Abnormal anti-thermal quenching of Mn2+ and reverse thermal response of Mn2+/Mn4+ luminescence in garnet phosphor for wide-range temperature sensing†
Annu
Balhara
ab,
Santosh K.
Gupta
 *ab,
G. D.
Patra
*ab,
G. D.
Patra
 c,
Boddu S.
Naidu
c,
Boddu S.
Naidu
 d and
Kathi
Sudarshan
d and
Kathi
Sudarshan
 ab
ab
aRadiochemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai-400085, India. E-mail: santoshg@barc.gov.in; santufrnd@gmail.com
bHomi Bhabha National Institute, Anushaktinagar, Mumbai-400094, India
cTechnical Physics Division, Bhabha Atomic Research Centre, Trombay, Mumbai-400085, India
dInstitute of Nano Science and Technology, Mohali-140306, India
First published on 18th October 2024
Abstract
Herein, we explored the diverse temperature-dependent luminescence, thermal quenching, and decay kinetics of Mn2+/Mn4+ emission centers in a Y3Al2Ga3O12 garnet host. Emission color tuning was achieved from red to deep red region as a function of the Mn doping amount. The tuning of the Mn valence state as a function of doping concentration in Y3Al2Ga3O12 was investigated in detail, and the mechanism of self-reduction of Mn4+ has been discussed. Interestingly, the temperature-dependent photoluminescence study of Y3Al2Ga3O12:Mn2+/4+ revealed the abnormal anti-thermal quenching of the Mn2+ emission band with increasing temperature. In contrast, Mn4+ emission displayed thermal quenching with increase in temperature. The interplay of self-reduction and defects in the anti-thermal quenching of Mn2+ has been discussed. Benefiting from the reverse temperature-dependent luminescence behaviour of Mn2+/4+, luminescence ratiometric and lifetime thermometry have demonstrated a promising wide temperature sensing range, from cryogenic conditions (10 K) to 490 K. A high relative sensitivity of 2.35% K−1 (190 K) and 3.10% K−1 (390 K) could be achieved for ratiometric and lifetime thermometry, respectively. The lifetime thermometer based on the lifetime of Mn2+ and Mn4+ ions offered good temperature resolution (<0.30 K). This work proposed the use of dual-emitting single-doped materials for highly sensitive wide sensing range thermometry and offers meaningful advancement in the design of orange–red emitting phosphors.
1. Introduction
Phosphor materials have emerged as potential sources of solid-state lighting technology for indoor/outdoor illumination, displays, optoelectronics, and light-emitting diodes (LEDs) applications.1–6 The transition metal manganese (Mn2+/4+ ions) is a well-known photoluminescence activator for various inorganic luminescent materials used in modern lighting sources owing to red emission with high luminous efficiency, low cost and non-rare earth-based red phosphors.7–11 Mn2+-based phosphors have been widely investigated to achieve multimode fluorescence for the design of white LEDs, thermal sensing, and anti-counterfeiting applications.12,13 Garnet phosphors (A3B2C3O12) are popular candidates for achieving the strong red to deep red emission of Mn2+/4+ ions as they can be stabilized in octahedral Al3+/Ga3+ sites.14,15 The high temperature solid-state synthesis of the garnet structure may result in the coexistence of Mn2+/4+ ions due to the defect-induced self-reduction of Mn4+ ions. The design of Mn2+/4+-activated materials offers the potential optimization of emission from red light (Mn2+) to deep red light (Mn4+) by controlling the composition and tuning the emission for indoor plant growth. Since the control of the Mn valence state is a great challenge for developing Mn-activated luminescent materials, few studies have reported dual-emitting Mn2+/4+ phosphors for suitable applications.14–20 Therefore, understanding the photophysical properties, defect-induced self-reduction, charge transfer dynamics, decay kinetics and thermal behaviour is essential for designing Mn-based phosphors for targeted applications utilizing the multiple valence states of Mn. The mixed valence of Mn offers the development of multifunctional phosphors with emission tunability, which is promising for the development of phosphors for indoor plant lighting, anti-counterfeiting, optoelectronics and temperature sensing applications.Benefitting from the multifunctionality of phosphor materials, optical temperature sensors based on the thermal response of luminescence intensity ratio (LIR), lifetime, and bandwidth have attracted scientific attention due to their high accuracy, non-contact thermometry, and fast response.21–26 Lifetime-based thermometers have gained popularity due to the low chances of erroneous temperature readout.22 Recently, dual-wavelength emission materials have emerged as perfect candidates for ratiometric or lifetime-based temperature sensing, which utilize temperature-dependent energy transfer between the dopant ions and charge transfer (CT) between the host matrix and dopant ions.27–29 Interestingly, the dual-wavelength emitting Mn2+/4+-activated phosphors can be studied for ratiometric and lifetime thermometry based on the diverse temperature-dependent photoluminescence (PL) properties and thermal quenching (TQ) of Mn2+/4+ ions.
Herein, we synthesized a series of Mn2+/4+-activated Y3Al2Ga3O12 (YAGG) phosphors using solid-state synthesis reported in our earlier work,3 which is detailed in the ESI.† We reported the stabilization of mixed valent states of Mn in the YAGG lattice and studied the photoluminescence properties of the dual-emitting Y3Al2Ga3O12:Mn2+/4+ phosphor at different excitation energies, doping amounts and temperature. The luminescence and application prospects of the dual-emitting Y3Al2Ga3O12:Mn2+/4+ phosphor have not been studied before to the best of our knowledge. The changes in the defects and oxidation state of Mn ions with composition were probed using positron annihilation lifetime spectroscopy (PALS) and electron paramagnetic resonance (EPR) spectroscopy. We investigated the temperature-dependent luminescence behaviour of Mn2+/4+ ions in a wide temperature range of 10 K to 490 K. The evolution of the Mn2+ emission band with temperature is discussed in detail with respect to temperature-dependent CT and defect-induced self-reduction of Mn4+ → Mn2+. The excitation wavelength-dependent decay kinetics was studied as a function of temperature to understand the depopulation mechanism and charge transfer dynamics in Mn2+/4+-activated YAGG system. We demonstrate that the dual-wavelength emitting YAGG:Mn phosphor (620 nm/675 nm) can be utilized for ratiometric and lifetime thermometry based on the diverse thermal response of luminescence and decay of Mn2+/4+ ions. The characterization details are provided in the ESI† file (Section S1.2).
2. Results and discussion
2.1 Structural and morphological analysis
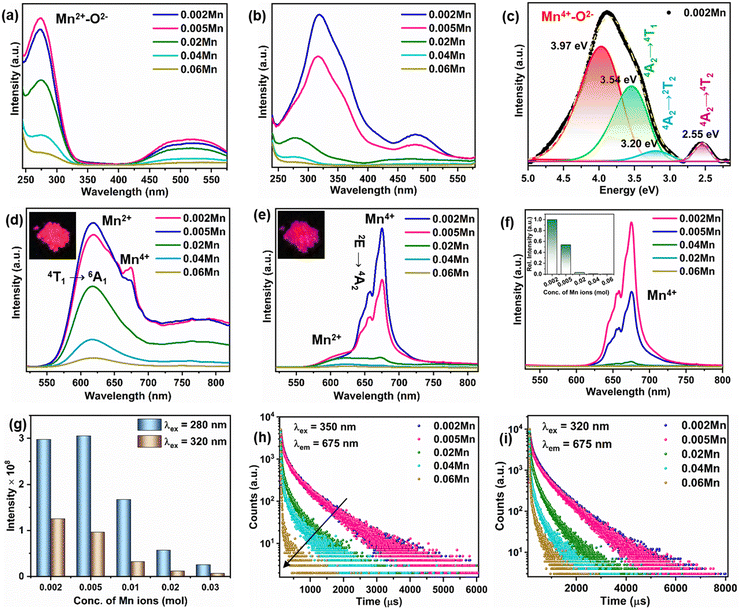
The XRD diffraction patterns of all the Mn-doped YAGG samples confirmed the phase purity and YAGG crystallized in a cubic system (Ia3d space group) (Fig. S1, ESI†). The garnet structure YAGG is composed of YO8 dodecahedron, AlO6/GaO6 octahedron, and AlO4/GaO4 tetrahedron (Fig. S2, ESI†). Based on the ionic size, the Mn2+/Mn4+ ions (0.67 Å/0.53 Å) preferably occupy the octahedral Al3+/Ga3+ (0.535 Å/0.62 Å) sites to form Mn2+/Mn4+ color centers.27 The FTIR spectra show the characteristic bands of Ga–O and Al–O stretching frequencies in the 580–850 cm−1 region (Fig. S3, ESI†).3 The TEM image of YAGG:0.02Mn shows that the sample crystallized as particles with irregular morphology and size ∼1 μm (Fig. S4, ESI†). The EDS spectra confirmed the presence of Y, Al, Ga, Mn, and O elements in the YAGG:0.02Mn sample (Fig. S5, ESI†).2.2 Photoluminescence study
The photoluminescence excitation (PLE) spectra show different profiles for 620 nm and 675 nm emissions (Fig. 1(a) and (b)). When excited at 280 nm, the emission spectra (Fig. 1(d)) show a broad red emission peaking at 620 nm along with weak sharp bands at 675 nm, which can be ascribed to the 4T1 → 6A1 transition of Mn2+ ions and the 2E → 4A2 transition of Mn4+ ions located in the AlO6/GaO6 octahedra, respectively.21,23 Mn2+ emission depends on the crystal field strength and red emission of Mn2+ ions in the YAGG lattice is in accordance with the octahedral coordination of Mn2+ ions.6,30 Mn4+ ions with 3d3 electronic configuration occupy the octahedral sites due to the preferred octahedral coordination and size.18,24 The absence of green emission of Mn2+ ions in the emission spectra negated the possible occupation of tetrahedral Al3+/Ga3+ sites. This suggested the presence of Mn2+/4+ ions in the YAGG:Mn samples, which was confirmed by EPR measurements. The presence of sextet EPR lines due to both Mn2+/4+ ions are marked in the EPR spectra (Fig. S6 and S7, ESI†). To further confirm the presence of Mn2+ and Mn4+ ions and investigate the relative amounts of Mn2+/4+ ions in the samples with different Mn doping contents, X-ray photoelectron spectroscopy (XPS) measurements were performed on two samples, i.e., YAGG:0.02Mn and YAGG:0.06Mn. The XPS survey spectra of the two samples and Mn 2p3/2 spectra are presented in Fig. S8a–c (ESI†). The Mn 2p3/2 spectra for YAGG:0.02Mn and YAGG:0.06Mn can be deconvoluted into two peaks of Mn2+ at 640.9 eV and Mn4+ at 644.9 eV.18,31 Though the peak positions remained similar, the relative peak areas of Mn2+ and Mn4+ show different order. In YAGG:0.02Mn, the area of the Mn2+ peak is much smaller than that of Mn4+, while in the YAGG:0.06Mn sample, the area of the Mn2+ peak is relatively higher than that of YAGG:0.02Mn. This indicated that the Mn2+ concentration increased compared to Mn4+ with the increase in the doping amounts of Mn ions in the YAGG matrix, which can be explained by the self-reduction of Mn4+ ions to Mn2+ at higher doping concentrations, as discussed later.The color tunability from the red emission of Mn2+ in YAGG:0.002Mn to the deep red emission of Mn4+ ions under different excitation wavelengths is depicted by the emission photographs captured by a camera (see inset of Fig. 1(d) and (e)). The emission spectra (λex = 320 nm) show the typical sharp band emission of Mn4+ ions with less intense Mn2+ emission (Fig. 1(e)). Interestingly, upon excitation at 280 and 320 nm, which can be ascribed to O2− → Mn2+ and O2− → Mn4+ CT transitions, the dual-band emission bands of both Mn2+ and Mn4+ were observed in the PL emission spectra of the samples at 620 nm (4T1 → 6A1) and 675 nm (2E → 4A2), respectively. Mn4+ emission dominates at λex = 320 nm, and Mn2+ emission is more intense at λex = 280 nm. The broad PLE spectrum (λem = 675 nm) of YAGG:0.002Mn is composed of four Gaussian bands centered at 312 nm (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 051 cm−1), 350 nm (28
051 cm−1), 350 nm (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 571 cm−1), 388 nm (25
571 cm−1), 388 nm (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 839 cm−1) and 487 nm (20
839 cm−1) and 487 nm (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533 cm−1) due to the O2− → Mn4+ CT, 4A2 → 4T1, 4A2 → 2T2 and 4A2 → 4T2 transitions of Mn4+ ions, respectively (Fig. 1(c)).5 The PL emission spectra of the samples under 350 nm excitation, i.e., the 4A2 → 4T1 transition of Mn4+ ions, showed single emission of Mn4+ ions at 675 nm only due to the 2E → 4A2 transition (Fig. 1(f)). The presence of dual-band emission and single-band emission under 320 and 350 nm excitations can be observed in Fig. S9 (ESI†). We evaluated the crystal field strength (Dq) and Racah parameter (B) of the Mn4+ sites in the YAGG:0.002Mn4+ sample using the following equations.
533 cm−1) due to the O2− → Mn4+ CT, 4A2 → 4T1, 4A2 → 2T2 and 4A2 → 4T2 transitions of Mn4+ ions, respectively (Fig. 1(c)).5 The PL emission spectra of the samples under 350 nm excitation, i.e., the 4A2 → 4T1 transition of Mn4+ ions, showed single emission of Mn4+ ions at 675 nm only due to the 2E → 4A2 transition (Fig. 1(f)). The presence of dual-band emission and single-band emission under 320 and 350 nm excitations can be observed in Fig. S9 (ESI†). We evaluated the crystal field strength (Dq) and Racah parameter (B) of the Mn4+ sites in the YAGG:0.002Mn4+ sample using the following equations.
| Dq/B = 15(x − 8)/x2 − 10x | (1) |
| Dq = E(4A2 − 4T2)/10 | (2) |
| x = E(4A2 − 4T1) − E(4A2 − 4T2)/Dq | (3) |
The crystal field strength (Dq), Racah parameters (B), and crystal field strength (Dq/B) for Mn4+ were calculated as 2053 cm−1, 798 cm−1, and 2.57, respectively.
The variation of emission intensity as a function of Mn concentration is displayed in Fig. 1(f) and (g) where the optimal concentration is 0.002 mol and 0.005 mol for 320/350 nm and 280 nm excitation, respectively. The tuning of Mn emission color with doping amounts can be visualized from the CIE diagram (Fig. S10 and S11, ESI†). The excitation wavelength-dependent color tunability of Mn emission is achieved due to the different absorption efficiency of Mn2+/4+ ions in the UV and near-UV regions (Fig. S12, ESI†). The concentration quenching resulted in the reduced emission intensity of both Mn2+/4+ ions at higher Mn doping amounts.32 The concentration quenching of both Mn2+/4+ ions is caused by electric dipole–dipole interactions as θ = 5.76 and 6.36 (close to 6) were obtained from the log(I/x) versus log(x) plot shown in Fig. S13a and b (discussion in ESI†), respectively.32
With the increase in the Mn ions content, Mn4+ emission is quenched significantly and the broad emission of Mn2+ is dominant in the YAGG samples with higher Mn content (Fig. S14 and S15, ESI†). This suggested the defect-induced self-reduction of Mn4+ → Mn2+ at higher Mn doping amounts.33 Due to charge imbalance, the substitution of Al3+/Ga3+ sites by Mn4+ ions will induce the formation of cation vacancies (VAl/Ga). The nature of defects with changing doping amount of Mn ions was probed using PALS technique (Fig. S16, ESI†). Typical positron annihilation lifetime spectra are shown in Fig. S16a (ESI†). All the lifetime spectra could be fitted as the sum of three exponentials or three positron annihilation lifetimes with longest lifetime of 1.5 to 2 ns with intensity <0.5%. The typical fitting of the lifetime spectra is given as inset of Fig. S16a (ESI†). The long-lived component of <0.5% intensity is due to positronium formation on the surface of the powder particles. The other two positron lifetimes (τ1 and τ2), intensity of the second positron lifetime (I2 and I1 ∼ 100 − I2) and the intensity-weighted average positron lifetime (τavg) are given in Fig. S16b (ESI†).
The first positron lifetime (τ1) is from the delocalised positron annihilation in the bulk of the sample while the second positron lifetime (τ2) is from defects. The first positron lifetime in all the samples is in the range from 167 to 173 ps and is close to the lifetimes of 147–160 ps reported for the YAG samples in the literature.34 With the doping of Mn, the first positron lifetime varied only marginally. A significant change is observed as a continuous reduction in the second positron lifetime, with the intensity of this component initially reducing and reaching a minimum at about 0.25–1% Mn doping and then increasing. Theoretical positron lifetimes for positron annihilations from various possible vacancies in YAG are reported.35 As per the calculations, Y vacancies are expected to give positron lifetime of 341 ps, Al vacancy gives a lifetime of 275–279 ps, and oxygen vacancies about 238 ps. The second positron lifetime suggested that cation vacancies are formed initially at low Mn doping amount and finally lead to oxygen vacancies at higher Mn doping. Mn can be doped in variable oxidation states. It is expected that the doping of Mn4+ would lead to the formation of cation vacancies while Mn2+ would enhance the formation of oxygen vacancies for charge compensation. These results indicated that Mn might be doped as Mn4+ at lower concentrations while Mn2+ is favoured at higher concentrations. This result is in line with the XPS analysis; hence, it can be concluded that more Mn4+ ions get reduced to Mn2+ with the increase in the Mn doping amounts.
The presence of both Mn4+ and Mn2+ emission indicated abnormal self-reduction in the YAGG lattice. The charge compensation model has been used in earlier reports to explain the abnormal self-reduction from Mn4+ → Mn2+ ions.15 The substitution of trivalent Al3+/Ga3+ sites by tetravalent Mn4+ ions in YAGG will induce the formation of positively charged 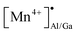 and negatively charged
and negatively charged 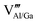 defects. To neutralize the excess positive charge, four Al3+/Ga3+ ions will be replaced by three Mn4+ ions (eqn (1)). The generated
defects. To neutralize the excess positive charge, four Al3+/Ga3+ ions will be replaced by three Mn4+ ions (eqn (1)). The generated 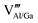 can act as electron donors, and the released electrons from the
can act as electron donors, and the released electrons from the 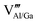 defects can be captured by the electron acceptor
defects can be captured by the electron acceptor 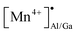 -defects. As a result, some of the Mn4+ ions are reduced to Mn2+. The above processes can be explained by the following equations.
-defects. As a result, some of the Mn4+ ions are reduced to Mn2+. The above processes can be explained by the following equations.
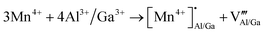 | (4) |
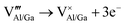 | (5) |
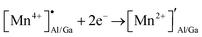 | (6) |
In addition, the YAGG:0.02Mn samples were synthesized under air atmosphere using MnCO3 and MnO2 as raw Mn source. The PLE and emission spectra (Fig. S17, ESI†) show similar spectral profiles, which confirm the self-reduction of Mn4+ to Mn2+ ions in the YAGG matrix.
The luminescence decay profiles for the Mn-doped YAGG phosphors for emission at 675 nm and 620 nm (λex = 320/350 nm and 280 nm) are presented in Fig. 1(h), (i) and Fig. S18 (ESI†), respectively. The average lifetimes (τavg) of Mn ions were calculated using a bi-exponential decay function.
| I(t) = I0 + A1e−t/τ1 + A1e−t/τ2 | (7) |
2.3 Temperature-dependent PL study
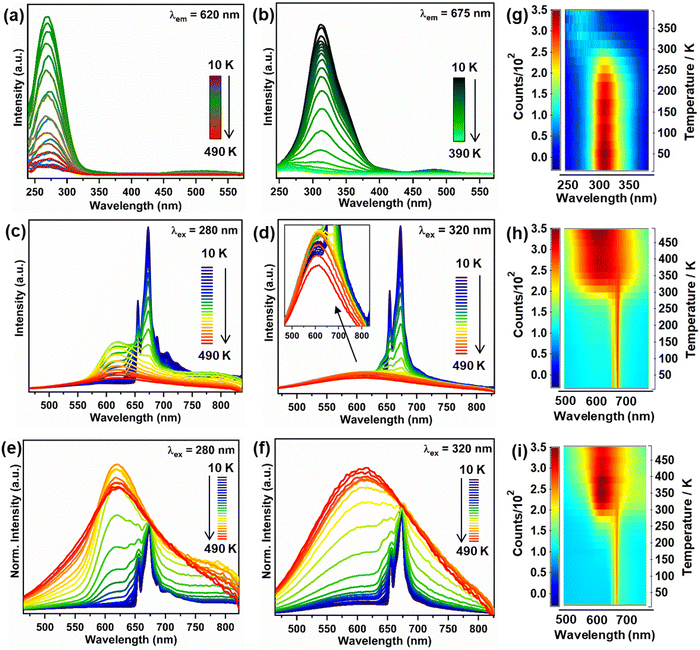
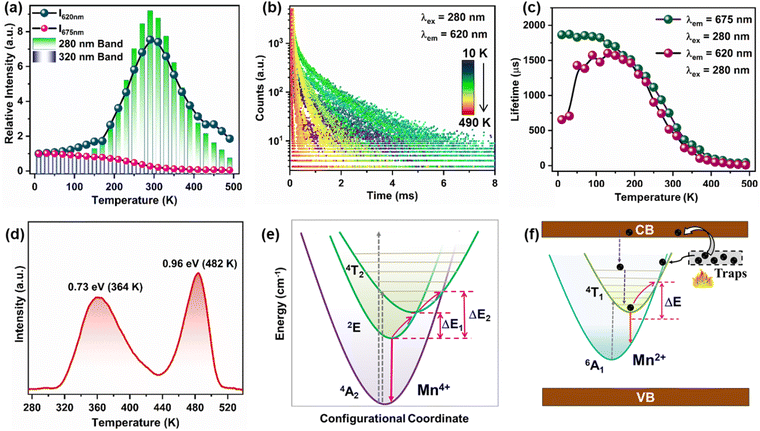
Since the excitations at 280 and 320 nm fall in the excitation range of both Mn4+ and Mn2+ ions, the temperature-dependent luminescence behaviour of the dual-emission bands of Mn2+/4+ ions can be investigated effectively using these two CT transitions. To understand the thermal behaviour of Mn2+/4+ luminescence, the temperature-dependent PLE and emission spectra of Mn2+/4+ under CT band excitation were recorded from 10 K to 490 K (Fig. 2(a)–(f)). The PLE spectra (λem = 620 nm) in Fig. 2(a) display an increase in the O2− → Mn2+ CT band intensity initially up to 290 K, followed by decreased intensity at elevated temperatures (Fig. 3(a)). On the contrary, the PLE spectra (λem = 675 nm) shows a continuous decrease in the intensity of the O2− → Mn4+ CT band with temperature (Fig. 3(a)). The temperature-dependent PL emission spectra (λex = 320 nm and 280 nm) demonstrated an abnormal trend in integral intensity change with temperature (Fig. 2(c) and (d)). The normalized emission spectra with respect to 675 nm emission shows that the spectral profile changes with temperature, where the Mn2+ emission becomes dominant at elevated temperatures (Fig. 2(e) and (f)). The broad emission at 620 nm (λex = 280 nm) due to Mn2+ ions increased with temperature till 290 K and shows TQ above 310 K; however, Mn4+ emission at 675 nm undergoes continuous TQ (Fig. 2(g) and 3(a)). The above observations can be visualized from the contour plots (Fig. 2(g)–(i)). The TQ in the Mn4+ ions can be caused by the crossover of 2E and 4T2 parabolas (ΔE1) or 4T2 and 4A2 parabolas (ΔE2), which results in non-radiative relaxation.37 The phonon-assisted crossover of 4T1 and 6A1 parabolas in the case of Mn2+ ions can result in non-radiative relaxation to the ground state (ΔE).38The trend of total integral intensity under different excitation wavelengths is shown in Fig. S20 (ESI†). The trend of the O2− → Mn2+ CT band is similar to the 620 nm peak intensity variation with temperature. This indicated that the release of electrons from the traps with increasing temperature may strength the O2− → Mn2+ CT, which led to the anti-TQ of Mn2+ emission (<290 K). The temperature dependence of the Mn2+ ion showing both anti-TQ and normal TQ can be fitted by the Shibata et al. model discussed in the ESI† (Fig. S21).39 The values of activation energies for the anti-TQ of Mn2+ emission below 290 K is 686 cm−1, normal TQ of Mn2+ emission after 310 K is 1955 cm−1, and energy barrier for TQ of Mn4+ ions is 774 cm−1. This suggested that the anti-TQ of Mn2+ ions and TQ of Mn4+ ions may be controlled by the thermal population of the 4T1 state of Mn2+ ions and the simultaneous depopulation of the 2E state of Mn4+ ions, respectively.
To get better insights into the depopulation and thermal quenching mechanisms, temperature-dependent decay curves were recorded at Mn2+/4+ emission wavelengths in the temperature range from 10 K to 490 K. The lifetime values of Mn2+ emission at 620 nm increases initially, which can be attributed to the thermal population of the 4T1 state of Mn2+ ions, which modulates the PL intensity of Mn2+ emission (Fig. 3(b) and (c) and Fig. S22, S23, ESI†).40 The lifetime increase indicated that with increasing temperature, the excited state population is increased by some thermally-activated processes. At higher temperature, the increased non-radiative pathways may result in the observed decrease in the lifetime values. In contrast, the lifetime values of 675 nm emission show a continuous decrease with temperature due to the increased non-radiative processes for the Mn4+ excited states (Fig. 3(c) and Fig. S22–S24, ESI†). The normal TQ of Mn4+ emission can be explained by the thermal cross-over of the excited states with the ground state of Mn4+ ions (Fig. 3(e)).
2.4 Mechanism of thermal quenching behaviour of Mn2+/4+ ions
To investigate the nature of defects, the thermoluminescence (TL) spectrum for the YAGG:0.002Mn2+/4+ was recorded sample upon irradiation with 280 nm light for 100 s. The TL spectra (Fig. 3(d)) shows two peaks at 364 K and 482 K, which can be attributed to the presence of VO and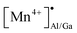 defects, respectively.33 The trap depths of these defects were calculated as 0.73 eV and 0.96 eV, using the equation E = T/500 (eV). Fig. 3(f) shows the proposed mechanism for the anti-TQ of Mn2+ emission. The shallow traps can capture the electrons during UV excitation, which can be released to the conduction band (CB) on increasing the temperature. The electrons in the CB can populate the excited 4T1 state of Mn2+. The increased thermal vibrations can also induce electron tunneling to the excited 4T1 level of Mn2+ ions from the trap levels around the excited energy levels of Mn2+. The above thermally-induced population of Mn2+ excited levels resulted in the enhancement of Mn2+ emission with temperature increase to 290 K. However, thermal quenching dominates at higher temperatures due to the thermal crossover of the 4T1 and 6A1 states. The observed trend can also be explained by the thermally-induced self-reduction of Mn4+ → Mn2+ ions as VGa/Al can act as electron donors.30,33 With the increase in temperature, the electrons are released from VGa/Al and transferred to the nearby Mn4+ ions.18
defects, respectively.33 The trap depths of these defects were calculated as 0.73 eV and 0.96 eV, using the equation E = T/500 (eV). Fig. 3(f) shows the proposed mechanism for the anti-TQ of Mn2+ emission. The shallow traps can capture the electrons during UV excitation, which can be released to the conduction band (CB) on increasing the temperature. The electrons in the CB can populate the excited 4T1 state of Mn2+. The increased thermal vibrations can also induce electron tunneling to the excited 4T1 level of Mn2+ ions from the trap levels around the excited energy levels of Mn2+. The above thermally-induced population of Mn2+ excited levels resulted in the enhancement of Mn2+ emission with temperature increase to 290 K. However, thermal quenching dominates at higher temperatures due to the thermal crossover of the 4T1 and 6A1 states. The observed trend can also be explained by the thermally-induced self-reduction of Mn4+ → Mn2+ ions as VGa/Al can act as electron donors.30,33 With the increase in temperature, the electrons are released from VGa/Al and transferred to the nearby Mn4+ ions.18
2.5 Temperature sensing application
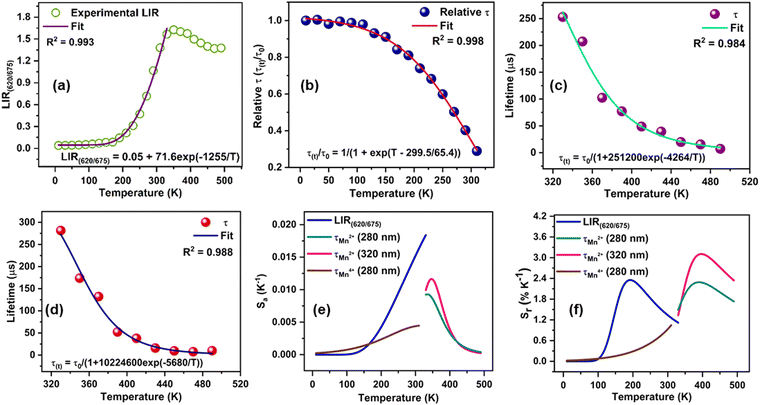
The diverse temperature-dependent behaviour of Mn2+/4+ emission upon 280 nm excitation can be utilized for LIR-based ratiometric and lifetime thermometry. The LIR (620 nm/675 nm) (Fig. 4(a)) from 10 K to 330 K can be fitted with the following exponential equation.41LIR(620/675) = A + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ΔE/kT) exp(−ΔE/kT) | (8) |
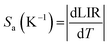 | (9) |
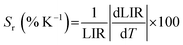 | (10) |
The Sa and Sr values as a function of temperature are shown in Fig. 4(e) and (f), respectively. The maximum Sa value of 0.018 K−1 (at 330 K) and high Sr of 2.35% K−1 (190 K) could be achieved for the Mn2+/4+ LIR-based thermometer. An Sr value higher than 1.0% K−1 was retained up to 330 K. The temperature uncertainty (δT) can be evaluated using the following expression.
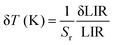 | (11) |
The thermal dependence of the lifetime values of 675 nm emission under 280 nm excitation wavelength can be utilized for wide-range temperature sensing from 10 to 310 K. The sigmoidal temperature dependences of the relative lifetime (τ(t)/τ0) of Mn4+ ions can be fitted using the Boltzmann function as follows.
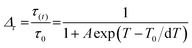 | (12) |
The temperature dependences of Mn2+ lifetime (λex = 280 nm/320 nm) can be fitted in the temperature range from 330 K to 490 K using an equation derived from the Mott–Seitz model.27
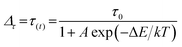 | (13) |
The Sa and Sr values for lifetime thermometry can be evaluated by the following relations.
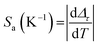 | (14) |
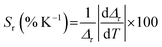 | (15) |
Lifetime thermometry based on the Boltzmann function using Mn4+ emission offers the maximum Sa value of 0.0045 K−1 (at 310 K), and Sr of 1.05% K−1 was attained at 310 K (Fig. 4(f)). Lifetime thermometry based on Mn2+ emission (λex = 280 nm) displays the maximum Sa value of 0.009 K−1 (at 330 K) and Sr of 2.29% K−1 (390 K). Lifetime thermometry based on Mn2+ emission (λex = 320 nm) displays the maximum Sa value of 0.012 K−1 (at 350 K) and Sr of 3.10% K−1 (390 K). The temperature uncertainty (δT) for lifetime thermometry can be evaluated using the following expression
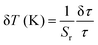 | (16) |
The thermometric performance of our thermometer was compared with similar thermometers in Table 1. Hence, the lifetime thermometry displayed by the YAGG:Mn2+/4+ phosphor is relatively higher than the earlier reported lifetime thermometers, and Sr greater than 1.0% K−1 can be achieved above 150 K. The reverse thermal response of Mn2+/4+ ions can be utilized for temperature sensing at low temperatures. The lifetime of the two valence states can be used in different temperature ranges for temperature detection with high precision. Hence, the mixed valence state YAGG:Mn2+/4+ phosphor is a potential candidate for a dual-mode temperature sensor. This work discussed the abnormal reverse thermal response of two ions, which can be utilized for targeted applications and provide useful insights into such mixed valent Mn2+/4+ orange-red emitting phosphors.
| System | Type | Temp. range | S r (% K−1) | Ref. |
|---|---|---|---|---|
| LaZnGa11O19(LZG):Cr3+/Ni2+ | LIR | 100–175 K, 200–475 K | 2.4% K−1 at 475 K | 42 |
| Sr2LuNbO6:Mn4+ | LIR | 12–523 K | 0.55% K−1 at 136 K | 43 |
| Lifetime | 12–523 K | 1.55% K−1 at 519 K | ||
| SrGdLiTeO6:Mn4+/Eu3+ | Lifetime | 300–600 K | 0.229% K−1 at 573 K | 44 |
| Ca3Ga2Ge3O12:Mn3+/Mn4+ | LIR | 5–75 K | 6% K−1 at 33 K | 40 |
| ZnGa2−yAlyO4:Mn2+/Mn4+ | LIR | 100–475 K | 4.345% K−1 at 350 K | 17 |
| Lu3Al5O12:Ce3+/Mn4+ | LIR | 250–350 K | 4.37% K−1 at 350 K | 27 |
| Lifetime | 250–350 K | 3.22% K−1 at 350 K | ||
| Mn4+-doped BaLaCa1−xMgxSbO6 | Lifetime | 303–503 K | 1.42% K−1 at 488 K | 9 |
| Y2O3:Ho3+/Mg2TiO4:Mn4+ | LIR | RT–100 °C | 4.6% °C−1 0.9 at 473 K | 45 |
| Lifetime | RT–100 °C | |||
| Gd3Al5O12:Mn3+/Mn4+ | Lifetime | 100–600 K | 2.08% K−1 at 249 K | 22 |
| Y2O3:Yb3+/Er3+ | Lifetime | 298–338 K | 0.50% K−1 at 310 K | 46 |
| Y3Al2Ga3O12:Mn2+/Mn4+ | LIR | 10–330 K | 2.35% K−1 at 190 K | This work |
| Lifetime (Mn4+) | 10 to 310 K | 1.05% K−1 at 310 K | ||
| Lifetime280 (Mn2+) | 330 to 490 K | 2.29% K−1 at 390 K | ||
| Lifetime320 (Mn2+) | 330 to 490 K | 3.10% K−1 at 390 K |
3. Conclusion
In conclusion, YAGG:Mn2+/4+ phosphors display different emission from red to deep red under different excitation wavelengths (280 and 320 nm). The luminescence tuning of orange-red emitting phosphors with doping concentration of Mn ions and excitation wavelength can be promising for designing orange-red emitting materials. The Mn2+/Mn4+ ions display distinctive luminescence and decay time behaviour, which can be utilized for potential wide range optical thermometry with high temperature sensitivity. The ratiometric thermometer offers a high maximum Sr value of 2.35% K−1 (190 K). Lifetime thermometry at two different excitation wavelengths, namely, 280 nm and 320 nm, presents temperature read-out with a high maximum Sr value of 3.10% K−1 (390 K) and 1.05% K−1 (310 K), respectively. This work reports a single metal (two oxidation states)-based dual emitting ratiometric and lifetime thermometer with wide sensing range. Additionally, this work demonstrated the self-reduction of Mn ions in Mn-doped garnet materials and abnormal temperature-dependent luminescence properties of Mn2+/4+ ions.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interests to declare.Acknowledgements
We would like to thank Dr Praveen Kumar, IACS, Kolkata, for help with TEM measurements and Dr Narender Rawat, BARC, for help in TL measurement. This work is funded by Government of India through Department of Atomic Energy. We would also like to thank Surajit Samui and Dr Ramendra Sundar Dey, INST Mohali for help in XPS measurements.References
- A. Balhara, S. K. Gupta, M. Abraham, B. Modak, S. Das, C. Nayak, H. V. Annadata and M. Tyagi, Trap engineering through chemical doping for ultralong X-ray persistent luminescence and anti-thermal quenching in Zn2GeO4, J. Mater. Chem. C, 2024, 12(5), 1728–1745 RSC.
- A. Balhara, S. K. Gupta, M. Abraham, A. K. Yadav, M. Jafar and S. Das, Negative thermal expansion in Sc2Mo3O12:Sm3+ for white LEDs and unveiling the impact of phase transition on cryogenic luminescence, J. Mater. Chem. C, 2024, 12(31), 11955–11966 RSC.
- A. Balhara, S. K. Gupta, B. Modak, M. Abraham, A. K. Yadav, H. V. Annadata, S. Das, N. S. Rawat and K. Sudarshan, Synergy between structural rigidity and cluster defects in a bright near-infrared Cr3+-based phosphor for excellent thermal stability and long afterglow, J. Mater. Chem. C, 2024, 12(26), 9716–9732 RSC.
- S. K. Gupta, B. Modak, M. Abraham, S. Das, R. Gupta, K. G. Girija, M. Mohapatra and K. Sudarshan, Defect induced tunable light emitting diodes of compositionally modulated zinc gallium germanium oxides, Chem. Eng. J., 2023, 474, 145595 CrossRef CAS.
- K. Li and R. Van Deun, Realizing a novel dazzling far-red-emitting phosphor NaLaCaTeO6:Mn4+ with high quantum yield and luminescence thermal stability via the ionic couple substitution of Na+ + La3+ for 2Ca2+ in Ca3TeO6:Mn4+ for indoor plant cultivation LEDs, Chem. Commun., 2019, 55(72), 10697–10700 RSC.
- Q. Zhou, L. Dolgov, A. M. Srivastava, L. Zhou, Z. Wang, J. Shi, M. D. Dramićanin, M. G. Brik and M. Wu, Mn2+ and Mn4+ red phosphors: synthesis, luminescence and applications in WLEDs. A review, J. Mater. Chem. C, 2018, 6(11), 2652–2671 RSC.
- S. Adachi, Mn4+vs. Mn2+—A comparative study as efficient activator ions in phosphor materials: A review, J. Lumin., 2023, 119993 CrossRef CAS.
- K. Chen, Z. Shao, C. Zhang, S. Jia, T. Deng, R. Zhou, Y. Zhou and E. Song, Illuminating the path to high-precision dual-mode optical thermometry: A Mn4+-activated oxyfluoride single crystal for a portable optical fiber thermometric platform, Chem. Eng. J., 2023, 477, 147165 CrossRef CAS.
- G. Li, G. Li, Q. Mao, L. Pei, H. Yu, M. Liu, L. Chu and J. Zhong, Efficient luminescence lifetime thermometry with enhancedMn4+-activated BaLaCa1−xMgxSbO6 red phosphors, Chem. Eng. J., 2022, 430, 132923 CrossRef CAS.
- C. Lu and W. You, Harnessing solid-state ion exchange for the environmentally benign synthesis of high-efficiency Mn4+-doped phosphors, Chem. Commun., 2024, 60(41), 5399–5402 RSC.
- Z. Zhou, N. Zhou, M. Xia, M. Yokoyama and H. B. Hintzen, Research progress and application prospects of transition metal Mn4+-activated luminescent materials, J. Mater. Chem. C, 2016, 4(39), 9143–9161 RSC.
- F. Fang, Y. Jin, H. Chen, H. Lin, Y. Li, Y. Xiong, F. Meng, L. Cao, F. Huang and L. Ma, Multiple encrypted dynamic fluorescent anti-counterfeiting and optical temperature detection of Ca2Ge7O16:Cr3+,Mn2+, J. Lumin., 2024, 269, 120514 CrossRef CAS.
- F. Fang, Y. Jin, W. Hu, Y. Chen, Y. Wei, Z. Zhang, C. Wang, F. Meng, L. Cao and F. Huang, Optical Information Transmission and Multimode Fluorescence Anticounterfeiting of Ca2−xMgxGe7O16:Mn2+, Inorg. Chem., 2024, 63(15), 6938–6947 CrossRef CAS PubMed.
- L. Dong, L. Zhang, Y. Jia, B. Shao, W. Lü, S. Zhao and H. You, Enhancing Luminescence and Controlling the Mn Valence State of Gd3Ga5−x−δAlx−y+δO12:yMn Phosphors by the Design of the Garnet Structure, ACS Appl. Mater. Interfaces, 2020, 12(6), 7334–7344 CrossRef CAS PubMed.
- L. Dong, L. Zhang, Y. Jia, B. Shao, W. Lü, S. Zhao and H. You, Site occupation and luminescence of novel orange-red Ca3M2Ge3O12:Mn2+,Mn4+ (M = Al, Ga) phosphors, ACS Sustainable Chem. Eng., 2020, 8(8), 3357–3366 CrossRef CAS.
- G. Doke, G. Krieke, A. Antuzevics, A. Sarakovskis and B. Berzina, Optical properties of red-emitting long afterglow phosphor Mg2Si1−xGexO4:Mn2+/Mn4+, Opt. Mater., 2023, 137, 113500 CrossRef CAS.
- L. Dong, L. Zhang, Y. Jia, Y. Xu, S. Yin and H. You, ZnGa2–yAlyO4:Mn2+,Mn4+ Thermochromic Phosphors: Valence State Control and Optical Temperature Sensing, Inorg. Chem., 2020, 59(21), 15969–15976 CrossRef CAS PubMed.
- L. Zheng, L. Zhang, L. Fang, H. Wu, H. Wu, G.-H. Pan, Y. Yang, Y. Luo, Z. Hao and J. Zhang, Be2+-Induced Red Emission Enhancement in CaAl12O19:Mn4+ for Wide Color Gamut Display: Suppressing Self-Reduction of Mn4+ to Mn2+, Adv. Opt. Mater., 2024, 12(3), 2301480 CrossRef CAS.
- D. Guo, Z. Wang, N. Wang, B. Zhao, Z. Li, J. Chang, P. Zhao, Y. Wang, X. Ma, P. Li and H. Suo, Doping-mediated thermal control of phase transition for supersensitive ratiometric luminescence thermometry, Chem. Eng. J., 2024, 492, 152312 CrossRef CAS.
- X. Zhang, Y. Xu, X. Wu, S. Yin, C. Zhong, C. Wang, L. Zhou and H. You, Optical thermometry and multi-mode anti-counterfeiting based on Bi3+/Ln3+ and Ln3+ doped Ca2ScSbO6 phosphors, Chem. Eng. J., 2024, 481, 148717 CrossRef CAS.
- W. Liu, D. Zhao, R.-J. Zhang, Q.-X. Yao and S.-Y. Zhu, Fluorescence lifetime-based luminescent thermometry material with lifetime varying over a factor of 50, Inorg. Chem., 2022, 61(41), 16468–16476 CrossRef CAS PubMed.
- L. Marciniak and K. Trejgis, Luminescence lifetime thermometry with Mn3+–Mn4+ co-doped nanocrystals, J. Mater. Chem. C, 2018, 6(26), 7092–7100 RSC.
- E. Song, M. Chen, Z. Chen, Y. Zhou, W. Zhou, H.-T. Sun, X. Yang, J. Gan, S. Ye and Q. Zhang, Mn2+-activated dual-wavelength emitting materials toward wearable optical fibre temperature sensor, Nat. Commun., 2022, 13(1), 2166 CrossRef CAS PubMed.
- M. Szymczak, W. Piotrowski, P. Woźny, M. Runowski and L. Marciniak, A highly sensitive lifetime-based luminescent manometer and bi-functional pressure–temperature sensor based on a spectral shift of the R-line of Mn4+ in K2Ge4O9, J. Mater. Chem. C, 2024, 12(19), 6793–6804 RSC.
- H. Zhang, J. Yao, K. Zhou, Y. Yang and H. Fu, Thermally activated charge transfer in dual-emission Mn2+-alloyed perovskite quantum wells for luminescent thermometers, Chem. Mater., 2022, 34(4), 1854–1861 CrossRef CAS.
- K. Li, Z. Zhang, D. Zhu and C. Yue, Excellent temperature sensitivities based on the FIR technique of up-conversion luminescence in a novel NaLaTi2O6:Yb3+,Tm3+ material, Inorg. Chem. Front., 2024, 11, 7464–7474 RSC.
- Y. Chen, J. He, X. Zhang, M. Rong, Z. Xia, J. Wang and Z.-Q. Liu, Dual-mode optical thermometry design in Lu3Al5O12:Ce3+/Mn4+ phosphor, Inorg. Chem., 2020, 59(2), 1383–1392 CrossRef CAS PubMed.
- F. Jahanbazi and Y. Mao, Recent advances on metal oxide-based luminescence thermometry, J. Mater. Chem. C, 2021, 9(46), 16410–16439 RSC.
- W. Piotrowski, K. Trejgis, K. Maciejewska, K. Ledwa, B. Fond and L. Marciniak, Thermochromic luminescent nanomaterials based on Mn4+/Tb3+ codoping for temperature imaging with digital cameras, ACS Appl. Mater. Interfaces, 2020, 12(39), 44039–44048 CrossRef CAS PubMed.
- Y. Xiao, P. Xiong, S. Zhang, Y. Sun, N. Yan, Z. Wang, Q. Chen, P. Shao, M. G. Brik and S. Ye, Cation-defect-induced self-reduction towards efficient mechanoluminescence in Mn2+-activated perovskites, Mater. Horiz., 2023, 10(9), 3476–3487 RSC.
- Y. Chen, J. Chen, J. Liang, J. He, Z.-Q. Liu and Y. Yin, Localized Charge Accumulation Driven by Li+ Incorporation for Efficient LED Phosphors with Tunable Photoluminescence, Chem. Mater., 2020, 32(22), 9551–9559 CrossRef CAS.
- M. Zhang, A. Sun, X. Li, S. Sun, W. Ding, D. Fang, B. Mi and Z. Q. Gao, Unveiling luminescence property of Li2MgGeO4:Mn4+ featuring tetrahedral crystallographic-site occupancy of Mn4+, Dalton Trans., 2024, 53, 10168–10177 RSC.
- P. Zhang, Z. Zheng, L. Wu, Y. Kong, Y. Zhang and J. Xu, Self-reduction-related defects, long afterglow, and mechanoluminescence in centrosymmetric Li2ZnGeO4:Mn2+, Inorg. Chem., 2021, 60(23), 18432–18441 CrossRef CAS PubMed.
- F. Selim, C. Varney, M. Tarun, M. Rowe, G. Collins and M. McCluskey, Positron lifetime measurements of hydrogen passivation of cation vacancies in yttrium aluminum oxide garnets, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88(17), 174102 CrossRef.
- A. Marinopoulos, First-principles study of the formation energies and positron lifetimes of vacancies in the Yttrium-Aluminum Garnet Y3Al5O12, Eur. Phys. J. B, 2019, 92, 1–9 CrossRef.
- D. Chen, Y. Zhou, W. Xu, J. Zhong, Z. Ji and W. Xiang, Enhanced luminescence of Mn4+:Y3Al5O12 red phosphor via impurity doping, J. Mater. Chem. C, 2016, 4(8), 1704–1712 RSC.
- T. Senden, R. J. A. van Dijk-Moes and A. Meijerink, Quenching of the red Mn4+ luminescence in Mn4+-doped fluoride LED phosphors, Light: Sci. Appl., 2018, 7(1), 8 CrossRef PubMed.
- A. J. van Bunningen, A. D. Sontakke, R. van der Vliet, V. G. Spit and A. Meijerink, Luminescence Temperature Quenching in Mn2+ Phosphors, Adv. Opt. Mater., 2023, 11(6), 2202794 CrossRef CAS.
- H. Shibata, Negative Thermal Quenching Curves in Photoluminescence of Solids, Jpn. J. Appl. Phys., 1998, 37(2R), 550 CrossRef CAS.
- Y. Wang, D. Włodarczyk, M. G. Brik, J. Barzowska, A. Shekhovtsov, K. Belikov, W. Paszkowicz, L. Li, X. Zhou and A. Suchocki, Effect of temperature and high pressure on luminescence properties of Mn3+ ions in Ca3Ga2Ge3O12 single crystals, J. Phys. Chem. C, 2021, 125(9), 5146–5157 CrossRef CAS.
- Z. Cheng, J. Huang, G. Lu, Y. Wu, M. Avdeev, T. Yang and P. Jiang, Mg4InSbO8 Spinel: Double 2:1 Ordering in Tetrahedral and Octahedral Sublattices and Perspective Application as an Optical Thermometer via Mn2+/4+ Doping, Chem. Mater., 2023, 35(22), 9669–9681 CrossRef CAS.
- Q. Zhang, G. Li, G. Li, D. Liu, P. Dang, L. Qiu, H. Lian, M. S. Molokeev and J. Lin, Optical Thermometer Based on Efficient Near-Infrared Dual-Emission of Cr3+ and Ni2+ in Magnetoplumbite Structure, Adv. Opt. Mater., 2024, 12(1), 2301429 CrossRef CAS.
- Z. Chen, S. Du, K. Zhu, Z. Tian, J. Zhang, F. Li, S. Zhang, S. Zhao, W. Cui and X. Yuan, Mn4+-activated double-perovskite-type Sr2LuNbO6 multifunctional phosphor for optical probing and lighting, ACS Appl. Mater. Interfaces, 2023, 15(23), 28193–28203 CrossRef CAS PubMed.
- L. Li, G. Tian, Y. Deng, Y. Wang, Z. Cao, F. Ling, Y. Li, S. Jiang, G. Xiang and X. Zhou, Constructing ultra-sensitive dual-mode optical thermometers: Utilizing FIR of Mn4+/Eu3+ and lifetime of Mn4+ based on double perovskite tellurite phosphor, Opt. Express, 2020, 28(22), 33747–33757 CrossRef CAS PubMed.
- M. Sekulić, V. Đorđević, Z. Ristić, M. Medić and M. D. Dramićanin, Highly sensitive dual self-referencing temperature readout from the Mn4+/Ho3+ binary luminescence thermometry probe, Adv. Opt. Mater., 2018, 6(17), 1800552 CrossRef.
- L. F. dos Santos, J. A. Galindo, K. D. O. Lima, A. R. Pessoa, A. M. Amaral, L. D. S. Menezes and R. R. Gonçalves, Lifetime-based luminescence thermometry from Yb3+/Er3+ codoped nanoparticles suspended in water, J. Lumin., 2023, 262, 119946 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc03650f |
| This journal is © The Royal Society of Chemistry 2024 |