivity. Using J-aggregate fluorescent molecules with narrowband absorption characteristics can address this issue. However, J-aggregates lack π–π interactions and exhibit low charge carrier mobility. Combining J-aggregates with graphene holds great promise. The graphene/h-BN/J-aggregate PTCDI-C13 heterojunction photodetector (Fig. 9j–l)137 achieves an ultra-narrow spectral response of 13 nm and a high responsivity of 103 A W−1. Growing PTCDI-C13 on an h-BN substrate ensures the device's narrowband absorption characteristics. This represents a significant advancement in the field of narrowband detection in 2019 and provides a new avenue for designing highly sensitive narrowband photodetectors. Taking the graphene/PDVF heterojunction photodetector presented by Yu et al. as an example,138 the researchers demonstrated a technique for tuning the detection wavelength using thermal annealing. This device can alter the position of the Fermi level in the PDVF film through thermal annealing, thereby changing the barrier height and the detection wavelength. The device achieves tunable measurements in the 350–850 nm range, exhibiting varying photosensitivity under different temperature treatments. This provides valuable support for understanding the complex electronic processes near the interface.
2D/organic heterojunctions have been the subject of extensive research, primarily confined to the VIS and NIR regions, with limited progress in DUV detection. The DUV spectrum is an integral component of sunlight with potential antiviral and sterilization applications, making DUV detection highly meaningful. SnO2, a wide-bandgap semiconductor material capable of sensing DUV light, can be combined with graphene to create high-performance DUV photodetectors. The PPy-NGr/SnO2 heterojunction photodetector exhibits an exceptionally high responsivity (4594.25 A W−1) and a high detectivity (6.47 × 1011 Jones).139 These characteristics result from the substantial built-in electric field (NGr/SnO2, PPy/SnO2, and PPy-NGr/SnO2), accelerating the efficient dissociation of photogenerated excitons. At the same time, NGr mitigates the valence band offset between PPy and SnO2. This work provides a design approach for cost-effective and easily manufacturable DUV photodetectors.
As mentioned earlier, pristine graphene exhibits a weak absorption rate as low as 2.3%, fundamentally limiting its applicability in photodetection. Chlorophyll is an organic semiconductor known for its exceptional light absorption properties. The graphene/chlorophyll phototransistor demonstrates high gain and responsivity through the integration of graphene and photosensitive chlorophyll molecules. This device's observed ultra-high light responsivity, reaching 106 A W−1,140 can be attributed to charge transfer at the interface and the efficient light effect within the chlorophyll layer. One of the most significant challenges in graphene-based electronics is the control of carrier types and the modulation of carrier concentration in graphene through doping. The method of regulating the electrical performance of graphene transistors using photoexcitation has the advantages of controllability and reversibility. It would be exciting and appealing if the carrier transport types (either n- or p-type) of an optically induced graphene transistor could be selectively controlled by choosing specific optical excitation wavelengths, enabling tunable carrier transport in a graphene-based photonic device. The TiOx/graphene/P3HT:PCBM heterojunction photodetector141 can determine whether the graphene transistor carries n-type or p-type carriers by choosing either UV or VIS light irradiation, achieving a degree of controllable and reversible n-type or p-type doping. The transistor device exhibits a negative shift in the charge neutrality point (CNP) value when illuminated with wavelengths less than 350 nm. Conversely, under illumination with wavelengths greater than 350 nm, a positive shift in the CNP value is observed, confirming the selective n-type and p-type carrier transport phenomenon in graphene transistors.
Methylammonium lead halide, that is, CH3NH3PbX3 (where X is a halogen), possesses excellent optoelectronic properties, and notably, these nanocrystals offer a simple manufacturing process and lower production costs. While CH3NH3PbX3/graphene heterojunctions have long been applied in solar cells, their utilization in photodetectors has also yielded excellent results. The graphene/CH3NH3PbI3 heterojunction photodetector142 demonstrates a photoresponsivity of 180 A W−1, an EQE of approximately 5 × 104%, a photodetection rate of around 109 Jones, and a broad spectral bandwidth in the VIS range, owing to efficient charge transfer from graphene to CH3NH3PbI3. The transfer of electrons from the graphene layer to the proximate CH3NH3PbI3 layer determines the occupied states in the CH3NH3PbI3 valence band generated by photon absorption. The recombination of photoexcited electron–hole pairs in CH3NH3PbI3 is restricted, allowing the photoexcited electrons to remain within the conduction band of CH3NH3PbI3 without decay. While previous studies have successfully combined organic semiconductor materials with graphene, high-gain phototransistors have not been achieved. The plasmonic effect of metal nanostructures can significantly improve photodetector performance. Compared to graphene/CH3NH3PbI3 heterojunction photodetectors, graphene/CH3NH3PbI3/NPs (NPs refers to nanoparticles) heterojunction photodetectors143 exhibit higher EQE and responsivity. This enhancement can be attributed to the surface plasmonic effects of NPs, which increase the light absorption cross-section of the surrounding CH3NH3PbI3 material, thereby improving light capture within the CH3NH3PbI3 layer. Introducing a combination of two distinct organic materials to form an organic heterojunction is a promising strategy for optimizing the performance of organic optoelectronic materials and expanding the range of applications for various optoelectronic devices. For example, doping PCBM into CH3NH3PbI3 can facilitate the separation of electron–hole pairs and selective charge capture, significantly enhancing the efficiency of electron–hole pair separation and the carrier lifetime in such organic heterojunctions. In the case of graphene/CH3NH3PbI3:PCBM (Fig. 10a and b),144 the electron lifetime is extended by four to five orders of magnitude compared to graphene/CH3NH3PbI3, resulting in a 30-fold increase in photoresponsivity, reaching 106 A W−1. Graphene/CH3NH3PbI3:PCBM represents the first application of doped organic heterojunctions in 2D/organic photodetectors, opening up a new design strategy for high-performance photodetectors. Subsequently, a continuous stream of high-performance photodetectors based on doped organic heterojunctions has emerged.
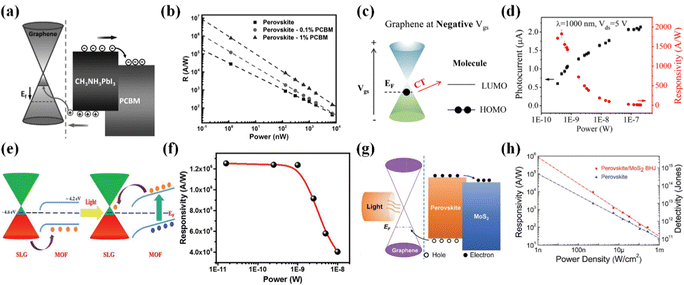 | ||
| Fig. 10 Enhancing the photodetector performance of graphene by combining it with complex organic materials. (a) The energy level diagram of graphene/CH3NH3PbI3:PCBM.144 (b) Responsivity as a function of the incident light power.144 Reproduced with permission.144 Copyright 2017, Wiley-VCH. (c) Schematic illustration of charge-transfer transitions of graphene/TCNQ.145 (d) Photocurrent and responsivity as a function of the incident light power.145 Reproduced with permission.145 Copyright 2018, American Chemical Society. (e) Energy band diagram of graphene/MOF.146 (f) Variation of responsivity as a function of illumination power.146 Reproduced with permission.146 Copyright 2018, Wiley-VCH. (g) Energy level diagram of graphene/PDA.147 (h) Dependence of responsivity and detectivity on the incident light power density.147 Reproduced with permission.147 Copyright 2018, Wiley-VCH. | ||
Charge-transfer (CT) complex tetrathiafulvalene-tetracyanoquinodimethane (TTF-TCNQ) was discovered in 1973, and it possesses unique properties related to intermolecular electron transitions, offering substantial applications in high-performance photodetectors. The graphene/TCNQ heterojunction photodetector (Fig. 10c and d)145 exhibits exceptionally high responsiveness between 600 and 2000 nm, with a peak exceeding 2000 A W−1. This can be attributed to TCNQ's unique low-energy intermolecular electronic transitions that extend into the NIR region. Interactions between molecules lead to efficient charge separation, resulting in an extremely high photocurrent gain, thus holding significant value in photodetection applications. MOFs are characterized by high porosity and intrinsic tunability, making them excellent materials for wearable devices. Meeting the global demand for wearable devices such as electronic skin, flexible optoelectronic devices, and biomedical equipment requires environmentally friendly, highly stable devices. The graphene/MOF heterojunction photodetector (Fig. 10e and f)146 is characterized by its non-toxic, stable, and environmentally friendly nature, making it highly suitable for wearable electronic devices. The high porosity of MOFs results in strong light absorption, while graphene exhibits excellent optoelectronic properties and flexibility. Consequently, graphene/MOF exhibits remarkable attributes such as ultrafast response (<150 ms), ultra-high responsivity (>106 A W−1), extremely high EQE (>5 × 108%), and extensibility. Combining MOFs with 2D materials paves the way for developing wearable and portable electronic products. Peng et al. introduced the graphene/CH3NH3PbI3:MoS2 heterojunction photodetector (Fig. 10g and h),147 further advancing the development of multi-heterojunction photodetectors. In this device, CH3NH3PbI3:MoS2 serves as the light-absorbing layer, graphene functions as the charge carrier transport layer, and MoS2 facilitates selective electron capture, thereby suppressing carrier recombination and increasing photocurrent. This photodetector exhibits a high responsivity (1.08 × 104 A W−1), a high detectivity (4.28 × 1013 Jones), a high EQE (2.0 × 106%), and a rapid light response time (45 ms). Moreover, it is crucial to note that the graphene/CH3NH3PbI3:MoS2 device maintains high performance even when applied to flexible substrates, opening up significant opportunities for application in wearable and portable electronic devices.
With the continuous advancement of technology, there is a growing demand for simple, lightweight, integrated electronic devices, especially those capable of converting light into electricity and generating light from electricity, serving as dual-function optoelectronic devices. Prior to this, research combining photodetectors with light-emitting diodes was limited, and such combinations typically exhibited subpar performance. The graphene/CH3NH3PbBr3 QDs/graphene heterojunction phototransistor (Fig. 11a and b)148 represents a dual-function device with both light emission and photodetection capabilities, featuring a vertical stacked “sandwich” structure. This device exhibits a rapid response time (∼20 μs), ultra-high responsivity (∼3 × 109 A W−1) (Fig. 11c), and a high EQE (∼1.2 × 1010%), with the magnitude and direction of the photocurrent being adjustable by applying gate voltage. Compared to lateral phototransistors, vertical phototransistors leverage an internal electric field to modulate charge separation directly, significantly increasing photocurrent and enhancing responsivity. Highly stable dual-function light-emitting phototransistors hold great promise in the fields of displays and optical communications. Commercial NIR photodetectors rely on semiconductor materials such as InGaAs and InAs. Therefore, developing high-performance emerging NIR photodetectors remains essential. The graphene/P3HT:F4 TCNQ heterojunction photodetector149 exhibits an ultra-high responsivity of 106 A W−1 in the NIR range and demonstrates broadband photoresponse from VIS to the NIR. This can be attributed to the photoinduced effects at the interface of charge transfer complexes and graphene molecules and the intermolecular electronic transitions of charge transfer complexes. Furthermore, the graphene/P3HT:F4 TCNQ structure exhibits tunable photoresponsivity, which can be flexibly adjusted through the electrical gating of graphene. The development of this device contributes to the advancement of low-cost, high-performance NIR photodetectors.
 | ||
| Fig. 11 Graphene/complex organic thin film photodetectors. (a) Schematic design of graphene/CH3NH3PbBr3 QDs/graphene.148 (b) Energy band representation.148 (c) Illumination light power dependent photoresponsivity curve of graphene/CH3NH3PbBr3 QDs/graphene.148 Reproduced with permission.148 Copyright 2019, American Chemical Society. (d) Schematic of graphene/ZnO/IEICO-4F:PTB7-Th.154 (e) The energy level alignment and working mechanism diagrams.154 (f) Responsivity as a function of input light power density.154 Reproduced with permission.154 Copyright 2021, Wiley-VCH. (g) Schematic device structure of graphene/PM6:Y6.155 (h) Schematic diagram of the device energy level.155 (i) Light power density dependent responsivity of graphene/PM6:Y6.155 Reproduced with permission.155 Copyright 2022, Wiley-VCH. | ||
The plasma effect can significantly enhance the responsivity of photodetectors. However, when combined with graphene, a single type of plasma structure is often used, which does not yield satisfactory results. Combining plasma nanomaterials with perovskites (MAPbI3) can result in ineffective synergistic effects and insufficient device performance. The graphene/gold nanostars (GNSs)/MAPbI3 heterojunction photodetector150 provides a perfect solution to both issues. This device incorporates spherical plasma gold nanoparticles (GNSs) and MAPbI3 materials. Due to the hybridization of tip and core plasma modes, GNSs generate a powerful local electric field, exhibiting a highly efficient light-capturing effect. The device demonstrates outstanding detection performance (R = 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 952 A W−1, D* = 1.31 × 1013 Jones, EQE = 1.59 × 107%), signifying the effective integration of MAPbI3 materials and GNSs into graphene. More importantly, even after undergoing 1000 repeated bends, the device maintains excellent performance and stability. Furthermore, the authors have fabricated a 10 × 10 array of photodetectors that exhibits high resolution in response to optical signals, laying the foundation for developing array detection devices. Tetrathiafulvalene–chloranil (TTF–CA) exhibits charge transfer electronic transitions down to 0.5 eV, making it an ideal absorber material for the shortwave infrared (SWIR) region. When combined with graphene as the charge transport layer, it produces an exceptionally high-performance graphene/TTF:CA photodetector (D* = 1013 Jones, R = 105 A W−1, τ = 2–12 ms).151 The photodetector's exceptional performance can be primarily attributed to optical photogating, which promotes delayed carrier recombination, leading to high gain and sensitivity. The critical contribution of this work is the discovery of charge transfer complex absorbers in the SWIR region, making low-cost and high-performance SWIR photodetectors feasible.
952 A W−1, D* = 1.31 × 1013 Jones, EQE = 1.59 × 107%), signifying the effective integration of MAPbI3 materials and GNSs into graphene. More importantly, even after undergoing 1000 repeated bends, the device maintains excellent performance and stability. Furthermore, the authors have fabricated a 10 × 10 array of photodetectors that exhibits high resolution in response to optical signals, laying the foundation for developing array detection devices. Tetrathiafulvalene–chloranil (TTF–CA) exhibits charge transfer electronic transitions down to 0.5 eV, making it an ideal absorber material for the shortwave infrared (SWIR) region. When combined with graphene as the charge transport layer, it produces an exceptionally high-performance graphene/TTF:CA photodetector (D* = 1013 Jones, R = 105 A W−1, τ = 2–12 ms).151 The photodetector's exceptional performance can be primarily attributed to optical photogating, which promotes delayed carrier recombination, leading to high gain and sensitivity. The critical contribution of this work is the discovery of charge transfer complex absorbers in the SWIR region, making low-cost and high-performance SWIR photodetectors feasible.
2D-COFs possess excellent properties such as thermal stability, permanent porosity, large surface area, diverse topological structures, and low mass density. Importantly, they feature a pre-designed π-electron framework and a highly ordered topological structure that enhances carrier transport. However, COFs are typically in solid powder form, making integration challenging. Xiong et al. successfully obtained COF thin films on graphene using chemical vapor deposition, forming an ordered graphene/COF heterojunction. Graphene/COF152 exhibits high responsivity (3.2 × 107 A W−1) and a fast response time (1.14 ms). Significantly, graphene/COF can be modulated by specific target molecules, attributed to COF's high surface area and polarity selectivity. The significant contribution of this work lies in providing a new research strategy for tunable multifunctional devices. Although perovskite materials have made substantial contributions in the field of solar cells, their use in photodetectors has been limited due to structural defects and grain boundaries often found in perovskite thin films, which hamper charge carrier transport capabilities. Additionally, perovskite materials have a low absorption coefficient in the NIR range, resulting in very small photocurrents. Hence, there is a need for a high-quality interface to effectively integrate 2D perovskite (BA)2(FA)n−1PbnI3n+1 with graphene. The utilization of hyperbolic gold nanostructures can address the issues above. Gold nanostructures exhibit light-capturing and electromagnetic field-enhancing effects, and the coupling effect between graphene, gold array, and (BA)2(FA)n−1PbnI3n+1 significantly enhances their light response capabilities in the UV and NIR spectral regions. The graphene/gold array/(BA)2(FA)n−1PbnI3n+1 heterojunction photodetector153 achieves a detectivity of 2.21 × 1013 Jones and features a low dark current of 1.0 × 10−10 A, with a high on/off ratio of up to 104. They utilized Au array/(BA)2(FA)n−1PbnI3n+1 as the photosensitive layer and graphene as the charge carrier transport layer, which maximizes light capture and photoinduced charge extraction. This represents an excellent strategy for combining two high-quality materials, namely, graphene and (BA)2(FA)n−1PbnI3n+1. Introducing zinc oxide into the charge carrier extraction layer similarly enhances the sensitivity of the photodetector in the NIR range. The graphene/ZnO/IEICO-4F:PTB7-Th heterojunction photodetector (Fig. 11d and e)154 proposed by our research group exhibits weak NIR light detection capabilities. It also features high-performance parameters (R = 6.1 × 106 A W−1 (Fig. 11f) and D* = 2.43 × 1013 Jones). The IEICO-4F:PTB7-Th heterojunction shows strong absorption in the NIR spectral range, and ZnO suppresses charge carrier recombination processes effectively. This ingenious design addresses the deficiency in weak light detection by photodetectors in the NIR region. Even more importantly, this device accomplishes the detection of human pulse signals and heart rate, representing another advancement in applying photodetectors in the medical field. While conventional organic heterojunctions have addressed issues such as the low quantum efficiency of a single absorption layer, they still suffer from poor dissociation efficiency and limited weak light detection capabilities. The PM6:Y6 heterojunction proposed by our research group cleverly overcomes these limitations, presenting a donor–acceptor interpenetrating bulk heterojunction structure. The graphene/PM6:Y6 heterojunction phototransistor (Fig. 11g and h)155 possesses the capability for weak light detection in the near-infrared region, along with high responsivity (2.86 × 106 A W−1) (Fig. 11i) and high detectivity (1.47 × 1014 Jones). This can be attributed to the high dissociation efficiency of the PM6:Y6 heterojunction and the alignment of energy bands at the interface. Furthermore, the device exhibits bidirectional photoresponse and a broadband photosensitive range. The authors also fabricated a retinal vision chip based on graphene/PM6:Y6, capable of handling visual images spanning over six orders of magnitude. This device can also be used for real-time monitoring of human pulses, holding significant promise in the field of medicine.
The production of highly conductive graphene films still faces significant challenges. Therefore, seeking alternative approaches for constructing highly conductive graphene films is a task of great significance and considerable challenges. Poly(3,4-ethylenedioxy thiophene):poly(styrene sulfonate) (PEDOT:PSS) (clevios PH1000) is a novel graphene solution that enables the fabrication of high-quality, large-area graphene films through spray coating. The graphene/PEDOT:PSS/P3HT:PC71BM heterojunction photodetector156 operates at an extremely low bias voltage, possibly working with low energy consumption. This photodetector exhibits characteristics of being “ultrathin” and “flexible” and can be attached to the surface of the human body, making it a promising candidate for wearable devices. The construction and utilization of multiple heterojunctions have substantially enhanced the performance of photodetectors. For instance, the graphene/P3HT/CH3NH3PbI3−xClx heterojunction phototransistor157 exhibited a responsivity of 4.3 × 109 A W−1 and a gain of 1010, surpassing previous CH3NH3PbI3−xClx phototransistors by several orders of magnitude. In this structure, CH3NH3PbI3−xClx is the light-absorbing layer, P3HT is the hole transport layer that effectively separates electrons and holes, and graphene is the charge transport layer. The ultrahigh responsivity and gain are attributed to intense light absorption and efficient photogenerated charge carrier separation. Moreover, this phototransistor can detect infrared light with wavelengths exceeding 1300 nm.
Phototransistors with only one semiconductor light-absorbing layer lack a built-in electric field, which reduces quantum efficiency and bandwidth. The graphene/PTCDA/pentacene heterojunction phototransistor (Fig. 12a and b)158 incorporates PTCDA/pentacene organic epitaxial heterostructures as the light-absorbing layer, resulting in the design of a high-performance phototransistor with optimized parameters such as responsivity (R) (Fig. 12c), carrier lifetime (τ), and EQE. The selection of PTCDA and pentacene as the light-absorbing layer is based on their complementary absorption spectra within the VIS light range. The high performance primarily stems from the inherent built-in electric field at the semiconductor interface that facilitates charge separation. This approach opens up a strategy for designing phototransistors, where organic heterojunctions as the light-absorbing layer can significantly enhance the performance of 2D/organic heterojunction phototransistors. Another promising design strategy is to create vertical Schottky barrier transistors by integrating two organic materials with graphene. The graphene/pentacene/PTCDI-C8 heterojunction photodetector (Fig. 12d and e)125 effectively suppresses dark current density and enhances the device's photocurrent, crucial for improving photodiode performance. PTCDI-C8 reduces the work function of graphene, preventing electron injection from graphene into pentacene and thus reducing dark current density. The reduced work function also improves the collection of photogenerated holes in pentacene, thereby increasing photocurrent density. As a result, the device's specific detectivity and responsivity (Fig. 12f) could be enhanced systematically by adjusting the gate bias. This represents the latest development in vertically stacked heterojunction devices integrated with graphene electrodes. The light response of 2D/organic heterojunction transistors is typically confined to a relatively narrow spectral range determined by the semiconductor's bandgap. Our research group introduced bidirectional (positive and negative) light response in organic heterojunctions for the first time. Previously reported graphene-based high-gain photodetectors had their spectral range typically limited to 400–700 nm. However, the graphene/C60/pentacene heterojunction transistor (Fig. 12g and h)126 extends the bandwidth to 405–1550 nm through bidirectional light response. It is important to note that the increased bandwidth is not achieved by altering the organic material's absorption unit. Instead, it leverages compassionate light responses in the visible spectrum. C60/pentacene, as the light-absorbing layer with a significant built-in electric field, efficiently promotes charge dissociation. Graphene, functioning as both light-absorbing and transport layer, exhibits a responsivity of 9127 A W−1 (EQE = 11.5%) in the visible range (Fig. 12i) and a responsivity exceeding 1800 A W−1 (EQE = 0.063%) in the NIR range, with a shortened response time of 275 μs. The exceptional performance of this photodetector is attributed to bidirectional carrier transport, providing a new mechanism for broadband detection using graphene and a novel approach for creating stable and ultra-fast photodetectors.
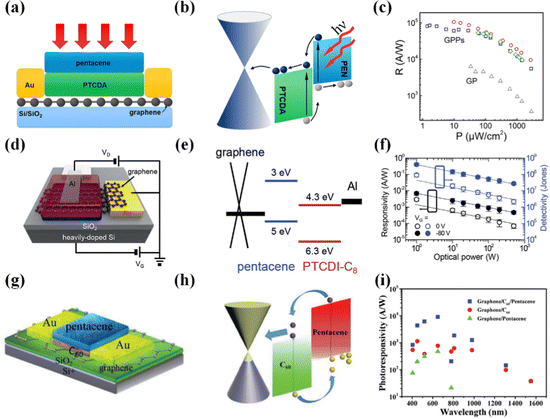 | ||
| Fig. 12 Enhancing the photodetector performance of graphene by combining it with bilayer organic thin films. (a) Schematic illustration of graphene/PTCDA/pentacene.158 (b) Energy band diagram.158 (c) Responsivity as a function of laser power density.158 Reproduced with permission.158 Copyright 2017, American Chemical Society. (d) Schematic of graphene/pentacene/PTCDI-C8.125 (e) Energy band alignment.125 (f) Responsivity and detectivity as a function of optical power.125 Reproduced with permission.125 Copyright 2017, Wiley-VCH. (g) 3D structure of graphene/C60/pentacene.126 (h) The photogenerated carrier transfer diagram of the whole device.126 (i) Responsivity as a function of wavelength.126 Reproduced with permission.126 Copyright 2018, Wiley-VCH. | ||
P3HT, a widely recognized conjugated polymer for photovoltaic applications, possesses high electrical conductivity and absorption coefficient, but it suffers from drawbacks such as poor mechanical strength and stability. By overlaying it with graphene and PEDOT:PSS, a high-performance photodetector with a detectivity of 1.8 × 108 Jones was obtained.159 It is worth noting that, similar to dark current, the photocurrent depends on the concentration of graphene, with the device's optimal performance achieved at a graphene concentration of 5%. This is because a small amount of graphene promotes charge carrier transport, while excess graphene can lead to particle aggregation, reducing the charge transfer rate. Furthermore, the device exhibits good photoresponse and bandwidth (0.624 MHz). A single absorption layer's limited spectral response bandwidth often constrains organic semiconductor/graphene photodetectors. This limitation can be effectively overcome by employing an organic heterojunction absorption layer strategy. PTCDA and C8-BTBT exhibit complementary optical absorption characteristics in the ultraviolet and visible spectra. Incorporating them as the absorption layer through van der Waals vapor growth significantly enhances the performance of photodetectors. The graphene/PTCDA/C8-BTBT heterojunction phototransistor160 achieves a high photoresponsivity of up to 5.76 × 105 A W−1 and a high optical gain of 1.38 × 109. Importantly, this device achieves ultra-broadband detection spanning the UV, VIS, and NIR regions, attributed to the ultraviolet photosensitivity of C8-BTBT and the high visible light absorption coefficient of PTCDA. The band alignment in the double heterojunction structure efficiently facilitates the transfer of photogenerated charge carriers (electrons) from the organic layer to the graphene layer. The high-quality interfaces produced through vacuum-based vapor deposition also contribute to efficient carrier transfer. This further advances the development of organic heterojunctions with complementary light absorption characteristics in photodetectors.
The response times of most 2D/organic heterojunction photodetectors are in the millisecond range. Lee et al. designed a graphene/ZnPc:C60/ZnPc heterojunction photodetector161 through the doping of organic semiconductor materials, achieving a response time in the microsecond range for the first time while maintaining high responsivity and high EQE. Electrons captured within C60 can recombine with holes in ZnPc, reducing the transient photocurrent decay time from 5.8 ms to 19 μs, resulting in a 300-fold performance improvement. This can be attributed to the shortened carrier lifetime within the light-absorbing layer C60, facilitating carrier mobility between C60 and the graphene layer. This work has officially elevated the photodetector's performance to the microsecond level, providing a strategy for designing ultra-high-performance photodetectors. PTAA, with a wide bandgap of approximately −5.2 eV, serves as the hole transport layer in photodetectors. The graphene/PTAA/CH3NH3PbI3−xClx heterojunction photodetector162 exhibits a high detectivity of 1013 Jones and a remarkable responsivity of 105 A W−1. This is attributed to the role of the hole transport layer PTAA, which facilitates the movement of photogenerated charge carriers from the CH3NH3PbI3−xClx layer to graphene while suppressing carrier recombination. The function of PTAA is akin to that of the ZnO/IEICO-4F:PTB7-Th heterojunction. However, the graphene/PTAA/CH3NH3PbI3−xClx structure is more streamlined. Additionally, introducing a hydrophobic protective layer (PMMA) significantly enhances the device's stability and demonstrates exceptional durability under bending, making it suitable for application in imaging and sensing fields.
There has been limited research on the impact of organic semiconductor thickness on the performance of photodetectors. To investigate the multi-heterojunction photodetector response mechanism and the effect of film thickness on performance, our research group proposed the graphene/C60/ZnPc heterojunction photodetector.163 By varying the thickness of the C60 film, an in-depth investigation was conducted into the relationship between film thickness and photocurrent, yielding two distinct heterojunction photodetector response mechanisms. This device exhibits a high responsivity of 6537 A W−1 and a rapid response time of 7.29 ms. Intriguingly, it exhibits bidirectional response characteristics attributed to the overlap in the responses of the graphene/C60 heterojunction and the C60/ZnPc heterojunction. The nodes between positive and negative responses are closely tied to the thickness of the C60 film, allowing for tuning the switching points for positive and negative light responses by adjusting the C60 film thickness. This work establishes a foundational understanding of the charge transfer mechanisms in optoelectronic devices. The graphene/C60/pentacene heterojunction photodetector,126 proposed by our research group in 2018, has been further advanced by Nguyen et al.,127 who introduced a self-assembled monolayer (SAM). SAM technology enhances the crystallinity of the C60 film, resulting in a fivefold performance improvement when compared to the direct deposition of C60 and pentacene on graphene. Image sensors are typically composed of at least four photodetectors, and the complexity of integration has posed limitations on the development of image sensors. In recent years, wearable optoelectronic devices have received extensive research interest; yet achieving versatile, single-polarization wearable optoelectronic technology remains challenging. Bera et al. introduced a graphene/MOF/graphene/PVDF heterojunction phototransistor,164 offering a core technology for wearable integrated optoelectronic devices. The graphene/MOF/graphene/PVDF device exhibits self-powered ultrahigh sensitivity (EQE ≈ 3 × 1010%) and ultrafast response (τ ≈ 220 μs), primarily attributed to the asymmetric Schottky junction formed between the MOF and graphene. Furthermore, this transistor offers tunable colors through electrical tuning. Additionally, the authors fabricated an optical strain sensor based on the graphene/MOF/graphene/PVDF heterojunction, operating on the principle of controlling the Dirac point of graphene by applying both upward and downward bending strains. This strain sensor exhibits excellent stretchability, making it highly suitable for wearable integrated optoelectronic devices. This work provides a promising direction for researching susceptible and ultrafast wearable devices. Typically, most graphene photodetectors exhibit lower responsivity in the near-infrared region. Graphene/C60/PbPc155 achieves tunable high photoresponsivity (2.2 × 103 A W−1) and broadband response (405–980 nm) in the NIR region. This is attributed to PbPc's strong absorption in the near-infrared region and the high exciton dissociation efficiency of the PbPc/C60 heterojunction. Moreover, this device exhibits bidirectional photoresponse due to the appropriate band alignment of graphene/C60. The authors further investigated the mechanism behind this bidirectional photoresponse, providing a novel approach for manufacturing broadband detection phototransistors with vast potential in the field of high-performance NIR photodetection.
Graphene-based 2D/organic heterojunction photodetectors have been the subject of approximately a decade of research, resulting in the continuous development of high-performance devices. In the future, these graphene-based 2D/organic heterojunctions still hold significant research value, given that graphene's broadband absorption characteristics are unparalleled by other 2D materials. Currently, there is limited research on long-wavelength detection, and graphene/organic heterojunction photodetectors are poised to excel in the field of long-wavelength detection.
4.2. MoS2-based photodetectors
Transition metal dichalcogenides (TMDs) are similarly endowed with high charge carrier mobility. However, unlike graphene, which exhibits a zero bandgap, TMDs possess tunable bandgaps, allowing for the suppression of dark current generation. Among TMDs, MoS2 stands out as a prominent representative, and photodetectors based on MoS2 typically exhibit exceptional performance. Nevertheless, MoS2 suffers from limited light absorption capabilities, making it an attractive candidate for synergistic combination with organic materials, opening up extensive development prospects (the summary of MoS2/organic devices is provided in Table 2).| 2D materials | Organic thin films | Published year | Active materials | Responsivity [A W−1] | Response time [ms] | Detection [nm] | Gain/EQE [%] | Detectivity [Jones] | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| MoS2 | Small molecule organic thin films | 2014 | MoS2/dye R6G | 1.17 | 5.1 × 10−3 | 405–980 | 280% | 1010 | 171 |
| 2015 | MoS2/rubrene | 0.5 | 5 | 450–700 | ≈60% | NA | 172 | ||
| 2016 | MoS2/pentacene | NA | NA | 500–700 | NA | NA | 173 | ||
| 2018 | MoS2/ZnPc | 1.4 × 104 | 8 | 532 | NA | 1011 | 174 | ||
| 2020 | MoS2/CuPc | 3 × 103 | 0.436 | 500–650 | 483% | 2 × 1010 | 175 | ||
| 2021 | MoS2/BTBT-SAM | 475 | 5500 | 300–700 | 1.4 × 105% | NA | 176 | ||
| 2022 | DPA/MoS2(DMDM) | 7.3 × 102 | NA | 365–550 | NA | 3.5 × 108 | 177 | ||
| 2022 | MoS2/2DPI | 390.5 | 400/430 | 405–1020 | 1.04 × 105 | 5.10 × 1012 | 178 | ||
| 2022 | PbPc/MoS2 | 1263.85 | NA | 808 | 194![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 066.7% 066.7% |
1.4 × 1013 | 179 | ||
| 2023 | MoS2/BTP-4F | 3.2 | 0.332/0.274 | 500–700 | 756% | 1.6 × 109 | 180 | ||
| Polymer and complex organic thin films | 2015 | MoS2/C8-BTBT | 0.022 | NA | Visible | ≈10% | NA | 181 | |
| 2015 | MoS2/P(VDF-Tr18FE) | 2570 | 1.8 | 500–1550 | NA | ≈2.2 × 1012 | 182 | ||
| 2016 | MoS2/P3HT:PCBM/metasurface | NA | <10−3 | 300–700 | NA | NA | 183 | ||
| 2017 | MoS2/g-C3N4 | 4 | 50 | 270–700 | ≈102% | 4 × 1011 | 184 | ||
| 2019 | MoS2/PDPP3T | 0.445 | 4 | 365–850 | NA | 3 × 108 | 185 | ||
| 2019 | MoS2/P(VDF-TrFE) BJT | 12 | 0.02 | 532 | 103 | 1013 | 186 | ||
| 2019 | MoS2/P(VDF-TrFE) | 0.14 | 5.5 | 375–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
NA | 9 × 1014 | 187 | ||
| 2022 | (C6H5C2H4NH3)2PbBr4/MoS2 | 7.98 × 10−3 | 42/38 | 405 | 103 | NA | 188 | ||
| 2022 | MAPbBr3/MoS2 | 0.368 | 252/260 | 532 | 8% | 3.74 × 1012 | 189 | ||
| 2023 | PSS/SnS2/MoS2 | 548.26/1389.08/457.47 | NA | 350/450/1150 | 1.94 × 105%/3.82 × 105%/4.72 × 104% | 2.49 × 1012/6.31 × 1012/2.07 × 1012 | 190 |
In 2014, Yu et al. pioneered the concept of 2D/organic heterojunction photodetectors based on MoS2, achieving noteworthy results. The MoS2/dye R6G heterostructure demonstrated a maximum photoresponsivity of 1.17 A W−1, a detectivity of 1.5 × 107 Jones, and an external quantum efficiency (EQE) of 280%.171 In comparison to the original MoS2 photodetector, the performance of the MoS2/dye R6G has been amplified by a factor of ten, primarily ascribed to the photoinduced charge transfer phenomena between MoS2 and dye R6G. Furthermore, this device has broadened its detection spectrum into the NIR (405–980 nm). This study marks the pioneering introduction of MoS2 into 2D/organic heterojunction photodetectors, ushering in a new era of research on MoS2/organic heterojunctions. Heterojunction photodetectors based on van der Waals (vdW) interactions typically exhibit excellent performance. Combining rubrene with MoS2 forms a vdW heterojunction photodetector,172 achieving a photoresponsivity of approximately 500 mA W−1 and a rectification ratio of up to 105, demonstrating commendable rectifying properties and gate-tunability. This device presents a promising avenue for low-cost, high-performance photodetectors. Previous research showed a lack of clear understanding of charge transport between organic materials and MoS2. Therefore, Jariwala et al. investigated the photodetection response of MoS2/pentacene heterojunction photodetectors.173 The results revealed that the MoS2/pentacene heterojunction exhibits a type II band alignment photovoltaic effect, giving rise to asymmetric photocurrent transfer characteristics. The smaller size of pentacene compared to SiO2 enhances the capture of photoexcited charge carriers, consequently accelerating the response time. This work underscores the potential of MoS2 as a receptor material in photovoltaic applications.
Traditional organic photovoltaic materials such as phthalocyanines (Pcs) and their metal derivatives exhibit excellent chemical stability and, when combined with 2D materials, possess the attribute of rapid charge transfer. The MoS2/ZnPc heterojunction photodetector (Fig. 13a and b)174 showcases a high responsivity (1.4 × 104 A W−1) (Fig. 13c), swift response time (8 ms), and high sensitivity (1011 Jones). The MoS2/ZnPc heterojunction establishes a van der Waals interface, enabling electron–hole pairs to spontaneously separate under illumination, with holes migrating to the ZnPc thin film layer. ZnPc is capable of utilizing the inherent capture states of MoS2. This work, rooted in the surface assembly of organic molecules, provides a novel design approach for developing 2D/organic photodetectors. The Pcs family can maintain an idealized energy offset for ultrafast exciton dissociation. CuPc stands out as a distinguished representative within the Pcs family. The MoS2/CuPc heterojunction constitutes an ideal type-II heterojunction, where CuPc can smooth the surface of MoS2, suppressing the generation of dark current and accelerating charge transfer. The MoS2/CuPc heterojunction photodetector175 exhibits a high responsivity (3 × 103 A W−1), a high detection rate (2 × 1010 Jones), a swift response time (0.436 ms), and an EQE of 483%. Compared to MoS2 photodetectors, the response time has been improved by four orders of magnitude. This work advances the development of highly sensitive VIS light photodetectors. Self-assembled monolayers (SAM) significantly enhance the interface quality by allowing precise film thickness control, reducing charge scattering and recombination, and simplifying solution adsorption processes. The MoS2/BTBT-SAM heterojunction proposed by Zhao et al.176 represents the first realization of all 2D organic–inorganic hybrid vdW heterojunction phototransistors. The MoS2/BTBT-SAM heterojunction photodetector demonstrates a high responsivity (475 A W−1) and EQE (1.45 × 105%), primarily attributed to the photovoltaic effect. BTBT-SAM promotes charge transfer through π–π interactions. This work advances the application of SAM technology in the field of photodetectors, providing more possibilities for the design of 2D atomic-molecular layers and fostering the development of novel heterostructures.
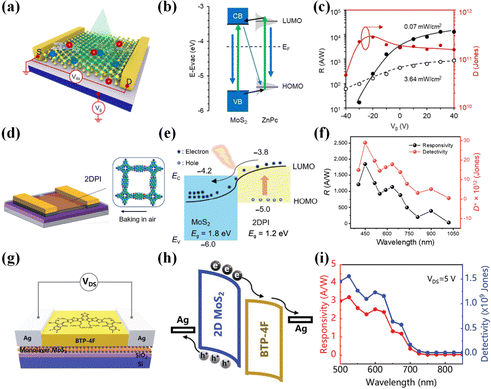 | ||
| Fig. 13 Enhancing the photodetector performance of MoS2 by combining it with small molecule organic materials. (a) Schematic illustration of MoS2/ZnPc.174 (b) Derived interfacial band alignment.174 (c) Responsivity and detectivity as a function of Vg.174 Reproduced with permission.174 Copyright 2018, American Chemical Society. (d) Schematic diagram of MoS2/2DPI.178 (e) Band alignment and the injection process of photogenerated carriers.178 (f) Responsivity and detectivity as a function of wavelength.178 Reproduced with permission.178 Copyright 2022, Nature Publishing Group. (g) Illustration of MoS2/BTP-4F.180 (h) Energy level matching.180 (i) Responsivity and detectivity as a function of wavelength.180 Reproduced with permission.180 Copyright 2023, Wiley-VCH. | ||
In 2022 and 2023, the performance of 2D/organic heterojunction photodetectors based on MoS2 has demonstrated remarkable advancements. Photodetectors with bipolar charge transport characteristics have been developed,177 and highly stable NIR photodetectors have been proposed.178,179 The suppression of dark current has seen recent developments, with dark currents reduced to the pA level.180 In contrast to heterojunctions, bilayer structures with independent channels can individually transport photogenerated excitons. 2,6-Diphenyl anthracene (DPA) is an outstanding representative in organic semiconductors, characterized by high hole mobility and electroluminescence properties. DPA/MoS2(DMDM)177 exhibits bipolar charge transport characteristics, with electrons and holes displaying distinct migration rates (holes at 0.13 cm2 V−1 s−1 and electrons at 1.1 cm2 V−1 s−1). The key to achieving balanced electron and hole transport lies in bilayer structures and independently layered configurations with “ideal” clean interfaces. Research findings indicate that large interfaces within bilayer structures and unique band alignments are advantageous for carrier accumulation and recombination. Furthermore, reduced abnormal photocurrent has been observed, attributed to the differing light absorption ranges and band alignment structures of DPA and MoS2. This work promotes the development of bipolar photodetectors, offering insights into the future development of organic–inorganic van der Waals heterojunction photodetectors. Achieving excellent and stable NIR photodetection is a meaningful yet challenging endeavor. MoS2/2DPI (Fig. 13d and e)178 accomplishes high-performance photodetection in the NIR region. The device exhibits high photoresponsivity (390.5 A W−1) (Fig. 13f), a high detection rate (5.10 × 1012 Jones), and a broad response range (405–1020 nm). This is attributed to the type-II heterojunction's rational band structure and 2D PI's strong NIR light absorption. This work provides critical technology for high-performance detection in the NIR range using monolayer TMDCs sensitized with 2D covalent organic polymers.
PbPc is an exemplary member of the phthalocyanine family, with most metal phthalocyanine materials responding to light in the UV to VIS range. However, PbPc demonstrates excellent photovoltaic response in the NIR region. Under a mere 0.061 μW cm−2 NIR illumination, PbPc/MoS2179 exhibits an exceptionally high photoresponsivity (1263.85 A W−1) and an extraordinarily high EQE (194![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 066.7%). This is attributed to the use of aluminum electrodes. From the perspective of energy level matching, the performance of gold electrodes should be far superior to that of aluminum electrodes. Paradoxically, this is because gold electrodes constitute Ohmic contacts, while aluminum electrodes form Schottky contacts. Schottky contacts have a significantly higher exciton dissociation efficiency than Ohmic contacts. This study elucidates the principles behind using materials such as Al, Au, Cu, and Ag as electrodes and analyzes the various factors contributing to exciton dissociation. It offers novel insights for enhancing the performance of 2D/organic devices. Organic materials with solubility properties are crucial for fabricating straightforward and high-performance photodetectors. BTP-4F possesses solubility characteristics and features a low exciton dissociation energy, facilitating effective exciton dissociation. The MoS2/BTP-4F heterojunction photodetector (Fig. 13g and h)180 not only exhibits shallow dark currents (at the pA level) but also demonstrates high responsivity (3.2 A W−1), elevated EQE (756%), remarkable detectivity (1.6 × 109 Jones) (Fig. 13i), and rapid response times (0.332/0.274 ms). This work advances the development of high-performance 2D/organic heterojunctions, providing a design paradigm for sensitive and rapidly responsive heterojunctions.
066.7%). This is attributed to the use of aluminum electrodes. From the perspective of energy level matching, the performance of gold electrodes should be far superior to that of aluminum electrodes. Paradoxically, this is because gold electrodes constitute Ohmic contacts, while aluminum electrodes form Schottky contacts. Schottky contacts have a significantly higher exciton dissociation efficiency than Ohmic contacts. This study elucidates the principles behind using materials such as Al, Au, Cu, and Ag as electrodes and analyzes the various factors contributing to exciton dissociation. It offers novel insights for enhancing the performance of 2D/organic devices. Organic materials with solubility properties are crucial for fabricating straightforward and high-performance photodetectors. BTP-4F possesses solubility characteristics and features a low exciton dissociation energy, facilitating effective exciton dissociation. The MoS2/BTP-4F heterojunction photodetector (Fig. 13g and h)180 not only exhibits shallow dark currents (at the pA level) but also demonstrates high responsivity (3.2 A W−1), elevated EQE (756%), remarkable detectivity (1.6 × 109 Jones) (Fig. 13i), and rapid response times (0.332/0.274 ms). This work advances the development of high-performance 2D/organic heterojunctions, providing a design paradigm for sensitive and rapidly responsive heterojunctions.
The epitaxial growth process offers notable advantages, including cleanliness, low-temperature operation, and precise morphological control. Photodetectors based on MoS2/C8-BTBT heterojunctions,181 fabricated via epitaxial growth techniques, achieve a photoresponsivity of 22 mA W−1 and a rectification ratio as high as 105, primarily due to the photovoltaic effect inherent in the MoS2/C8-BTBT heterojunction. Both 2D and organic materials have a rich library of materials, which lays a solid foundation for developing 2D heterostructures. In traditional photodetectors, gate voltage and source–drain voltage are employed to enhance sensitivity, albeit at the cost of increased losses due to self-heating. P(VDF-TrFE) presents the ability to fine-tune the transport characteristics of the channel, thereby improving the detector's performance. The MoS2/P(VDF-TrFE) heterojunction photodetector (Fig. 14a and b)182 exhibits a high detectivity (2.2 × 1012 Jones) and a remarkable photoresponsivity (2570 A W−1) (Fig. 14c), primarily attributed to P(VDF-TrFE) suppression of dark current in MoS2 and the enhanced detector sensitivity brought about by stable residual polarization. Furthermore, this device can achieve detection in the NIR region (500–1550 nm). Plasma metasurface structures can enhance the light absorption of 2D materials. Petoukhoff et al. applied metasurface to MoS2/P3HT:PCBM heterojunction photodetectors,183 resulting in a six-fold increase in charge density and a broadening of the absorption bandwidth by 90 nm. This enhancement is attributed to the metasurface expediting the hole transfer from MoS2 to P3HT:PCBM. This study underscores the synergistic effect between 2D/organic heterojunctions and metasurface, promising substantial applications in thin-film solar cells. 2D materials typically exhibit varying bandgaps and dark current characteristics. The cost-effective control and mitigation of dark current pose intriguing challenges. g-C3N4 is considered a promising candidate for integration with 2D materials due to its ability to suppress dark current in these materials. Furthermore, g-C3N4 facilitates rapid charge transfer between itself and 2D materials, improving the efficiency of photogenerated exciton dissociation. The MoS2/g-C3N4 heterojunction photodetector184 demonstrates a photoresponsivity of 4 A W−1, a response time of 50 ms, 100% EQE, a detectivity of 4 × 1011 Jones, and broadband responsiveness (270–700 nm). Notably, this device exhibits excellent flexibility, maintaining high performance even after hundreds of continuous bends, laying a solid foundation for developing low-cost, flexible photodetectors based on MoS2.
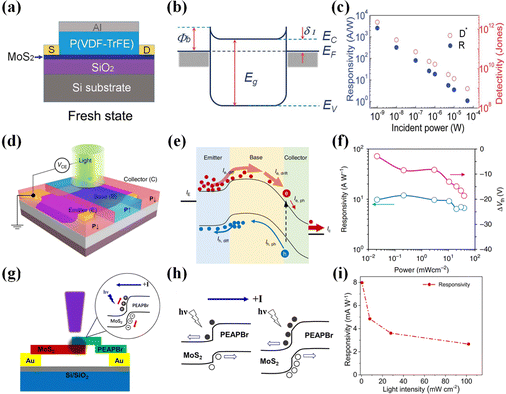 | ||
| Fig. 14 Enhancing the photodetector performance of MoS2 by combining it with organic polymers or complex materials. (a) The cross section structures of MoS2/P(VDF-TrFE).182 (b) Equilibrium energy band diagrams.182 (c) Photoresponsivity and detectivity as a function of incident power.182 Reproduced with permission.182 Copyright 2015, Wiley-VCH. (d) Schematic illustration of MoS2/P(VDF-TrFE).186 (e) Energy band diagram.186 (f) Responsivity and extracted threshold voltage shift as a function of incident power.186 Reproduced with permission.186 Copyright 2019, Nature Publishing Group. (g) Schematic illustrations of (C6H5C2H4NH3)2PbBr4/MoS2.188 (h) Energy band diagrams of (C6H5C2H4NH3)2PbBr4/MoS2.188 (i) Responsivity vs. light intensity.188 Reproduced with permission.188 Copyright 2022, American Chemical Society. | ||
While most of these heterojunctions primarily operate in the UV to VIS range, PDPP3T exhibits photosensitivity in the NIR region. The amalgamation of MoS2 with PDPP3T promises to yield high-performance broadband photodetectors. The MoS2/PDPP3T heterojunction phototransistor185 demonstrates self-powered photoresponsivity at zero bias voltage, with high responsivity and a rapid response time of 4 ms. This is attributed to forming the MoS2/PDPP3T heterojunction and creating an asymmetric source–drain configuration. Furthermore, PDPP3T's solubility facilitates its use in flexible photodetectors, rendering MoS2/PDPP3T a highly flexible option with substantial potential for integration into wearable optical-electronic devices, presenting significant application value. Ferroelectric materials, such as P(VDF-TrFE) copolymers, exhibit exceptional properties in fabricating simple rotation layers. The MoS2/P(VDF-TrFE) BJT heterojunction photodetector proposed by Lv et al. (Fig. 14d–f)186 demonstrates self-powering and high gain characteristics, marking the first instance of improving the response time of MoS2-based photodetectors to the microsecond level (20 μs). What's more, this device can easily tailor its reconfigurable features through finite element polarization switching. This reconfigurable device holds the potential to advance intelligent image window sensors. Its application in high-stability array detection is feasible. Likewise utilizing P(VDF-TrFE) copolymers, Wang et al. introduced MoS2/P(VDF-TrFE),187 achieving ultrabroad detection ranging from UV to MIR with a high on–off ratio (103) and a fast response time (5.5 ms). This marks a groundbreaking development in 2D/organic heterojunction photodetectors, expanding the detection wavelength by 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nm for the first time. This achievement is attributed to the thermoelectric effect of the MoS2/P(VDF-TrFE) heterojunction and the significant enhancement of MoS2's photodetection performance by the P(VDF-TrFE) polar field. P(VDF-TrFE) can suppress MoS2's dark current and narrow MoS2's bandgap, thus broadening the detection spectrum. This work provides a reliable approach for long-wave detection.
000 nm for the first time. This achievement is attributed to the thermoelectric effect of the MoS2/P(VDF-TrFE) heterojunction and the significant enhancement of MoS2's photodetection performance by the P(VDF-TrFE) polar field. P(VDF-TrFE) can suppress MoS2's dark current and narrow MoS2's bandgap, thus broadening the detection spectrum. This work provides a reliable approach for long-wave detection.
2D Ruddlesden–Popper (RP) phase perovskites exhibit excellent stability, and (C6H5C2H4NH3)2PbBr4/MoS2 proposed by Xu et al. (Fig. 14g–i)188 demonstrates an ultra-low dark current (0.1 pA), owing to the low carrier density of (C6H5C2H4NH3)2PbBr4 and the reasonable band structure of the heterojunction. Furthermore, this device maintains excellent photodetection performance after over a thousand switching cycles. Notably, the device can operate at zero bias voltage, showcasing self-powering and self-driven capabilities. At illumination intensities below 0.5 mW cm−2, the device exhibits excellent photoresponsivity even under zero bias conditions. This work underscores the significant potential of the 2D RP perovskite in weak light detection. MAPbBr3/MoS2 and (C6H5C2H4NH3)2PbBr4/MoS2 demonstrate self-powered characteristics, maintaining excellent photodetection performance under zero bias voltage. MAPbBr3 possesses favorable attributes such as low trap density, high absorption coefficient, and high photovoltaic conversion efficiency. With MoS2 as the n-type semiconductor and MAPbBr3 as the p-type semiconductor, the MAPbBr3/MoS2 heterojunction exhibits robust photovoltaic properties. Under zero bias conditions, MAPbBr3/MoS2189 showcases a photoresponsivity of 368 mA W−1 and a photodetection rate of 3.74 × 1012 Jones. This work lays the foundation for the development of high-performance photovoltaic devices.
4.3. Photodetectors with other 2D materials
In addition to graphene and MoS2, numerous 2D materials have been successfully employed in the fabrication of high-performance photodetectors (summarized in Table 3).191–200 For instance, WS2/poly-TPD:PCBM192 has demonstrated self-powered capabilities, while SWCNTs/PQT-12:F4TCNQ,194 Bi2Se3/MOF(Ni-CAT-1),195 ReS2/2D perovskite ((CH3(CH2)3NH3)2(CH3NH3)4Pb5I16),196 IEICO-4F/WSe2,197 3DG/P3HT:PC71BM,199 and graphite/C60/BP,200 among other devices, exhibit infrared detection capabilities. Notably, 3DG/P3HT:PC71BM199 can detect wavelengths exceeding 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nm, expanding the detection range into the MIR region.
000 nm, expanding the detection range into the MIR region.
| 2D materials | Published year | Active materials | Responsivity [A W−1] | Response time [ms] | Detection [nm] | Gain/EQE [%] | Detectivity [Jones] | Ref. |
|---|---|---|---|---|---|---|---|---|
| Other 2D materials except graphene and MoS2 | 2017 | WS2/PTCDA | NA | NA | 400–600 | NA | NA | 191 |
| 2019 | WS2/poly-TPD:PCBM | 1.02 | <181 | 450 | 8% | 1.1 × 1011 | 192 | |
| 2020 | Bi2Se3/rubrene | 124 | 58 | 532 | >103% | NA | 193 | |
| 2021 | SWCNTs/PQT-12:F4TCNQ | 2.75 × 106 | 9.7 | 400–2600 | 108% | 3.12 × 1014 | 194 | |
| 2021 | Bi2Se3/MOF(Ni-CAT-1) | 4725 | 130 | 600–2000 | 2.3 × 104% | 3.5 × 1013 | 195 | |
| 2022 | ReS2/(CH3(CH2)3NH3)2(CH3NH3)4Pb5I16 | 2.2 | 0.443/0.72 | 2000 | 68% | 1.8 × 1014 | 196 | |
| 2022 | IEICO-4F/WSe2 | 8.32 | 3.39/4.24 | 532–808 | NA | 4.65 × 1011 | 197 | |
| 2022 | MoTe2/PBDB-T:ITIC | 0.706 | NA | 656 | 133% | 2.62 × 1010 | 198 | |
| 2022 | 3DG/P3HT:PC71BM | 5.8 × 105 | 24 | 300–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
>106% | 3 × 1015 | 199 | |
| 2022 | Graphite/C60/BP | 100 | 31.4 | 400–1060 | NA | NA | 200 |
In 2017, the first 2D organic heterojunction photodetector based on WS2 was introduced. WS2/PTCDA191 achieved an EQE of 1.8 ± 0.2% and an IQE of 11 ± 1%. The hybrid charge transfer excitons, formed through the interaction of Frenkel excitons in PTCDA and Wannier excitons in WS2, subsequently dissociate into free charges, enhancing quantum efficiency. While the device may not exhibit outstanding hydrogen sensitivity, this work has stimulated the extensive utilization of 2D materials in 2D/organic heterojunctions. In 2019, leveraging WS2, Huang et al. introduced the WS2/poly-TPD:PCBM heterojunction photodetector.192 This device maintained a high responsivity (17 A W−1), a high detection rate (1.1 × 1011 Jones), and a high on/off ratio even under zero bias voltage. The poly-TPD:PCBM heterojunction enhanced the light absorption coefficient, facilitating efficient dissociation of photogenerated excitons. Appropriate band structures and energy gaps also favored the separation of electron–hole pairs. Furthermore, the authors generated high-quality poly-TPD/PCBM and WS2 films through a straightforward solution-coating technique and chemical vapor deposition. This provides relevant insights and design examples for creating high-performance 2D/organic heterojunctions. Prolonged photoconductivity is the primary challenge in high-speed imaging, which Pei et al. effectively addressed using the 2D material Bi2Se3. The Bi2Se3/rubrene heterojunction photodetector193 exhibits a high responsivity (124 A W−1) and fast response time (54 ms). This can be attributed to the rational band alignment structure and the built-in electric field in the Bi2Se3/rubrene heterojunction. Interestingly, the device also demonstrates a negative differential resistance effect and gate-tunable backward-like diode behavior, leading to the inversion of the Fermi level position. This work can potentially advance the application of 2D/organic devices in high-speed imaging. Infrared photodetection in the military sector is of paramount importance. The SWCNTs/PQT-12:F4TCNQ heterojunction photodetector proposed by Yang et al.194 achieves broadband detection up to 2600 nm, with a high responsivity (2.75 × 106 A W−1), a high detection rate (3.12 × 1014 Jones), and a high EQE (108%). The broadband detection can be attributed to the ultra-low intermolecular electronic transition energy in the SWCNT layer and the ultra-broadband absorption characteristics of the PQT-12:F4TCNQ layer. This device demonstrates outstanding stability, robustness, and flexibility, maintaining excellent optoelectronic performance even after 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 seconds of cyclic testing. Additionally, the authors demonstrated a noise reduction array, which holds significant potential for application in the field of biological synapses. Bi2Se3/MOF(Ni-CAT-1) (Fig. 15a and b)195 exhibits broad-spectrum absorption characteristics (600–2000 nm). Unlike the reasons behind the broad-spectrum absorption in SWCNTs/PQT-12:F4TCNQ, Bi2Se3/MOF(Ni-CAT-1) achieves this due to the intense light absorption by the MOF(Ni-CAT-1) layer and the high charge carrier mobility of the Bi2Se3 layer. Specifically, this is attributed to the synergistic effect of Schottky barriers and the photovoltaic effect. The device maintains a high responsivity (4725 A W−1) (Fig. 15c) and a high detectivity (3.5 × 1013 Jones) at 1500 nm, surpassing Bi2Se3 photodetectors by 2–3 orders of magnitude. This work highlights the enormous potential of MOF materials in infrared detection.
000 seconds of cyclic testing. Additionally, the authors demonstrated a noise reduction array, which holds significant potential for application in the field of biological synapses. Bi2Se3/MOF(Ni-CAT-1) (Fig. 15a and b)195 exhibits broad-spectrum absorption characteristics (600–2000 nm). Unlike the reasons behind the broad-spectrum absorption in SWCNTs/PQT-12:F4TCNQ, Bi2Se3/MOF(Ni-CAT-1) achieves this due to the intense light absorption by the MOF(Ni-CAT-1) layer and the high charge carrier mobility of the Bi2Se3 layer. Specifically, this is attributed to the synergistic effect of Schottky barriers and the photovoltaic effect. The device maintains a high responsivity (4725 A W−1) (Fig. 15c) and a high detectivity (3.5 × 1013 Jones) at 1500 nm, surpassing Bi2Se3 photodetectors by 2–3 orders of magnitude. This work highlights the enormous potential of MOF materials in infrared detection.
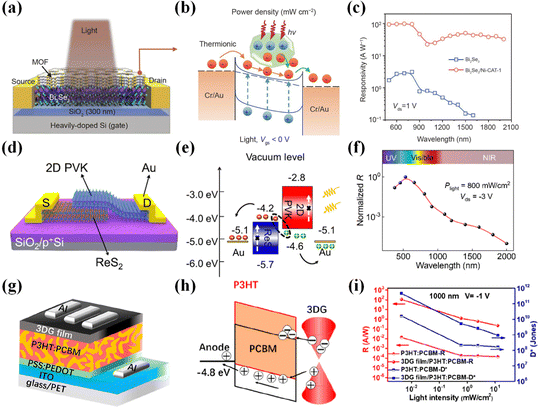 | ||
| Fig. 15 Enhancing the photodetector performance of other 2D materials by combining them with organic thin films. (a) Schematic diagram of Bi2Se3/MOF(Ni-CAT-1).195 (b) The carrier transport mechanism and band energy diagram at Vgs < 0.195 (c) Responsivity as a function of wavelength.195 Reproduced with permission.195 Copyright 2021, Nature Publishing Group. (d) Schematic image of ReS2/(CH3(CH2)3NH3)2(CH3NH3)4Pb5I16.196 (e) Interlayer excitation under infrared light.196 (f) Normalized wavelength-dependent responsivity.196 Reproduced with permission.196 Copyright 2022, American Chemical Society. (g) Schematic of 3DG/P3HT:PC71BM.199 (h) Working mechanism under infrared illumination.199 (i) Power-dependent R and D*.199 Reproduced with permission.199 Copyright 2022, American Chemical Society. | ||
2D perovskites possess a wide bandgap, leading to a relatively poor spectral response. Surprisingly, ReS2/(CH3(CH2)3NH3)2(CH3NH3)4Pb5I16 has achieved exceptional photodetector performance. ReS2/(CH3(CH2)3NH3)2(CH3NH3)4Pb5I16 (Fig. 15d–f)196 exhibits characteristics of broad-spectrum response (2000 nm) due to the heterojunction's direct type-II bandgap of 0.4 eV. Furthermore, this device features self-powered capabilities, maintaining high detectivity (1.8 × 1014 Jones), high EQE (68%), and ultra-fast response times (0.443/0.72 ms) even at zero bias voltage. The sub-millisecond response time is attributed to the significant band offset at the heterojunction interface, ensuring efficient charge carrier separation. Moreover, the device demonstrates outstanding stability, retaining excellent photodetection performance after seven days of operation. This work provides an effective design strategy for high-performance and highly stable photodetectors. Zhu et al. designed an IEICO-4F/WSe2 heterojunction photodetector.197 By investigating the heterojunction's photoluminescence quenching behavior, they achieved a high-performance photodetector in the near-infrared range (808 nm) with a responsivity of 8.32 A W−1 and a detectivity of 4.65 × 1011 Jones. The photodetection performance of this device is attributed to the effective separation of photogenerated charge carriers and charge transfer at the type-II heterojunction interface. While NIR detection has significantly developed, research on MIR detection remains limited. MIR detection has crucial value in various fields, such as biometrics and military applications, making it a meaningful and challenging endeavor. In 2022, Ge et al. introduced a 3DG/P3HT:PC71BM heterojunction photodetector (Fig. 15g and h)199 capable of ultra-broadband detection extending beyond 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nm in the MIR spectrum. This device exhibits an exceptionally high responsivity (5.8 × 105 A W−1) and a high detectivity (3 × 1015 Jones) (Fig. 15i). The PCBM layer and functional groups within the 3DG layer serve as electron trapping sites, extending the lifetime of charge carriers and enhancing the device's photoresponse and photocurrent. Furthermore, this device demonstrates outstanding performance in weak light detection, easily detecting light at the pw level. It maintains excellent flexibility and mechanical stability, providing a core technology for designing highly stable wearable integrated optoelectronic devices.
000 nm in the MIR spectrum. This device exhibits an exceptionally high responsivity (5.8 × 105 A W−1) and a high detectivity (3 × 1015 Jones) (Fig. 15i). The PCBM layer and functional groups within the 3DG layer serve as electron trapping sites, extending the lifetime of charge carriers and enhancing the device's photoresponse and photocurrent. Furthermore, this device demonstrates outstanding performance in weak light detection, easily detecting light at the pw level. It maintains excellent flexibility and mechanical stability, providing a core technology for designing highly stable wearable integrated optoelectronic devices.
4.4. Applications of 2D/organic heterojunctions in flexible photodetectors
Research into applying 2D/organic heterostructures in flexible devices is increasingly gaining attention, particularly in developing photodetectors. These photodetectors combine the exceptional electronic and optical properties of 2D materials with the flexibility and tunability of organic materials, offering an efficient and revolutionary photonic conversion solution in the realm of flexible electronics.115,135,146,147,156,184,185,194,199In recent years, scientists have been advancing research on flexible photodetectors based on 2D/organic heterostructures to better meet the demand for flexible sensing and imaging technologies in electronic skin and flexible electronics applications. For instance, Gao et al. developed an organic dye/Zn0.9Mg0.1O NPs/graphene photodetector (Fig. 16a),115 showcasing exceptional photoresponse and mechanical flexibility by combining an organic dye, magnesium-doped zinc oxide, and graphene. Additionally, the graphene/MOF photodetector designed by Bera et al. (Fig. 16b)146 demonstrated significant advantages such as non-toxicity, environmental friendliness, and stability, making it highly suitable for wearable and stretchable electronic devices. Furthermore, researchers have successfully developed various flexible 2D/organic heterostructure photodetectors, such as the cost-effective graphene/CH3NH3PbI3:MoS2 (Fig. 16c),147 ultra-thin graphene/PEDOT:PSS/P3HT:PC71BM (Fig. 16d),156 exceptionally durable SWCNTs/PQT-12:F4TCNQ (Fig. 16e and f),194 and low-light performing 3DG/P3HT:PC71BM photodetectors (Fig. 16g and h).199 These 2D/organic material photodetectors have demonstrated outstanding performance and high adaptability in flexible wearable devices.
 | ||
| Fig. 16 Flexible 2D/organic heterojunction photodetectors. (a) Flexible graphene/Zn0.9Mg0.1O NPs/BET narrowband photodetector.115 Reproduced with permission.115 Copyright 2017, Wiley-VCH. (b) Schematic illustration of graphene/MOF under bending strain.146 Reproduced with permission.146 Copyright 2018, Wiley-VCH. (c) Flexible graphene/CH3NH3PbI3:MoS2 hybrid photodetector.147 Reproduced with permission.147 Copyright 2018, Wiley-VCH. (d) Ultrathin and flexible graphene/PEDOT:PSS/P3HT:PC71BM photodetector.156 (e) Photocurrent of SWCNTs/PQT-12:F4TCNQ under different bending radii.194 (f) Flexible SWCNTs/PQT-12:F4TCNQ phototransistor.194 Reproduced with permission.194 Copyright 2021, Wiley-VCH. (g) Flexible 3DG/P3HT:PC71BM photodetector.199 (h) Photocurrent for flexible 3DG/P3HT:PC71BM as a function of cycle number.199 Reproduced with permission.199 Copyright 2022, American Chemical Society. | ||
Developing 2D/organic heterostructure photodetectors generally represents a significant breakthrough in materials science and electronic engineering. It opens up new possibilities for application in flexible electronic devices, particularly in areas like electronic skin, health monitoring, and environmental sensing. As future research progresses and technologies mature, these 2D/organic heterostructure photodetectors are poised to play an increasingly pivotal role in innovative materials and the next generation of electronic devices.
5. Summary of device performance
Photodetectors of organic materials suffer from the disadvantages of low photoelectron mobility, material instability, low photo-quantum efficiency, temperature dependence, short lifetime, and restricted sensitivity range.201–203 Photodetectors of 2D materials suffer from drawbacks such as limited spectral response range, weak light detection capability, high thermal noise, poor stability, and physical size constraints.204–206 Combining 2D materials with organic materials brings new development opportunities.38,41,207 It is undeniable that after a decade of development, 2D/organic heterojunction photodetectors have significantly improved the performance of photodetectors. However, taking 2D/organic heterojunction photodetectors to large-scale applications is still a long way to go. To date, graphene remains the best and most widely used 2D material, and graphene-based devices generally outperform other devices in terms of responsivity and detectivity (shown in Fig. 17a). The ultra-high responsivity and ultra-high detectability are mainly attributed to graphene's broadband spectral response, high carrier mobility, superior electrical conductivity, good mechanical flexibility, and excellent resistance to light fatigue.208–210 MoS2 has a tunable bandgap, which means that MoS2 can achieve controllable switching and small dark current.211,212 Moreover, MoS2 possesses excellent chemical stability and can be grown over large areas using chemical vapor deposition.213 Graphene-based 2D/organic heterojunction photodetectors typically have higher EQE, and MoS2-based ones are usually excellent regarding response speed (shown in Fig. 17b). Some 2D materials have shown excellent performance in MIR wavelength detection up to 300 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 nm (shown in Fig. 17e). Organic/2D heterojunction photodetectors can achieve bandwidths up to 105 Hz and still reach 104 Hz while having a response rate of 109 A W−1 (Fig. 17c).
600 nm (shown in Fig. 17e). Organic/2D heterojunction photodetectors can achieve bandwidths up to 105 Hz and still reach 104 Hz while having a response rate of 109 A W−1 (Fig. 17c).
Graphene-based 2D/organic heterojunction photodetectors peaked their development rate in 2021 and have reached a relatively mature bottleneck. 2D/organic heterojunction photodetectors based on MoS2 and other 2D materials are still developing rapidly (Fig. 17d). Devices187,199 are available for detection in the NIR (Fig. 17e) but not in the FIR or longer wavelengths. Detection in the UV to NIR bands is well developed, but detection at long wavelengths is still understudied, and 2D/organic heterojunctions still have great potential for development in the field of long-wavelength detection.48,214
6. Future development
6.1. Long-wave and narrow-band detection
Long-wavelength and narrow-band detection are two dark clouds over 2D/organic heterojunction photodetectors and directions that must be vigorously researched and developed. Detection technology in the VIS to NIR bands is very mature, but the detection of FIR needs to be improved. The energy of photons is proportional to the frequency of electromagnetic radiation and inversely proportional to the wavelength. The photon energy can be expressed by the equation E = hv = hc/λ. The MIR and FIR energies are minimal, and the carrier energies are close to the molecular vibration or scattering, which are not easily collected and dissociated. 2D/charge transfer complex heterojunctions are considered an effective strategy to address long-wave detection, where the effect of donor–acceptor bonding and acceptor-induced negative anions in charge transfer complexes creates a new narrow bandgap, thus broadening the detection range.215 The method can now easily detect the NIR and MIR bands, and further innovations and developments are needed for the detection of FIR.Narrow-band detectors are typically used to detect or receive signals in a specific frequency range and are widely used in communication, radar, and medical systems.216–218 Narrowband detection has triggered much research, but previous related strategies have more obvious drawbacks, such as reduced responsivity, complex configuration, and non-tunability. Although narrowband detection has been achieved in 2D/organic heterojunction photodetectors such as graphene/Zn0.9Mg0.1O NPs/BET dye,115 graphene/h-BN/J-aggregate PTCDI-C13,137 graphene/PDVF,138etc., the performance of these devices is not satisfactory (R ≈ 102–103 A W−1, τ ≈ 1000 ms). Therefore, it is still challenging to realize high-performance narrowband photodetectors, and more 2D/organic heterojunction photodetectors with advanced materials, functions, and structures are needed in the future.
6.2. New organic materials and structure
Currently, 2D materials commonly used in 2D/organic heterojunction photodetectors include graphene and TMDs, and organic materials mainly include COFs, MOFs, ferroelectrics (P(VDF-TrEE)),182,186,187 semiconductors (pentacene),125–127,129,158,165,173 insulators (PVCN)45 and photochromic molecules (DAE). More 2D and organic, exceedingly narrow bandgap materials must be developed to develop high-performance long-wave and narrowband detectors. Another way to reduce the bandgap is through modulation: for example, inter-doping TCNQ with MOF Ru2(C6H5COO)4Cl to reduce the bandgap of the MOF from 0.625 eV to 0.175 eV.219 Narrow bandgap is the basis for long-wave detection, and materials such as MOFs, COFs, topological Dirac semimetals, and topological insulators have good prospects for development.Heterostructures, mainly in stacking and doping, usually contain only 1–2 organic materials as the absorbing layer and one 2D material as the absorbing layer. In the future, more complex heterostructures are needed to enhance the performance of photodetectors, such as s-scheme heterojunctions,220 double charge transfer heterojunctions221,222 and heterojunctions with more layers. In addition, higher-performance photodetectors can be obtained by combining several approaches, such as local field coupling combined with built-in electric fields. Overall, the effective trapping and efficient dissociation of carriers should be enhanced, the energy loss should be reduced, and the charge decomposition efficiency should be improved.223 Also, high-quality heterogeneous interfaces that are easy to implement should also be emphasized.224
6.3. Exploring the 2D/organic interface: new horizons in materials science
In materials science, heterostructures constitute systems composed of different material layers, with interfaces between these layers possessing unique electronic, optical, and chemical properties.225 Research on interfaces in 2D/organic heterojunctions has long been a focal point. Effective interface formation between 2D materials and organic materials enables efficient charge transfer, thereby enhancing the efficiency of solar cells or photodetectors. Additionally, investigating interfaces in 2D/organic heterojunctions involves studying molecular arrangements, charge recombination, band alignment, and other microscale mechanisms at these interfaces, all representing current focal points in materials science.33,226In organic photovoltaic materials, the high binding energy of excitons often stands as a crucial limiting factor in their performance. Combining 2D materials with organic materials enables the formation of novel electronic states at the interface, reducing excitons' binding energy and promoting their separation—an essential aspect for enhancing photovoltaic conversion efficiency.50,227 For instance, Shao et al. employed edge-on C12TBP, stacked in a π–π configuration with graphene, inducing orbital coupling and hybridization to improve electron transfer at the interface.122 Liu et al. achieved high-quality interfaces by epitaxially depositing several layers of the organic small molecule C8-BTBT onto graphene, resulting in devices exhibiting high internal quantum efficiency (41%) and responsivity (1.8 × 105 A W−1).133 Similarly, heterojunctions such as the self-assembled BTBT with MoS2 have been explored.176 However, the exciton dissociation efficiency in planar 2D/organic heterojunctions still falls short of the requirements, necessitating a further enhancement of the built-in electric field. Vertical P–N structures (combination of p-type and n-type semiconductors) have emerged as a solution.172–174,181 These structures can generate a strong built-in electric field at their interface, aiding in the effective separation of excitons. When an external voltage is applied, this field can be further strengthened, leading to more efficient separation of electrons and holes and enhancing charge collection efficiency. In practical applications, this implies that designing and optimizing the interface structure between 2D materials and organic materials and introducing suitable P–N structures can improve the performance of photodetectors and other optoelectronic devices. For instance, effective P–N structures can be formed by introducing appropriate doping layers between 2D and organic layers or by introducing additional semiconductor materials. Such structural designs enhance the built-in electric field and improve the transport and collection of charge carriers. In the future, integrating the unique properties of 2D materials with the advantages of traditional P–N structures could lead to the development of more efficient and higher-performance photovoltaic systems.
The research on highly ordered 2D atomic/organic molecular interfaces holds significant importance in developing new nanoelectronic devices, sensors, optoelectronic materials, and more.48,49 Precise control over molecular arrangement and interactions at interfaces enables manipulating the electronic properties of the materials, facilitating the design and optimization of device performance at the nanoscale. Moreover, this research domain offers insights into understanding and simulating complex biological interfaces; for instance, similar π–π stacking phenomena exist in protein–cell membrane interactions.228–230 Therefore, in-depth exploration of interfaces between graphene-like 2D materials and organic substances holds significance for materials science and nanotechnology. It offers novel perspectives and applications in various biochemistry and molecular biology fields.
6.4. Development and application of 2D/organic devices
In recent years, the development of organic solar cells has been rapid, and more organic systems with higher conversion efficiencies and more excellent stability have been proposed, which will continue to promote the development of graphene/organic heterojunction detectors and even a more extensive range of 2D/organic heterojunctions.237–241 In addition, novel biosynaptic, low-energy functional photodetector devices, photonic chips for on-chip computing, and susceptible biomonitoring detectors have been the trend in recent years.242–246 At present, more and more new structures and materials better reduce the gap between the energy bands of organic materials and reduce the energy loss in transmission, such as heterojunctions with double donor–acceptor groups and supramolecular systems with double donor–acceptor groups, and in the future, these new materials and structures will be combined with graphene for in-depth exploration in order to improve the performance of graphene/organic photodetectors (Fig. 18).Although graphene/organic charge transfer complexes have made some progress in long-wave detection, their responsivity is still at a low level, which is because the new energy bands generated by organic charge transfer complexes have a small band gap, which makes it challenging to match the energy bands, leading to difficulties in the efficient dissociation process. Therefore, the overall efficiency of most organic charge transfer complexes at long wavelengths is low, which limits their further application and development. In the future, optimizing the long-wave detection depth of organic charge transfer complexes and improving the structure and energy band matching to prepare high-performance graphene/organic charge transfer complex heterojunction detectors is necessary.
While 2D/organic heterojunction photodetectors have had some success, more research into applications is still needed. This is mainly attributed to the fact that the design and preparation of 2D/organic heterojunction photodetectors involves a wide range of materials and processes and often requires highly sophisticated techniques and equipment. This complexity may make research and development even more challenging. In addition, some organic materials may be unstable or prone to degradation, limiting the devices' long-term stability. Future research and development will likely focus on optimizing materials, improving preparation processes, enhancing performance, and reducing costs to facilitate the widespread use of these technologies.
Author contributions
All authors contributed to the writing of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by the National Key Research and Development Program of China (Grant No. 2023YFB3611400) and the National Natural Science Foundation of China (NSFC) (No. 62175026, 62305047).References
- F. N. Xia, T. Mueller, Y. M. Lin, A. Valdes-Garcia and P. Avouris, Nat. Nanotechnol., 2009, 4, 839–843 CrossRef CAS PubMed.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- O. Lopez-Sanchez, D. Lembke, M. Kayci, A. Radenovic and A. Kis, Nat. Nanotechnol., 2013, 8, 497–501 CrossRef CAS PubMed.
- V. N. Fomin, V. M. Nikitin, E. B. Zhbakov, V. A. Sautkin and E. K. Suyazova, Phys. Wave Phenom., 2014, 22, 125–131 CrossRef.
- C. H. Chen, Z. Y. Li and L. Fu, Light: Sci. Appl., 2023, 12, 77 CrossRef CAS PubMed.
- S. Watson, W. K. Zhang, J. Tavares, J. Figueiredo, H. Cantu, J. Wang, E. Wasige, H. Salgado, L. Pessoa and A. Kelly, Microw Opt. Technol. Lett., 2019, 61, 1121–1125 CrossRef.
- P. D. Grant, S. R. Laframboise, R. Dudek, M. Graf, A. Bezinger and H. C. Liu, Electron. Lett., 2009, 45, 952–953 CrossRef CAS.
- Y. Zhou, X. Qiu, Z. A. Wan, Z. H. Long, S. Poddar, Q. P. Zhang, Y. C. Ding, C. L. J. Chan, D. Q. Zhang, K. M. Zhou, Y. J. Lin and Z. Y. Fan, Nano Energy, 2022, 100, 107516 CrossRef CAS.
- T. Dong, J. Simoes and Z. C. Yang, Adv. Mater. Interfaces, 2020, 7, 1901657 CrossRef CAS.
- T. Zhang, C. C. Ling, X. M. Wang, B. X. Feng, M. Cao, X. Xue, Q. Z. Xue, J. Q. Zhang, L. Zhu, C. A. K. Wang, H. P. Lu and W. P. Liu, Adv. Mater. Technol., 2022, 7, 2200250 CrossRef CAS.
- P. Buechele, M. Richter, S. F. Tedde, G. J. Matt, G. N. Ankah, R. Fischer, M. Biele, W. Metzger, S. Lilliu, O. Bikondoa, J. E. Macdonald, C. J. Brabec, T. Kraus, U. Lemmer and O. Schmidt, Nat. Photonics, 2015, 9, 843–848 CrossRef CAS.
- W. W. Moses, Nucl. Instrum. Methods Phys. Res., Sect. A, 2009, 610, 11–15 CrossRef CAS PubMed.
- J. L. Wang, H. H. Fang, X. D. Wang, X. S. Chen, W. Lu and W. D. Hu, Small, 2017, 13, 1700894 CrossRef PubMed.
- W. Lang, Mater. Sci. Eng. R-Rep., 1996, 17, 1–55 CrossRef.
- B. Jalali and S. Fathpour, J. Lightwave Technol., 2006, 24, 4600–4615 CAS.
- R. E. Camacho-Aguilera, Y. Cai, N. Patel, J. T. Bessette, M. Romagnoli, L. C. Kimerling and J. Michel, Opt. Express, 2012, 20, 11316–11320 CrossRef CAS PubMed.
- R. I. Macdonald, Appl. Opt., 1981, 20, 591–594 CrossRef CAS PubMed.
- N. Koguchi, S. Takahashi and T. Chikyow, J. Cryst. Growth, 1991, 111, 688–692 CrossRef CAS.
- D. B. Li, X. J. Sun, H. Song, Z. M. Li, Y. R. Chen, H. Jiang and G. Q. Miao, Adv. Mater., 2012, 24, 845–849 CrossRef CAS PubMed.
- M. Ruff, H. Mitlehner and R. Helbig, IEEE Trans. Electron Devices, 1994, 41, 1040–1054 CrossRef CAS.
- B. Zhao, F. Wang, H. Y. Chen, L. X. Zheng, L. X. Su, D. X. Zhao and X. S. Fang, Adv. Funct. Mater., 2017, 27, 1700264 CrossRef.
- D. L. Smith and C. Mailhiot, J. Appl. Phys., 1987, 62, 2545–2548 CrossRef CAS.
- M. Casalino, G. Coppola, M. Iodice, I. Rendina and L. Sirleto, Sensors, 2010, 10, 10571–10600 CrossRef PubMed.
- Y. K. Su, C. L. Lin, S. M. Chen, J. R. Chang and D. H. Jaw, J. Appl. Phys., 1997, 81, 7529–7532 CrossRef CAS.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- A. H. Castro Neto, F. Guinea, N. M. R. Peres, K. S. Novoselov and A. K. Geim, Rev. Mod. Phys., 2009, 81, 109–162 CrossRef CAS.
- S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS PubMed.
- S. Gopalan, M. L. van de Put, G. Gaddemane and M. V. Fischetti, Phys. Rev. Appl., 2022, 18, 054062 CrossRef CAS.
- K. F. Mak, C. Lee, J. Hone, J. Shan and T. F. Heinz, Phys. Rev. Lett., 2010, 105, 136805 CrossRef PubMed.
- Y. Kim, S. S. Cruz, K. Lee, B. O. Alawode, C. Choi, Y. Song, J. M. Johnson, C. Heidelberger, W. Kong, S. Choi, K. Qiao, I. Almansouri, E. A. Fitzgerald, J. Kong, A. M. Kolpak, J. Hwang and J. Kim, Nature, 2017, 544, 340–343 CrossRef CAS PubMed.
- S. J. Kang, D. H. Lee, J. Kim, A. Capasso, H. S. Kang, J. W. Park, C. H. Lee and G. H. Lee, 2D Mater., 2020, 7, 022003 CrossRef CAS.
- J. R. An, X. Y. Zhao, Y. N. Zhang, M. X. Liu, J. Yuan, X. J. Sun, Z. Y. Zhang, B. Wang, S. J. Li and D. B. Li, Adv. Funct. Mater., 2022, 32, 2110119 CrossRef CAS.
- F. X. Yang, S. S. Cheng, X. T. Zhang, X. C. Ren, R. J. Li, H. L. Dong and W. P. Hu, Adv. Mater., 2018, 30, 1702415 CrossRef PubMed.
- X. T. Gan, D. Englund, D. Van Thourhout and J. L. Zhao, Appl. Phys. Rev., 2022, 9, 021302 CAS.
- J. S. He, L. L. Tao, H. Zhang, B. Zhou and J. B. Li, Nanoscale, 2019, 11, 2577–2593 RSC.
- L. Liu, Z. Q. Cheng, B. Jiang, Y. X. Liu, Y. L. Zhang, F. Yang, J. H. Wang, X. F. Yu, P. K. Chu and C. Ye, ACS Appl. Mater. Interfaces, 2021, 13, 30797–30805 CrossRef CAS PubMed.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147–150 CrossRef CAS PubMed.
- J. Y. Han, F. K. Wang, S. Han, W. J. Deng, X. Y. Du, H. Yu, J. Gou, Q. J. Wang and J. Wang, Adv. Funct. Mater., 2022, 32, 2205150 CrossRef CAS.
- L. S. Li, Y. Y. Huang, J. B. Peng, Y. Cao and X. B. Peng, J. Mater. Chem. C, 2014, 2, 1372–1375 RSC.
- Y. Higashi, K. S. Kim, H. G. Jeon and M. Ichikawa, J. Appl. Phys., 2010, 108, 034502 CrossRef.
- J. Wang, J. Y. Han, X. Q. Chen and X. R. Wang, InfoMat, 2019, 1, 33–53 CrossRef CAS.
- L. Song, L. J. Ci, H. Lu, P. B. Sorokin, C. H. Jin, J. Ni, A. G. Kvashnin, D. G. Kvashnin, J. Lou, B. I. Yakobson and P. M. Ajayan, Nano Lett., 2010, 10, 3209–3215 CrossRef CAS PubMed.
- T. Miyake, K. Nakamura, R. Arita and M. Imada, J. Phys. Soc. Jpn., 2010, 79, 044705 CrossRef.
- C. Pramanik and G. P. Miller, Molecules, 2012, 17, 4625–4633 CrossRef CAS PubMed.
- J. Y. Jang, S. H. Kim, S. Nam, D. S. Chung, C. W. Yang, W. M. Yun, C. E. Park and J. B. Koo, Appl. Phys. Lett., 2008, 92, 143306 CrossRef.
- J. S. Jung, J. W. Lee, K. Kim, M. Y. Cho, S. G. Jo and J. Joo, Chem. Mater., 2010, 22, 2219–2225 CrossRef CAS.
- S. Shaari, S. Naka and H. Okada, J. Photopolym. Sci. Technol., 2016, 29, 363–368 CrossRef CAS.
- H. Y. Wang, Z. X. Li, D. Y. Li, P. Chen, L. J. Pi, X. Zhou and T. Y. Zhai, Adv. Funct. Mater., 2021, 31, 2103106 CrossRef CAS.
- M. Gobbi, E. Orgiu and P. Samorì, Adv. Mater., 2018, 30, 1706103 CrossRef PubMed.
- J. Y. Han and J. Wang, Chin. Phys. B, 2019, 28, 017103 CrossRef CAS.
- H. W. Li, Z. Dong, Y. Zhang, L. Q. Li, Z. C. Wang, C. Wang, K. Zhang and H. Zhang, 2D Mater., 2021, 8, 012001 CrossRef CAS.
- X. Q. Chen, K. Shehzad, L. Gao, M. S. Long, H. Guo, S. C. Qin, X. M. Wang, F. Q. Wang, Y. Shi, W. D. Hu, Y. Xu and X. R. Wang, Adv. Mater., 2020, 32, 1902039 CrossRef CAS PubMed.
- K. Pei and T. Y. Zhai, Cell Rep. Phys. Sci., 2020, 1, 100166 CrossRef CAS.
- P. Cheng, G. Li, X. W. Zhan and Y. Yang, Nat. Photonics, 2018, 12, 131–142 CrossRef CAS.
- N. Gasparini, A. Salleo, I. McCulloch and D. Baran, Nat. Rev. Mater., 2019, 4, 229–242 CrossRef.
- P. Sonar, J. P. F. Lim and K. L. Chan, Energy Environ. Sci., 2011, 4, 1558–1574 RSC.
- Y. Liu, R. Cheng, L. Liao, H. L. Zhou, J. W. Bai, G. Liu, L. X. Liu, Y. Huang and X. F. Duan, Nat. Commun., 2011, 2, 579 CrossRef PubMed.
- X. C. Miao, S. Tongay, M. K. Petterson, K. Berke, A. G. Rinzler, B. R. Appleton and A. F. Hebard, Nano Lett., 2012, 12, 2745–2750 CrossRef CAS PubMed.
- G. Rao, M. Freitag, H. Y. Chiu, R. S. Sundaram and P. Avouris, ACS Nano, 2011, 5, 5848–5854 CrossRef CAS PubMed.
- M. Buscema, J. O. Island, D. J. Groenendijk, S. I. Blanter, G. A. Steele, H. S. J. van der Zant and A. Castellanos-Gomez, Chem. Soc. Rev., 2015, 44, 3691–3718 RSC.
- P. L. Richards, J. Appl. Phys., 1994, 76, 1–24 CrossRef CAS.
- W. Z. Yu, S. J. Li, Y. P. Zhang, W. L. Ma, T. Sun, J. Yuan, K. Fu and Q. L. Bao, Small, 2017, 13, 1700268 CrossRef PubMed.
- J. C. Norman, D. Jung, Y. T. Wan and J. E. Bowers, Appl. Photon., 2018, 3, 030901 CrossRef.
- H. Li, P. L. Xu, D. Liu, J. Y. He, H. L. Zu, J. J. Song, J. Zhang, F. H. Tian, M. J. Yun and F. Y. Wang, Nanotechnology, 2021, 32, 375202 CrossRef CAS PubMed.
- M. Czerniak-Reczulska, A. Niedzielska and A. Jedrzejczak, Adv. Mater. Sci., 2015, 15, 67 CrossRef CAS.
- T. Sattar, Top. Curr. Chem., 2019, 377, 1–45 CrossRef CAS PubMed.
- T. Low and P. Avouris, ACS Nano, 2014, 8, 1086–1101 CrossRef CAS PubMed.
- Z. Y. Wang and B. X. Mi, Environ. Sci. Technol., 2017, 51, 8229–8244 CrossRef CAS PubMed.
- A. Splendiani, L. Sun, Y. B. Zhang, T. S. Li, J. Kim, C. Y. Chim, G. Galli and F. Wang, Nano Lett., 2010, 10, 1271–1275 CrossRef CAS PubMed.
- H. L. Zeng, J. F. Dai, W. Yao, D. Xiao and X. D. Cui, Nat. Nanotechnol., 2012, 7, 490–493 CrossRef CAS PubMed.
- K. F. Mak, K. L. He, C. Lee, G. H. Lee, J. Hone, T. F. Heinz and J. Shan, Nat. Mater., 2013, 12, 207–211 CrossRef CAS PubMed.
- H. A. Li, Y. Li, A. Aljarb, Y. M. Shi and L. J. Li, Chem. Rev., 2018, 118, 6134–6150 CrossRef CAS PubMed.
- C. Knieke, A. Berger, M. Voigt, R. N. K. Taylor, J. Röhrl and W. Peukert, Carbon, 2010, 48, 3196–3204 CrossRef CAS.
- H. J. Park, J. Meyer, S. Roth and V. Skákalová, Carbon, 2010, 48, 1088–1094 CrossRef CAS.
- Y. X. Yan, F. Z. Nashath, S. R. Chen, S. Manickam, S. S. Lim, H. T. Zhao, E. Lester, T. Wu and C. H. Pang, Nanotechnol. Rev., 2020, 9, 1284–1314 CrossRef CAS.
- J. G. Song, X. Z. Wang and C. T. Chang, J. Nanomater., 2014, 2014, 6 Search PubMed.
- A. Dodabalapur, Solid State Commun., 1997, 102, 259–267 CrossRef CAS.
- S. Reineke, M. Thomschke, B. Lüssem and K. Leo, Rev. Mod. Phys., 2013, 85, 1245–1293 CrossRef CAS.
- G. M. Farinola and R. Ragni, Chem. Soc. Rev., 2011, 40, 3467–3482 RSC.
- H. Sirringhaus, T. Kawase, R. H. Friend, T. Shimoda, M. Inbasekaran, W. Wu and E. P. Woo, Science, 2000, 290, 2123–2126 CrossRef CAS PubMed.
- T. Yasuda, T. Goto, K. Fujita and T. Tsutsui, Appl. Phys. Lett., 2004, 85, 2098–2100 CrossRef CAS.
- H. Sirringhaus, N. Tessler and R. H. Friend, Science, 1998, 280, 1741–1744 CrossRef CAS PubMed.
- C. D. Sheraw, L. Zhou, J. R. Huang, D. J. Gundlach, T. N. Jackson, M. G. Kane, I. G. Hill, M. S. Hammond, J. Campi, B. K. Greening, J. Francl and J. West, Appl. Phys. Lett., 2002, 80, 1088–1090 CrossRef CAS.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed.
- S. Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC.
- S. Bhunia, K. A. Deo and A. K. Gaharwar, Adv. Funct. Mater., 2020, 30, 2002046 CrossRef CAS.
- Z. J. Lin, J. Lu, M. Hong and R. Cao, Chem. Soc. Rev., 2014, 43, 5867–5895 RSC.
- G. Maurin, C. Serre, A. Cooper and G. Féreyd, Chem. Soc. Rev., 2017, 46, 3104–3107 RSC.
- M. S. Yao, W. H. Li and G. Xu, Coord. Chem. Rev., 2021, 426, 213479 CrossRef CAS.
- T. Agostinelli, M. Campoy-Quiles, J. C. Blakesley, R. Speller, D. D. C. Bradley and J. Nelson, Appl. Phys. Lett., 2008, 93, 203305 CrossRef.
- F. Stuker, C. Baltes, K. Dikaiou, D. Vats, L. Carrara, E. Charbon, J. Ripoll and M. Rudin, IEEE Trans. Med. Imaging, 2011, 30, 1265–1273 Search PubMed.
- C. M. M. Paschoal, D. D. Souza and L. A. P. Santos, IEEE Trans. Nucl. Sci., 2013, 60, 735–738 Search PubMed.
- G. Llosá, N. Belcari, M. G. Bisogni, G. Collazuol, S. Marcatili, P. Barrillon, C. de la Taille, S. Bondil-Blin, N. Dinu, M. Melchiorri, A. Tarolli, C. Piemonte and A. Del Guerra, IEEE Trans. Nucl. Sci., 2009, 56, 2586–2593 Search PubMed.
- E. Suematsu and N. Imai, IEEE Trans. Microw Theory Technol., 1996, 44, 133–143 CrossRef.
- B. Zhang, Y. Q. Guan, L. H. Xia, D. X. Dong, Q. Chen, C. Xu, C. Wu, H. X. Huang, L. B. Zhang, L. Kang, J. Chen and P. H. Wu, Supercond. Sci. Technol., 2021, 34, 034005 CrossRef.
- X. Huang, Y. L. Guo and Y. Q. Liu, Chem. Commun., 2021, 57, 11429–11442 RSC.
- B. H. Park, S. D. Baek, J. Y. Kim, J. Bae, H. Han and O. Kwon, Opt. Eng., 2002, 41, 1339–1345 CrossRef.
- J. F. Huang, J. Lee, J. Vollbrecht, V. V. Brus, A. L. Dixon, D. X. Cao, Z. Y. Zhu, Z. F. Du, H. B. Wang, K. Cho, G. C. Bazan and T. Q. Nguyen, Adv. Mater., 2020, 32, 1906027 CrossRef CAS PubMed.
- Y. Zhai, G. R. Gu and X. J. Lu, Micromachines, 2019, 10, 4 CrossRef PubMed.
- X. F. Yin, G. Y. Liu and S. L. Cao, J. Nanoelectron. Optoelectron., 2020, 15, 1508–1517 CrossRef CAS.
- J. Xue, Z. F. Zhu, X. B. Xu, Y. Gu, S. L. Wang, L. M. Xu, Y. S. Zou, J. Z. Song, H. B. Zeng and Q. Chen, Nano Lett., 2018, 18, 7628–7634 CrossRef CAS PubMed.
- Z. Alaie, S. M. Nejad and M. H. Yousefi, Mater. Sci. Semicond. Process., 2015, 29, 16–55 CrossRef CAS.
- M. Ding, Z. Guo, X. H. Chen, X. R. Ma and L. Q. Zhou, Nanomaterials, 2020, 10, 362 CrossRef CAS PubMed.
- F. Zhong, H. Wang, Z. Wang, Y. Wang, T. He, P. S. Wu, M. Peng, H. L. Wang, T. F. Xu, F. Wang, P. Wang, J. S. Miao and W. D. Hu, Nano Res., 2021, 14, 1840–1862 CrossRef CAS.
- F. C. Liu, H. Shimotani, H. Shang, T. Kanagasekaran, V. Zólyomi, N. Drummond, V. I. Fal'ko and K. Tanigaki, ACS Nano, 2014, 8, 752–760 CrossRef CAS PubMed.
- M. Yang, J. Wang, J. Y. Han, J. W. Ling, C. H. Ji, X. Kong, X. C. Liu, Z. H. Huang, J. Gou, Z. J. Liu, F. X. Xiu and Y. D. Jiang, ACS Photonics, 2018, 5, 3438–3445 CrossRef CAS.
- V. Mackowiak, J. Peupelmann, Y. Ma and A. Gorges, Thorlabs, Inc, 2015, 56.
- Z. X. Zou, J. W. Liang, X. H. Zhang, C. Ma, P. Xu, X. Yang, Z. X. S. Zeng, X. X. Sun, C. G. Zhu, D. L. Liang, X. J. Zhuang, D. Li and A. L. Pan, ACS Nano, 2021, 15, 10039–10047 CrossRef CAS PubMed.
- Z. Q. Xu, Y. P. Zhang, Z. Y. Wang, Y. T. Shen, W. C. Huang, X. Xia, W. Z. Yu, Y. Z. Xue, L. T. Sun, C. X. Zheng, Y. R. Lu, L. Liao and Q. L. Bao, 2D Mater., 2016, 3, 041001 CrossRef.
- J. Gilot, M. M. Wienk and R. A. J. Janssen, Adv. Funct. Mater., 2010, 20, 3904–3911 CrossRef CAS.
- K. T. Ly, R. W. Chen-Cheng, H. W. Lin, Y. J. Shiau, S. H. Liu, P. T. Chou, C. S. Tsao, Y. C. Huang and Y. Chi, Nat. Photonics, 2017, 11, 63–68 CrossRef.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- Y. Lee, S. H. Yu, J. Jeon, H. Kim, J. Y. Lee, H. Kim, J.-H. Ahn, E. Hwang and J. H. Cho, Carbon, 2015, 88, 165–172 CrossRef CAS.
- X. Yu, Z. Dong, J. K. W. Yang and Q. J. Wang, Optica, 2016, 3, 979–984 CrossRef CAS.
- L. Gao, C. Ge, W. Li, C. Jia, K. Zeng, W. Pan, H. Wu, Y. Zhao, Y. He, J. He, Z. Zhao, G. Niu, X. Guo, F. P. G. de Arquer, E. H. Sargent and J. Tang, Adv. Funct. Mater., 2017, 27, 1702360 CrossRef.
- G. F. Jones, R. M. Pinto, A. De Sanctis, V. K. Nagareddy, C. D. Wright, H. Alves, M. F. Craciun and S. Russo, Adv. Mater., 2017, 29, 1702993 CrossRef PubMed.
- S. Qin, X. Chen, Q. Du, Z. Nie, X. Wang, H. Lu, X. Wang, K. Liu, Y. Xu, Y. Shi, R. Zhang and F. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 38326–38333 CrossRef CAS PubMed.
- J. Liu, K. Zhou, J. Liu, J. Zhu, Y. Zhen, H. Dong and W. Hu, Adv. Mater., 2018, 30, 1803655 CrossRef PubMed.
- Y. Lee, H. Kim, S. Kim, D. Whang and J. H. Cho, ACS Appl. Mater. Interfaces, 2019, 11, 23474–23481 CrossRef CAS PubMed.
- S. Chugh, N. Adhikari, J. H. Lee, D. Berman, L. Echegoyen and A. B. Kaul, ACS Appl. Mater. Interfaces, 2019, 11, 24349–24359 CrossRef CAS PubMed.
- R. Pan, J. Han, X. Zhang, Q. Han, H. zhou, X. Liu, J. Gou, Y. Jiang and J. Wang, Carbon, 2020, 162, 375–381 CrossRef CAS.
- M.-Y. Shao, M. Yu, M. Suzuki, H. Yamada, Y.-P. Wang, Y. Chen, C. Lu, D. Wang and Z.-Y. Yang, ACS Appl. Electron. Mater., 2020, 2, 3459–3467 CrossRef CAS.
- J. Han, C. Zhang, S. Peng, X. Zhang, X. Liu, H. Zhou, Z. Wu, H. Yu and J. Wang, J. Mater. Chem. C, 2021, 9, 13963–13971 RSC.
- Y. J. Choi, S. B. Jo and J. H. Cho, Adv. Mater., 2021, 33, 2102725 CrossRef CAS PubMed.
- J. S. Kim, Y. J. Choi, H. J. Woo, J. Yang, Y. J. Song, M. S. Kang and J. H. Cho, Adv. Funct. Mater., 2017, 27, 1704475 CrossRef.
- J. Han, J. Wang, M. Yang, X. Kong, X. Chen, Z. Huang, H. Guo, J. Gou, S. Tao, Z. Liu, Z. Wu, Y. Jiang and X. Wang, Adv. Mater., 2018, 30, 1804020 CrossRef PubMed.
- N. N. Nguyen, H. C. Lee, K. Baek, M. S. Yoo, H. Lee, H. Lim, S. Choi, C. J. Kim, S. Nam and K. Cho, Adv. Funct. Mater., 2020, 31, 2008813 CrossRef.
- Y. Gan, S. Qin, Q. Du, Y. Zhang, J. Zhao, M. Li, A. Wang, Y. Liu, S. Li, R. Dong, L. Zhang, X. Chen, C. Liu, W. Wang and F. Wang, Adv. Sci., 2022, 9, 2204332 CrossRef CAS PubMed.
- S. Qin, X. Qin, Q. Du, Y. Gan, Y. Zhang, A. Wang, X. Yan, R. Dong, Y. Liu, S. Li, C. Liu, W. Wang and F. Wang, J. Mater. Chem. C, 2022, 10, 11710–11718 RSC.
- J. Zhao, Q. Du, X. Zheng, Y. Liu, S. Li, W. Wang, F. Wang and S. Qin, ACS Photonics, 2023, 10, 2955–2963 CrossRef CAS.
- W. C. Tan, W. H. Shih and Y. F. Chen, Adv. Funct. Mater., 2014, 24, 6818–6825 CrossRef CAS.
- E. H. Huisman, A. G. Shulga, P. J. Zomer, N. Tombros, D. Bartesaghi, S. Z. Bisri, M. A. Loi, L. J. Koster and B. J. van Wees, ACS Appl. Mater. Interfaces, 2015, 7, 11083–11088 CrossRef CAS PubMed.
- X. Liu, X. Luo, H. Nan, H. Guo, P. Wang, L. Zhang, M. Zhou, Z. Yang, Y. Shi, W. Hu, Z. Ni, T. Qiu, Z. Yu, J. B. Xu and X. Wang, Adv. Mater., 2016, 28, 5200–5205 CrossRef CAS PubMed.
- P.-H. Chang, Y.-C. Tsai, S.-W. Shen, S.-Y. Liu, K.-Y. Huang, C.-S. Li, H.-P. Chang and C.-I. Wu, ACS Photonics, 2017, 4, 2335–2344 CrossRef CAS.
- Q.-M. Wang and Z.-Y. Yang, Carbon, 2018, 138, 90–97 CrossRef CAS.
- O. Yakar, O. Balci, B. Uzlu, N. Polat, O. Ari, I. Tunc, C. Kocabas and S. Balci, ACS Appl. Nano Mater., 2019, 3, 409–417 CrossRef.
- B. Sun, G. Zhou, Y. Wang, X. Xu, L. Tao, N. Zhao, H. K. Tsang, X. Wang, Z. Chen and J. B. Xu, Adv. Opt. Mater., 2021, 9, 2100158 CrossRef CAS.
- M. Yu, Y. Chen, Y. G. Chen, Z. Y. Yang, W. F. Zhang and G. Yu, Adv. Mater. Interfaces, 2021, 8, 2100770 CrossRef CAS.
- S. Fu, R. Song, Y. Wang, Y. Han, C. Gao, J. Ma, H. Xu, B. Li, A. Shen and Y. Liu, Adv. Mater. Interfaces, 2023, 10, 2202488 CrossRef CAS.
- S. Y. Chen, Y. Y. Lu, F. Y. Shih, P. H. Ho, Y. F. Chen, C. W. Chen, Y. T. Chen and W. H. Wang, Carbon, 2013, 63, 23–29 CrossRef CAS.
- P. H. Ho, S. S. Li, Y. T. Liou, C. Y. Wen, Y. H. Chung and C. W. Chen, Adv. Mater., 2015, 27, 282–287 CrossRef CAS PubMed.
- Y. Lee, J. Kwon, E. Hwang, C. H. Ra, W. J. Yoo, J. H. Ahn, J. H. Park and J. H. Cho, Adv. Mater., 2015, 27, 41–46 CrossRef CAS PubMed.
- Z. Sun, L. Aigouy and Z. Chen, Nanoscale, 2016, 8, 7377–7383 RSC.
- L. Qin, L. Wu, B. Kattel, C. Li, Y. Zhang, Y. Hou, J. Wu and W. L. Chan, Adv. Funct. Mater., 2017, 27, 1704173 CrossRef.
- M. Cui, Y. Guo, Y. Zhu, H. Liu, W. Wen, J. Wu, L. Cheng, Q. Zeng and L. Xie, J. Phys. Chem. C, 2018, 122, 7551–7556 CrossRef CAS.
- K. P. Bera, G. Haider, M. Usman, P. K. Roy, H. I. Lin, Y. M. Liao, C. R. P. Inbaraj, Y. R. Liou, M. Kataria, K. L. Lu and Y. F. Chen, Adv. Funct. Mater., 2018, 28, 1804802 CrossRef.
- Z. Y. Peng, J. L. Xu, J. Y. Zhang, X. Gao and S. D. Wang, Adv. Mater. Interfaces, 2018, 5, 1800505 CrossRef.
- K. P. Bera, G. Haider, Y. T. Huang, P. K. Roy, C. R. Paul Inbaraj, Y. M. Liao, H. I. Lin, C. H. Lu, C. Shen, W. Y. Shih, W. H. Shih and Y. F. Chen, ACS Nano, 2019, 13, 12540–12552 CrossRef CAS PubMed.
- M. A. Iqbal, M. Cui, A. Liaqat, R. faiz, M. Hossain, X. Wang, S. Hussain, C. Dang, H. Liu, W. Wen, J. Wu and L. Xie, Nanotechnology, 2019, 30, 254003 CrossRef CAS PubMed.
- Y. H. Lee, S. Park, Y. Won, J. Mun, J. H. Ha, J. H. Lee, S. H. Lee, J. Park, J. Yeom, J. Rho, H. Ko and J. H. Oh, NPG Asia Mater., 2020, 12, 79 CrossRef CAS.
- M. A. Iqbal, A. Liaqat, S. Hussain, X. Wang, M. Tahir, Z. Urooj and L. Xie, Adv. Mater., 2020, 32, 2002628 CrossRef CAS PubMed.
- Y. Xiong, Q. Liao, Z. Huang, X. Huang, C. Ke, H. Zhu, C. Dong, H. Wang, K. Xi, P. Zhan, F. Xu and Y. Lu, Adv. Mater., 2020, 32, 1907242 CrossRef CAS PubMed.
- F. Feng, T. Wang, J. Qiao, C. Min, X. Yuan and M. Somekh, ACS Appl. Mater. Interfaces, 2021, 13, 61496–61505 CrossRef CAS PubMed.
- Z. He, J. Han, X. Du, L. Cao, J. Wang, C. Zheng, H. Lin and S. Tao, Adv. Funct. Mater., 2021, 31, 2103988 CrossRef CAS.
- C. Han, X. Liu, X. Han, M. He, J. Han, H. Zhang, X. Hou, H. Zhou, H. Yu, Z. Wu, J. Gou and J. Wang, Adv. Funct. Mater., 2022, 32, 2209680 CrossRef CAS.
- Z. Liu, K. Parvez, R. Li, R. Dong, X. Feng and K. Mullen, Adv. Mater., 2015, 27, 669–675 CrossRef CAS PubMed.
- C. Xie and F. Yan, ACS Appl. Mater. Interfaces, 2017, 9, 1569–1576 CrossRef CAS PubMed.
- X. Chen, X. Liu, B. Wu, H. Nan, H. Guo, Z. Ni, F. Wang, X. Wang, Y. Shi and X. Wang, Nano Lett., 2017, 17, 6391–6396 CrossRef CAS PubMed.
- A. Yadav, A. Upadhyaya, S. K. Gupta, A. S. Verma and C. M. S. Negi, Thin Solid Films, 2019, 675, 128–135 CrossRef CAS.
- X. Liu, X. Chen, J. Yi, Z. Luo, H. Nan, H. Guo, Z. Ni, Y. Ding, S. Dai and X. Wang, Org. Electron., 2019, 64, 22–26 CrossRef CAS.
- Y. Lee, S. Cha and C. Kim, Adv. Mater. Interfaces, 2021, 8, 2100478 CrossRef CAS.
- G. Zhou, R. Sun, Y. Xiao, G. Abbas and Z. Peng, Adv. Electron. Mater., 2021, 7, 2000522 CrossRef CAS.
- M. He, J. Han, X. Han, J. Gou, M. Yang, Z. Wu, Y. Jiang and J. Wang, Carbon, 2021, 178, 506–514 CrossRef CAS.
- Q. Dai, G. Hu, W. Lv, S. Xu, L. Sun, G. F. Schneider, L. Jiang and Y. Peng, Adv. Mater. Interfaces, 2022, 9, 2200116 CrossRef CAS.
- M. Li, S. Qin, X. Zheng, Q. Du, Y. Liu, S. Li, H. Li, W. Wang and F. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 48442–48451 CrossRef CAS PubMed.
- Y. J. Choi, H. J. Woo, S. Kim, J. Sun, M. S. Kang, Y. J. Song and J. H. Cho, J. Ind. Eng. Chem., 2020, 89, 233–238 CrossRef CAS.
- Y. Yao, Q. Ou, K. Wang, H. Peng, F. Fang, Y. Shi, Y. Wang, D. I. Asperilla, Z. Shuai and P. Samori, Nat. Commun., 2021, 12, 3667 CrossRef CAS PubMed.
- D. U. Lim, S. B. Jo and J. H. Cho, J. Am. Chem. Soc., 2021, 143, 879–890 CrossRef CAS PubMed.
- K. P. Bera, Y.-G. Lee, M. Usman, R. Ghosh, K.-L. Lu and Y.-F. Chen, ACS Appl. Electron. Mater., 2022, 4, 2337–2345 CrossRef CAS.
- G. Hu, H. Zhu, Q. Dai, C. Jiang, Y. Peng, W. Lv, S. Xu, L. Sun, L. Jiang and G. F. Schneider, Appl. Phys. Lett., 2022, 121, 123501 CrossRef CAS.
- S. H. Yu, Y. Lee, S. K. Jang, J. Kang, J. Jeon, C. Lee, J. Y. Lee, H. Kim, E. Hwang, S. Lee and J. H. Cho, ACS Nano, 2014, 8, 8285–8291 CrossRef CAS PubMed.
- F. Liu, W. L. Chow, X. He, P. Hu, S. Zheng, X. Wang, J. Zhou, Q. Fu, W. Fu, P. Yu, Q. Zeng, H. J. Fan, B. K. Tay, C. Kloc and Z. Liu, Adv. Funct. Mater., 2015, 25, 5865–5871 CrossRef CAS.
- D. Jariwala, S. L. Howell, K. S. Chen, J. Kang, V. K. Sangwan, S. A. Filippone, R. Turrisi, T. J. Marks, L. J. Lauhon and M. C. Hersam, Nano Lett., 2016, 16, 497–503 CrossRef CAS PubMed.
- Y. Huang, F. Zhuge, J. Hou, L. Lv, P. Luo, N. Zhou, L. Gan and T. Zhai, ACS Nano, 2018, 12, 4062–4073 CrossRef CAS PubMed.
- Z. H. Xu, L. Tang, S. W. Zhang, J. Z. Li, B. L. Liu, S. C. Zhao, C. J. Yu and G. D. Wei, Mater. Today Phys., 2020, 15, 100273 CrossRef.
- B. Zhao, Z. Gan, M. Johnson, E. Najafidehaghani, T. Rejek, A. George, R. H. Fink, A. Turchanin and M. Halik, Adv. Funct. Mater., 2021, 31, 2105444 CrossRef CAS.
- J. Li, S. Ding, X. Ren, Q. Sun, L. Sun, L. Zheng, F. Li, W. Zhu and W. Hu, ACS Mater. Lett., 2022, 4, 1483–1492 CrossRef CAS.
- Q. Luo, G. Feng, Y. Song, E. Zhang, J. Yuan, D. Fa, Q. Sun, S. Lei and W. Hu, Nano Res., 2022, 15, 8428–8434 CrossRef CAS.
- S. Xu, D. Yang, L. Sun, W. Lv, X. Wu, Y. Wei, X. Fang, X. Song, Y. Wang, Y. Tang and Y. Peng, ACS Appl. Electron. Mater., 2022, 4, 2777–2786 CrossRef CAS.
- Z. Xu, M. He, Q. Wu, C. Wu, X. Li, B. Liu, M. C. Tang, J. Yao and G. Wei, Adv. Sci., 2023, 10, 2207743 CrossRef CAS PubMed.
- D. He, Y. Pan, H. Nan, S. Gu, Z. Yang, B. Wu, X. Luo, B. Xu, Y. Zhang, Y. Li, Z. Ni, B. Wang, J. Zhu, Y. Chai, Y. Shi and X. Wang, Appl. Phys. Lett., 2015, 107, 183103 CrossRef.
- X. Wang, P. Wang, J. Wang, W. Hu, X. Zhou, N. Guo, H. Huang, S. Sun, H. Shen, T. Lin, M. Tang, L. Liao, A. Jiang, J. Sun, X. Meng, X. Chen, W. Lu and J. Chu, Adv. Mater., 2015, 27, 6575–6581 CrossRef CAS PubMed.
- C. E. Petoukhoff, M. B. Krishna, D. Voiry, I. Bozkurt, S. Deckoff-Jones, M. Chhowalla, D. M. O'Carroll and K. M. Dani, ACS Nano, 2016, 10, 9899–9908 CrossRef CAS PubMed.
- D. B. Velusamy, M. A. Haque, M. R. Parida, F. Zhang, T. Wu, O. F. Mohammed and H. N. Alshareef, Adv. Funct. Mater., 2017, 27, 1605554 CrossRef.
- M. Sun, P. Yang, D. Xie, Y. Sun, J. Xu, T. Ren and Y. Zhang, Adv. Electron. Mater., 2018, 5, 1800580 CrossRef.
- L. Lv, F. Zhuge, F. Xie, X. Xiong, Q. Zhang, N. Zhang, Y. Huang and T. Zhai, Nat. Commun., 2019, 10, 3331 CrossRef PubMed.
- X. Wang, H. Shen, Y. Chen, G. Wu, P. Wang, H. Xia, T. Lin, P. Zhou, W. Hu, X. Meng, J. Chu and J. Wang, Adv. Sci., 2019, 6, 1901050 CrossRef PubMed.
- Y. Hao, C. He, J. Xu, Y. Bao, H. Wang, J. Li, H. Luo, M. An, M. Zhang, Q. Zhang, J. Qiu and Y. Yang, J. Phys. Chem. C, 2022, 126, 16349–16356 CrossRef CAS.
- Z. Xu, Y. Zeng, F. Meng, S. Gao, S. Fan, Y. Liu, Y. Zhang, S. Wageh, A. A. Al-Ghamdi, J. Xiao, Z. Guo and H. Zhang, Adv. Mater. Interfaces, 2022, 9, 2200912 CrossRef CAS.
- A. K. Dwivedi, S. Jit and S. Tripathi, IEEE Trans. Electron Devices, 2023, 70, 4694–4699 CAS.
- X. Liu, J. Gu, K. Ding, D. Fan, X. Hu, Y. W. Tseng, Y. H. Lee, V. Menon and S. R. Forrest, Nano Lett., 2017, 17, 3176–3181 CrossRef CAS PubMed.
- F. Huang, J. Li, Z. Xu, Y. Liu, R. Luo, S. W. Zhang, P. Nie, Y. Lv, S. Zhao, W. Su, W. D. Li, S. Zhao, G. Wei, H. C. Kuo and F. Kang, Nanomaterials, 2019, 9, 1312 CrossRef CAS PubMed.
- K. Pei, F. Wang, W. Han, S. Yang, K. Liu, K. Liu, H. Li and T. Zhai, Small, 2020, 16, 2002312 CrossRef CAS PubMed.
- B. Yang, Y. Wang, L. Li, J. Zhang, J. Wang, H. Jiao, D. Hao, P. Guo, S. Zeng, Z. Hua and J. Huang, Adv. Funct. Mater., 2021, 31, 2103787 CrossRef CAS.
- F. Wang, J. Wu, Y. Zhang, S. Yang, N. Zhang, H. Li and T. Zhai, Sci. China-Mater., 2021, 65, 451–459 CrossRef.
- W. Su, S. Zhang, C. Liu, Q. Tian, X. Liu, K. Li, Y. Lv, L. Liao and X. Zou, Nano Lett., 2022, 22, 10192–10199 CrossRef CAS PubMed.
- Q. Zhu, Y. Chen, T. Chen, L. Zuo, Y. Sun, R. Wang and M. Xu, Nano Res., 2022, 15, 8595–8602 CrossRef CAS.
- D. Vikraman, S. Hussain, H. Liu, S. H. Abbas Jaffery, K. Karuppasamy, J.-H. Lee, A. Kathalingam, J. Jung and H.-S. Kim, J. Mater. Res. Technol., 2022, 17, 2875–2887 CrossRef CAS.
- Z. Ge, N. Xu, Y. Zhu, K. Zhao, Y. Ma, G. Li and Y. Chen, ACS Photonics, 2022, 9, 59–67 CrossRef CAS.
- T. K. Yun, Y. Lee, M. J. Kim, J. Park, D. Kang, S. Kim, Y. J. Choi, Y. Yi, B. Shong, J. H. Cho and K. Kim, Small, 2022, 18, 2105916 CrossRef CAS PubMed.
- D. Z. Yang and D. G. Ma, Adv. Opt. Mater., 2019, 7, 1800522 CrossRef.
- L. L. Shi, Q. B. Liang, W. Y. Wang, Y. Zhang, G. H. Li, T. Ji, Y. Y. Hao and Y. X. Cui, Nanomaterials, 2018, 8, 713 CrossRef PubMed.
- J. L. Miao and F. J. Zhang, Laser Photon. Rev., 2019, 13, 1800204 CrossRef.
- J. S. Miao and C. Wang, Nano Res., 2021, 14, 1878–1888 CrossRef.
- Q. X. Qiu and Z. M. Huang, Adv. Mater., 2021, 33, 2008126 CrossRef CAS PubMed.
- A. Bablich, S. Kataria and M. C. Lemme, Electronics, 2016, 5, 13 CrossRef.
- J. Sun, Y. Choi, Y. J. Choi, S. Kim, J. H. Park, S. Lee and J. H. Cho, Adv. Mater., 2019, 31, 1803831 CrossRef PubMed.
- D. H. Shin and S. H. Choi, Micromachines, 2018, 9, 350 CrossRef PubMed.
- M. A. Khan, A. Kumar, J. Zhang and M. Kumar, J. Mater. Chem. C, 2021, 9, 8129–8157 RSC.
- H. J. Geng, D. Yuan, Z. Yang, Z. J. Tang, X. W. Zhang, K. Yang and Y. Su, J. Mater. Chem. C, 2019, 7, 11056–11067 RSC.
- A. Taffelli, S. Dirè, A. Quaranta and L. Pancheri, Sensors, 2021, 21, 2758 CrossRef CAS PubMed.
- H. S. Nalwa, RSC Adv., 2020, 10, 30529–30602 RSC.
- M. Chhowalla, H. S. Shin, G. Eda, L. J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed.
- K. Ulman and S. Y. Quek, Nano Lett., 2021, 21, 8888–8894 CrossRef CAS PubMed.
- M. T. Fontana, D. A. Stanfield, D. T. Scholes, K. J. Winchell, S. H. Tolbert and B. J. Schwartz, J. Phys. Chem. C, 2019, 123, 22711–22724 CrossRef CAS.
- K. H. Li, Y. Lu, X. K. Yang, L. C. Fu, J. G. He, X. T. Lin, J. J. Zheng, S. C. Lu, C. Chen and J. Tang, InfoMat, 2021, 3, 1145–1153 CrossRef CAS.
- X. C. Tan, H. Zhang, J. Y. Li, H. W. Wan, Q. S. Guo, H. B. Zhu, H. Liu and F. Yi, Nat. Commun., 2020, 11, 5245 CrossRef CAS PubMed.
- L. L. Li, Y. H. Deng, C. X. Bao, Y. J. Fang, H. T. Wei, S. Tang, F. J. Zhang and J. S. Huang, Adv. Opt. Mater., 2017, 5, 1700672 CrossRef.
- J. H. Kim, A. Liess, M. Stolte, A. M. Krause, V. Stepanenko, C. W. Zhong, D. Bialas, F. C. Spano and F. Würthner, Adv. Mater., 2021, 33, 2100582 CrossRef CAS PubMed.
- Q. L. Xu, L. Y. Zhang, B. Cheng, J. J. Fan and J. G. Yu, Chem, 2020, 6, 1543–1559 CAS.
- J. S. Kim, Y. J. Choi, H. J. Woo, J. Yang, Y. J. Song, M. S. Kang and J. H. Cho, Adv. Funct. Mater., 2017, 27, 1704475 CrossRef.
- N. N. Nguyen, H. C. Lee, K. Baek, M. S. Yoo, H. Lee, H. Lim, S. Choi, C. J. Kim, S. Nam and K. Cho, Adv. Funct. Mater., 2021, 31, 2008813 CrossRef CAS.
- S. L. Zhang, Q. J. Wang, P. Zhang, J. Wang, Y. Li, C. Lu, M. T. Sarwar, X. B. Dong, Q. H. Zhao, A. D. Tang, L. J. Fu and H. M. Yang, Small, 2023, 19, 2300770 CrossRef CAS PubMed.
- B. Wu, Y. H. Zhao, H. Y. Nan, Z. Y. Yang, Y. H. Zhang, H. J. Zhao, D. W. He, Z. L. Jiang, X. L. Liu, Y. Li, Y. Shi, Z. H. Ni, J. L. Wang, J. B. Xu and X. R. Wang, Nano Lett., 2016, 16, 3754–3759 CrossRef CAS PubMed.
- R. H. Dong, T. Zhang and X. L. Feng, Chem. Rev., 2018, 118, 6189–6235 CrossRef CAS PubMed.
- Y. L. Huang, Y. J. Zheng, Z. B. Song, D. Z. Chi, A. T. S. Wee and S. Y. Quek, Chem. Soc. Rev., 2018, 47, 3241–3264 RSC.
- X. P. Xu, Y. Li and Q. Peng, Adv. Mater., 2022, 1, 311–326 Search PubMed.
- W. R. Zhuang, Y. Wang, P. F. Cui, L. Xing, J. Lee, D. Kim, H. L. Jiang and Y. K. Oh, J. Controlled Release, 2019, 294, 311–326 CrossRef CAS PubMed.
- C. D. M. Churchill, L. R. Rutledge and S. D. Wetmore, Phys. Chem. Chem. Phys., 2010, 12, 14515–14526 RSC.
- P. G. Song, Z. G. Xu, Y. P. Wu, Q. F. Cheng, Q. P. Guo and H. Wang, Carbon, 2017, 111, 807–812 CrossRef CAS.
- Z. X. Liu Lau, G. Q. Zhou, S. X. Li, J. Ren, S. K. Das, R. X. Xia, G. Wu, H. M. Zhu, X. H. Lu, L. Yip, H. Z. Chen and C. Z. Li, Nano Energy, 2019, 63, 103807 CrossRef.
- A. Wadsworth, Z. Hamid, J. Kosco, N. Gasparini and I. McCulloch, Adv. Mater., 2020, 32, 2001763 CrossRef CAS PubMed.
- Q. Zhang, B. Kan, F. Liu, G. K. Long, X. J. Wan, X. Q. Chen, Y. Zuo, W. Ni, H. J. Zhang, M. M. Li, Z. C. Hu, F. Huang, Y. Cao, Z. Q. Liang, M. T. Zhang, T. P. Russell and Y. S. Chen, Nat. Photonics, 2015, 9, 35–41 CrossRef CAS.
- N. N. Liang, D. Meng, Z. T. Ma, B. Kan, X. Y. Meng, Z. Zheng, W. Jiang, Y. Li, X. J. Wan, J. H. Hou, W. Ma, Y. S. Chen and Z. H. Wang, Adv. Energy Mater., 2017, 7, 1601664 CrossRef.
- L. L. Zhan, S. X. Li, T. K. Lau, Y. Cui, X. H. Lu, M. M. Shi, C. Z. Li, H. Y. Li, J. H. Hou and H. Z. Chen, Energy Environ. Sci., 2020, 13, 635–645 RSC.
- J. Yao, B. B. Qiu, Z. G. Zhang, L. W. Xue, R. Wang, C. F. Zhang, S. S. Chen, Q. J. Zhou, C. K. Sun, C. Yang, M. Xiao, L. Meng and Y. F. Li, Nat. Commun., 2020, 11, 2726 CrossRef CAS PubMed.
- L. Liu, H. R. Xiao, K. Jin, Z. Xiao, X. Y. Du, K. Y. Yan, F. Hao, Q. Y. Bao, C. Y. Yi, F. Y. Liu, W. T. Wang, C. T. Zuo and L. M. Ding, Nano-Micro Lett., 2023, 15, 23 CrossRef CAS PubMed.
- B. Minnaert and P. Veelaert, Materials, 2012, 5, 1933–1953 CrossRef.
- T. Zhu, L. Y. Zheng, Z. Xiao, X. Y. Meng, L. Liu, L. M. Ding and X. Gong, Sol. RRL, 2019, 3, 1900322 CrossRef CAS.
- P. P. Kumavat, P. Sonar and D. S. Dalal, Renewable Sustainable Energy Rev., 2017, 78, 1262–1287 CrossRef.
- X. Fan, M. L. Zhang, X. D. Wang, F. H. Yang and X. M. Meng, J. Mater. Chem. A, 2013, 1, 8694–8709 RSC.
- Z. H. Zhang, S. Y. Wang, C. S. Liu, R. Z. Xie, W. D. Hu and P. Zhou, Nat. Nanotechnol., 2022, 17, 27–32 CrossRef CAS PubMed.
- L. J. Pi, P. F. Wang, S. J. Liang, P. Luo, H. Y. Wang, D. Y. Li, Z. X. Li, P. Chen, X. Zhou, F. Miao and T. Y. Zhai, Nat. Electron., 2022, 5, 248–254 CrossRef CAS.
- P. S. Wu, T. He, H. Zhu, Y. Wang, Q. Li, Z. Wang, X. Fu, F. Wang, P. Wang, C. X. Shan, Z. Y. Fan, L. Liao, P. Zhou and W. D. Hu, InfoMat, 2022, 4, 12275 CrossRef.
- N. Ilyas, J. Y. Wang, C. M. Li, D. Y. Li, H. Fu, D. E. Gu, X. D. Jiang, F. C. Liu, Y. D. Jiang and W. Li, Adv. Funct. Mater., 2022, 32, 2110976 CrossRef CAS.
- Y. Z. Luo, Y. Q. Xie, X. Ye and Y. Wang, Phys. Chem. Chem. Phys., 2019, 21, 7613–7617 RSC.
| This journal is © The Royal Society of Chemistry 2024 |