Open Access Article
Open Access ArticleSustainable synthesis of activated porous carbon from lignin for enhanced CO2 capture: a comparative study of physicochemical activation routes†
Himanshu
Patel
 ab,
Amar
Mohanty
ab,
Amar
Mohanty
 ab and
Manjusri
Misra
ab and
Manjusri
Misra
 *ab
*ab
aBioproducts Discovery and Development Centre, Department of Plant Agriculture, University of Guelph, Crop Science Building, Guelph, Ontario N1G 2W1, Canada. E-mail: mmisra@uoguelph.ca
bSchool of Engineering, University of Guelph, Thornbrough Building, 80 South Ring Road E, Guelph, Ontario N1G 2W1, Canada
First published on 30th July 2024
Abstract
A sustainable and readily available material, lignin protobind 2400, was upcycled to activated porous carbon (APC) compatible with post-combustion CO2 capture. The effectiveness of the novel two-step physicochemical activation using KOH + CO2 and ZnCl2 + CO2 was compared with that of the respective physical (only CO2) and chemical activation (only KOH or ZnCl2). The effect of carbonization conditions (N2 or CO2 purging) on the resulting APC properties and CO2 adsorption performance was studied. The maximum BET surface area of 1480 m2 g−1 and the best CO2 adsorption capacity of 5.68, 3.66, and 2.67 mmol g−1 were observed at 0, 25, and 40 °C/1 bar, respectively. From the precursor to the final product, the APC yield falls within the range of 14.5–40.8 wt%. The APC derived from lignin exhibited better CO2/N2 selectivity. The isosteric heat of adsorption for all the APCs remained below 40 kJ mol−1, which suggested a lower energy requirement during the regeneration. The excellent reusability with fluctuations of only 0.51% in the amount of CO2 adsorbed over ten consecutive adsorption/desorption cycles highlights the APC's outstanding recyclability.
1. Introduction
Climate change is closely tied to the continuous increase in anthropogenic greenhouse gas (GHG) emissions and poses a significant threat to human existence. The energy sector, driven by hefty dependence on fossil fuels, is accountable for 72% of GHGs, with CO2 contributing 76% to the overall GHG emissions.1 Despite a noteworthy increase in renewables, fossil fuels are projected to maintain a substantial global dependence of 74% by 2040, underscoring the critical need to decarbonize the energy sector.2 This highlights the significance of CO2 as the most crucial greenhouse gas (GHG) contributing to catastrophic events, such as climate change associated with global warming, increased ocean acidity, and rising sea levels. The World Meteorological Organization's report highlights that the socioeconomic consequences of climate change are worsening, with unprecedented GHG concentrations pushing global temperatures perilously closer to critical levels.3 The primary aim of the Paris Climate Change Agreement is to achieve a 40% reduction in GHG emissions by 2030.4 To address the immediate challenges posed by the escalating levels of global CO2 concentration, there is an ongoing exploration of carbon capture, utilization, and storage (CCUS) as a potential short-term solution to curb the release of this specific greenhouse gas. Fundamentally, carbon capture involves the application of technologies to either directly extract CO2 from the air/atmosphere or to capture it during the formation process (large point source). Specifically, the removal of CO2 from a flue gas stream is termed post-combustion CO2 capture, which can be a cost-effective approach for addressing emissions from current coal and gas-fired power plants, as well as coal-intensive industries, such as cement, oil refineries, chemicals, and steel.5 Approximately one-third of the existing coal and gas-fired power plants have been constructed in the last decade. Retrofitting these facilities with post-combustion CO2 capture enables them to remain operational, thereby avoiding the expenses associated with early phase shutdowns.6Several methods such as amine absorption, membrane separation, cryogenic distillation, and physical adsorption have been developed for CO2 capture from industrial flue gases. The adsorption-based method, utilizing solid adsorbents such as porous aluminosilicate or zeolites, metal organic frameworks, silica gel, and activated porous carbons (APCs), offers excellent performance and energy efficiency compared to other separation techniques.7,8 In this context, APCs present numerous advantages over other CO2 adsorbents. Their affordability, well-developed pore structure, exceptional thermal and chemical stability, heightened efficiency under humid conditions, straightforward surface functionalization, and lower energy requirements during regeneration highlight their significance as CO2 adsorbents coupled with commendable multi-cyclic stability.9,10 Presently, large-scale production of APCs predominantly relies on carbon-intensive precursors, such as coal, as well as carbon-neutral alternatives, such as wood and coconut shells.11 Researchers are exploring economically viable and efficient replacements to mitigate the environmental impact of this sector and fortify the supply chain.12
Lignin is the Earth's second most abundant naturally occurring complex organic material. Lignocellulosic wastes (forest and agricultural waste) and pulp paper mills are two potential sources of lignin, generating 225 and 130 Mt of lignin per annum, respectively.13 Projections indicate that this figure will surge by 225 Mt per year by 2030, driven by the increasing annual production of lignin as a by-product of bioethanol production mandated by the Renewable Fuel Standard (RFS) program aimed at 60 billion gallons of biofuel.14 Hence, the development of cost-effective valorisation technologies is crucial to ensure the long-term stability and vitality of biorefineries. Traditionally regarded as a low-value waste by-product, recent studies have demonstrated its potential in producing high-value commodities.15–19 Despite an expanding body of research on the conversion of lignin into commercial commodities, a large fraction of lignin produced by the paper industry is currently incinerated as a low-value fuel for electricity and heat generation (with a value of <$50 per dry ton).20,21 Less than 2% is used to produce specialty chemicals and other value-added products. The advancement of value-added lignin-derived co-products has the potential to enhance the profitability of second-generation biorefineries and the paper industry by valorising their lignin by-products.
Physical and chemical activation are the most common and extensively studied techniques for producing APCs. Their individual effects on the performance and properties of APCs prepared from a wide range of carbonaceous materials have been investigated extensively. A hybrid synthesis route of physicochemical activation that simultaneously utilizes both physical and chemical activating agents is less explored. Previously, some researchers investigated the effect of physicochemical activation on other applications, such as Cu adsorption,22 dye (i.e., crystal violate) removal,23 and CH4 storage.24 The effect of physicochemical activation on the CO2 adsorption performance is even less studied among other applications of APCs. Previous studies have reported the preparation of APC via physicochemical activation using ZnCl2 + CO225 and KOH + CO226,27 for CO2 adsorption. However, these studies lacked a comparison of the effectiveness of physicochemical activation with the respective physical and chemical reagents used. In our previous study, we compared the CO2 adsorption performance of APCs derived through physicochemical activation of pine sawdust with those derived through chemical activation (using only KOH) and physical activation (using only CO2).28 Physicochemical activation (KOH + CO2) resulted in APCs with 68% and 586% more BET surface area compared to APCs derived using only KOH and only CO2 activation, respectively. However, the CO2 adsorption capacity of physicochemically activated carbon was 26.5% lower than that of APCs produced using only KOH and 53.3% higher than that of APCs produced using only CO2 activation. However, a limitation of this study was that it tested only one combination of physical and chemical activating reagents. The results may be significantly different for different combinations and feedstocks.
In this study, the combined effects of chemical activating agents (such as KOH and ZnCl2) and a physical activating agent (CO2) on the textural properties and CO2 adsorption performance of the resulting APC were investigated. In addition, we compared the effectiveness of physicochemical activation with that of individual chemical and physical activating agents. The effect of carbonization conditions on APC performance was also studied. Lignin protobind 2400, a sustainable material, was used as a precursor to derive APC for CO2 capture applications.
2. Experimental
2.1. Feedstock and material
Lignin protobind 2400 (L) was purchased from ALM Private Limited, Hoshiarpur, Punjab, India. The chemical and thermal properties of L have been reported previously.29 KOH pellets (P250-3) with a purity of ≥85.0%, anhydrous ZnCl2 with a purity of ≥98.0%, and 1 N HCl solution (SA48-1) from Fisher Scientific were used.2.2. Synthesis of activated porous carbon
A horizontal fixed-bed annealing furnace (Carbolite GERO, GLO 10/11-1G) was used for carbonization and activation. More details on the furnace used have been published elsewhere.28 APCs were produced by direct activation as well as two-step activation. KOH, ZnCl2, CO2, and their combinations were used as the activating agents. Based on the type of activating agent used, the activation can be classified into three categories: (i) physical activation using CO2, (ii) chemical activation utilizing KOH and ZnCl2, and (iii) physicochemical activation using KOH + CO2 and ZnCl2 + CO2. Fig. 1 illustrates the methodology followed during the APC preparation. The precursors were dried in a hot-air oven at 80 °C for 24 h, prior to carbonization and/or activation. Lignin protobind 2400 was carbonized at 600 °C, with a heating rate of 7.5 °C min−1 of a heating rate and a residence time of 1.5 h in an N2 atmosphere (at 50 NLPH). The reactor was allowed to cool down naturally and the resulting biochar was labelled as L N. Pore formation via CO2 mainly occurs via the Boudouard reaction, which is activated above 700 °C.30 Considering the poor thermal conductivity of the feedstock used and the heat transfer limitations of the reactor housing, all activation experiments were performed at 800 °C. Direct physical activation of lignin protobind 2400 was performed at 800 °C, at a heating rate of 7.5 °C min−1, and with 1.5 h of residence time in a CO2 atmosphere (at 75 NLPH). The reactor was cooled naturally, and the resulting biochar was labelled L CD. For all CO2 related activations, the precursors were heated from room temperature to 800 °C in an N2 environment (at 50 NLPH). After achieving 800 °C, N2 purging was stopped, and CO2 was purged (at 75 NLPH) throughout the holding time of 1.5 h and until the reactor temperature dropped below 700 °C during the natural cool-down cycle. From 700 °C to room temperature, N2 was purged.Directly activated APC (i.e., L CD) and biochar (i.e., L N) were pulverized in a ball mill (Fritsch, Pulverisette 5) for 1 h and sieved through 300 μm mesh. Prior to chemical and physicochemical activation, 25 g of the pulverized precursor was impregnated with 50 g of KOH or ZnCl2, and 150 ml of deionized water was added. The slurry was stirred for 24 h at 500 rpm followed by overnight drying in a hot-air oven at 105 °C. The dried and impregnated samples were activated at 800 °C, with a heating rate of 5 °C min−1 for 1.5 h. Adhering KOH or ZnCl2 was removed from the derived APCs via hot water washing, followed by acid soaking in 0.1 M HCl, and then further hot water washing until a neutral pH was achieved. The washed APCs were dried at 105 °C for 24 h, labelled, and stored. The yield was calculated based on the initial and final weight differences. The combined yield of the carbonization and activation steps was considered as the APC yield.
L X–Y denotes the lignin protobind 2400 based activated porous carbon. Where X denotes the type of purging gas used during carbonization: X = N indicates carbonization was executed in N2 and X = CD indicates carbonization was performed in a CO2 atmosphere. Y denotes the type of activating agent used in the two-step activation process: Y = K indicates KOH is used as an activating agent, Y = CD denotes CO2 is used as an activating agent, Y = Z means ZnCl2 is used as an activating agent, Y = KCD means KOH + CO2 is used as the activating agent, and Y = ZCD indicates ZnCl2 + CO2 is used as the activating agent. For example, L N–KCD represents lignin protobind 2400 based activated carbon derived via a two-step activation method from lignin protobind 2400 biochar prepared in a N2 atmosphere, followed by activation using KOH + CO2 as an activating agent. The details of the characterization protocol have been explained elsewhere.28,31
3. Results and discussion
3.1. Composition and structural characterization
The elemental compositions of lignin protobind 2400, biochar, and APCs are listed in Table 1. Carbonization significantly increased the carbon content and reduced the hydrogen and oxygen content. Chemical activation using KOH and physical activation using CO2 produced APC with slightly higher carbon content than biochar. However, chemical activation using ZnCl2 and physicochemical activation (KOH + CO2 and ZnCl2 + CO2) significantly reduced the carbon content compared to biochar. Similar trends were observed during physicochemical activation (using KOH + CO2) of pine sawdust.28 Compared to the feedstock, the reduced H/C molar ratio for the biochar and APCs indicated that the aromatization reaction was dominant during carbonization and activation.| Material | C, wt% | H, wt% | N, wt% | O,a wt% | H/C | C/O | H/O |
|---|---|---|---|---|---|---|---|
| a By difference. SD = standard deviation. | |||||||
| L | 63.03 | 6.20 | 1.23 | 29.54 | 1.18 | 2.85 | 3.36 |
| SD = 0.23 | SD = 0.01 | SD = 0.00 | SD = 0.22 | ||||
| L N | 84.84 | 2.45 | 1.13 | 11.58 | 0.35 | 9.77 | 3.39 |
| SD = 0.09 | SD = 0.01 | SD = 0.98 | SD = 0.95 | ||||
| L N–K | 85.05 | 0.32 | 0.68 | 13.95 | 0.05 | 8.13 | 0.37 |
| SD = 0.30 | SD = 0.03 | SD = 0.02 | SD = 0.27 | ||||
| L CD–K | 87.25 | 1.02 | 0.78 | 10.95 | 0.14 | 10.62 | 1.49 |
| SD = 0.63 | SD = 0.01 | SD = 0.01 | SD = 0.62 | ||||
| L N–CD | 86.17 | 1.15 | 1.12 | 11.56 | 0.16 | 9.94 | 1.59 |
| SD = 1.79 | SD = 0.10 | SD = 0.13 | SD = 1.95 | ||||
| L CD | 85.91 | 0.79 | 1.30 | 12.00 | 0.11 | 9.55 | 1.05 |
| SD = 1.05 | SD = 0.09 | SD = 0.15 | SD = 1.27 | ||||
| L N–KCD | 81.86 | 0.51 | 1.22 | 16.41 | 0.07 | 6.65 | 0.50 |
| SD = 1.41 | SD = 0.16 | SD = 0.22 | SD = 1.77 | ||||
| L CD–KCD | 81.10 | 1.16 | 1.20 | 16.53 | 0.17 | 6.54 | 1.12 |
| SD = 1.14 | SD = 0.03 | SD = 0.01 | SD = 1.13 | ||||
| L N–Z | 76.24 | 0.84 | 0.66 | 22.25 | 0.13 | 4.57 | 0.61 |
| SD = 6.36 | SD = 0.15 | SD = 0.02 | SD = 6.53 | ||||
| L N–ZCD | 76.11 | 0.93 | 0.61 | 22.35 | 0.15 | 5.93 | 0.90 |
| SD = 4.67 | SD = 0.13 | SD = 0.04 | SD = 4.80 | ||||
| L CD–Z | 80.22 | 1.01 | 0.74 | 18.03 | 0.15 | 5.93 | 0.90 |
| SD = 1.55 | SD = 0.09 | SD = 0.02 | SD = 1.65 | ||||
| L CD–ZCD | 80.37 | 1.06 | 0.67 | 17.90 | 0.16 | 5.99 | 0.95 |
| SD = 2.81 | SD = 0.13 | SD = 0.05 | SD = 2.94 | ||||
Scanning electron microscopy (SEM) was utilized to investigate the morphology of the lignin protobind 2400 based APCs. SEM micrographs of all lignin-based APCs are presented in Fig. S1 (ESI†), indicating a blocky and sharp edge morphology. Random cracks and cavities were observed on the external surface. The porous structure and detailed morphology of L N–K were further investigated using transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HR-TEM). As portrayed in Fig. 2a, the overlapping of multiple carbon sheets resulted in a relatively dense TEM image. The HR-TEM image of L N–K depicted in Fig. 2b shows disordered worm-like micropores randomly distributed on the surface of L N–K, indicating a microporous nature, which was further confirmed by N2 adsorption isotherms.
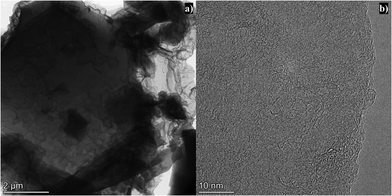 | ||
| Fig. 2 (a) Transmission electron micrograph and (b) high-resolution transmission electron micrograph of L N–K. | ||
Raman spectra were recorded to evaluate crystallographic disorders in the carbon structure. Only the key features of the Raman spectra within the Raman shift range of 800–2000 cm−1 are highlighted in Fig. 3. Two broad overlapping peaks were deconvoluted using the Fourier–Gaussian peak-fitting method. The first peak, a maxima occurring at ∼1300 cm−1, represents a defect or disorder in the carbon structure originating from local defects (D-band). The second peak at ∼1600 cm−1 can be attributed to the graphitic crystallites of sp2 hybridized carbon (G-band). The ID/IG ratio is the intensity ratio of the D and G peaks, which quantifies the degree of graphitization. This signifies the ratio of disordered carbon structure to ordered carbon structure. A lower ID/IG ratio indicates a more ordered or crystalline carbon structure. Fig. 3k portrays the ID/IG ratios of all ten APCs. Physical activation resulted in the most ordered carbon structure morphologically, which is consistent with a previous study using different feedstocks.28 Chemical or physicochemical activation leads to pronounced deformation of the aromatic rings, which reduces the degree of graphitization. During activation, ZnCl2 was more reactive towards the aromatic rings of biochar produced in a CO2 atmosphere than in a N2 atmosphere. This can be inferred from the higher ID/IG ratio for L N–Z than for L CD–Z and L N–ZCD than for L CD–ZCD. Conversely, KOH was more reactive towards aromatic rings of biochar produced in a N2 atmosphere than in a CO2 atmosphere. The higher ID/IG ratio for L N–K than for L CD–K, L N–KCD and L CD–KCD support this statement.
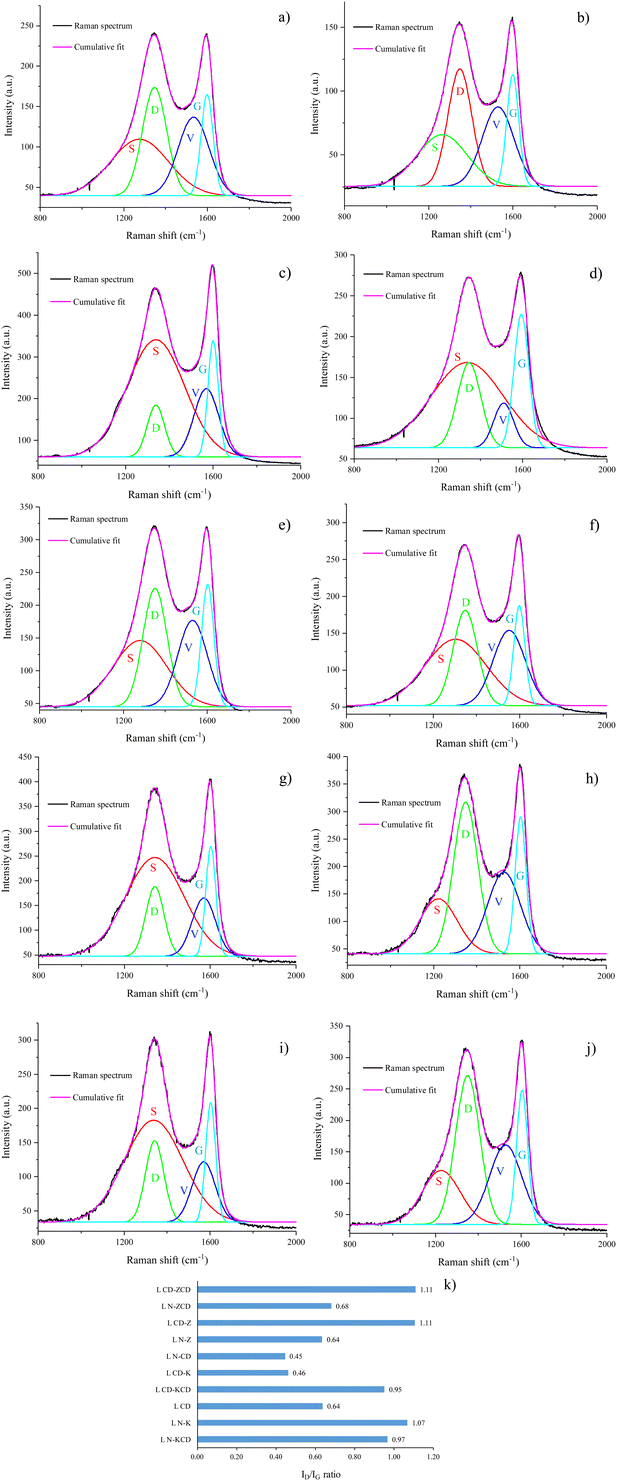 | ||
| Fig. 3 Curve fitting of Raman spectra (a) L N–K, (b) L CD–K, (c) L N–CD, (d) L CD, (e) L N–KCD, (f) L CD–KCD, (g) L N–Z, (h) L CD–Z, (i) L N–ZCD, (j) L CD–ZCD, and (k) ID/IG ratio. | ||
3.2. Textural properties
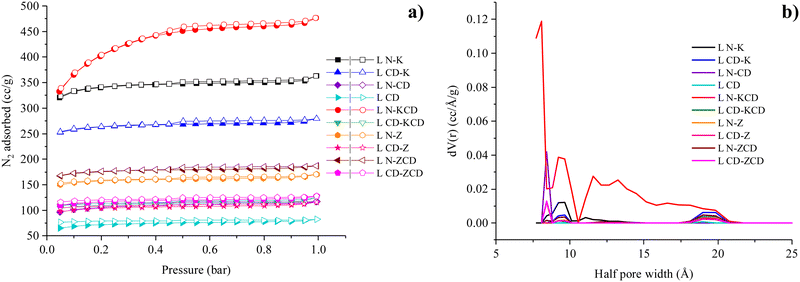
The textural properties of the carbonaceous adsorbent significantly affect its CO2 adsorption performance. Therefore, to evaluate the porosity, N2 adsorption isotherms were recorded at −196 °C up to a relative pressure of 1.0 bar. Fig. 4a shows the N2 adsorption isotherms of lignin-based APCs prepared using a wide range of activating agents. For all the samples, a steep rise in N2 adsorption was observed at P/P0 < 0.05. The rise ranged from 65 to 333 cc g−1, which could be attributed to micropore filling due to better adsorbent–adsorbate interactions at lower pressures.32,33 Thereafter, beyond P/P0 = 0.05, the N2 adsorption increased gradually, except for L N–KCD. For L N–KCD, N2 adsorption continued to rise exponentially up to a P/P0 of 0.45 and reached a plateau. For all the samples, hysteresis at P/P0 > 0.4 suggested a type IV isotherm according to IUPAC classification. The existence of hysteresis is linked to the capillary condensation of N2. Hysteresis is a characteristic of mesoporous adsorbents and confirms the presence of micro and mesopores.34,35 The pore size distribution curve presented in Fig. 4b (and Fig. S2, ESI†) further confirms the microporous and mesoporous nature of all APCs. The figure illustrates pore width maxima occurring at 8.4/9.7/11.1/19.0 Å for L N–K, 9.7/12.1/19.8 Å for L CD–K, 8.4 Å for L N–CD, 19.0 Å for L CD, 8.1/9.2/11.6 Å for L N–KCD, 9.2/19.0 Å for L CD–KCD, 8.4/9.7/19.0 Å for L N–Z, 9.7/19.0 Å for L CD–Z, 8.4/9.7/19.0 Å for L N–ZCD and 8.4/9.2/19.0 Å for L CD–ZCD. For all the APCs, the pore diameter of ∼8 Å available in the pore size distribution curves was large enough to accommodate CO2 with a kinetic diameter of 3.3 Å.36 This is crucial for efficient CO2 capture. Pore width maxima centred at 19 Å were common in L CD and APCs derived from the secondary activation of L CD, such as L CD–Z and L CD–ZCD. This indicated the absence of a pore-widening effect during secondary chemical activation using ZnCl2 and physicochemical activation using ZnCl2 + CO2. In contrast, the secondary chemical activation of L CD using KOH resulted in pore widening, and the maxima shifted to 19.8 Å. The pore size distribution curves suggested that the secondary activation of L CD via both chemical and physicochemical activation resulted in the formation of additional micropores.Table 2 lists the yields and key textural properties of the L-based APCs. The conversion yield of the raw biomass to APC ranged within 14.5–40.8 wt%. The highest SBET of 1480 m2 g−1 and maximum total pore volume of 0.737 cm3 g−1 were obtained for the APC produced via the physicochemical activation of L N using KOH + CO2. In contrast, the chemical activation of L N using KOH promoted the formation of micropores, achieving a micro-porosity (φmicro) as high as 97.74%. Physicochemical or chemical treatment of L N led to better porosity than the identical treatment of L CD did. For example, chemical activation of L N using KOH resulted in SBET of 1108 m2 g−1, which is considerably higher than that for the chemical activation of L CD using KOH. This could be attributed to the relatively higher processing temperature of L CD (i.e., 800 °C), leading to the formation of a sturdy aromatic structure, which could be less susceptible to further activation. For L N, combined physical and chemical activation (using KOH + CO2 and ZnCl2 + CO2) produced better porosity than physical activation (using CO2) and chemical activation (using KOH and ZnCl2) alone. Compared to KOH activation, physicochemical activation with KOH + CO2 increased the SBET by 33% and the total pore volume by 31%. However, the SBET and total pore volume were improved by ∼12% and 10%, respectively, for physicochemical activation using ZnCl2 + CO2 compared to activation with ZnCl2 only. A similar trend was reported in ref. 24, 28, and 37. For lignin-based APCs, the percentage of micropore volume (fmicro) ranged from 81 to 93%, indicating the dominance of micropores. Micropores are desired for better CO2 capture.
| Yield, wt% | S BET, m2 g−1 | S micro, m2 g−1 | φ micro, % | R avg, nm | V T, cm3 g−1 | V micro, cm3 g−1 | f micro, % | |
|---|---|---|---|---|---|---|---|---|
| S BET = specific BET surface area; Smicro = micro-pore area using t-plot method; φmicro = micro-porosity, % = (Smicro/SBET) × 100; Ravg = average pore radius; VT = estimated at a relative pressure P/P0 = 0.99; Vmicro = micropore volume estimated using t-plot method; fmicro = percentage of micro-pore volume, % = (Vmicro/VT) × 100. | ||||||||
| L N–KCD | 14.54 | 1480 | 1383 | 93.45 | 0.995 | 0.737 | 0.597 | 81.00 |
| L N–K | 28.96 | 1108 | 1083 | 97.74 | 1.014 | 0.562 | 0.515 | 91.64 |
| L CD | 40.82 | 269 | 257 | 95.53 | 0.946 | 0.127 | 0.107 | 84.25 |
| L CD–K | 35.28 | 844 | 826 | 97.87 | 1.024 | 0.432 | 0.400 | 92.59 |
| L N–CD | 35.36 | 399 | 385 | 96.49 | 0.908 | 0.181 | 0.159 | 87.84 |
| L CD–KCD | 30.09 | 353 | 338 | 95.75 | 1.093 | 0.193 | 0.163 | 84.46 |
| L N–Z | 38.93 | 504 | 491 | 97.42 | 1.045 | 0.264 | 0.237 | 89.77 |
| L CD–Z | 31.82 | 334 | 322 | 96.40 | 1.071 | 0.180 | 0.155 | 86.11 |
| L N–ZCD | 37.19 | 564 | 551 | 97.70 | 1.026 | 0.290 | 0.266 | 91.72 |
| L CD–ZCD | 31.82 | 377 | 364 | 96.55 | 1.051 | 0.198 | 0.175 | 88.38 |
3.3. CO2 adsorption characteristics
CO2 and N2 adsorption isotherms were recorded at 0, 25 and 40 °C up to 1 bar (Table 3). CO2 uptake values at 0.1–0.15 bar are also listed in Table 3. Low-pressure CO2 uptake at 0.1–0.15 bar is vital as it represents the realistic partial pressure of CO2 in the flue gas.38Fig. 5a–c demonstrate CO2 adsorption isotherms at three different temperatures. At 1 bar, saturation in terms of CO2 uptake was absent for L N–K, L CD–K and L N–KCD, indicating more CO2 uptake at elevated adsorption pressure. This was consistent over the range of tested adsorption temperatures. The absence of hysteresis indicated that CO2 adsorption followed type I isotherms, which further advocates the microporous nature of the APCs.| Sample | CO2 adsorption capacity, mmol g−1 | N2 adsorption capacity, mmol g−1 | q st, kJ mol−1 | CO2/N2 selectivity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 bar | 0.1 (0.15) bar | ||||||||||||
| 0 °C | 25 °C | 40 °C | 0 °C | 25 °C | 40 °C | 0 °C | 25 °C | 40 °C | 0 °C | 25 °C | 40 °C | ||
| L N–K | 5.68 | 3.66 | 2.67 | 1.38 (1.84) | 0.72 (1.00) | 0.46 (0.66) | 0.88 | 0.52 | 0.34 | 25.53 | 13.52 | 12.56 | 12.50 |
| L CD–K | 5.16 | 3.41 | 2.56 | 1.73 (2.17) | 0.85 (1.12) | 0.54 (0.73) | 0.75 | 0.46 | 0.30 | 29.99 | 18.51 | 16.09 | 16.14 |
| L N–CD | 2.77 | 2.00 | 1.57 | 1.16 (1.40) | 0.61 (0.79) | 0.40 (0.54) | 0.50 | 0.29 | 0.18 | 31.20 | 18.20 | 18.10 | 20.39 |
| L CD | 2.64 | 1.96 | 1.52 | 1.07 (1.31) | 0.61 (0.79) | 0.39 (0.53) | 0.47 | 0.28 | 0.18 | 30.31 | 17.69 | 17.88 | 18.79 |
| L N–KCD | 4.40 | 2.87 | 2.08 | 0.99 (1.31) | 0.52 (0.73) | 0.34 (0.48) | 0.66 | 0.40 | 0.26 | 24.53 | 12.97 | 11.86 | 11.97 |
| L CD–KCD | 2.80 | 2.02 | 1.55 | 1.11 (1.34) | 0.61 (0.78) | 0.39 (0.53) | 0.47 | 0.28 | 0.17 | 30.74 | 18.21 | 18.58 | 19.92 |
| L N–Z | 3.06 | 2.03 | 1.50 | 1.00 (1.26) | 0.51 (0.67) | 0.32 (0.44) | 0.49 | 0.29 | 0.17 | 20.29 | 16.45 | 15.15 | 17.17 |
| L CD–Z | 2.67 | 1.84 | 1.39 | 0.97 (1.20) | 0.50 (0.66) | 0.32 (0.43) | 0.44 | 0.26 | 0.16 | 32.51 | 17.71 | 16.50 | 17.82 |
| L N–ZCD | 3.14 | 2.04 | 1.51 | 0.97 (1.24) | 0.50 (0.65) | 0.31 (0.43) | 0.48 | 0.29 | 0.18 | 29.38 | 16.53 | 14.57 | 15.84 |
| L CD–ZCD | 2.73 | 1.87 | 1.41 | 0.98 (1.21) | 0.51 (0.67) | 0.33 (0.44) | 0.45 | 0.27 | 0.17 | 31.10 | 17.46 | 16.13 | 17.17 |
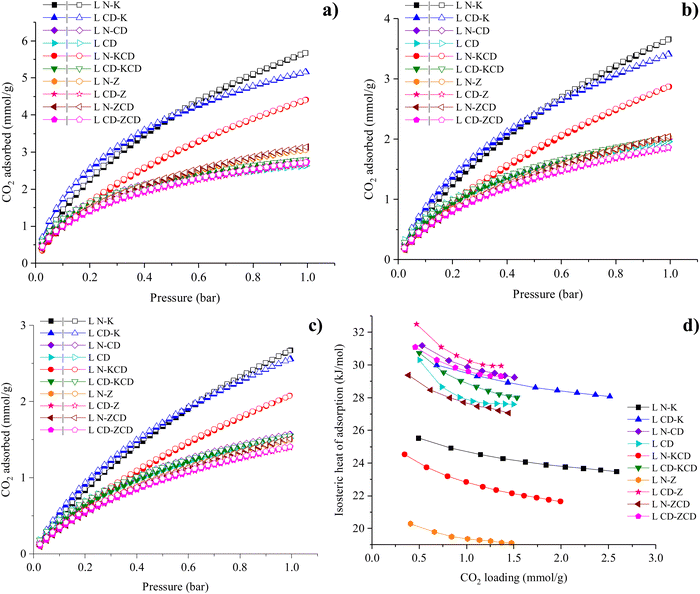 | ||
| Fig. 5 CO2 adsorption isotherms at (a) 0 °C, (b) 25 °C, and (c) 40 °C (solid symbols represent adsorption, and hollow symbols indicate desorption), and (d) isosteric heat of CO2 adsorption. | ||
Despite having higher SBET for physicochemically activated APC (i.e., L N–KCD), it indicated lower CO2 adsorption capacity than chemically activated APC (i.e., L N–K). It indicates non-linearity between SBET and CO2 adsorption capacity, which was commonly observed.7,28,39,40 Conversely, combined physical and chemical activation using ZnCl2 + CO2 slightly improved the CO2 uptake compared to individual physical or chemical activation using CO2 or ZnCl2, respectively. APCs derived via chemical activation using KOH resulted in the highest CO2 adsorption capacity. The maximum CO2 adsorption capacity of 5.68 mmol g−1 at 0 °C/1 bar was observed for L N–K. At lower adsorption pressure, L CD–K exhibited better CO2 adsorption than L N–K. However, the CO2 adsorption capacity of L N–K starts surpassing that of L CD–K above an adsorption pressure of 0.5 bar. The observation was consistent over the range of adsorption temperatures studied. This could be attributed to the superior microporosity of L CD–K as micropores are likely to get occupied first at lower adsorption pressure. Two-step physical activation yields a slightly better CO2 adsorption performance than one-step physical activation. Energy penalty during two-step physical activation is yet to be justified by a marginal increase in CO2 adsorption. From a better CO2 uptake perspective, the performance of the type of activating agents can be ranked as: KOH > KOH + CO2 > ZnCl2 + CO2 > ZnCl2 > CO2. Table 4 compares the CO2 adsorption capacity of APCs derived from various biomass precursors.
| Precursor (P) | Activating agent (AA) | Impregnation ratio (P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA) AA) |
Carbonization (C) & activation conditions (A) (T (°C)/residence time (min)) | Yield (wt%) | S BET (m2 g−1) | V t (cm3 g−1) | CO2 adsorption conditions | CO2 adsorption capacity (mmol g−1) | q st (kJ mol−1) | Selectivity (CO2/N2)e | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | P (bar) | |||||||||||
| a Direct activation. b Two-step activation. c Pyrolytic carbonization. d Hydrothermal carbonization. e At 25 °C. | ||||||||||||
| Bamboo shoot shellsb | CaCl2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Cc-600/120 | — | 541 | 0.24 | 0/25 | 1/1 | 3.14/2.39 | 35.0 | — | 41 |
| A-700/120 | ||||||||||||
| Bamboo shoot shells + ureab | K2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Cc-500/120 | — | 1958 | 0.83 | 0/25 | 1/1 | 7.52/3.60 | 33.0 | 14.0 | 42 |
| A-800/— | ||||||||||||
| Chitosana | KOH | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
A-700/60 | — | 1506 | 0.64 | 0/25 | 1/1 | 6.91/4.40 | 32.5 | 21.0 | 43 |
| Coconut shells b | K2S2O3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Cc — | — | 1188 | 0.47 | 0/25 | 1/1 | 5.31/3.59 | 35.0 | 20.0 | 44 |
| A-700/60 | ||||||||||||
| Coconut shellsb | K2S2O8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Cc-500/120 | — | 581 | 0.26 | 0/25 | 1/1 | 3.77/2.56 | 39.0 | 17.0 | 45 |
| A-750/120 | ||||||||||||
| Coconut shellsb | KOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Cc-500/120 | — | 1172 | 0.44 | 0/25 | 1/1 | 6.04/4.23 | 37.0 | 22.0 | 46 |
| A-600/60 | ||||||||||||
| Corn cobb | KOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Cd-230/480 | 16.3 | 2716 | 0.71 | 15 | 1 | 4.50 | 24.1 | — | 47 |
| ZnCl2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
A-600/60 | 18.9 | 1567 | 0.48 | 15 | 1 | 3.64 | 17.7 | |||
| H3PO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
17.3 | 2314 | 0.59 | 15 | 1 | 2.95 | 9.3 | ||||
| Cotton ballsb | KOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Cc-500/60 | 23.3 | 1381 | — | 25 | 1 | 5.14 | — | — | 48 |
| A-700/90 | ||||||||||||
| Garlic peelb | KOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Cd-200/1440 | — | 1262 | 0.70 | 0/25 | 1/1 | 4.33/2.82 | — | — | 49 |
| A-800/60 | ||||||||||||
| Lotus seedsb | KOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Cc-600/120 | — | 2230 | 0.96 | 0/25 | 1/1 | 6.80/3.10 | 30.4 | — | 50 |
| A-800/120 | ||||||||||||
| Macadamia nutshellb | KOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
Cc-500/120 | — | 1417 | 0.75 | 0/25/40 | 1/1/1 | 6.58/3.94/3.15 | 27.0 | — | 51 |
| A-771/150 | ||||||||||||
| Slash pinea | KOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
A-580/120 | 19.5 | 1185 | 0.35 | 0/15 | 1 | 4.93/3.86 | 39.7 | — | 52 |
| Pine sawdustb | KOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Cc-600/90 | 19.89 | 1319 | 0.66 | 0/25/40 | 1/1/1 | 6.35/3.82/2.81 | 24.9 | 11.8 | 28 |
| A-800/90 | ||||||||||||
| Lignin protobind 2400b | KOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Cc-600/90 | 28.96 | 1108 | 0.56 | 0/25/40 | 1/1/1 | 5.68/3.66/2.67 | 25.5 | 12.6 | Present study |
| A-800/90 | ||||||||||||
A linear relationship between CO2 uptake and textural properties (i.e., SBET, Smicro, φmicro, Ravg, VT, Vmicro, and fmicro) is presented in Fig. S3 (ESI†) to better understand the parameters affecting the CO2 adsorption performance of APC. No clear trend was observed between any of the textural properties and CO2 uptake. This implied that CO2 adsorption on the APC surface is affected by multiple parameters.53
3.4. Selectivity and heat of adsorption
For practical on-field gas separation applications, better CO2 uptake along with higher CO2/N2 selectivity is desired. A CO2/N2 volume ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 was assumed, and ideal adsorption solution theory was applied to determine CO2 over N2 selectivity at 0, 25, and 40 °C/1 bar. Adsorption isotherms of a single component were fitted into the Langmuir–Freundlich isotherm model in eqn (1) and the resulting parameters were fitted to eqn (2).
85 was assumed, and ideal adsorption solution theory was applied to determine CO2 over N2 selectivity at 0, 25, and 40 °C/1 bar. Adsorption isotherms of a single component were fitted into the Langmuir–Freundlich isotherm model in eqn (1) and the resulting parameters were fitted to eqn (2).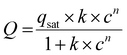 | (1) |
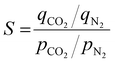 | (2) |
Table 3 lists CO2/N2 selectivity for all the APCs, which ranged within 11.9–20.4. A clear trend between selectivity and adsorption temperature was absent. Higher polarizability, quadruple moment, critical temperature, and lower kinetic diameter for CO2 than N2, favoured better adsorption of CO2 on the APC surface.54 Also, being an electrophilic molecule, CO2 strongly interacts with the heteroatom-containing functional groups of APCs.
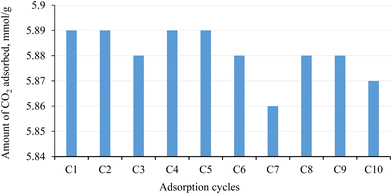
The isosteric heat of adsorption (qst) measures the strength of adhesion between adsorbent and adsorbate, which is linked to the ease of regeneration of the adsorbent. Table 3 lists qst values of all the APCs at lower CO2 loading, which ranged within ∼20–33 kJ mol−1. The qst value is inversely proportional to the energy required during the regeneration of the adsorbent. For all the APCs, qst < 40 kJ mol−1 indicated that adsorption predominantly occurred via physisorption through dipole–quadrupole interaction between CO2 and the APC surface. Secondary activation of biochar prepared in CO2 indicated higher CO2/N2 selectivity and qst than that of biochar prepared in the N2 atmosphere. The observation was consistent for all the tested combination of activating agents considered in the study. For example, L N–K vs. L CD–K, L N–KCD vs. L CD–KCD, L N–Z vs. L–CD Z and L N–ZCD vs. L CD–ZCD. CO2 carbonization/activation may have modified the surface chemistry of the material or introduced functional groups, leading to stronger interactions with CO2 molecules. This enhanced interaction can result in a higher heat of adsorption. A similar trend observed for APCs derived from pine sawdust indicates this effect is not much more feedstock sensitive.28Fig. 5d represents variation in qst with CO2 loading. The elevated qst observed at the minimum CO2 loading may be explained by a preference for occupying ultrafine pores and/or the adhesion of CO2 to surface heterogeneity. As CO2 loading increases, the diminishing trend in the isosteric heat of adsorption can be ascribed to the saturation of strong binding sites. For most of the APCs, a consistently strong correlation with increasing CO2 loading suggests uniformity in adsorption sites in terms of binding energy. The moderate qst value indicates convenient regeneration, supporting their suitability for multi-cycle CO2 adsorption applications. Multi-cyclic CO2 adsorption at 0 °C was executed for the best-performing APC in terms of CO2 adsorption i.e., L N–K up to 10 cycles. As indicated in Fig. 6, a fluctuation of 0.51% in the amount of CO2 adsorbed was observed during 10 consecutive adsorption/desorption cycles. The remarkable stability and reusability of L N–K for CO2 adsorption could be attributed to non-destructive uptake and release. However, its stability towards the actual flue gas scenario has yet to be tested.
As of 2023, approximately 40 commercial CO2 capture facilities are actively operating worldwide, with a total annual capture capacity exceeding 45 Mt of CO2. Despite the announcement of over 50 upcoming capture facilities scheduled to commence operations by 2030, declared since January 2022, the current project pipeline only represents roughly one-third of the anticipated requirement for achieving net-zero emissions by 2030. Noteworthy advancements have also been observed in the application of CCUS within the industrial sector. In 2022, several new projects were commissioned, encompassing the integration of CCUS into sectors such as iron, steel, fertilizer, and other chemical production processes. Projections indicate that approximately 25 biomass and waste-fired combined heat and power plants could be responsible for capturing around 30 Mt of CO2 by the year 2030. Considering the ongoing project pipeline, it is anticipated that by 2030, the annual capture capacity, derived from both new constructions and retrofits, could reach around 90 Mt of CO2 from hydrogen production, approximately 80 Mt from power generation, and roughly 35 Mt from various industrial facilities, including cement and steel production.55
In the wider spectrum, utilization of APC for post-combustion CO2 capture presents invaluable benefits such as compatibility to current energy systems, easy scalability, and steady operation. APC can be used in packed-bed adsorption systems, known for their simplicity and straightforward scalability. APC derived from green and renewable sources can effectively capture CO2 emissions from industries heavily reliant on coal, including cement, chemical, oil refineries, and steel. As per the technical report from the National Energy Technology Laboratory (NETL), an adsorbent proves practical and economically viable for CO2 capture if it demonstrates an isothermal CO2 adsorption capacity exceeding 3 mmol g−1 at 25 °C and 1 bar.56 The L N–K, as indicated, exhibits an isothermal CO2 adsorption capacity of 3.66 mmol g−1 at 25 °C and 1 bar, signifying its suitability for commercial operations. Along with CO2 capture applications, the conversion of lignin into a stable form of APC restricts the release of carbon into the atmosphere.
4. Conclusion
Lignin protobind 2400 was converted into a value-added product, activated porous carbon (APC), using combined activating agents KOH + CO2 and ZnCl2 + CO2. Also, the effectiveness of the combined physicochemical activation with individual chemical and physical activating agents was compared. Although KOH + CO2 activation improved the BET surface area by 33% compared to only KOH activation, CO2 uptake for KOH + CO2 activation decreased by 23%, compared to only KOH activation. On the other hand, physicochemical activation using ZnCl2 + CO2 slightly improved the BET surface area as well as CO2 uptake compared to only ZnCl2 and only CO2 activation. From a better CO2 uptake perspective, the performance of this type of activating agent can be ranked as: KOH > KOH + CO2 > ZnCl2 + CO2 > ZnCl2 > CO2. Secondary activation of biochar carbonized in the N2 atmosphere offered a better BET surface area and increased CO2 uptake compared to that of biochar carbonized in the CO2 atmosphere. Considerably higher CO2/N2 selectivity and isosteric heat of CO2 adsorption were observed for APC derived from secondary activation of biochar carbonized in a CO2 environment than in a N2 environment. The combination of better CO2 adsorption capacity, lower heat of adsorption, reasonably good CO2/N2 selectivity, and excellent reusability/stability of lignin-based APC supported its utilization for post-combustion CO2 capture.Nomenclature
| GHGs | Greenhouse gases |
| CCUS | Carbon capture, utilization, and storage |
| APC | Activated porous carbon |
| BET | Brunauer–Emmett–Teller |
| L | Lignin protobind 2400 |
| NLPH | Normal litres per hour |
| L X–Y | Lignin protobind 2400 based activated porous carbon, where X represents gas purged during carbonization and Y denotes activating agent used; X = N indicates N2 purging, X = CD indicates CO2 purging; Y = K denotes KOH activation, Y = CD denotes CO2 activation, Y = Z denotes ZnCl2 activation, Y = KCD denotes KOH + CO2 activation, Y = ZCD denotes ZnCl2 + CO2 activation |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| HR-TEM | High-resolution transmission electron microscopy |
| NLDFT | Non-local density functional theory |
| S BET | Specific BET surface area, m2 g−1 |
| S micro | Micro-pore area using a t-plot method, m2 g−1 |
| φ micro | Micro-porosity (Smicro/SBET) × 100, % |
| R avg | Average pore radius, nm |
| V T | Estimated at a relative pressure P/P0 = 0.99, cm3 g−1 |
| V micro | Micropore volume estimated using a t-plot method, cm3 g−1 |
| f micro | Percentage of micro-pore volume (Vmicro/VT) × 100, % |
| q st | Isosteric heat of adsorption, kJ mol−1 |
Author contributions
Himanshu Patel: methodology, validation, formal analysis, investigation, and writing – original draft. Amar Mohanty: conceptualization, resources, supervision, investigation, validation, and writing – review & editing. Manjusri Misra: conceptualization, methodology, funding acquisition, project administration, resources, supervision, investigation, validation, and writing – review & editing.Data availability
Data for this article, including Raw/processed/metadata files will be available from the corresponding author on reasonable request.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors would like to acknowledge the financial support from (i) the Ontario Agri-Food Innovation Alliance – Bioeconomy for Industrial Uses Research Program (Project No. 030671); (ii) the Natural Sciences and Engineering Research Council of Canada (NSERC), Canada Discovery Grants Program Project No. 401716; (iii) NSERC Alliance Grants Program (Project No. 401769) along with the partner industry Competitive Green Technologies, Lamington, Ontario, Canada (Project No. 055427); and (iv) the Agriculture and Agri-Food Canada (AAFC)/Bioindustrial Innovation Canada (BIC) Project No. 056369 to carry out this work.References
- CDIAC, Carbon Dioxide Information Analysis Center, 2011.
- B. P. E. Outlook, London, United Kingdom, 2019.
- W. M. Organization, WMO Statement on the State of the Global Climate in 2019, 2020.
- T. R. K. C. Doddapaneni, R. Praveenkumar, H. Tolvanen, J. Rintala and J. Konttinen, Appl. Energy, 2018, 213, 272–284 CrossRef.
- H. Patel, A. Mohanty and M. Misra, Renewable Sustainable Energy Rev., 2024, 199, 114484 CrossRef CAS.
- X. Yuan, J. Wang, S. Deng, M. Suvarna, X. Wang, W. Zhang, S. T. Hamilton, A. Alahmed, A. Jamal and A.-H. A. Park, et al. , Renewable Sustainable Energy Rev., 2022, 162, 112413 CrossRef CAS.
- L. Rao, S. Liu, L. Wang, C. Ma, J. Wu, L. An and X. Hu, Chem. Eng. J., 2019, 359, 428–435 CrossRef CAS.
- A. Mukherjee, J. A. Okolie, A. Abdelrasoul, C. Niu and A. K. Dalai, J. Environ. Sci., 2019, 83, 46–63 CrossRef CAS PubMed.
- L. Jiang, A. Gonzalez-Diaz, J. Ling-Chin, A. P. Roskilly and A. J. Smallbone, Appl. Energy, 2019, 245, 1–15 CrossRef CAS.
- Y. Guo, C. Tan, J. Sun, W. Li, J. Zhang and C. Zhao, Chem. Eng. J., 2020, 381, 122736 CrossRef CAS.
- G. Singh, K. S. Lakhi, S. Sil, S. V. Bhosale, I. Kim, K. Albahily and A. Vinu, Carbon, 2019, 148, 164–186 CrossRef CAS.
- A. K. Mohanty, S. Vivekanandhan, O. Das, L. M. Romero Millán, N. B. Klinghoffer, A. Nzihou and M. Misra, Nat. Rev. Methods Primers, 2024, 4, 19 CrossRef CAS.
- J. Becker and C. Wittmann, Biotechnol. Adv., 2019, 37, 107360 CrossRef CAS PubMed.
- U. S. EPA, United States Environ. Prot. Agency.
- Z. Wu, J. Zou, Y. Zhang, X. Lin, D. Fry, L. Wang and J. Liu, Chem. Eng. J., 2022, 427, 131547 CrossRef CAS.
- P. Sirous-Rezaei, D. Creaser and L. Olsson, Appl. Catal., B, 2021, 297, 120449 CrossRef CAS.
- L. Yu, L. Liang, I. Bajaj, K. Seabright, D. J. Keffer, I. N. Ivanov, H. Chen, S. Dai, A. J. Ragauskas, C. T. Maravelias and D. P. Harper, Carbon, 2023, 213, 118285 CrossRef CAS.
- Y.-Y. Wang, C. E. Wyman, C. M. Cai and A. J. Ragauskas, ACS Appl. Polym. Mater., 2019, 1, 1672–1679 CrossRef CAS.
- J. Zhang, L. Yu, Z. Wang, Y. Tian, Y. Qu, Y. Wang, J. Li and H. Liu, J. Chem. Technol. Biotechnol., 2011, 86, 1177–1183 CrossRef CAS.
- M. Y. Balakshin, E. A. Capanema, I. Sulaeva, P. Schlee, Z. Huang, M. Feng, M. Borghei, O. J. Rojas, A. Potthast and T. Rosenau, ChemSusChem, 2021, 14, 1016–1036 CrossRef CAS PubMed.
- H. Luo and M. M. Abu-Omar, Encyclopedia of Sustainable Technologies, 2017, vol. 3, pp. 573–585 Search PubMed.
- D. V. Cuong, N.-L. Liu, V. A. Nguyen and C.-H. Hou, Sci. Total Environ., 2019, 692, 844–853 CrossRef CAS PubMed.
- D. V. Cuong and C.-H. Hou, J. Taiwan Inst. Chem. Eng., 2022, 139, 104533 CrossRef CAS.
- K. Adlak, R. Chandra, V. K. Vijay and K. K. Pant, J. Anal. Appl. Pyrolysis, 2021, 155, 105102 CrossRef CAS.
- S. Balou, S. E. Babak and A. Priye, ACS Appl. Mater. Interfaces, 2020, 12, 42711–42722 CrossRef CAS PubMed.
- A. D. Igalavithana, S. W. Choi, P. D. Dissanayake, J. Shang, C.-H. Wang, X. Yang, S. Kim, D. C. W. Tsang, K. B. Lee and Y. S. Ok, J. Hazard. Mater., 2020, 391, 121147 CrossRef CAS.
- J. Serafin, B. Dziejarski, X. Vendrell, K. Kiełbasa and B. Michalkiewicz, Biomass Bioenergy, 2023, 175, 106880 CrossRef CAS.
- H. Patel, H. Weldekidan, A. Mohanty and M. Misra, Carbon Capture Sci. Technol., 2023, 100128 CrossRef CAS.
- S. Sahoo, M. Ö. Seydibeyoğlu, A. K. Mohanty and M. Misra, Biomass Bioenergy, 2011, 35, 4230–4237 CrossRef CAS.
- N. A. Ahmad, K. A. Al-attab, Z. A. Zainal and P. Lahijani, Bioresour. Technol. Rep., 2021, 15, 100785 CrossRef CAS.
- H. Weldekidan, H. Patel, A. Mohanty and M. Misra, Carbon Capture Sci. Technol., 2024, 10, 100149 CrossRef CAS.
- S. Rani, E. Padmanabhan and B. K. Prusty, J. Pet. Sci. Eng., 2019, 175, 634–643 CrossRef CAS.
- W. Wu, C. Wu, G. Zhang, J. Liu, Y. Li and G. Li, Fuel, 2023, 332, 126107 CrossRef CAS.
- S. Charola, H. Patel, S. Chandna and S. Maiti, J. Cleaner Prod., 2019, 223, 969–979 CrossRef CAS.
- J. Rouquerol, D. Avnir, C. W. Fairbridge, D. H. Everett, J. M. Haynes, N. Pernicone, J. D. F. Ramsay, K. S. W. Sing and K. K. Unger, Pure Appl. Chem., 1994, 66, 1739–1758 CrossRef CAS.
- Y.-S. Bae and C.-H. Lee, Carbon, 2005, 43, 95–107 CrossRef CAS.
- K. Kiełbasa, Ş. Bayar, E. A. Varol, J. Sreńscek-Nazzal, M. Bosacka and B. Michalkiewicz, Ind. Crops Prod., 2022, 187, 115416 CrossRef.
- B. Ashourirad, P. Arab, T. Islamoglu, K. A. Cychosz, M. Thommes and H. M. El-Kaderi, J. Mater. Chem. A, 2016, 4, 14693–14702 RSC.
- M. Singh, N. Borkhatariya, P. Pramanik, S. Dutta, S. K. Ghosh, P. Maiti, S. Neogi and S. Maiti, J. CO2 Util., 2022, 60, 101975 CrossRef CAS.
- B. Zhao, M. Borghei, T. Zou, L. Wang, L.-S. Johansson, J. Majoinen, M. H. Sipponen, M. Österberg, B. D. Mattos and O. J. Rojas, ACS Nano, 2021, 15, 6774–6786 CrossRef CAS PubMed.
- W. Wu, C. Wu, G. Zhang and J. Liu, J. Anal. Appl. Pyrolysis, 2022, 168, 105742 CrossRef CAS.
- W. Wu, C. Wu, J. Liu, H. Yan, G. Zhang, G. Li, Y. Zhao and Y. Wang, Fuel, 2024, 363, 130937 CrossRef CAS.
- J. Shao, J. Wang, Q. Yu, F. Yang, M. Demir, O. C. Altinci, A. Umay, L. Wang and X. Hu, Sep. Purif. Technol., 2024, 333, 125891 CrossRef CAS.
- J. Bai, J. Shao, Q. Yu, M. Demir, B. Nazli Altay, T. Muhammad Ali, Y. Jiang, L. Wang and X. Hu, Chem. Eng. J., 2024, 479, 147667 CrossRef CAS.
- C. Liu, Y. Zhi, Q. Yu, L. Tian, M. Demir, S. G. Colak, A. A. Farghaly, L. Wang and X. Hu, ACS Appl. Nano Mater, 2024, 7(5), 5434–5441 CrossRef CAS.
- J. Yang, L. Yue, X. Hu, L. Wang, Y. Zhao, Y. Lin, Y. Sun, H. DaCosta and L. Guo, Energy Fuels, 2017, 31, 4287–4293 CrossRef CAS.
- A. Sarwar, M. Ali, A. H. Khoja, A. Nawar, A. Waqas, R. Liaquat, S. R. Naqvi and M. Asjid, J. CO2 Util., 2021, 46, 101476 CrossRef CAS.
- H. Patel, H. Mangukiya, P. Maiti and S. Maiti, J. Cleaner Prod., 2020, 125738 Search PubMed.
- G. Huang, Y. Liu, X. Wu and J. Cai, New Carbon Mater., 2019, 34, 247–257 CrossRef CAS.
- G. Singh, K. S. Lakhi, K. Ramadass, C. I. Sathish and A. Vinu, ACS Sustainable Chem. Eng., 2019, 7, 7412–7420 CrossRef CAS.
- C. Wu, G. Zhang, J. Liu, H. Yan and Y. Lv, Int. J. Energy Res., 2022, 46, 17204–17219 CrossRef CAS.
- M. B. Ahmed, M. A. H. Johir, J. L. Zhou, H. H. Ngo, L. D. Nghiem, C. Richardson, M. A. Moni and M. R. Bryant, J. Cleaner Prod., 2019, 225, 405–413 CrossRef CAS.
- C. Wu, J. Liu, Y. Wang, Y. Zhao, G. Li and G. Zhang, Sep. Purif. Technol., 2024, 329, 125188 CrossRef CAS.
- P. Pramanik, H. Patel, S. Charola, S. Neogi and S. Maiti, J. CO2 Util., 2021, 45, 101450 CrossRef CAS.
- I. 2023, Tracking Clean Energy Progress 2023, Paris, 2023.
- H. W. Pennline, Sorbent Research for the Capture of Carbon Dioxide, 2016 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ya00305e |
| This journal is © The Royal Society of Chemistry 2024 |