Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The effects of lead, copper, and iron corrosion products on antibiotic resistant bacteria and antibiotic resistance genes†
Veronika
Folvarska
 a,
San Marie
Thomson
a,
Zihao
Lu
a,
San Marie
Thomson
a,
Zihao
Lu
 a,
Maya
Adelgren
a,
Adam
Schmidt
b,
Ryan J.
Newton
a,
Maya
Adelgren
a,
Adam
Schmidt
b,
Ryan J.
Newton
 c,
Yin
Wang
c,
Yin
Wang
 b and
Patrick J.
McNamara
b and
Patrick J.
McNamara
 *a
*a
aDept. of Civil, Construction, Environmental Engineering, Marquette University, USA. E-mail: patrick.mcnamara@marquette.edu
bDepartment of Civil and Environmental Engineering, University of Wisconsin-Milwaukee, Milwaukee, WI 53201, USA
cSchool of Freshwater Sciences, University of Wisconsin-Milwaukee, Milwaukee, Wisconsin, USA
First published on 24th April 2024
Abstract
Antibiotic resistance is a public health crisis. Antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARGs) are present in drinking water distribution systems. Metals are known selective pressures for antibiotic resistance, and metallic corrosion products are found within drinking water distribution systems due to the corrosion of metal pipes. While corrosion products are a source of metals, the impact of specific corrosion products on antibiotic resistance has not been investigated. The objective of this study was to determine the impact of six corrosion products—CuO, Cu2O, Pb5(PO4)3OH, β-PbO2, Fe3O4, and α-FeOOH—on the abundance of ARB and ARGs. Lab-scale microcosms were seeded with source water from Lake Michigan and amended with individual corrosion products. In general, copper and lead corrosion products increased antibiotic resistance, although not universally across different ARB and ARG types. Concentration and speciation of copper and lead corrosion products were found to have an impact on antibiotic resistance profiles. Meanwhile, iron corrosion products had minimal impact on antibiotic resistance. Overall, this study sheds light on how pipe materials may impact antibiotic resistance as a result of corrosion products.
Environmental significanceDrinking water distribution pipes are often made out of metals including iron, copper, and lead. Corrosion of these metal pipes generates corrosion products that are released into the water. Our study found that both copper and lead corrosion products increased abundance of antibiotic resistant bacteria and antibiotic resistance genes, while iron corrosion products had minimal impact. Many different considerations go into selecting pipe material, such as durability, availability, cost, etc., and this research highlights that pipe material also impacts antibiotic resistance. |
1. Introduction
According to the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO), antibiotic resistance is a growing global public health crisis.1,2 Antibiotic resistance has a wide range of negative impacts at the patient, healthcare, and societal levels.3 For instance, ineffective antibiotics bring greater risk to modern medical practices, such as C-sections, transplants, and chemotherapy, all of which rely on antibiotics to manage infections.4,5 Treating infections caused by antibiotic resistant bacteria (ARB) also adds significant monetary cost to health care systems. In 2014, antibiotic resistance added $1383 per patient to the cost of treating a bacterial infection in the United States.6 On a global scale, the economic impact of drug-resistant infections is expected to be greater than $1 trillion in output losses annually after 2030.7 Deaths from antibiotic resistant infections also remain high. In 2019, 1.27 million deaths globally were attributed directly to drug-resistant infections.8 In order to combat antibiotic resistance, it is necessary to identify factors that trigger resistance to antibiotics and reduce pressures created by human activities that select for antibiotic resistance.9–11A variety of environmental factors act as selective pressures for antibiotic resistance.12 Metals can exert a selective pressure for antibiotic resistance. For example, metal and antibiotic resistance conferring genes can be located on the same genetic element.13 Additionally, there are cross-resistance mechanisms, where one biochemical system provides resistance to both toxicants; and co-regulatory resistance, where a linkage between regulatory systems allows one toxicant to trigger the removal of another toxicant.13 As a result of these relationships, antibiotic resistance genes (ARGs) have been found to co-occur with metal resistance genes (MRGs) in a variety of environments.14,15 The drinking water distribution system (DWDS) is one such environment.16,17 Metals are prominent in the DWDS and enter the system through source water, corrosion inhibitors, and the corrosion of metal pipes.18 The metals then have ample opportunity to interact with microorganisms, which are seeded from source water and water treatment processes and develop biofilms that line the vast interior surfaces of the DWDS.19
Corrosion is the deterioration of metal surfaces through electrochemical cell formation.20 Corrosion is a commonly occurring process within metal drinking water pipes as all parts required to complete the formation of the electrochemical cell are present. As part of the corrosion process, corrosion products, which are reprecipitates of metal ions released from corrosion, develop on the surface of pipes and can be released into the drinking water.21 Lead, copper, and iron pipes are often used in DWDSs. Common corrosion products include cuprous oxide (Cu2O) and cupric oxide (CuO) from copper pipes;22 goethite (α-FeOOH), lepidocrocite (γ-FeOOH), and magnetite (Fe3O4) from iron pipes;23 and lead dioxides (e.g., plattnerite (β-PbO2)), lead(II) carbonates (e.g., hydrocerussite (Pb3(CO3)2(OH)2)), and lead(II) phosphates (e.g., hydroxylpyromorphite (Pb5(PO4)3OH)) from lead pipes.24,25 These corrosion products have all been found within DWDSs. It is possible corrosion products can be an inducer for antibiotic resistance due to their metal contents. Although research regarding the impact of DWDS metal sources on antibiotic resistance has been done, such as evaluating the impacts of corrosion inhibitors,26,27 no specific research regarding the impacts of corrosion products on antibiotic resistance has been conducted.
The main goal of this study was to elucidate the impacts of common DWDS corrosion products on antibiotic resistance. This study's novelty lies in its examination of the relationship between corrosion products and antibiotic resistance, a connection unexplored in previous research. The specific objective was to determine how representative metal corrosion products that include plattnerite (β-PbO2), hydroxylpyromorphite (Pb5(PO4)3OH), cuprous oxide (Cu2O), cupric oxide (CuO), magnetite (Fe3O4), and goethite (α-FeOOH) impacted the abundance of ARB and ARGs. This research was accomplished through lab-scale microcosms in which water from a drinking water source was exposed to corrosion products. Subsequent heterotrophic plate count (HPC) and quantitative polymerase chain reaction (qPCR) methods were utilized to investigate changes in resistance. Knowing the impacts of different corrosion products will shed light on how pipe material can impact antibiotic resistance in DWDSs, which can be used to inform decisions on pipe material choice in future installations.
2. Methods
2.1 Microcosm set-up
Lab-scale microcosms were used to determine the impact of six different corrosion products on antibiotic resistance (Fig. 1). Specifically, twelve different experimental conditions were tested by varying corrosion products and concentrations (Table 1). More specifically, two lead, two copper, and two iron corrosion products were tested. Each corrosion product was tested individually at 5 mg L−1 and 50 mg L−1 added. The typical amount of corrosion products released from pipe walls is difficult to establish as it can vary based upon the changes in water chemistry, hydraulic conditions, and pipe age.28 The concentrations used for this study were chosen based on previous toxicity studies which indicate that 5 mg L−1 of metals can cause oxidative stress and affect bacterial growth.29,30 An additional three experimental conditions were tested to investigate the impacts of dissolved metal species. All conditions were tested in triplicate.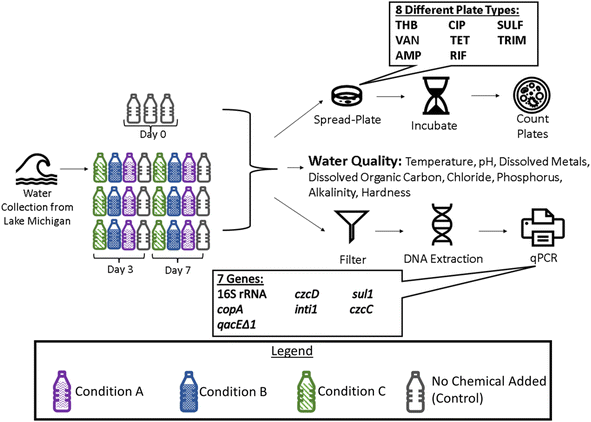 | ||
| Fig. 1 Overview of microcosm experimental set-up. Water collected from Lake Michigan was distributed amongst autoclaved bottles. Three experimental conditions along with one control condition were tested in triplicate in each experimental set. Conditions tested in each set are specified in Table 1. Bottles were sampled for water quality, bacterial plating, and qPCR analysis. | ||
| Set | Start date | Condition tested | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 1 | 02/01/2022 | 5 mg per L Pb5(PO4)3OH | 5 mg per L β-PbO2 | 5 mg per L α-FeOOH | Control |
| 2 | 05/31/2022 | 5 mg per L CuO | 5 mg per L Cu2O | 5 mg per L Fe3O4 | Control |
| 3 | 03/29/2022 | 50 mg per L Pb5(PO4)3OH | 50 mg per L β-PbO2 | 50 mg per L α-FeOOH | Control |
| 4 | 06/14/2022 | 50 mg per L CuO | 50 mg per L Cu2O | 50 mg per L Fe3O4 | Control |
| 5 | 08/16/2022 | 50 mg per L PbCl2 | 50 mg per L CuCl2 | 50 mg per L FeCl2 | Control |
On day 0 of each set, water was collected from Lake Michigan at Atwater Beach, Milwaukee, WI, USA in a disinfected 40 L carboy, as described previously.27 Water was brought back to the lab and transferred into autoclaved 1 L bottles. Six bottles were operated for each condition: three of the bottles were sampled on day 3 and three of the bottles were sampled on day 7 for each condition tested. Impacts of corrosion products on ARB and ARGs were monitored on both day 3 and day 7 to gain an understanding of how fast- and slow-growing bacteria may be affected, respectively. Corrosion products were added to bottles at concentrations listed in Table 1. Set 5 of the microcosms, also shown in Table 1, was designed to test dissolved metal conditions and serve as a comparison point to the corrosion products. The chemical compounds used for the dissolved metal conditions were considered appropriate as long as they were more soluble in water than the corrosion products and contained the same metals as the corrosion products (Cu, Pb, and Fe). It is noted that, although 50 mg L−1 of the metal compounds were added, the actual dissolved metal ion concentration is likely less due to precipitation of the metals once in contact with the water matrix (ESI Table 3†). Controls, also in triplicate, were run for each set. Control bottles consisted solely of source water and were used to monitor changes in antibiotic resistance with no metals added (no added corrosion product). All bottles were mixed at 150 rpm on an orbital shaker at room temperature (20 ± 2.0 °C) and covered with caps.
2.2 Water quality
Water quality measurements were taken on days 0, 3, and 7 as specified in Table 2. Initial water quality measurements of source water from Lake Michigan are presented in ESI Table 1.† The corrosion products tested are fairly insoluble, however the exact solubility of any corrosion product varies depending on water quality parameters, such as pH, natural organic matter, carbonate concentration, etc.31–34 Dissolved metals released from the corrosion products are biologically available to the microbes and were measured. However, due to the heterogeneous nature of corrosion products and their solubility, the focus of this research was to first determine if the added corrosion products impacted antibiotic resistance. Note, dissolved (soluble) metal concentrations of samples were measured by initially passing the samples through 0.45 μm filters as defined by Standard Method 3030 B.35 This operational definition of dissolved may vary from the conceptual definition of dissolved, a solute surrounded by water molecules, if particulates smaller than 0.45 μm pass through the filter.| Measurement | Day taken | Method used |
|---|---|---|
| a Chloride was tested only on day 0 for the first four microcosms, but tested on day 0, 3, and 7 for the fifth microcosm because the metals added were chloride-based. | ||
| pH | 0, 3, 7 | Thermo Fisher Scientific pH probe |
| Phosphate | 0, 3, 7 | Ascorbic acid method (4500 P)35 |
| Dissolved metal concentrations | 0, 3, 7 | Agilent 7700s inductively coupled plasma mass spectrometer (ICP-MS) with acid digestion |
| Dissolved organic carbon (DOC) | 0, 3, 7 | Shimadzu TOC analyzer (Shimadzu, Kyoto, Japan) |
| Temperature | 0, 3, 7 | Thermometer |
| Alkalinity | 0 | Hach kit (no. 24443, concentration range 5–400 mg L−1) |
| Hardness | 0 | Hach kit (no. 1457–01, concentration range 20–400 mg L−1) |
| Ammonia | 0 | Hach kit (no. 2241–00, concentration range 0–3 mg L−1) |
| Chloride | 0a | Hach kit (no. 144001, concentration range 0–400 mg L−1) |
2.3 Corrosion product preparation
Cuprous oxide (Cu2O), cupric oxide (CuO), and magnetite (Fe3O4) were obtained from Sigma-Aldrich. Goethite (α-FeOOH), plattnerite (β-PbO2), and hydroxylpyromorphite (Pb5(PO4)3OH) were synthesized based on modification of reported approaches.31,36,37 The crystalline phases of all corrosion products were confirmed using X-ray diffraction (XRD) performed on a Bruker D8 Discover A25 diffractometer (ESI Fig. 1†).Preparation of goethite was performed through a temperature-controlled nucleation process under alkaline condition.37 In a typical synthesis, approximately 4.85 g of Fe(NO3)3·9H2O was added to 50 mL of water and precipitated by dropwise addition of a 4.8 M KOH solution. The solution was stirred vigorously until the pH reached 12. The suspension was sonicated for 30 minutes at room temperature, followed by oven drying at 100 °C for 70 minutes. The solids were then collected, washed, air dried, and preserved for use.
Hydroxylpyromorphite was prepared through a homogeneous precipitation method at pH 7.5 with the use of lead(II) acetate (Pb(CH3COO)2) and ammonium dihydrogen phosphate (NH4H2PO4) as precursors.36 250 mL of a 4.4 M CH3COONH4 buffer solution was mixed with 250 mL of a 0.4 M Pb(CH3COO)2 solution under stirring, followed by the rapid addition of 500 mL of a 0.12 M NH4H2PO4 solution under mixing. The resulting suspension was adjusted to pH 7.5, stirred for 10 minutes at ambient temperature, and then aged for 48 hours at 100 °C. The obtained precipitates were then collected, washed, air dried, and preserved for use.
Plattnerite was synthesized through homogeneous oxidation of dissolved Pb(II) with free chlorine under alkaline condition.31 Briefly, an aliquot of a NaOCl solution (5 wt%) was added to a 1 L polypropylene reactor consisting of 5 mM Pb(NO3)2 under rapid mixing to reach a free chlorine concentration of 800 mg L−1 as Cl2. The reactor was covered with aluminum foil to prevent photo-induced decay of free chlorine. The solution was stirred, and pH was adjusted and maintained at 11 for 48 hours. The solids were then collected, washed, air dried, and preserved for use.
2.4 Quantification of total heterotrophic bacteria and ARB
Samples were plated from bottles on days 0, 3, and 7 using the spread plate method. R2A served as the base medium to which seven different antibiotics were added individually as previously described.26,27,38 Plates were prepared under aseptic conditions for the following antibiotics: ampicillin (AMP) at 32 μg mL−1, sulfamethoxazole (SULF) at 100 μg mL−1, rifampicin (RIF) at 4 μg mL−1, ciprofloxacin (CIP) at 4 μg mL−1, trimethoprim (TRIM) at 16 μg mL−1, tetracycline (TET) at 16 μg mL−1, and vancomycin (VAN) at 32 μg mL−1. These concentrations are based on diagnostic concentrations inferring clinical resistance from the Clinical and Laboratory Standards Institute (CLSI) dilution method.39 All plates had a 100 μg mL−1 of cycloheximide addition for fungal growth prevention. R2A plates with no antibiotic addition were also used to enumerate total heterotrophic bacteria (THB). Once samples were plated, the plates were stored in an incubator at 30 °C following growth plate protocol as done in previous studies.26,27,38 Colony forming units (CFUs) were manually counted after five days of incubation, as recommended for low nutrient (R2A) media40 and done in previous studies.26,39 Any CFUs observed on antibiotic plates after incubation were assumed to be antibiotic resistant bacteria. Since ARB were counted on day 3 and day 7 for seven different antibiotic plate types, there were fourteen possible scenarios where the concentration of ARB could have changed due to the addition of a corrosion product at a given concentration in our experimental setup. Raw ARB data are presented in ESI Table 4.†2.5 Quantification of metal and antibiotic resistant genes
Water samples were filtered through 0.22 μm filters using aseptic techniques, on days 0, 3, and 7. The volume of water filtered varied between 290–500 mL to obtain the highest possible volume without breaking the filter in order to maximize DNA collected. The filters were put in individual, sterile 2 mL tubes and stored at −20 °C until DNA extraction. For DNA extraction, each filter was crushed using a sterile pipette tip, and underwent three liquid nitrogen freeze/thaw cycles to achieve cell lysis. This minor modification to the DNA extraction protocol was used previously to aid in cell lysis.41 DNA extraction was then completed using the FastDNA® SPIN Kit (MP Biomedicals, Solon, OH) following the manufacturers protocol. qPCR was run on the extracted DNA samples from corrosion product sets to quantify the following genes: 16S rRNA, intI1, sul1, copA, czcC, czcD, and qacEΔ1. These genes were chosen for quantification after they were found to be in high abundance in a gene screening assay. ESI Table 2† provides more information regarding primers for each gene, qPCR reactions, preparation of the standard curves, and confirmation of amplicon identity via melt curve analysis. qPCR was run with technical duplicates on each sample pulled from the triplicate reactors with technical duplicates averaged for statistical analysis. All qPCR was run on a Light Cycler 96 (Roche Molecular Systems Inc., USA). qPCR gene count data for each sample is presented in ESI Table 5.†2.6 Statistical analysis
Two-way ANOVA analysis along with Tukey's honest significant differences test for post hoc multiple comparison was done using GraphPad Prism 7® v9.3.1 (GraphPad Software, La Jolla, CA) to statistically evaluate results. For THB and ARB, two categorical variables, corrosion product and antibiotic type, were compared against colony forming unit concentration. For gene analysis, two categorical variables, corrosion product and gene type, were compared against gene concentration. P-values < 0.05 were considered to be statistically significant.3. Results and discussion
3.1 Copper corrosion products generally increased antibiotic resistance
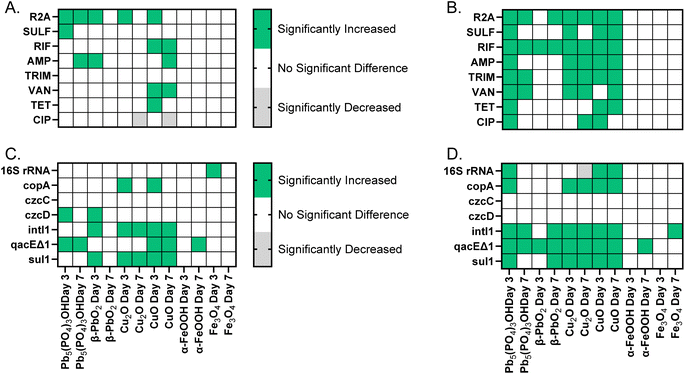
The addition of copper corrosion products to Lake Michigan water increased the absolute abundance of ARB on day 3 and day 7 (Fig. 2). Also, a higher corrosion product concentration increased ARB concentration more than a lower corrosion product concentration (Fig. 2). More specifically, CuO addition resulted in 6 scenarios of increased ARB at 5 mg L−1 and 12 scenarios of increased ARB at 50 mg L−1. Cu2O addition resulted in no scenarios of increased ARB at 5 mg L−1 and 10 scenarios of increased ARB at 50 mg L−1. Differences in the number of scenarios with increased ARB, as well as the types of ARB that increased, between the different types of copper corrosion products tested indicate that speciation of the corrosion products also impacts antibiotic resistance.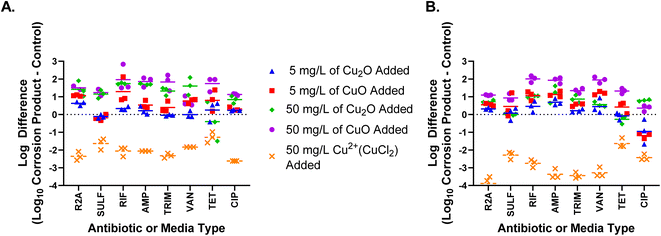 | ||
| Fig. 2 Log difference in the absolute abundance of bacteria, measured as colony forming units after a 5 day incubation on R2A or R2A supplemented with an antibiotic vs. a no addition control on (A) day 3 and (B) day 7. Copper corrosion product type and concentration added on day 0 to each microcosm are indicated by the color–symbol combination. Grow out condition abbreviations for added antibiotics are: R2A = total heterotrophic bacteria (no antibiotic), SULF = sulfamethoxazole, RIF = rifampicin, AMP = ampicillin, TRIM = trimethoprim, VAN = vancomycin, TET = tetracycline, and CIP = ciprofloxacin. For all graphs, points above the dashed line indicate more growth than in the control, while points below dashed line indicate growth was less than in the control. The statistical significance of each of the corrosion product scenarios shown is indicated in Fig. 5. | ||
Although the addition of copper corrosion products increased ARB, this addition also increased the absolute abundance of THB (p-values < 0.05; Fig. 2). The increase in THB suggests that the increased absolute abundance of ARB was due to the promotion of overall bacterial growth, most likely caused by the increased presence of a limiting nutrient. Although copper is commonly known as an antimicrobial that decreases bacterial growth, copper toxicity varies based on multiple factors including bacterial tolerance and copper ion concentrations.42,43 Copper, in trace amounts, assists in biochemical transformations as a cofactor for enzymes and is therefore necessary for bacterial growth.44,45 In this case, it is likely that the addition of copper corrosion products increased ARB abundance by increasing the amount of bioavailable copper within the systems. When increases in THB were taken into account, there were far fewer scenarios that resulted in a significant relative abundance change in ARB following the addition of a corrosion product. Increased relative abundance of ARB was observed for only three scenarios, 50 mg L−1 of CuO on day 7 for RIF, AMP, and VAN (p-values < 0.05) as shown in ESI Fig. 2.† It appears the effect of copper on ARB abundance is multi-faceted. With the compounds and concentrations examined, copper was more likely to increase resistance through bacterial growth stimulation than selection for resistant phenotypes, but both scenarios occurred at the high concentration. Further work is needed to determine if a corrosion product concentration threshold must be reached for resistance selection.
While copper corrosion products at both concentrations tested increased absolute abundance of ARB, the dissolved copper condition resulted in decreased THB and ARB concentrations. The addition of 50 mg L−1 of CuCl2 significantly decreased the absolute abundance of THB and all types of ARB on both days 3 and 7 (p-values < 0.05). Cu2+ has been found to inhibit bacterial growth at levels ranging from 0.635 μg L−1 to 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 546 μg L−1 depending on copper sensitivity of bacteria.43 It is likely that the dissolved copper condition resulted in ARB and THB death because it increased copper ion concentrations to toxic levels. At 50 mg L−1, CuO and Cu2O resulted in dissolved copper concentrations on average of 1427 μg L−1 and 607 μg L−1 respectively, while CuCl2 resulted in higher dissolved copper concentrations, on average of 5331 μg L−1. As mentioned in the methods, dissolved copper concentrations were measured using the operational definition of passing the sample through a 0.45 μm filter prior to ICP analysis. Variability in dissolved copper concentrations amongst triplicates, particularly from corrosion product bottles (see ESI Table 3†), indicate small corrosion product particles passed through the filters and resulted in the unexpectedly high dissolved metal concentrations measured under some conditions. Thus, although dissolved metal concentrations were operationally defined, these values are not an accurate representation of theoretical dissolved metal concentration. Further research should investigate whether concentrations of dissolved metal ions released from metal corrosion products, rather than the corrosion products themselves, directly correspond to shifts in antibiotic resistance due to dissolved metal bioavailability. Further work should also explore whether potential biofilm formation on the non-dissolved corrosion products contribute to any changes in antibiotic resistance. Additionally, more work is needed to determine whether copper corrosion products increase antibiotic resistance even if copper ion concentrations released are below regulated levels, such as those set by the US EPA Lead and Copper Rule (LCR).
546 μg L−1 depending on copper sensitivity of bacteria.43 It is likely that the dissolved copper condition resulted in ARB and THB death because it increased copper ion concentrations to toxic levels. At 50 mg L−1, CuO and Cu2O resulted in dissolved copper concentrations on average of 1427 μg L−1 and 607 μg L−1 respectively, while CuCl2 resulted in higher dissolved copper concentrations, on average of 5331 μg L−1. As mentioned in the methods, dissolved copper concentrations were measured using the operational definition of passing the sample through a 0.45 μm filter prior to ICP analysis. Variability in dissolved copper concentrations amongst triplicates, particularly from corrosion product bottles (see ESI Table 3†), indicate small corrosion product particles passed through the filters and resulted in the unexpectedly high dissolved metal concentrations measured under some conditions. Thus, although dissolved metal concentrations were operationally defined, these values are not an accurate representation of theoretical dissolved metal concentration. Further research should investigate whether concentrations of dissolved metal ions released from metal corrosion products, rather than the corrosion products themselves, directly correspond to shifts in antibiotic resistance due to dissolved metal bioavailability. Further work should also explore whether potential biofilm formation on the non-dissolved corrosion products contribute to any changes in antibiotic resistance. Additionally, more work is needed to determine whether copper corrosion products increase antibiotic resistance even if copper ion concentrations released are below regulated levels, such as those set by the US EPA Lead and Copper Rule (LCR).
Although THB and ARB concentration decreased after CuCl2 addition, ARB selection did occur. On day 3, the relative abundance of bacteria resistant to SULF and TET was higher with the CuCl2 addition than with the control. By day 7, this additionally became true for bacteria resistant to RIF and CIP. So, although CuCl2 addition was toxic, the ARB survived better than the THB as a whole.
qPCR results, shown in Fig. 3, indicate that the addition of 5 mg L−1 of either copper corrosion product did not alter the bacterial abundance as a whole. The absolute abundance of the 16S rRNA gene did not significantly change on either day 3 or day 7 compared to the control group at 5 mg L−1 for either CuO or Cu2O indicating the total number of bacteria within the system was relatively unchanged. It is important to note that the lack of change in 16S rRNA gene abundance does not mean that the bacteria community structure was unchanged. Future work is needed to elucidate the impact of corrosion products on microbial community structure as some microorganisms are more tolerant to metals than others. Moreover, the absolute abundances of three efflux pumps associated with metal resistance (copA, czcC, and czcD) were not significantly different compared to the control group, which also indicates that copper corrosion products did not result in relative increases in antibiotic resistance bacteria at 5 mg L−1.
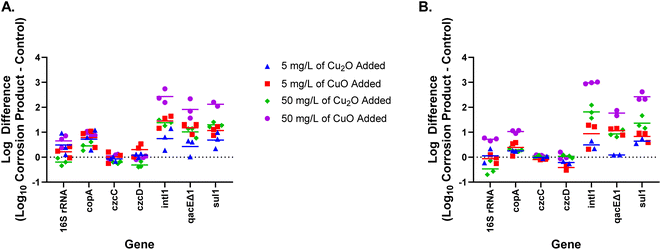 | ||
| Fig. 3 Log difference in gene counts, measured with qPCR, between corrosion product conditions vs. the no addition control on (A) day 3 and (B) day 7. Copper corrosion product type and concentration added on day 0 to each microcosm are indicated by the color–symbol combination. Genes quantified: 16S rRNA = total bacteria, copA, czcC, and czcD = metal resistances, intI1 = integron-integrase known to carry resistances, qacEΔ1 = antiseptic resistance, and sul1 = antibiotic, sulfonamide resistance. For all graphs, points above the dashed line indicate more gene abundance than in the control, while points below dashed line indicate gene abundance was less than in the control. The statistical significance of each of the scenarios shown is indicated in Fig. 5. | ||
Despite no significant change in bacterial abundance in the copper corrosion product microcosms, low concentrations of copper corrosion products did promote the increase of some ARGs. CuO significantly increased the absolute abundance and relative abundance of sulfonamide resistance gene (sul1), efflux pump (qacEΔ1) and class I integron (intI1). Previous studies proposed that the qacEΔ1 gene (the attenuated variant of qacE), and sul1, were frequently present in the 3′ conserved segment of most class I integrons.49,50 Thus, all three functional genes may be co-transmitted if the microorganisms are exposed to a selective stress. There are two potential pathways that may allow CuO to promote the development of antibiotic resistance at low concentrations. On one hand, sub-inhibitory concentration (0.01 mg L−1) of cupric ion (Cu2+) can increase conjugative transfer of ARGs via an increase in cell membrane permeability and altered expression of conjugation regulatory genes (korA, korB, and trbA).51,52 On the other hand, the selective stress from CuO at non-toxic concentrations may be related to the copper acquisition and utilization by microorganisms. A higher number of copper importers, increased permeability of porins, or a stronger ability to reduce Cu2+ can provide advantages to bacteria by increasing their rate of copper acquisition and utilization when copper is limited in a system.45–48 The microorganisms with these advantageous may also carry intI1, qacEΔ1, and sul1, leading these genes to be indirectly amplified. In other words, intI1, qacEΔ1, and sul1 could have increased because the bacteria carrying these genes had greater copper acquisition and utilization capabilities allowing them to become more dominant, rather than the genes themselves being expressed and providing the advantage. More research is needed on the co-occurrence and potential relationship between ARGs, mobile genetic elements, and metal utilization-related genes. In contrast, the effect of Cu2O on ARGs was smaller compared to CuO at 5 mg L−1. Cu2O had almost no effect on ARGs at 5 mg L−1 (Fig. 3).
At the higher concentration of 50 mg L−1, qPCR results indicate that CuO influences antibiotic resistance by stimulating bacterial growth, including growth of some antibiotic resistant bacteria, while Cu2O both decreases the total number of bacteria and provides a selective pressure for the maintenance of resistance. In the CuO treatment, the absolute abundance of copper resistance gene (copA), ARGs (sul1 and qacEΔ1), and intI1 increased significantly with the increase in total bacteria. The relative abundance of ARGs (sul1 and qacEΔ1) and intI1 were likely further increased under the selection pressure associated with copper utilization, similar to what occurred with CuO at 5 mg L−1. The addition of 50 mg L−1 of Cu2O, however, decreased the total bacteria by day 7 and resulted in a significant increase in the relative abundance of all ARGs and MRGs tested by day 7. Previous studies also showed that Cu2O was more effective in contact killing than CuO.53 Overall, at 50 mg L−1 the mechanisms by which CuO and Cu2O result in increased antibiotic resistance seem to be different as CuO increases resistance concurrently with total bacterial abundance, but Cu2O increases resistance while decreasing bacterial abundance. Different microbes within these systems likely have different tolerance levels to metals. This study focused on the impacts of the corrosion products on the bacterial community as a whole, but future research should consider impacts on specific microbes through microbial community analysis.
In summary, CuO and Cu2O microcosm additions resulted in greater antibiotic resistance. Copper corrosion products generally increased the absolute abundance of ARB and ARGs, and 50 mg L−1 of copper corrosion products typically yielded more antibiotic resistance than 5 mg L−1. Differences in antibiotic resistance profiles suggested that the speciation of corrosion products influenced the absolute and relative abundance of ARB and ARGs.
3.2 Lead corrosion products resulted in multiple ARB and ARG increases
Overall, the lead corrosion product concentration added to the microcosms had a significant impact on the ARB concentration. At 5 mg L−1, only 3 experimental scenarios resulted in a significant increase in ARB concentrations. Pb5(PO4)3OH resulted in 2 scenarios of increased ARB, and β-PbO2 resulted in 1 scenario of increased ARB (Fig. 4). At 50 mg L−1, 12 experimental scenarios resulted in a significant change in ARB concentration, and this result was highly dependent on the lead corrosion product added (Fig. 4). Pb5(PO4)3OH resulted in 10 scenarios of increased ARB while β-PbO2 resulted in only 2 scenarios of increased ARB.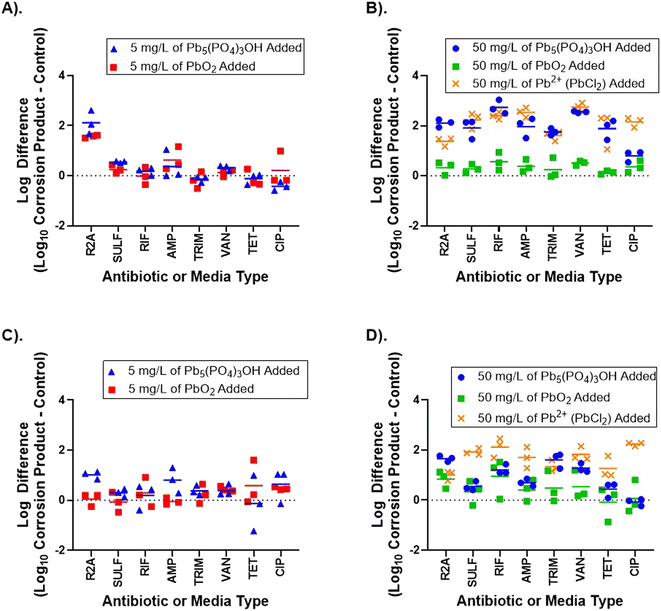 | ||
| Fig. 4 Impact of lead corrosion products on THB and ARB. Log difference in the absolute abundance of bacteria, measured as colony forming units after a 5 day incubation on R2A or R2A supplemented with an antibiotic, for the no addition control vs. (A) 5 mg L−1 corrosion product on day 3 and (C) 5 mg L−1 corrosion product on day 7. Log difference in the absolute abundance of bacteria, measured as colony forming units after a 5 day incubation on R2A or R2A supplemented with antibiotic, for the no addition control vs. (B) 50 mg L−1 corrosion product or dissolved lead on day 3 and (D) 50 mg L−1 corrosion product or dissolved lead on day 7. Lead corrosion product and dissolved lead type and concentration added on day 0 to each microcosm are indicated by the color–symbol combination. Grow out condition abbreviations for added antibiotics are: R2A = total heterotrophic bacteria (no antibiotic), SULF = sulfamethoxazole, RIF = rifampicin, AMP = ampicillin, TRIM = trimethoprim, VAN = vancomycin, TET = tetracycline, and CIP = ciprofloxacin. For all graphs, points above the dashed line indicate more ARB growth than in the control, while points below dashed line indicate ARB growth was less than in the control. The statistical significance of each of the corrosion product scenarios shown is indicated in Fig. 5. | ||
These results also indicate dissolved lead (e.g., Pb2+) is a contributor to increased ARB absolute abundance (Fig. 4). The addition of 50 mg L−1 PbCl2 resulted in significantly increased absolute abundance of bacteria resistant to all antibiotics tested on both day 3 and 7 (p-values < 0.05). Additionally, the PbCl2 addition significantly increased THB relative to the control on both days 3 and day 7 (p-values < 0.05). The increases in THB and ARB absolute abundance following various Pb additions was surprising as Pb is not typically beneficial to bacteria. Unlike Cu, Pb is not usually considered a trace nutrient and has limited impacts on physiological functions in most bacteria.54 The benefit Pb provides to bacteria may be more indirect as a result of how the community structure changes in response to Pb.
Although lead corrosion products resulted in increased ARB absolute abundance, neither of the two lead corrosion products increased the relative abundance of ARB. In fact, both Pb5(PO4)3OH and β-PbO2 significantly decreased the relative abundance of all ARB types tested on day 3 (p-values < 0.05). By day 7, the lead corrosion products had no significant impacts on ARB relative abundance, with the exception of 5 mg per L Pb5(PO4)3OH increasing the proportion of bacteria resistant to RIF. In contrast, the dissolved lead condition increased the relative abundance in 9 out of 14 possible scenarios (ESI Fig. 3†).
Gene quantification indicated that lead corrosion products increased the absolute and relative abundance of ARGs, but only in a few instances (ESI Fig. 4†). At 5 mg L−1, Pb5(PO4)3OH increased the absolute and relative abundance of qacEΔ1. At 50 mg L−1 both lead corrosion products increased the absolute and relative abundance of qacEΔ1 and intI1. Similar to ARB, the impact of lead corrosion products on ARGs varied depending on lead corrosion product concentration and type.
3.3 Iron corrosion products had minimal impact on antibiotic resistance
The addition of iron corrosion products did not result in increases in the absolute or relative abundance of ARB at either 5 mg L−1 or 50 mg L−1, but the FeCl2 addition increased ARB as compared to the no addition control (see ESI Fig. 5†). Iron corrosion product additions resulted in lower dissolved iron concentrations compared to FeCl2 additions (ESI Table 3†). Because of the low solubility of the iron corrosion products,55 it is likely that the dissolved iron species released by the corrosion products are not high enough to increase ARB under tested conditions or that the dissolved iron released from the corrosion products are converted to insoluble iron oxyhydroxide/hydroxide.Iron corrosion products also generally did not increase the absolute or relative abundance of ARGs at the concentrations tested (ESI Fig. 6†). Only the absolute and relative abundance of the qacEΔ1 gene was increased in the α-FeOOH addition on day 7 at both 5 and 50 mg L−1. Fe3O4 did not increase the relative abundance of any ARGs tested at 5 or 50 mg L−1. Compared to the copper and lead corrosion products tested, the iron corrosion products resulted in the fewest scenarios of increased antibiotic resistance (Fig. 5). As various forms of iron have been used and are still used as water piping materials,56 the finding that iron corrosion products evaluated do not increase ARB or ARG abundance provides favor to the continual use of iron materials in DWDSs.
4. Conclusions
The addition of copper and lead corrosion products to Lake Michigan water, which is a major drinking water source, resulted in more instances of increased ARB and ARG abundance relative to the controls (no corrosion product added) and to the iron corrosion product additions. Although further research is needed to evaluate the risk of corrosion products increasing antibiotic resistance, reducing the development of antibiotic resistance in engineered systems is one avenue for combating the global rise in antibiotic resistance. Thus, the use of copper and lead pipes to carry drinking water should be further evaluated in comparison to other materials for real-world effects on antibiotic resistance. Lead service lines are already being replaced around the United States to minimize lead toxicity to drinking water consumers. In addition to showcasing another reason lead pipes may be harmful, this research indicates that copper pipes, which in some cases are being used to replace lead pipes, may have ramifications that iron pipes do not.Additionally, the corrosion product concentration added and the speciation of the corrosion product impacted the levels of antibiotic resistance over a seven-day period. Typically, higher concentrations of copper and lead corrosion products resulted in greater antibiotic resistance. Moreover, Pb5(PO4)3OH and CuO resulted in more increases in antibiotic resistance profiles than β-PbO2 and Cu2O respectively.
Although this study provided new information regarding the effects of corrosion products on antibiotic resistance, further work is needed to fully understand these effects within drinking water pipes, which are complex and dynamic systems consisting of environmental factors and engineering controls that may act individually or in combination to shape the microbial community. Exploring the impacts of water quality on corrosion products and antibiotic resistance would improve understanding of the water conditions most likely to promote antibiotic resistance in the DWDS. Determining the role of microbial community shifts in changing antibiotic resistance profiles would provide further insight into the impacts of corrosion products in drinking water pipes. Longer incubation of corrosion products within a pipe biofilm setup would also be useful in understanding antibiotic resistance in real systems. Furthermore, due to corrosion product particles passing through 0.45 μm filters, variability amongst measured metal ion concentrations made it difficult to assess the role of metal ions as a function of concentration in impacting antibiotic resistance. Future research that controls metal ion concentration is needed to investigate the relationship between metal ion concentrations in DWDSs and the development of antibiotic resistance among resident microorganisms.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This material is based upon work supported by the National Science Foundation under grant numbers 2027288 and 2027233. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.References
- World Health Organization, Data Global Action Plan on Antimicrobial Resistance, 2015, available from: https://www.who.int/publications/i/item/9789241509763 Search PubMed.
- CDC, Antibiotic Resistance Threats in the United States, 2019, Atlanta, Georgia, 2019 Nov, available from: https://stacks.cdc.gov/view/cdc/82532 Search PubMed.
- N. D. Friedman, E. Temkin and Y. Carmeli, The negative impact of antibiotic resistance, Clin. Microbiol. Infect., 2016, 22, 416–422 CrossRef CAS PubMed.
- S. C. Davies and C. Oxlade, Innovate to secure the future: the future of modern medicine, Future Healthc. J., 2021, 8(2), e251–e256 CrossRef PubMed.
- L. C. Ventola, The Antibiotic Resistance Crisis Part 1: Causes and Threats, Pharm. Ther., 2015, 40(4), 277–283 Search PubMed.
- K. E. Thorpe, P. Joski and K. J. Johnston, Antibiotic-resistant infection treatment costs have doubled since 2002, now exceeding $2 billion annually, Health Aff., 2018, 37(4), 662–669 CrossRef PubMed.
- World Bank, Drug-Resistant Infections a Threat to Our Economic Future, 2017, available from: https://www.worldbank.org Search PubMed.
- Antimicrobial Resistance Collaborators, Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis, Lancet, 2022, 399(10325), 629–655 CrossRef PubMed.
- A. Pruden, R. E. Alcalde, P. J. J. Alvarez, N. Ashbolt, H. Bischel, N. L. Capiro, E. Crossette, D. Frignon, K. Grimes, C. N. Haas, K. Ikuma, A. Kappell, T. LaPara, L. Kimbell, M. Li, X. Li, P. McNamara, Y. Seo, M. D. Sobsey, E. Sozzi, T. Navab-Daneshmand, L. Raskin, M. V. Riquelme, P. Vikesland, K. Wigginton and Z. Zhou, An Environmental Science and Engineering Framework for Combating Antimicrobial Resistance, Environ. Eng. Sci., 2018, 35(10), 1005–1011 CrossRef CAS.
- T. R. Burch, R. J. Newton, L. K. Kimbell, E. L. LaMartina, K. O’Malley, S. M. Thomson, C. W. Marshall and P. J. McNamara, Targeting current and future threats: recent methodological trends in environmental antimicrobial resistance research and their relationships to risk assessment, Environ. Sci.: Water Res. Technol., 2022, 8, 1787–1802 RSC.
- S. Hernando-Amado, T. M. Coque, F. Baquero and J. L. Martínez, Defining and combating antibiotic resistance from One Health and Global Health perspectives, Nat. Microbiol., 2019, 4, 1432–1442 CrossRef CAS PubMed.
- United Nations Environment Programme, Bracing for Superbugs Strengthening Environmental Action in the One Health Response to Antimicrobial Resistance, 2023, available from: https://www.unep.org/resources/superbugs/environmental-action Search PubMed.
- C. Baker-Austin, M. S. Wright, R. Stepanauskas and J. V. McArthur, Co-selection of antibiotic and metal resistance, Trends Microbiol., 2006, 14, 176–182 CrossRef CAS PubMed.
- L. G. Li, Y. Xia and T. Zhang, Co-occurrence of antibiotic and metal resistance genes revealed in complete genome collection, ISME J., 2017, 11(3), 651–662 CrossRef CAS PubMed.
- C. W. Knapp, S. M. McCluskey, B. K. Singh, C. D. Campbell, G. Hudson and D. W. Graham, Antibiotic resistance gene abundances correlate with metal and geochemical conditions in archived Scottish soils, PLoS One, 2011, 6(11), e27300 CrossRef CAS PubMed.
- L. K. Kimbell, Y. Wang and P. J. McNamara, The impact of metal pipe materials, corrosion products, and corrosion inhibitors on antibiotic resistance in drinking water distribution systems, Appl. Microbiol. Biotechnol., 2020, 104, 7673–7688 CrossRef CAS PubMed.
- L.K. Kimbell, E. L. LaMartina, A. D. Kappell, J. Huo, Y. Wang, R. J. Newton and P. J. McNamara, Cast iron drinking water pipe biofilms support diverse microbial communties containing antibiotic resistance genes, metal resistance genes, and class 1 integrons, Environ. Sci.: Water Res. Technol., 2021, 7, 584–598 RSC.
- E. Sanganyado and W. Gwenzi, Antibiotic resistance in drinking water systems: occurrence, removal, and human health risks, Sci. Total Environ., 2019, 669, 785–797 CrossRef CAS PubMed.
- V. Rilstone, L. Vignale, J. Craddock, A. Cushing, Y. Filion and P. Champagne, The role of antibiotics and heavy metals on the development, promotion, and dissemination of antimicrobial resistance in drinking water biofilms, Chemosphere, 2021, 282, 131048 CrossRef CAS PubMed.
- V. L. Snoeyink, Oxidation-Reduction Reactions, Water Chemistry, John Wiley & Sons, 1980, pp. 363–374 Search PubMed.
- J. Świetlik, U. Raczyk-Stanisławiak, P. Piszora and J. Nawrocki, Corrosion in drinking water pipes: the importance of green rusts, Water Res., 2012, 46(1), 1–10 CrossRef PubMed.
- W. Xiao, S. Hong, Z. Tang, S. Seal and J. S. Taylor, Effects of blending on surface characteristics of copper corrosion products in drinking water distribution systems, Corros. Sci., 2007, 49(2), 449–468 CrossRef CAS.
- H. Zhang, D. Liu, L. Zhao, J. Wang, S. Xie, S. Liu, P. Lin, X. Zhang and C. Chen, Review on corrosion and corrosion scale formation upon unlined cast iron pipes in drinking water distribution systems, J. Environ. Sci., 2022, 117, 173–189 CrossRef CAS PubMed.
- E. J. Kim and J. E. Herrera, Characteristics of lead corrosion scales formed during drinking water distribution and their potential influence on the release of lead and other contaminants, Environ. Sci. Technol., 2010, 44(16), 6054–6061 CrossRef CAS PubMed.
- J. Tully, M. K. Desantis and M. R. Schock, Water quality-pipe deposit relationships in Midwestern lead pipes, AWWA Water Sci., 2019, 1(2), e1127 CrossRef CAS.
- A. D. Kappell, K. R. Harrison and P. J. McNamara, Effects of zinc orthophosphate on the antibiotic resistant bacterial community of a source water used for drinking water treatment, Environ. Sci., 2019, 5(9), 1523–1534 CAS.
- L. K. Kimbell, E. L. LaMartina, S. Kohls, Y. Wang, R. J. Newton and P. J. McNamara, Impact of corrosion inhibitors on antibiotic resistance, metal resistance, and microbial communities in drinking water, mSphere, 2023, 8, e00307 CrossRef PubMed.
- Y. He, L. Pan, R. Chen and B. Shi, Field studies of aluminum release and deposition in drinking water distribution systems, Chemosphere, 2021, 271, 130067 CrossRef PubMed.
- H. Cornejo-Garrido, D. Kibanova, A. Nieto-Camacho, J. Guzmán, T. Ramírez-Apan and P. Fernández-Lomelín, Oxidative stress, cytoxicity, and cell mortality induced by nano-sized lead in aqueous suspensions, Chemosphere, 2011, 84(10), 1329–1335 CrossRef CAS PubMed.
- V. Aruoja, H. C. Dubourguier, K. Kasemets and A. Kahru, Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata, Sci. Total Environ., 2009, 407(4), 1461–1468 CrossRef CAS PubMed.
- Y. Wang, Y. Xie, W. Li, Z. Wang and D. E. Giammar, Formation of lead(IV) oxides from lead(II) compounds, Environ. Sci. Technol., 2010, 44(23), 8950–8956 CrossRef CAS PubMed.
- A. E. Broo, B. Berghult and T. Hedberg, Copper Corrosion in Water Distribution Systems--the Influence of Natural Organic Matter (NOM) on the Solubility of Copper Corrosion Products, 1998, available from: https://www.archive.org Search PubMed.
- Y. Xie, Y. Wang, V. Singhal and D. E. Giammar, Effects of pH and carbonate concentration on dissolution rates of the lead corrosion product PbO2, Environ. Sci. Technol., 2010, 44(3), 1093–1099 CrossRef CAS PubMed.
- J. D. Noel, Y. Wang and D. E. Giammar, Effect of water chemistry on the dissolution rate ofthe lead corrosion product hydrocerussite, Water Res., 2014, 54, 237–246 CrossRef CAS PubMed.
- APHA, Standard Methods for the Examination of Water and Wastewater, 1998 Search PubMed.
- Y. Zhu, B. Huang, Z. Zhu, H. Liu, Y. Huang, X. Zhao and M. Liang, Characterization, dissolution and solubility of the hydroxypyromorphite-hydroxyapatite solid solution [(PbxCa1-x)5(PO4)3OH] at 25 °C and pH 2-9, Geochem. Trans., 2016, 17(1), 1–18 CrossRef PubMed.
- A. Singh, K. U. Ulrich and D. E. Giammar, Impact of phosphate on U(VI) immobilization in the presence of goethite, Geochim. Cosmochim. Acta, 2010, 74(22), 6324–6343 CrossRef CAS.
- K. R. Harrison, A. D. Kappell and P. J. McNamara, Benzalkonium chloride alters phenotypic and genotypic antibiotic resistance profiles in a source water used for drinking water treatment, Environ. Pollut., 2020, 257, 113472 CrossRef CAS PubMed.
- CLSI, Performance standards for antimicrobial susceptibility testing, Clinical and Laboratory Standards Institute, Wayne, PA, 28th edn, 2018 Search PubMed.
- S. Ćirić, O. Petrović and D. Milenković, Low-nutrient R2A medium in monitoring microbiological quality of drinking water, Chem. Ind. Chem. Eng. Q., 2010, 16(1), 39–45 CrossRef.
- K. O’Malley, P.J. McNamara and W. McDonald, Seasonal and spatial patterns differ between intracellular and extracellular antibiotic resistance genes in urban stormwater runoff, Environ. Sci.: Adv., 2022, 1, 380–390 Search PubMed.
- E. Ladomersky and M. J. Petris, Copper tolerance and virulence in bacteria, Metallomics, 2015, 7(6), 957–964 CrossRef CAS PubMed.
- L. P. T. M. Zevenhuizen, J. Dolfing, E. J. Eshuis and I. J. Scholten-Koerselman, Inhibitory effects of copper on bacteria related to the free ion concentration, Microb. Ecol., 1979, 5(2), 139–146 CrossRef CAS PubMed.
- J. T. Trevors and C. M. Cotter, Review copper toxicity and uptake in microorganisms, J. Ind. Microbiol., 1990, 6(2), 77–84 CrossRef CAS , available from: https://academic.oup.com/jimb/article/6/2/77/5987461.
- J. M. Argüello, D. Raimunda and T. Padilla-Benavides, Mechanisms of copper homeostasis in bacteria, Front. Cell. Infect. Microbiol., 2013, 3 DOI:10.3389/fcimb.2013.00073.
- A. Andrei, Y. Öztürk, B. Khalfaoui-Hassani, J. Rauch, D. Marckmann and P. I. Trasnea, Cu homeostasis in bacteria: the ins and outs, Membranes, 2020, 10, 1–45 CrossRef PubMed.
- A. Hyre, K. Casanova-Hampton and S. Subashchandrabose, Copper Homeostatic Mechanisms and Their Role in the Virulence of Escherichia coli and Salmonella enterica, EcoSal Plus, 2021, 9(2), eESP00142020 CrossRef PubMed.
- G. E. Kenney, L. M. K. Dassama, M. E. Pandelia, A. S. Gizzi, R. J. Martinie and P. Gao, The biosynthesis of methanobactin, Science, 2018, 359(6382), 1411–1416 CrossRef CAS PubMed.
- I. T. Paulsen, T. G. Littlejohn, P. Radstrom, L. Sundstrom, O. Skold and G. Swedberg, et al., The 3’ Conserved Segment of Integrons Contains a Gene Associated with Multidrug Resistance to Antiseptics and Disinfectants, Antimicrob. Agents Chemother., 1993, 761–768 CrossRef CAS PubMed , available from: https://journals.asm.org/journal/aac.
- J. Bengtsson-Palme, R. Hammarén, C. Pal, M. Östman, B. Björlenius and C. F. Flach, Elucidating selection processes for antibiotic resistance in sewage treatment plants using metagenomics, Sci. Total Environ., 2016, 572, 697–712 CrossRef CAS PubMed.
- Q. Wang, L. Liu, Z. Hou, L. Wang, D. Ma, G. Yang, S. Guo, J. Luo, L. Qi and Y. Luo, Heavy metal copper accelerates the conjugative transfer of antibiotic resistance genes in freshwater microcosms, Sci. Total Environ., 2020, 717, 137055 CrossRef CAS PubMed.
- Y. Zhang, A. Z. Gu, T. Cen, X. Li, M. He and D. Li, Sub-inhibitory concentrations of heavy metals facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes in water environment, Environ. Pollut., 2018, 237, 74–82 CrossRef CAS PubMed.
- M. Hans, A. Erbe, S. Mathews, Y. Chen, M. Solioz and F. Mücklich, Role of copper oxides in contact killing of bacteria, Langmuir, 2013, 29(52), 16160–16166 CrossRef CAS PubMed.
- C. Seiler and T. U. Berendonk, Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture, Front. Microbiol., 2012, 3 DOI:10.3389/fmicb.2012.00399.
- D. R. Lide, G. Baysinger, L. I. Berger, R. N. Goldberg, H. V. Kehiaian, and K. Kuchitsu, Thermochemistry, Electrochemistry, and Solution Chemistry, CRC Handbook of Chemistry and Physics, Taylor and Francis Group, 2014, pp. 1–210 Search PubMed.
- Office of Ground Water and Drinking Water Standards and Risk Management Division, Deterioirating Buried Infrastructure Management Challenges and Strategies, US Environmental Protection Agency, 2004 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00026a |
| This journal is © The Royal Society of Chemistry 2024 |