Open Access Article
Open Access ArticleLignin cationization for the removal of phosphates and nitrates from effluents of wastewater treatment plants†
Fannyuy V.
Kewir
 a,
Carlos E.
Astete
a,
Carlos E.
Astete
 a,
Divine B.
Nde
b,
Jessica R.
Eberhard
c,
W. David
Constant
a and
Cristina M.
Sabliov
a,
Divine B.
Nde
b,
Jessica R.
Eberhard
c,
W. David
Constant
a and
Cristina M.
Sabliov
 *a
*a
aBiological & Agricultural Engineering Department, Louisiana State University, LSU Ag Center, Baton Rouge, Louisiana 70803, USA. E-mail: csabliov@lsu.edu
bDepartment of Chemistry, Louisiana State University, Baton Rouge, Louisiana 70803, USA
cDepartment of Biological Sciences, Museum of Natural Science, Louisiana State University, Baton Rouge, LA 70803, USA
First published on 30th September 2024
Abstract
The removal of phosphates and nitrates from wastewater treatment plant (WWTP) effluents is important for preventing pollution of receiving waters. In this study, we chemically modified alkaline lignin (aLN) with quaternary ammonium groups to obtain biodegradable cationic lignin (cLN). We characterized the cLN and tested its efficacy for removing phosphates and nitrates in a lab setting and on field-collected WWTP samples. Adsorption isotherm and kinetic studies were performed in aqueous media, and the effects of several variables (contact time, pH, initial concentration, and adsorbent dose) were investigated. The Langmuir isotherm described phosphate and nitrate adsorption well, with R2 values of 0.97 and 0.84, and maximum adsorption capacities of 0.59 mg g−1 and 2 mg g−1 respectively. For phosphate, the data fit the Freundlich isotherm model with an R2 of 0.95, suggesting that both homogenous and heterogeneous adsorbent surfaces were involved in phosphate adsorption. Adsorption kinetics revealed that both phosphate and nitrate sorption onto cLN was better described by the pseudo-second-order model, with a correlation coefficient of 1. Furthermore, a 2-dimension Doehlert matrix was used to model the effect of initial concentration and adsorbent dose on the phosphate and nitrate removal. The results showed that cLN 1516 mol% was most effective for low phosphate and nitrate concentrations. With an obtained optimum adsorbent dose of 10 mg mL−1, we achieved a successful reduction of nutrient loads of WWTP effluent from 0.42 mg L−1 to 0.18 mg L−1 (adsorption capacity of 0.6 mg g−1) and from 4.1 mg L−1 to 2.3 mg L−1 (adsorption capacity of 4.5 mg g−1), corresponding to the removal of 57.7% and 43.9% for phosphates and nitrates respectively.
Environmental significanceThe research paper “Lignin cationization for the removal of phosphates and nitrates from effluents of wastewater treatment plants” addresses the important problem of water pollution from a point source. Our approach involved repurposing a waste stream from the paper and pulp industry, lignin, to reduce the nutrient loads of final effluents of wastewater treatment plants. This approach was intended to follow and close the circular economy loop whereby waste material is used for environmental remediation with the potential for application as phosphate and nitrate fertilizer for plants. |
1. Introduction
Water treatment is a key component of sustainable water resource management. However, wastewater treatment plants (WWTPs) constitute a major point source of pollution, with effluent loads often greater than those of nonpoint sources.1 Nitrates and phosphates are among the pollutants present in the water taken in by WWTPs and originate from various sources, including agricultural, industrial, and household waste.2,3 The efficient removal of these pollutants before the release of WWTP effluents into the natural environment is important, since high phosphorus and nitrogen concentrations (between 20–100 and 500–1000 μg L−1 respectively) can cause excessive algal and plant growth, leading to eutrophication in aquatic habitats.4 An increase in mortality of aquatic life due to eutrophication causes an imbalance of aquatic ecosystems while the continued supply of nutrient loads upsets the biogeochemical cycles of nitrogen and phosphorus.5 In this regard, the United States Environmental Protection Agency (USEPA) has set strict ecoregion-specific concentration ranges for total nitrogen (0.12–2.18 mg L−1) and total phosphorus (0.076–0.1 mg L−1) on WWTPs, to reduce effluent nutrient concentrations and protect receiving waters.1Biological and chemical methods have been researched and implemented for wastewater treatment, but their use is often limited due to high costs and instability.6 Adsorption is generally preferred to chemical (such as precipitation with iron salts, alum, or lime) and biological (aerobic and anaerobic) treatments due to convenience, flexibility, ease of operation, simplicity of design, and reduced production of sludge.2,7,8 Metal-based adsorbents and composite materials are some conventional adsorbents that have been utilized with some success but they have drawbacks such as low sorption capacity, limited surface area/active sites, and short adsorption–regeneration cycles.9 Activated carbon has proven to be an effective adsorbent but its high cost limits large-scale use.10,11 In recent years, research has been oriented toward finding cheaper, more efficient, and sustainable adsorbent materials, with a great deal of this research focused on lignin-based materials.12
Lignin is the second most abundant biopolymer on Earth13 after cellulose. It exhibits several characteristics such as biodegradability/biocompatibility, aromaticity, high thermal stability, renewability, easy functionalization, durability, and low cost12,14,15 that make it attractive for the synthesis of biomaterials. Lignin contains a large number of active functional groups such as aliphatic and phenolic hydroxyl groups, carbonyl groups, methoxy groups, and phenyl groups,16 which make it suitable as a chelating agent for a variety of contaminants in water/wastewater.12 Lignin functionalization and chemical and physical modification have received a great deal of attention in efforts to increase its efficiency as a removal agent for wastewater applications. Although a considerable amount of research has been conducted on the use of lignin-based materials as adsorbents for removing heavy metals, dyes, and sulfates,12,13,16–23 fewer studies23,24 have explored the ability of raw lignin to remove phosphates and nitrates from wastewater. After experimenting with phosphate and nitrate removal using lignin, Sajjadi's work12 suggested that the ineffectiveness of lignin may be due to its coiled 3D structure and overall negative charge, which would induce double-layer electrostatic repulsion with the phosphate and nitrate anions during adsorption.
Because adsorption is a surface phenomenon, the efficiency of the process is intrinsically tied to adsorbent properties such as surface charge and surface area. A large surface area provides maximum available sites for interaction, thereby increasing adsorption capacity. Both Zong's23 and Li's13 groups demonstrated the capability and versatility of lignin-based adsorbents for contaminant removal from wastewater. However, like the materials they used, the literature reveals that the focus has been on inorganic composites with components (for example, positively charged polyethylenimine) that may be toxic to the environment due to their potential for bioaccumulation and environmental persistence. Moreover, some of these adsorbents are non-recyclable.
In this study, we designed a positively charged biodegradable lignin polymer for improved phosphate and nitrate removal and with potential recyclability through agricultural applications. We adopted a circular economy approach, wherein biodegradable lignin was derived from waste biomass, chemically modified, and used in environmental remediation, leaving a minimal environmental footprint. The circular economy can be defined as a system that is restorative or regenerative by intention and design, replacing the ‘end-of-life’ concept and aiming to eliminate waste and the use of toxic chemicals that impair reuse. These goals are met through the superior design of materials, products, and systems.25 Specifically, we chemically modified alkaline lignin (aLN) with quaternary ammonium groups following methods previously studied by Chauhan et al., Gogoi et al., and Pinto et al.26–28 to modify biomass. The resulting cationic lignin (cLN) was tested in a laboratory setting and on field-collected WWTP samples, as a novel biobased system29 for the adsorption of phosphates and nitrates from aqueous media. Following chemical characterization of the cLN, we performed isotherm and kinetic studies in batch adsorption experiments and investigated the effects of several variables (contact time, pH of the solution, initial ion concentration, competing anions, and adsorbent dose) on adsorption efficacy. To our knowledge, the materials synthesized and tested in this study have not previously been used for phosphate and nitrate removal.
2. Materials and methods
2.1. Materials
The alkaline lignin used was purchased from TCI (Portland, OR), sodium hydroxide (NaOH) and hydrochloric acid (HCl) were supplied by VWR International (West Chester, PA), and sulfuric acid (H2SO4) was purchased from Ricca Chemical Company (Arlington, TX). Glycidyl triethylammonium chloride (GTAC), poly[lactic-co-glycolic acid] (PLGA), oxalyl chloride, Amicon Ultra 0.5 mL centrifugal filters and 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (TMDP) were purchased from Sigma Aldrich (St. Louis, MO). Anhydrous dimethyl sulfoxide (DMSO) was purchased from Alfa Aesar (Ward Hill, MA) while regular DMSO was from Thermo Fisher Scientific (Waltham, MA). Dichloromethane (DCM), dimethylformamide (DMF), pyridine, chloroform-d, chromium(III) 2,4-pentanedionate (Cr(acac)3), cyclohexanol, potassium dihydrogen phosphate (KH2PO4), ammonium molybdate ((NH4)Mo7O24·4H2O), potassium antimonyl tartrate trihydrate (K(SbO)C4H4O6·1/2H2O), ascorbic acid, and sodium nitrate (NaNO3) were also purchased from Thermo Fisher Scientific (Waltham, MA), while NitraVer X nitrate reagent set and test ‘N Tubes’ were supplied by HACH Company (Loveland, CO, USA). All solutions were prepared with deionized water DI H2O (resistivity 18.2 MΩ cm, Millipore, Milli-Q, Barnstead International, Dubuque, IA).2.2. Synthesis of cationic lignin
The cationic synthesis protocol was adapted from Kong's30 and Wahlström's20 works. Briefly, 2 g of lignin was dissolved in 100 mL of DI H2O (DI H2O; resistivity 18.2 MΩ cm, Millipore, Milli-Q, Barnstead International, Dubuque, IA) and the pH was adjusted to 12.5 using a 0.2 M NaOH solution. The solution was then transferred to a 250 mL three-neck round-bottom flask and placed in an oil bath at 60 °C and 220 rpm. Next, we added glycidyl triethylammonium chloride (GTAC) to the lignin at volumes corresponding to the desired mol percent dosages. After 24 h, the solution was submerged in a cold-water bath for 30 min and then transferred into dialysis tubing (MWCO 12–14 kD), and submerged in deionized water for 48 h to remove any unreacted GTAC and other impurities. The dialysis water was replaced every 6 h for 48 h. After dialysis, the solution containing the cationic lignin was freeze-dried (Labconco, MO, USA), and the samples were stored at −20 °C for further analysis.2.3. Characterization of cationic lignin
The amounts of various OH groups (aliphatic-OH, carboxylic-OH, and phenolic-OH [para-OH phenyl, condensed and syringyl-OH, guaiacyl-OH, and catechol-OH groups]) in the unmodified and cLN, lignin were estimated using 31P NMR after phosphitylation with 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (TMDP), as described in various published protocols (e.g., ref. 31–33). The chemical shift ranges integrated for various OH group types in the quantification of lignin substructures were adapted from Araneda.34 We estimated the degree of substitution (DS) as the ratio of substituted phenolic-OH groups (original minus derivatized amounts of OH groups given by 31P NMR spectra) divided by the original number of OH groups.20We measured the zeta potential of our synthesized cLN dispersed in DI H2O using a PMX-430 ZetaView QUATT Laser analyzer (Particle Matrix, Ammersee, Germany). A Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) was used to obtain the point of zero charges of the cLN by titrating NaOH solutions against the cLN and observing the variation of zeta potential with pH and size.20
We obtained FT-IR spectra in the range of 4000–400 cm−1 by the attenuated total reflectance (ATR) method at room temperature using a spectrometer (Bruker Alpha P, Germany). We acquired scanning electron microscope (SEM) images and elemental mapping by energy dispersive X-ray spectrometry (SEM/EDS) using a JSM-6700F (JEOL Ltd, Tokyo, Japan) of cLN that had been sputter-coated with a thin layer of platinum.
2.4. Phosphate and nitrate adsorptions
The adsorption capacity of the synthesized cLN was evaluated in a batch system. Using a protocol adapted from Jiao35 and Zong.23 100 mg L−1 stock phosphate and nitrate solutions were prepared by dissolving 143.25 mg of KH2PO4 and 137.10 mg respectively, in DI water and diluted accordingly to obtain the desired concentrations. Each adsorption experiment was done with an adsorbent dose of 2 mg L−1 at room temperature and a stirring speed of 300 rpm. The pH was maintained at neutral pH. The samples were centrifuged using a JXN-26 centrifuge (Beckman Coulter Inc., CA, USA) at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 10 °C for 30 min and collected in 2 mL Amicon Ultra 0.5 centrifugal filter tubes. The ion concentrations of the filtrate were determined as described in Section 2.6. For each experimental run, the amount of adsorbate per unit weight of adsorbent at time t, qt (mg g−1), was calculated according to eqn (1):
000 rpm and 10 °C for 30 min and collected in 2 mL Amicon Ultra 0.5 centrifugal filter tubes. The ion concentrations of the filtrate were determined as described in Section 2.6. For each experimental run, the amount of adsorbate per unit weight of adsorbent at time t, qt (mg g−1), was calculated according to eqn (1):| qt = (C0 − Ct)V/m | (1) |
The sorption percentage was estimated from eqn (2):
| Sorption (%) = (Ci − Ce)/Ci × 100 | (2) |
To investigate the effects of the adsorbent dose and initial ion concentration on the removal percentage, a 2-factor Doehlert's matrix was used to plan the experiments and optimize the process.36,37 For 2 factors, the Doehlert design consists of seven experiments comprising a central point (0) and six others represented in a coded value matrix (Table 1). Adsorption experiments were carried out at room temperature with an agitation speed of 300 rpm, pH 7, and contact time of 25 min. Aliquots of 1.5 mL were withdrawn from the mixture at predetermined time intervals and centrifuged using a JXN-26 centrifuge (Beckman Coulter Inc., CA, USA) at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 10 °C for 30 min. The resulting supernatant was used to determine the phosphate and nitrate concentrations. These experiments were carried out in duplicates.
000 rpm and 10 °C for 30 min. The resulting supernatant was used to determine the phosphate and nitrate concentrations. These experiments were carried out in duplicates.
| Expt # | Coded variables | |
|---|---|---|
| X 1 | X 2 | |
| 1 | 0 | 0 |
| 2 | 1 | 0 |
| 3 | −1 | 0 |
| 4 | 0.5 | 0.866 |
| 5 | −0.5 | −0.866 |
| 6 | 0.5 | −0.866 |
| 7 | −0.5 | 0.866 |
For this design, Lagrange's criterion for the two-variable function was used to model the response (eqn (3)):
| Y = a + b(X1) + c(X2) + d(X12) + e(X22) + f(X1X2) | (3) |
As a realistic test of the adsorption capacity of our adsorbent, we conducted adsorption experiments as described above using final effluent samples from one WWTP in Baton Rouge, LA, USA; South Baton Rouge (SBR) plant. Samples were collected in 1 liter bottles and stored in an ice chest with ice while being transported from the plants to the lab. After filtration with a P8 qualitative filter paper (Fisher Scientific, Pittsburgh, PA), we tested the samples for the presence of phosphates and nitrates as described in Section 2.6, and carried out adsorption experiments using optimum adsorption conditions that were selected based on the initial lab experiments described above.
2.5. Adsorption kinetics and isotherm models
Kinetic models and adsorption isotherms are often used to assess the efficiency and adsorption capacity of adsorbent material. The most commonly used isotherms are the linear Langmuir and Freundlich models.38 We determined the linear Langmuir and Freundlich adsorption isotherms by varying the initial concentrations38 of phosphate (from 0.5 to 1 mg L−1) and nitrates (3.6 to 15.8 mg L−1) by diluting the stock solutions accordingly; we used an adsorbent dose of 2 mg mL−1 with a contact time of 90 min at room temperature and neutral pH. The linear Langmuir model, which can be used to describe adsorbate–adsorbent interaction at the solid–liquid (monolayer) interface,4 is summarized by eqn (4):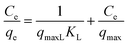 | (4) |
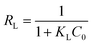 | (5) |
The linear Freundlich model, which describes multilayer sorption (chemisorption) on heterogeneous surfaces,40 is described by eqn (6):
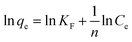 | (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce to ln
Ce to ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe are used to determine n and KF.
qe are used to determine n and KF.
The linearized pseudo-first- and second-order (PFO and PSO) expressions are described by eqn (7) and (8) respectively:
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − K1t qe − K1t | (7) |
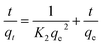 | (8) |
2.6. Determination of phosphate and nitrate concentration
To measure the concentration of phosphates in our samples, we adapted a protocol used with an automatic AQ300 Discrete Analyzer (SEAL Analytical). The original protocol is equivalent to USEPA Method 365.1, Rev 2.0, and is approved for the Clean Water Act (CWA) for use in wastewater compliance monitoring under the National Pollutant Discharge Elimination System (NPDES). Our modified protocol was used to conduct the analyses manually and phosphate concentrations reported refer to orthophosphate–phosphorus (PO4–P).We prepared five different stock solutions using DI water: 2.5 M H2SO4, a 4% (w/v) ammonium molybdate (CAS: 12054-85-2) solution, a 3 g L−1 potassium antimonyl tartrate trihydrate (CAS: 28300-74-5) solution, and a 15 g L−1 ascorbic acid (CAS: 50-81-7) solution spiked with 0.12 mg P/L standard. With these reagents, we prepared a working phosphate color reagent using the following proportions: 65 mL (2.5 M H2SO4) + 22 mL (4% w/v ammonium molybdate) + 7.5 mL (3 g L−1 potassium antimonyl tartrate trihydrate), brought to 100 mL with DI water. We added 140 μL of the color reagent and 40 μL of ascorbic acid to 400 μL of the sample. After an 8 minute reaction time to allow for the color change (reduced phosphomolybdate blue complex formation) to occur, we placed 200 μL of the mixture in a well plate. We then measured light absorbance at a wavelength of 880 nm using a BioTek Cytation 3 plate reader photo spectrometer (BioTek Instruments Inc., VT, USA). We generated and used a phosphate standard curve to estimate orthophosphates in subsequent adsorption studies.
Nitrate concentrations were tested according to the nitrate chromotropic acid TNT method using a DR-900 colorimeter (HACH Company, Loveland, CO, USA) at 420 nm. Nitrate concentrations reported refer to nitrate–nitrogen (NO3−–N).
3. Results and discussion
3.1. Lignin chemical modification/structure confirmation
Commercial alkaline lignin (TCI America) was used for cationization and further analyses. The cationization was done following various GTAC dosages calculated based on the total initial number of phenolic-OH groups in the uncationized lignin. The alkaline lignin had a total phenolic OH group of 2.32 mmol g−1 (see Table 2) and was rich in guaiacyl units (1.30 mmol g−1) with fewer syringyl units (0.29 mmol g−1). Hence, it can be concluded that the lignin source (not specified by the company, TCI America) is softwood lignin. However, within the circular economy framework, it would be advisable to use hardwood (not softwood) waste biomass as source material for lignin extraction because it contains a higher proportion of phenolic OH groups, in particular syringyl units with methoxy groups that are susceptible to modification, specifically cationization in our case.| GTAC vol. (mL) | GTAC dosage (mol%) | Aliphatic OH (mmol g−1) | Phenolic OH (mmol g−1) | COOH (mmol g−1) | Zeta potential (mV) | pH |
|---|---|---|---|---|---|---|
| 0.0 | 0.0 | 1.5 ± 0.4 | 2.3 ± 0.7 | 0.7 ± 0.3 | −44.0 | 2.3 |
| 3.2 | 461.0 | 2.2 ± 0.4 | 0.8 ± 0.0 | 0.5 ± 0.1 | −15.0 | 7.1 |
| 3.5 | 505.5 | 2.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.0 | −9.9 | 6.7 |
| 5.3 | 768.3 | 2.3 ± 0.9 | 0.7 ± 0.3 | 0.4 ± 0.2 | −7.8 | 6.8 |
| 6.4 | 922.0 | 2.0 ± 1.1 | 0.5 ± 0.2 | 0.4 ± 0.2 | 6.4 | 7.0 |
| 10.5 | 1516.5 | 1.1 ± 0.7 | 0.2 ± 0.1 | 0.2 ± 0.1 | 18.5 | 6.8 |
3.2. Characterization of cationic lignin
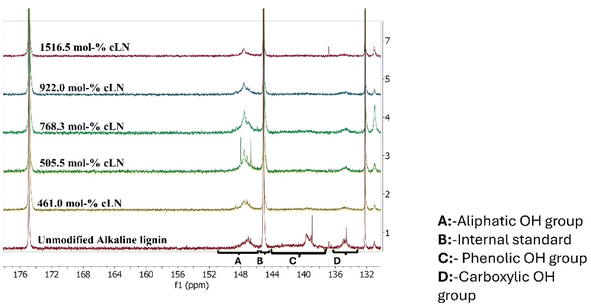
The variation in the peak intensities of the various OH groups, for both the unmodified and cLN, is illustrated in the stacked 31P NMR spectra (Fig. 1), for sections of the spectra that correspond to those OH groups. The variation observed with the OH-group types quantified in mmol g−1 (up to a 1516 mol% dosage) was consistent with published studies, with an increase in the aliphatic-OH groups due to the opening of the epoxy ring,20 a decrease in the phenolic-OH groups, and only a slight reduction of carboxylic-OH groups with varied GTAC dosage.
Estimated amounts of the various OH groups in the lignin modifications are summarized in Table 2. The observed reduction in the amount of phenolic-OH groups with GTAC dosage confirms that the reaction site for the substitution by the quaternary ammonium salt of GTAC was the phenolic group of lignin. This agrees with Kong et al.30 (2015) and Acurio Cerda et al.42 (2023). After this series of chemical modifications, the zeta potentials were determined (Table 2). The 922 and 1516 mol% cLN were chosen for further testing because of their positive zeta potentials at ≈neutral pHs. ALN was used as a control in adsorption studies.
The variation of zeta potential with pH of cLN 922 and 1516 was used to determine the points of zero charge (pHpzc). For cLN 922, the zeta potential ranged from +31.6 mV at pH 2.3 to −10.4 mV at pH 10.1, and the point of zero charge was at pH 6.6. For cLN 1516, the zeta potential ranged from +38.2 mV at pH 2.6 to 2.1 mV at pH 10.0, and the pHpzc was not attained within this range (Fig. 2).
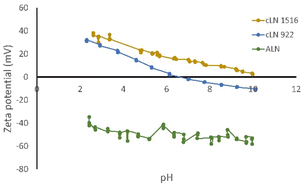 | ||
| Fig. 2 Zeta potential measured across a range of pH values for aLN, cLN 922, and cLN 1516 mol% cationized lignin. | ||
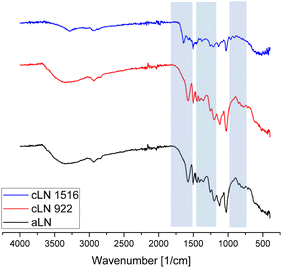
The fact that the surface charge of cLN 1516 remains positive shows that there was no pH environment where the surface charge of the adsorbent was neutralized and therefore would remove the adsorbate over a wide pH range. The zeta potential of the unmodified aLN was ≈−44 at neutral pH and showing that the chemical modification of the lignin was successful. Hence, it was concluded that the charge of the cLN resulting from GTAC derivatization is permanent and not pH dependent, as later confirmed by the FTIR (Fig. 3); this is consistent with conclusions drawn in similar research by Wahlström.20
Absorption peaks at 1583 cm−1 in both lignin types are attributed to the C–H vibration of the aromatic ring. This illustrates the existence of the aromatic skeletal structure in both lignin types. The appearance of new absorption peaks at 1465 cm−1 in cLN 1516 was attributed to the stretching of the symmetric-CH3 in the quaternary ammonium compounds,42 while the IR peaks at 968 cm−1, and 910 cm−1 for the cLN are assigned to methyl and methylene groups linked to quaternary ammonium atoms.27 This further demonstrates the successful grafting of GTAC on lignin, similar to Kong30 who studied the cationization of kraft lignin. Furthermore, the peak at 1653 cm−1 in the cLN reflects the N–H bending in the ammonium salt, while the peak at 814 cm−1 is attributed to C–H deformations out-of-plane in positions 2 and 6 of the syringyl units.
3.3. Phosphate and nitrate adsorption
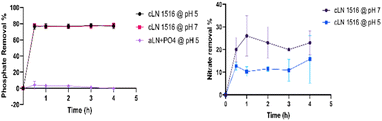 | ||
| Fig. 4 Phosphate (left) and nitrate (right) adsorption by aLN at pH 5, and by cLN 1516 at pH 5 and 7. | ||
Based on these removal rates and titration results, cLN 1516, which conserved a positive charge within the target pH range of final effluent discharges,6–8 was selected as the lignin modification for further adsorption experiments in the study.
| Adsorbate | Isotherms | Kinetics | ||||||
|---|---|---|---|---|---|---|---|---|
| Langmuir | Freundlich | PFO | PSO | |||||
| Parameter | Value | Parameter | Value | Parameter | Value | Parameter | Value | |
| Nitrates | q max (mg g−1) | 2 | K F | 0.26 | q e (mg g−1) | 0.82 | q e (mg g−1) | 0.82 |
| K L (mL mg−1) | 0.09 | 1/n | 0.53 | q e,cal (mg g−1) | 0.23 | q e,cal (mg g−1) | 0.67 | |
| R L | 0.92 | R 2 | 0.78 | K 1 (min−1) | −4.18 × 10−6 | K 2 (g (mg min−1)−1) | 0.13 | |
| R 2 | 0.84 | R 2 | 0.05 | R 2 | 0.99 | |||
| Phosphates | q max (mg g−1) | 0.59 | K F | 0.56 | q e (mg g−1) | 0.25 | q e (mg g−1) | 0.25 |
| K L (mL mg−1) | 2.87 | 1/n | 0.6 | q e,cal (mg g−1) | 0.05 | q e,cal (mg g−1) | 0.22 | |
| R L | 0.26 | R 2 | 0.95 | K 1 (min−1) | −7.97 × 10−5 | K 2 (g (mg min−1)−1) | 5.05 | |
| R 2 | 0.97 | R 2 | 0.6 | R 2 | 1 | |||
We assessed the pseudo-first- and second-order kinetic models with cLN 1516 at room temperature and neutral pH. The adsorption process was fast over the first 30 minutes but then flattened, possibly due to the saturation of adsorption sites. The adsorption kinetics revealed that phosphate and nitrate sorption onto cLN was better described by the pseudo-second-order model, which had a correlation coefficient close to unity as shown in Table 3. This was further supported by the fact that the estimated qe values were close to the experimentally determined qe,cal values. Hence, the adsorption process was governed by electron exchange on the adsorbent surface with the adsorbate (chemisorption).
The adsorption capacities for phosphates and nitrates by various materials modified under various conditions is summarized in Table 4. The adsorbents that have not been modified with inorganic metallic components3,4,45 display comparable adsorption capacities in the same range as our cationic lignin. The advantage of our system is that these adsorption capacities were obtained at only 2 mg mL−1 adsorbent dosage. This demonstrates the feasibility of the application of the proposed material for tertiary-stage wastewater treatment and the potential as a plant fertilizer source following the circular economy approach. With optimization, the adsorption capacities can be attained as shown in Section 3.3.3.
| Adsorbent material | Adsorption capacity (mg g−1) | Adsorbent dosage (mg mL−1) | Reference | |
|---|---|---|---|---|
| PO43− | NO3− | |||
| Mg–Fe oxide | 28.3 | NA | NA | 46 |
| Local clay | NA | 5.1 | 20 | 3 |
| Lanthanum-modified platanus biochar | 148.11 | 0.4 | 47 | |
| Hybrid hydrated metal oxides | ||||
| HFeO | −111 | NA | NA | 48 |
| HZrO | −92 | |||
| HCuO | −74 | |||
| Lanthanum-incorporated porous zeolite | 17.2 | NA | 2 | 49 |
| AL–PEI–La | 65.79 | NA | 1.25 | 23 |
| Tablet precipitation material (used cement) | 3.81 | NA | 15 | 50 |
| Solid waste residue (SWR) generated from Awash Melkassa Aluminium Sulphate and Sulphuric Acid Factory | 0.962 | 0.071 | 20 | 4 |
| Raw corn stalks (RCS) | 22.8833 | 13.6054 | 1 | 38 |
| Hybrid anion exchange (HA520E-Fe) | 12.45 | 58.6 | 1 | 51 |
| La-biochar | 36.06 | NA | 2 | 52 |
| Amorphous lanthanum hydroxide | 107.53 | NA | 2.5 | 53 |
| Nanosize-dispersed ZrO2 particles on zirconia-functionalized graphite oxide (ZrAGO) | 131.6 | NA | 0.5 | 54 |
| Mg oak biochar (in situ) | 64.6 | NA | NA | 55 |
| Mg GHW biochar (in situ) | 65.1 | NA | NA | 55 |
| MgO-functionalized lignin-based bio-charcoal (MFLC) | 295.91 | NA | 2 | 35 |
| Zirconium(IV) loaded lignocellulosic butanol residue (LBR-Zr) | 8.75 | NA | NA | 24 |
| Hydrous zirconium oxide-based nanocomposite HZO-201 | 28 | NA | 0.5 | 56 |
| Magnetic bio-activated carbons (MBACs) | 21.18 | NA | 6.67 | 57 |
| Magnetic lignin-based nanoparticles (M/ALFe) | 0.09 | NA | 20 | 13 |
| Dolomite | 0.30 | NA | 60 | 45 |
| Hydroxyapatite | 1.10 | NA | 20 | 45 |
| Fe2O3-contained lignocellulose nanocomposite | NA | 26.12 | 4 | 10 |
| Polymeric adsorbent (NDQ) | NA | 221.8 | 2 | 2 |
| Pyrolyzed biochar | NA | 35.59 | 200 | 58 |
| B/Mg400 | 138 | NA | 16.6 | 59 |
| Cationic alkaline lignin (present study) | 0.59 | 2 | 2 | Present study |
| Exp't # | Phosphates | Nitrates | |||
|---|---|---|---|---|---|
| Ads dose (mg mL−1) | Conc. (mg L−1) | Removal% | Conc. (mg L−1) | Removal% | |
| 1 | 6 | 50.25 | 10.30 ± 2.37 | 13.6 | 32.10 ± 3.13 |
| 2 | 10 | 50.25 | 24.93 ± 0.94 | 13.6 | 52.40 ± 7.83 |
| 3 | 2 | 50.25 | 4.76 ± 1.57 | 13.6 | 13.28 ± 2.61 |
| 4 | 8 | 100.00 | 10.26 ± 0.18 | 24.1 | 28.42 ± 3.23 |
| 5 | 4 | 0.50 | 82.91 ± 0.76 | 3.0 | 35.00 ± 2.36 |
| 6 | 8 | 0.50 | 90.06 ± 3.28 | 3.0 | 76.67 ± 4.71 |
| 7 | 4 | 100.00 | 0.92 ± 0.31 | 24.1 | 21.78 ± 2.64 |
Multiple regression analysis applied to the experimental data showed that the model was statistically significant with p-values less than 0.05 (Table 5) for both phosphates and nitrates. The following equations were generated to represent phosphates (eqn (9)) and nitrates (eqn (10)) removal percentages:
| YP = 84.12 − 1.31X1 − 2.25X2 + 0.28X12 + 0.01X22 + 0.01X1X2 | (9) |
| YN = 1.62 + 10.34X1 − 0.96X2 + 0.05X12 + 0.07X22 − 0.42X1X2 | (10) |
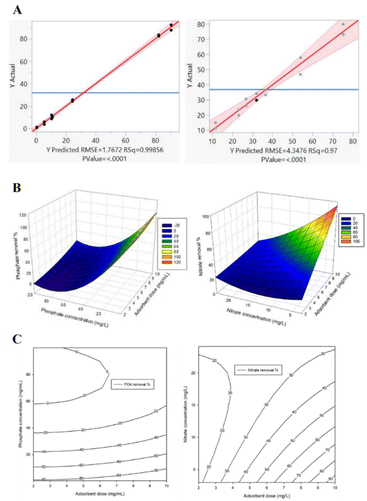
Clustering points around the diagonal indicate a good fit and validate the model with the plot of predicted versus experimental values60 for both phosphates and nitrates (Fig. 5A) with regression coefficient R2 values of 1.00 and 0.97 respectively.
The significance of the model coefficients at the 95% confidence interval was determined using the Student's t-test as summarized in Table 6. On the one hand, the interaction between the adsorbent dose and phosphate concentration is insignificant. However, the phosphate concentration is significant in the first and second order, while the adsorbent dose is only significant at the second order. This means that the adsorbent dose does not affect phosphate removal significantly when considered linearly unlike the phosphate concentration where a small variation would influence the phosphate removal considerably. On the other hand, the interaction between the adsorbent dose and nitrate concentration is significant. Variations in adsorbent dose would affect the nitrate removal considerably when considered linearly but not at the second-order level. The nitrate concentration is significant only at the second-order level.
| Parameter | Phosphates | Nitrates | ||||
|---|---|---|---|---|---|---|
| Coefficient | STD error | p-Value | Coefficient | STD error | p-Value | |
| a Significant at p < 0.05. | ||||||
| Intercept | 84.12 | 3.874 | 2.13 × 10−8 | 1.62 | 11.266 | 0.8894 |
| X 1 | −1.31 | 1.204 | 0.3066 | 10.34 | 3.0241 | 0.0091a |
| X 2 | −2.25 | 0.061 | 3.29 × 10−10a | −0.96 | 0.8277 | 0.2804 |
| X 1 2 | 0.28 | 0.096 | 0.0179a | 0.05 | 0.2353 | 0.8495 |
| X 2 2 | 0.01 | 5 × 10−4 | 1.59 × 10−9a | 0.07 | 0.0254 | 0.0200a |
| X 1 X 2 | 0.01 | 0.006 | 0.4074 | −0.42 | 0.0728 | 0.0005a |
| Overall p-value | 3.87 × 10−11 | 4.86 × 10−6 | ||||
| Adjusted R2 | 1 | 0.96 | ||||
From the response surface plots, it can be deduced that adsorption sites reached saturation quickly, with little or no adsorbate retention as the exchange sites were overwhelmed (Fig. 5B). Exploiting the vicinity of optimum from the contour plots (Fig. 5C), the optimum conditions for phosphate removal were an adsorbent dose of 5 mg mL−1 and a phosphate concentration of 0.5 mg L−1 while the corresponding optimum conditions for nitrates were 10 mg mL−1 and 4.1 mg L−1. The theoretical removal percentages corresponding to these optimum points for phosphates and nitrates were 83.54% and 89.94% respectively while the experimental removal percentages were 92.66% and 80.95% respectively. After verification, the percentage difference between the theoretical and experimental data for phosphates was 9.842% and for nitrates was 9.996%. The fact that both differences were <10% validated the model and further confirmed that cLN 1516 mol% is highly effective at low phosphate and nitrate concentrations with adsorption capacities of 1.15 mg g−1 and 8.5 mg g−1. Since our target is final effluent, which usually has lower ion concentrations after primary and secondary treatments (TN: 15–35 mg L−1 and TP: 4–10 mg L−1),1 our cLN functions well within that range as a tertiary treatment but would not be effective for systems with higher concentrations.
Results from the adsorption experiments carried out on the SBR WWTP using the experimentally determined (see Section 3.3.3) optimum adsorbent dose of 10 mg mL−1 showed a significant reduction of nutrient loads of 57.7% and 43.9% for phosphates and nitrates respectively. These effluent samples, present contain several types of pollutants such as chlorides, sulfates, and carbonates at varied concentrations; they probably competed with phosphates and nitrates for the adsorption sites, thereby limiting the adsorption of the phosphates and nitrates to the observed 57.7% and 43.9%. We could not report the averages because the subsequent set of SBR WWTP samples collected had low initial phosphate and nitrate concentrations (0.03 mg L−1 and 0.67 mg L−1 respectively) and were below detection limits after adsorption. This aligns with conclusions from the Doehlert experiments that the lower the concentration, the higher the removal percentage.
3.4. Morphological and elemental analysis
SEM imaging of aLN (Fig. 6A) and cLN 1516 (Fig. 6B) was performed to highlight their characteristic morphological differences. Compared to aLN, cLN 1516 had more irregular, porous, and random shapes with more available surfaces for interaction with adsorbates.Elemental mapping by SEM/EDS of the adsorbent after adsorption revealed its surface elemental composition (Fig. 7). As expected, the typical chemical constituent elements in wastewater as reported by Chang's group63 are present on the adsorbent surface displayed by the EDS mapping. Most of these elements (Ca, Mg, Na, K, S) are common in the natural environment while trace elements such as Cu come from domestic wastewater. The confirmation of the presence of other anions on the adsorbent surface further confirms that they compete for adsorption sites thereby limiting the adsorption of phosphates and nitrates.
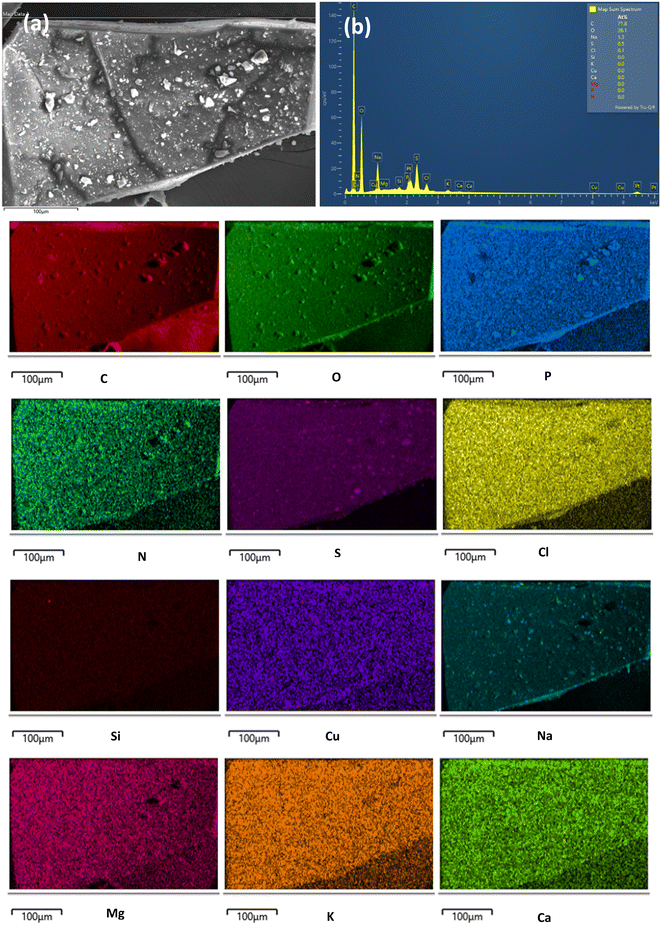 | ||
| Fig. 7 SEM/EDS image of elemental mapping of cLN 1516 after adsorption showing (a) electron image and (b) area EDS spectrum of the labelled elements. | ||
4. Conclusions
High nutrient loads of wastewater treatment plant effluents released into natural waters hinder the attainment of water quality standards. In our study, alkaline lignin was chemically modified to provide the positive charge needed for removing anionic pollutants from wastewater. The structure of the functionalized lignin polymer was confirmed with NMR, FTIR, and SEM/EDS. The most promising cationic lignin modification variant was further tested in adsorption experiments to determine its efficacy for removing phosphates and nitrates from wastewater. Nitrates and phosphates were chosen because not only they are predominant pollutants in effluents from water treatment plants but also macronutrients that can be applied as fertilizers after removal. A 2-dimensional Doehlert matrix was used to model the effects of initial concentration and adsorbent dose on phosphate and nitrate removal, and we showed that cLN 1516 was most effective at low phosphate and nitrate concentrations. With an experimentally determined optimum adsorbent dose of 10 mg mL−1, we reduced nutrient loads with removal of 57.7% and 43.9% for phosphates and nitrates respectively. Our research demonstrates that lignin, a byproduct of the pulp and paper industry, can be repurposed as a wastewater remediation material with possible application as a plant nutrient source, thereby closing the circular economy loop.Data availability
Data for this article, including tables of phosphate and nitrate kinetics, isotherms, Doehlert's matrix design, and adsorption data are available at Science Data Bank at https://doi.org/10.57760/sciencedb.09760.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Thomas P. Blanchard of the Department of Oceanography and Coastal Sciences, College of the Coast and Environment, Louisiana State University for his help and guidance with the phosphate measurement protocols. We thank Dr Dorin Boldor and Dr Yongchan Kwon of the Biological & Agricultural Engineering Department, Louisiana State University for permission to use their centrifuge. We thank Dr Chandra Theegala of the Biological & Agricultural Engineering Department, Louisiana State University for his assistance with the spectrophotometer. We also thank Mr Michael Lowe and Ms Cynthia Thomas, both wastewater treatment lab supervisors, for their help with wastewater samples and characteristic data. We also acknowledge financial support from the Louisiana Board of Regents and the USDA NIFA.References
- R. O. Carey and K. W. Migliaccio, Contribution of wastewater treatment plant effluents to nutrient dynamics in aquatic systems, Environ. Manage., 2009, 44(2), 205–217 CrossRef PubMed.
- Y. Wu, Y. Wang, J. Wang, S. Xu, L. Yu and C. Philippe, et al., Nitrate removal from water by new polymeric adsorbent modified with amino and quaternary ammonium groups: batch and column adsorption study, J. Taiwan Inst. Chem. Eng., 2016, 66, 191–199, DOI:10.1016/j.jtice.2016.06.019.
- A. Battas, A. El Gaidoumi, A. Ksakas and A. Kherbeche, Adsorption study for the removal of nitrate from water using local clay, Sci. World J., 2019, 2019, 1–10, DOI:10.1155/2019/9529618.
- Y. W. Berkessa, S. T. Mereta and F. F. Feyisa, Simultaneous removal of nitrate and phosphate from wastewater using solid waste from factory, Appl. Water Sci., 2019, 9(2), 1–10, DOI:10.1007/s13201-019-0906-z.
- T. Wu, S. S. Yang, L. Zhong, J. W. Pang, L. Zhang and X. F. Xia, et al., Simultaneous nitrification, denitrification and phosphorus removal: what have we done so far and how do we need to do in the future?, Sci. Total Environ., 2023, 856, 158977, DOI:10.1016/j.scitotenv.2022.158977.
- F. Mahoo, Methods for phosphorus recovery from waste water: a review, J. Biodiversity Environ. Sci., 2018, 13(2), 315–323 Search PubMed.
- A. Bhatnagar and M. Sillanpää, A review of emerging adsorbents for nitrate removal from water, Chem. Eng. J., 2011, 168(2), 493–504, DOI:10.1016/j.cej.2011.01.103.
- J. Xie, Z. Wang, S. Lu, D. Wu, Z. Zhang and H. Kong, Removal and recovery of phosphate from water by lanthanum hydroxide materials, Chem. Eng. J., 2014, 254, 163–170 CrossRef CAS.
- A. A. Werkneh and E. R. Rene, Applications of Nanotechnology and Biotechnology for Sustainable Water and Wastewater Treatment, Energy Environ. Sustainability, 2019, 405–430 Search PubMed.
- S. Abdolmaleki, S. M. Amininasab and M. Ghadermazi, Modification of Fe2O3-contained lignocellulose nanocomposite with silane group to remove nitrate and bacterial contaminations from wastewater, Iran. Polym. J., 2019, 28(10), 859–872, DOI:10.1007/s13726-019-00749-9.
- N. Öztürk and T. E. Bektaş, Nitrate removal from aqueous solution by adsorption onto various materials, J. Hazard. Mater., 2004, 112(1–2), 155–162 CrossRef PubMed.
- M. Sajjadi, F. Ahmadpoor, M. Nasrollahzadeh and H. Ghafuri, Lignin-derived (nano)materials for environmental pollution remediation: current challenges and future perspectives, Int. J. Biol. Macromol., 2021, 178, 394–423, DOI:10.1016/j.ijbiomac.2021.02.165.
- T. Li, S. Lü, Z. Wang, M. Huang, J. Yan and M. Liu, Lignin-based nanoparticles for recovery and separation of phosphate and reused as renewable magnetic fertilizers, Sci. Total Environ., 2021, 765, 142745, DOI:10.1016/j.scitotenv.2020.142745.
- D. Wang, S. H. Lee, J. Kim and C. B. Park, “Waste to Wealth”: Lignin as a Renewable Building Block for Energy Harvesting/Storage and Environmental Remediation, ChemSusChem, 2020, 13(11), 2807–2827 CrossRef CAS PubMed.
- J. G. Lee, L. L. Larive, K. T. Valsaraj and B. Bharti, Binding of Lignin Nanoparticles at Oil-Water Interfaces: An Ecofriendly Alternative to Oil Spill Recovery, ACS Appl. Mater. Interfaces, 2018, 10(49), 43282–43289 CrossRef CAS PubMed.
- Q. Tang, Y. Qian, D. Yang, X. Qiu and Y. Qin, Lignin-Based Nanoparticles: A Review on Their Preparations and Applications, Polymers, 2020, 12–16 Search PubMed.
- L. Hu, C. Guang, Y. Liu, Z. Su, S. Gong and Y. Yao, et al., Adsorption behavior of dyes from an aqueous solution onto composite magnetic lignin adsorbent, Chemosphere, 2020, 246, 125757, DOI:10.1016/j.chemosphere.2019.125757.
- H. W. Kwak, H. Lee and K. H. Lee, Surface-modified spherical lignin particles with superior Cr(VI) removal efficiency, Chemosphere, 2020, 239, 124733, DOI:10.1016/j.chemosphere.2019.124733.
- A. Naseer, A. Jamshaid, A. Hamid, N. Muhammad, M. Ghauri and J. Iqbal, et al., Lignin and lignin based materials for the removal of heavy metals from waste water - an overview, Z. Phys. Chem., 2019, 233(3), 315–345 CrossRef CAS.
- R. Wahlström, A. Kalliola, J. Heikkinen, H. Kyllönen and T. Tamminen, Lignin cationization with glycidyltrimethylammonium chloride aiming at water purification applications, Ind. Crops Prod., 2017, 104(February), 188–194, DOI:10.1016/j.indcrop.2017.04.026.
- H. Guo, Y. Chen, S. Yang, R. Li, X. Zhang and Q. Dong, et al., Lignin-based adsorbent-catalyst with high capacity and stability for polychlorinated aromatics removal, Bioresour. Technol., 2021, 337(June), 125453, DOI:10.1016/j.biortech.2021.125453.
- T. Zhao, K. Zhang, J. Chen, X. Shi, X. Li and Y. Ma, et al., Changes in heavy metal mobility and availability in contaminated wet-land soil remediated using lignin-based poly(acrylic acid), J. Hazard. Mater., 2019, 368(January), 459–467 CrossRef CAS PubMed.
- E. Zong, G. Huang, X. Liu, W. Lei, S. Jiang and Z. Ma, et al., A lignin-based nano-adsorbent for superfast and highly selective removal of phosphate, J. Mater. Chem. A, 2018, 6(21), 9971–9983 RSC , available from: http://xlink.rsc.org/?DOI=C8TA01449C..
- E. Zong, X. Liu, J. Jiang, S. Fu and F. Chu, Preparation and characterization of zirconia-loaded lignocellulosic butanol residue as a biosorbent for phosphate removal from aqueous solution, Appl. Surf. Sci., 2016, 387, 419–430, DOI:10.1016/j.apsusc.2016.06.107.
- P. Ekins, T. Domenech, P. Drummond, R. Bleischwitz, N. Hughes and L. Lotti, The Circular Economy: What, Why, How and Where, Managing Environmental and Energy Transitions for Regions and Cities, 2019, pp. 1–89, available from: https://www.oecd.org/cfe/regionaldevelopment/Ekins-2019-Circular-Economy-What-Why-How-Where.pdf Search PubMed.
- H. Gogoi, T. Leiviskä, J. Rämö and J. Tanskanen, Production of aminated peat from branched polyethylenimine and glycidyltrimethylammonium chloride for sulphate removal from mining water, Environ. Res., 2019, 175(January), 323–334, DOI:10.1016/j.envres.2019.05.022.
- P. I. F. Pinto, S. Magina, E. Budjav, P. C. R. Pinto, F. Liebner and D. Evtuguin, Cationization of Eucalyptus Kraft LignoBoost Lignin: Preparation, Properties, and Potential Applications, Ind. Eng. Chem. Res., 2022, 61(10), 3503–3515 CrossRef CAS PubMed.
- S. Chauhan, B. Kumari, G. S. Chauhan, K. Chauhan, S. Ranote and R. Kumar, et al., A highly efficient and green adsorbent for anionic dyes synthesized from whole pine needles modified with glycidyltrimethylammonium chloride: synthesis, kinetic, and thermodynamic investigation, Biomass Convers. Biorefin., 2023, 1, 3, DOI:10.1007/s13399-023-04776-8.
- H. Zhang, K. Xue, B. Wang, W. Ren, D. Sun and C. Shao, et al., Advances in lignin-based biosorbents for sustainable wastewater treatment, Bioresour. Technol., 2024, 395(January), 130347, DOI:10.1016/j.biortech.2024.130347.
- F. Kong, K. Parhiala, S. Wang and P. Fatehi, Preparation of cationic softwood kraft lignin and its application in dye removal, Eur. Polym. J., 2015, 67, 335–345, DOI:10.1016/j.eurpolymj.2015.04.004.
- D. S. Argyropoulos, N. Pajer and C. Crestini, Quantitative 31P NMR Analysis of Lignins and Tannins, J. Visualized Exp., 2021, 174, e62696, DOI:10.3791/62696.
- A. Granata and D. S. Argyropoulos, 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane, a Reagent for the Accurate Determination of the Uncondensed and Condensed Phenolic Moieties in Lignins, J. Agric. Food Chem., 1995, 1538–1544 CrossRef CAS.
- X. Meng, C. Crestini, H. Ben, N. Hao, Y. Pu and A. J. Ragauskas, et al., Determination of hydroxyl groups in biorefinery resources via quantitative 31P NMR spectroscopy, Nat. Protoc., 2019, 14(9), 2627–2647, DOI:10.1038/s41596-019-0191-1.
- J. F. Araneda, I. W. Burton, M. Paleologou, S. D. Riegel and M. C. Leclerc, Analysis of lignins using 31P benchtop NMR spectroscopy: quantitative assessment of substructures and comparison to high-field NMR, Can. J. Chem., 2022, 100(11), 799–808 CrossRef CAS.
- G. J. Jiao, J. Ma, Y. Li, D. Jin, Y. Guo and J. Zhou, et al., Enhanced adsorption activity for phosphate removal by functional lignin-derived carbon-based adsorbent: optimization, performance and evaluation, Sci. Total Environ., 2021, 761, 143217, DOI:10.1016/j.scitotenv.2020.143217.
- S. L. C. Ferreira, W. N. L. Dos Santos, C. M. Quintella, B. B. Neto and J. M. Bosque-Sendra, Doehlert matrix: a chemometric tool for analytical chemistry - review, Talanta, 2004, 63(4), 1061–1067 CrossRef CAS PubMed.
- C. Resendiz-Moctezuma, A. P. L. Fonville, B. N. Harsh, M. J. Stasiewicz and M. J. Miller, Use of Doehlert Matrix as a Tool for High-Throughput Screening of Organic Acids and Essential Oils on Miniaturized Pork Loins, Followed by Lab-Scale Validation That Confirmed Tested Compounds Do Not Show Synergistic Effects against Salmonella Typhimurium, Foods, 2023, 12(21), 4034, DOI:10.3390/foods12214034.
- C. Fan and Y. Zhang, Adsorption isotherms, kinetics and thermodynamics of nitrate and phosphate in binary systems on a novel adsorbent derived from corn stalks, J. Geochem. Explor., 2018, 188(April 2017), 95–100, DOI:10.1016/j.gexplo.2018.01.020.
- P. Saha, S. Datta and S. K. Sanyal, Application of Natural Clayey Soil as Adsorbent for the Removal of Copper from Wastewater, J. Environ. Eng., 2010, 136(12), 1409–1417 CrossRef CAS.
- O. S. H. Santos, M. Coelho da Silva, V. R. Silva, W. N. Mussel and M. I. Yoshida, Polyurethane foam impregnated with lignin as a filler for the removal of crude oil from contaminated water, J. Hazard. Mater., 2017, 324, 406–413, DOI:10.1016/j.jhazmat.2016.11.004.
- K. Y. Foo and B. H. Hameed, Insights into the modeling of adsorption isotherm systems, Chem. Eng. J., 2010, 156(1), 2–10 CrossRef CAS.
- K. Acurio Cerda, M. Kathol, G. Purohit, E. Zamani, M. D. Morton and O. Khalimonchuk, et al., Cationic Lignin as an Efficient and Biorenewable Antimicrobial Material, ACS Sustainable Chem. Eng., 2023, 11(28), 10364–10379, DOI:10.1021/acssuschemeng.3c01414.
- Y. Wang, W. Liu, L. Zhang and Q. Hou, Characterization and comparison of lignin derived from corncob residues to better understand its potential applications, Int. J. Biol. Macromol., 2019, 134, 20–27, DOI:10.1016/j.ijbiomac.2019.05.013.
- E. L. Ungureanu, A. L. Mocanu, C. A. Stroe, C. M. Panciu, L. Berca and R. M. Sionel, Agricultural Byproducts Used as Low-Cost Adsorbents for Removal of Potentially Toxic Elements from Wastewater: A Comprehensive Review, Sustainability, 2023, 15(7), 5999, DOI:10.3390/su15075999.
- S. P. Boeykens, M. N. Piol, L. Samudio Legal, A. B. Saralegui and C. Vázquez, Eutrophication decrease: phosphate adsorption processes in presence of nitrates, J. Environ. Manage., 2017, 203, 888–895 CrossRef CAS PubMed.
- Y. Xu, T. Liu, Y. Huang, J. Zhu and R. Zhu, Role of phosphate concentration in control for phosphate removal and recovery by layered double hydroxides, Environ. Sci. Pollut. Res., 2020, 27(14), 16612–16623 CrossRef CAS PubMed.
- Z. Jia, W. Zeng, H. Xu, S. Li and Y. Peng, Adsorption removal and reuse of phosphate from wastewater using a novel adsorbent of lanthanum-modified platanus biochar, Process Saf. Environ. Prot., 2020, 140, 221–232, DOI:10.1016/j.psep.2020.05.017.
- N. Y. Acelas, B. D. Martin, D. López and B. Jefferson, Selective removal of phosphate from wastewater using hydrated metal oxides dispersed within anionic exchange media, Chemosphere, 2015, 119, 1353–1360 CrossRef CAS PubMed.
- Y. He, H. Lin, Y. Dong and L. Wang, Preferable adsorption of phosphate using lanthanum-incorporated porous zeolite: characteristics and mechanism, Appl. Surf. Sci., 2017, 426, 995–1004, DOI:10.1016/j.apsusc.2017.07.272.
- S. Yang, P. Jin, X. Wang, Q. Zhang and X. Chen, Phosphate recovery through adsorption assisted precipitation using novel precipitation material developed from building waste: behavior and mechanism, Chem. Eng. J., 2016, 292, 246–254, DOI:10.1016/j.cej.2016.02.006.
- S. Wiriyathamcharoen, S. Sarkar, P. Jiemvarangkul, T. T. Nguyen, W. Klysuban and S. Padungthon, Synthesis optimization of hybrid anion exchanger containing triethylamine functional groups and hydrated Fe(III) oxide nanoparticles for simultaneous nitrate and phosphate removal, Chem. Eng. J., 2020, 381(May 2019), 122671, DOI:10.1016/j.cej.2019.122671.
- Q. Xu, Z. Chen, Z. Wu, F. Xu, D. Yang and Q. He, et al., Novel lanthanum doped biochars derived from lignocellulosic wastes for efficient phosphate removal and regeneration, Bioresour. Technol., 2019, 289(April), 121600, DOI:10.1016/j.biortech.2019.121600.
- J. Xie, Z. Wang, S. Lu, D. Wu, Z. Zhang and H. Kong, Removal and recovery of phosphate from water by lanthanum hydroxide materials, Chem. Eng. J., 2014, 254, 163–170, DOI:10.1016/j.cej.2014.05.113.
- E. Zong, D. Wei, H. Wan, S. Zheng, Z. Xu and D. Zhu, Adsorptive removal of phosphate ions from aqueous solution using zirconia-functionalized graphite oxide, Chem. Eng. J., 2013, 221, 193–203, DOI:10.1016/j.cej.2013.01.088.
- C. A. Takaya, L. A. Fletcher, S. Singh, U. C. Okwuosa and A. B. Ross, Recovery of phosphate with chemically modified biochars, J. Environ. Chem. Eng., 2016, 4(1), 1156–1165, DOI:10.1016/j.jece.2016.01.011.
- L. Chen, X. Zhao, B. Pan, W. Zhang, M. Hua and L. Lv, et al., Preferable removal of phosphate from water using hydrous zirconium oxide-based nanocomposite of high stability, J. Hazard. Mater., 2015, 284, 35–42 CrossRef CAS PubMed.
- T. Han, X. Lu, Y. Sun, J. Jiang, W. Yang and P. G. Jönsson, Magnetic bio-activated carbon production from lignin via a streamlined process and its use in phosphate removal from aqueous solutions, Sci. Total Environ., 2020, 708, 135069, DOI:10.1016/j.scitotenv.2019.135069.
- S. Li, T. Galoustian and H. Trejo, Biochar pyrolyzed with concentrated solar radiation for enhanced nitrate adsorption, J. Anal. Appl. Pyrolysis, 2023, 174(July), 106131, DOI:10.1016/j.jaap.2023.106131.
- M. de Carvalho Eufrásio Pinto, D. David da Silva, A. L. Amorim Gomes, R. M. Menezes dos Santos, R. A. Alves de Couto and R. Ferreira de Novais, et al., Biochar from carrot residues chemically modified with magnesium for removing phosphorus from aqueous solution, J. Cleaner Prod., 2019, 222, 36–46 CrossRef.
- S. B. Imandi, V. V. R. Bandaru, S. R. Somalanka and H. R. Garapati, Optimization of medium constituents for the production of citric acid from byproduct glycerol using Doehlert experimental design, Enzyme Microb. Technol., 2007, 40(5), 1367–1372 CrossRef CAS.
- H. Xu, H. W. Paerl, B. Qin, G. Zhu, N. S. Hall and Y. Wu, Determining critical nutrient thresholds needed to control harmful cyanobacterial blooms in eutrophic Lake Taihu, China, Environ. Sci. Technol., 2015, 49(2), 1051–1059 CrossRef CAS PubMed.
- Q. Zhou, H. Sun, L. Jia, W. Wu and J. Wang, Simultaneous biological removal of nitrogen and phosphorus from secondary effluent of wastewater treatment plants by advanced treatment: a review, Chemosphere, 2022, 296(December 2021), 134054, DOI:10.1016/j.chemosphere.2022.134054.
- A. C. Chang, A. L. Page and F. T. Bingham, Re-utilization of municipal wastewater sludges - metals and nitrate, J. Water Pollut. Control Fed., 1981, 53(2), 237–245 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00312h |
| This journal is © The Royal Society of Chemistry 2024 |