Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A multi-modal mass spectrometry approach for the detection and mapping of date rape drugs in fingermarks†
R. Krishna a,
K. Hamera,
R. Bradshawa,
S. Bleay
a,
K. Hamera,
R. Bradshawa,
S. Bleay b,
L. M. Colea,
E. Claudec,
J. Langridgec,
J. Bucekd and
S. Francese
b,
L. M. Colea,
E. Claudec,
J. Langridgec,
J. Bucekd and
S. Francese *a
*a
aBiomolecular Research Centre, Centre for Mass Spectrometry Imaging, Sheffield Hallam University, Sheffield, UK. E-mail: s.francese@shu.ac.uk
bLondon South Bank University, London, UK
cWaters Corporation, Wilmslow, UK
dPlasmion GmbH, Augsburg, Germany
First published on 27th May 2025
Abstract
Although drug facilitated sexual assault (DFSA) is an old issue, current statistics on the frequency of this crime have led to growing concerns. As these drugs are metabolised very quickly, toxicological evidence from biological fluids, corroborating the victim's statement, is challenging to recover, especially with late reports. We are proposing an additional method involving the analysis of the victim's fingermarks recovered at the scene(s) of the crime, which may contain the parent drug and its metabolite. As a case study, a multi-modal mass spectrometry-based approach has been developed and explored to detect and image both risperidone and its pharmacologically active metabolite, paliperidone, after contamination of a fingertip at very low concentrations and deposition of a fingerprint on a surface. In particular Matrix Assisted Laser Desorption Ionisation Mass Spectrometry Imaging (MALDI MSI), Desorption Electrospray Ionisation Mass Spectrometry Imaging (DESI MSI) and Soft Ionisation by Chemical Reaction In Transfer, (SICRIT®) have been used in different combinations to both detect the drugs and reconstruct the fingerprint ridge pattern; this approach enables the simultaneous provision of both chemical information (circumstances surrounding the crime) and biometric information. A forensic operational scenario has also been simulated whereby the contaminated fingerprint is deposited on paper and enhanced with a routine fingerprint enhancement technique prior to analysis via mass spectrometry imaging. Overall, our investigation indicates that this additional approach is feasible and is worth exploring further.
1. Introduction
Drug facilitated sexual assault (DFSA) refers to sexual violence (SV) perpetrated against an individual under the influence of prescription, licit or illicit substances.1 Proactive DFSA is the intentional incapacitation of the victim through the covert administration of such drugs to perpetrate SV.2 Whilst the practice of incapacitating victims to sexually assault them is not at all a modern age issue,3,4 proactive DFSA, through drink or needle spiking, has become an escalating concern due to the frequency with which it occurs and the long term mental health impact on the victims and their families, as well as the deriving social cost of drug-related harms and interventions.5,6In 2022, in the UK, the Home Affairs Select Committee (HASC) issued a survey receiving more than 3000 spiking victims or witnesses’ responses,4 though it was accepted that victims’ numbers are probably higher due to under-reporting. HASC reports that between May 2022 and April 2023, the police received 6732 reports of spiking, averaging 561 reports a month.4 In 2021, The Australian Bureau of Statistics (ABS) published a report on SV from which it emerged that in the previous 10 years, 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 women had experienced and reported DFSA, with an estimated lack of reporting of 321
900 women had experienced and reported DFSA, with an estimated lack of reporting of 321![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 cases.7 In the US, a national study from 2007 found that 2.3% of surveyed women (2.6 million) reported alcohol/drug-facilitated rape after covert administration.8 In a subsequent survey conducted between 2010 and 2012, including women and males subjected to SV through DFSA in their lifetime, 32% of women (7229) and 30.3% responders (5575) reported DFSA through drugs or a combination of drugs and alcohol.9
800 cases.7 In the US, a national study from 2007 found that 2.3% of surveyed women (2.6 million) reported alcohol/drug-facilitated rape after covert administration.8 In a subsequent survey conducted between 2010 and 2012, including women and males subjected to SV through DFSA in their lifetime, 32% of women (7229) and 30.3% responders (5575) reported DFSA through drugs or a combination of drugs and alcohol.9
Sexual assaults are a challenging crime to prosecute due to several reasons including lack of/late reporting and independent witness testimony, difficulties in proving lack of consent, amnesia,10 but also lack of solid physical evidence. In the case of DFSA, lack of toxicological evidence is also critical; the vast majority of DFSA drugs most commonly used for DFSA, also colloquially (and perhaps disingenuously) referred as to “date rape drugs”, are rapidly metabolised; their short half-life time has resulted in false negatives and/or often renders their quantification in biological fluids, analysed days after the crime has occurred, a prohibitive challenge.4,11–13
Other types of biological specimens have therefore become desirable to attempt the detection of these substances; fingermarks (ridge pattern impressions accidentally deposited on a surface) may potentially be such a specimen. Through touch, the victim may in fact accidentally deposit their fingermarks on objects after the drug spiking, and/or during the subsequent sexual assault, when the drugs are being metabolised. The drug and their metabolites could therefore be excreted through sweat and transferred to the touched surface within deposited mark. It may be possible, as observed for other types of drugs and metabolites that, even if the recovery of the fingermark and analysis occurs after several days, these species can still be detected in this specimen.14,15 The attention towards drugs is justified by the fact that whilst consumption of alcohol remains the most prevalent means to perpetrate SV,4,10 a range of substances, covertly administered to the victim, has also been documented in the literature, including (in a non-exhaustive list) cannabinoids, opioids, benzodiazepines, amphetamines, GHB, antidepressants, cathinones and antipsychotics.4,16
This paper explores a multi-modal mass spectrometry approach for the detection and mapping of risperidone in fingermarks. Risperidone is a benzisoxazole and it is a widely prescribed atypical antipsychotic drug for the treatment of schizophrenia and other psychosis. However, its use has also been reported in DFSA.16,17–19 The combination of its intended use as an antipsychotic and misuse as an DFSA drug, makes for an interesting case study due to (1) the potential transferability of the method to the detection and mapping of other DFSA drugs; and (2) the intelligence that is possible to provide around an individual to either narrow down the pool of suspects (fingermark of the perpetrator taking the prescribed medication) or to provide circumstantial or corroborating evidence assisting the victim's claims (from the fingermark of the victim covertly administrated with this drug in the context of DFSA). Furthermore, risperidone has been detected in a large range of biological specimens20 using LC MS/MS; interestingly, a non-invasive specimen, close to fingermarks, namely fingertip smears, was employed to detect a range of antipsychotics in patients in the context of drug adherence, through LC MS/MS.21 However, the antipsychotics investigated did not include risperidone, the analytical specimen had no ridge detail and use of LC MS/MS required destruction of the fingermark sample.
The in situ mass spectrometric approaches employed in the work presented here have never been developed or applied to fingermarks with respect to the detection and mapping of risperidone and its main metabolite (individually and in mixture) and, in contrast to the application of LC MS/MS methods, they have the benefit of preserving, at large, the integrity of the fingermark. As such, they are particularly interesting from a forensic standpoint as well as from a drug adherence viewpoint as they are potentially faster, not requiring extensive sample preparation.
Here we have employed an array of mass spectrometry techniques and developed methods for the detection and/or mapping of risperidone and its pharmacologically active metabolite 9-hydroxy-risperidone (paliperidone) in intact fingerprints (ridge impressions deliberately deposited on a surface), with some of these methods used in tandem to maximise the forensic intelligence retrievable. In particular, one vacuum and two ambient ionisation techniques were employed, namely Matrix Assisted Laser Ionisation Mass Spectrometry Imaging (vacuum MALDI MSI), Desorption Electrospray Ionisation Mass Spectrometry Imaging (DESI MSI) and thermal desorption soft ionisation through Chemical Reaction In Transfer, (SICRIT®), (Plasmion GmbH, Augsburg, Germany), based on a dielectric barrier discharge ionisation (DBDI) source.22 The inclusion of SICRIT® in the workflow was due to documented success in detecting cocaine, heroin and fentanyl from fingerprints, in the picogram range.22 In this study, MALDI MSI and DESI MSI were employed to detect and map risperidone and paliperidone in fingerprints, whereas SICRIT® was employed to preliminarily and quickly profile the presence of these species and to detect them at a higher sensitivity. Multi-modal workflows involved the sequential use (in separate instances) of (i) DESI MSI and MALDI MSI; and (ii) thermal ionisation profiling through SICRIT® followed by MALDI MSI or by DESI MSI and then MALDI MSI.
Finally, considering the operational perspective, risperidone and paliperidone-contaminated fingermarks were also preliminarily treated using fingermark enhancement techniques, following the protocols reported by the Fingermark Visualisation Manual (FVM)23 prior to sequential analysis by MALDI MSI and DESI MSI, to demonstrate feasibility of these mass spectrometric techniques in an operational workflow. We have elected to visualise fingermarks on paper, as one of the possible surfaces of deposition scenarios; 1,2-indandione was initially used as it is a Cat A recommended technique for fingermark enhancement on paper (FVM3) and subsequently 4-dimethylaminocinnamaldehyde (DMAC) was employed as a Cat B process. We up-scaled the challenge by using red paper in addition to white paper as deposition surfaces; red paper has been reported as a challenging substrate for fingermark visualisation, both anecdotally, during research24 and also in operational work.25 This is because the colour and the background fluorescence of the substrate make it difficult to visualise marks with the commonly used reagents for porous surfaces, ninhydrin and indandione. Ninhydrin develops purple-red marks giving very low colour contrast with the coloured paper, and the red dyes used in the paper produce background fluorescence at the wavelengths used to examine 1,2-indandione.
Therefore, this experiment intended to demonstrate the compatibility of mass spectrometry imaging-based approaches with 1,2-indandione and DMAC, and the deriving biometric and chemical intelligence that can be provided.
Overall, this research has shown promise for an alternative approach in supporting DFSA allegations by detecting the spiked/injected drug in the fingermark of the victim that may be recovered after the incident. The detection and mapping of these species using in situ mass spectrometry-based methods, as standalone or as a multimodal approach, provide toxicological, biometric and circumstantial evidence to the investigators.
2. Materials and methods
2.1. Materials
Ultra-pure methanol (MeOH), acetonitrile (ACN), and formic acid (FA) for DESI MSI were sourced from ROMIL, UK Ltd. The Milli-Q water was obtained from an in-house Millipore water purification system (Merck KGaA, Darmstadt, Germany) for MALDI MS based analyses or from ROMIL for DESI MS based analyses. The MALDI matrix α-CHCA, red phosphorus, trifluoroacetic acid and the standards for risperidone (RIS) and paliperidone (PAL) were sourced from Merck Life Science, UK Ltd. Double-sided conductive carbon tape was purchased from TAAB (Aldermaston, UK). ALUGRAM SIL G/UV254, silica pre-coated aluminium sheets were purchased from Macherey-Nagel GmbH, Germany. MeOH, zinc chloride and acetic acid for 1,2-indandione enhancement were obtained from Fisher Chemical (Loughborough, UK). 1,2-Indandione was supplied by BVDA, (Haarlem, Netherlands) and DMAC by Sigma (Gillingham, UK). Ethanol was purchased from JT Baker (Lutterworth, UK). Ethyl acetate was purchased from Acros Organics (Loughborough, UK) and petroleum ether (BP 40–60) from Honeywell (Muskegon, USA).2.2. Instrumentation and instrumental settings
The DESI XS source on the Xevo TQ-XS was operated in positive mode and as it follows: the capillary and the cone voltages were optimised and then set to 1.0 kV, and 30 V respectively. The positioning of the DESI source was set as follows: the x-axis and the y-axis at 2 a.u., the z-axis at 4 a.u. and the angle of the DESI emitter was set at 72°. The solvent composition used was 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (MeOH
5 (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, 0.1% FA) and the solvent flow rate was set at 2 μL min−1. For profiling experiments, ions were scanned over the m/z range 250–450 at a scan rate of 1 scan per second.
water, 0.1% FA) and the solvent flow rate was set at 2 μL min−1. For profiling experiments, ions were scanned over the m/z range 250–450 at a scan rate of 1 scan per second.
To confirm the presence of risperidone and paliperidone through MS/MS on the TQ-XS, the instrument was operated in MRM mode using the transitions at nominal m/z of 411 → 191 and 427 → 191, respectively. For MS/MS experiments, collision energy was optimised and set to 40 eV.
Imaging experiments were set up using the DESI method editor (Waters, Wilmslow, UK) using a scan rate of 10 scans per second and a step size of 50 μm × 50 μm. Collision gas flow rate was set to 0.14 mL min−1 for MS/MS profiling and imaging experiments.
The DESI XS source on the cIMS was operated in positive ionisation mode with the following optimised instrument settings: capillary voltage, 0.7 kV; cone voltage, 40 V; heated Transfer Line (HTL) temperature, 100 °C; source temperature, 120 °C; API gas pressure, 0.08 mPa; DESI emitter angle, 72°; solvent flow rate, 1.00 μL min−1, scan rate, 450 μm s−1 and and a step size of 50 μm × 50 μm for imaging experiments. The cIMS was operated in MS mode (sensitivity), with data acquired from m/z 50–1200. The manual quadrupole profile employed was (M1 = m/z 250, mass M2 = m/z 300, mass M3 = m/z 400). The solvent composition used was 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (MeOH
5 (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, 0.1% FA) including 100 pg μL−1 LEU-ENK for continuous lock mass. The instrument was calibrated with polyalanine prior to every analysis with an average mean prediction error of 0.7 ppm.
water, 0.1% FA) including 100 pg μL−1 LEU-ENK for continuous lock mass. The instrument was calibrated with polyalanine prior to every analysis with an average mean prediction error of 0.7 ppm.
A step size of 50 μm × 50 μm was used for imaging experiments. The manual quadrupole profile employed was (M1 = m/z 250, mass M2 = m/z 300, mass M3 = m/z 400). The instrument was calibrated with red phosphorus prior to every analysis with an average mean prediction error of 0.2 ppm.
Mass spectra were processed either directly in Masslynx v4.2 (respective releases as above) or opened in Masslynx and exported into mMass, an open-source mass spectral analysis platform.27,28 MSI experiments were set up using DESI Method Editor v2.3.8 for the Xevo TQ-XS, and Quartz (v2.12.1, v2.7.1) for cIMS, and MRT respectively.
All the DESI-cIMS and MALDI-MRT imaging experiments were processed and visualised using HDI v1.8 (Waters Corporation, Wilmslow UK) with the following settings: MS resolution of (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for cIMS, and 200
000 for cIMS, and 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for MRT), m/z window of (0.02 for cIMS, and 0.005 for MRT), and the number of most intense peaks set to 1000. The DESI-Xevo TQ-XS MRM images were auto processed from Masslynx and visualised in HDI v1.8. Except for those generated by the Xevo TQ-XS, all the images were TIC (Total Ion Count), normalised unless specified otherwise. Relevant regions of interests (ROI) were exported to MassLynx™ v4.2 (respective releases as above) software for spectral analysis.
000 for MRT), m/z window of (0.02 for cIMS, and 0.005 for MRT), and the number of most intense peaks set to 1000. The DESI-Xevo TQ-XS MRM images were auto processed from Masslynx and visualised in HDI v1.8. Except for those generated by the Xevo TQ-XS, all the images were TIC (Total Ion Count), normalised unless specified otherwise. Relevant regions of interests (ROI) were exported to MassLynx™ v4.2 (respective releases as above) software for spectral analysis.
2.3 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) and 10 μL were deposited on a glass slide and allowed to evaporate prior to fingertip contamination and contaminated fingermark generation as described above.
1 ratio) and 10 μL were deposited on a glass slide and allowed to evaporate prior to fingertip contamination and contaminated fingermark generation as described above.Risperidone and paliperidone were also analysed on a DESI XS source coupled to cIMS to compare the performance with that of the Xevo TQ-XS. A risperidone and paliperidone-contaminated fingerprint was prepared according to Groeneveld et al.29 and as described for the same type of experiment on the DESI-Xevo TQ-XS, except 50 μL of risperidone and paliperidone were mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio each at a concentration of 1 × 104 ng mL−1 rather than 5 × 103 ng mL−1.
1 ratio each at a concentration of 1 × 104 ng mL−1 rather than 5 × 103 ng mL−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ACN
30 ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1% TFAaq. Following optimisation, the sprayer was operated as follows: nozzle temperature, 75 °C, pressure at 0.07 MPa (10 psi), flowrate 100 μL min−1 and a velocity of 1200 mm min−1.
0.1% TFAaq. Following optimisation, the sprayer was operated as follows: nozzle temperature, 75 °C, pressure at 0.07 MPa (10 psi), flowrate 100 μL min−1 and a velocity of 1200 mm min−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v ACN
30 v/v ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1% TFAaq in a 1
0.1% TFAaq in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and 0.5 μL of each resulting solution was spotted across the fingermark. Risperidone and paliperidone-contaminated ungroomed fingerprints were prepared using the method of Groeneveld et al.,29 as for the DESI-cIMS contaminated fingerprint experiment described in section 2.3.1., prior to be imaged.
1 ratio and 0.5 μL of each resulting solution was spotted across the fingermark. Risperidone and paliperidone-contaminated ungroomed fingerprints were prepared using the method of Groeneveld et al.,29 as for the DESI-cIMS contaminated fingerprint experiment described in section 2.3.1., prior to be imaged.In the second process, the 4-dimethylaminocinnamaldehyde (DMAC) contact transfer process was used. DMAC impregnated sheets were first prepared by dipping 80gsm white copy paper in a solution of 0.25 g DMAC dissolved in 100 mL ethanol and allowing them to dry. These sheets were kept in plastic wallets in a freezer until ready for use. To visualise the fingermarks, the paper samples to be treated were placed between 2 sheets of impregnated paper, placed in a plastic wallet and left for 24 hours at room temperature.
Examination of the samples treated with both 1,2-indandione and DMAC was carried out using fluorescence examination using Crimelite 82S (Foster + Freeman, Evesham, UK) forensic light sources at 3 wavelengths namely (i) blue (420–470 nm) in conjunction with GG495 viewing goggles/camera filter, (ii) blue/green (445–510 nm) in conjunction with OG550 viewing goggles/camera filter and (iii) green (480–560 nm) in conjunction with OG590 viewing goggles/camera filter.
Photography of the fingermarks was carried out using a Canon EOS 800D DSLR camera with EF-S 18–55 mm lens.
| Combination no. | Nature of the sequential process | CNT mark | RIS mark | RIS/PAL mark |
|---|---|---|---|---|
| 1 | SICRIT® Ionisation → DESI MSI | — | — | √ |
| 2 | SICRIT® ionisation → MALDI MSI | — | — | √ |
| 3 | SICRIT® ionisation → DESI MSI-MALDI MSI (with dry wet method29) | — | √ | √ |
| 4 | 1,2-Indandione → DESI MSI | √ | √ | √ |
| 5 | 1,2-Indandione → MALDI MSI | √ | √ | √ |
| 6 | DMAC → DESI MSI/MALDI MSI | — | — | √ |
2.3.6.1 SICRIT ionisation → DESI MSI. For this mass spectrometry based sequential experiment, a natural fingerprint was deposited on a glass slide after contamination of the fingertip with a dried solution of 10 μL of 5 × 103 ng mL−1 risperidone and 75 μL of 5 × 103 ng mL−1 paliperidone following the method of Groeneveld et al.29 The SICRIT source was set to 300 °C and once inserted in the heating cartridge, MS spectra were acquired for 30 seconds after which the glass slide was submitted to DESI MS/MS Imaging at 50 μm × 50 μm spatial resolution on a Xevo TQ-XS mass spectrometer using the settings described in section 2.2.
2.3.6.2 Chemical enhancement → MALDI MSI/DESI MSI of risperidone and paliperidone in fingermarks. Two sets of marks were prepared and submitted to this workflow. For the first set, a total of 6 marks, including 2 control, 2 risperidone- and 2 risperidone and paliperidone contaminated-fingermarks were prepared. Specifically, control marks were natural marks (i.e. deposited with no prior contamination of the fingertip with the drug(s)). Risperidone-contaminated marks were generated preliminarily by spotting a 10 μL of a 1 × 104 ng mL−1 solution of risperidone on a glass slide and risperidone/paliperidone-contaminated marks were generated spotting a mixed solution of 5 μL of risperidone 1 × 104 ng mL−1 and 5 μL of paliperidone 1 × 104 ng mL−1. The fingerprints were generated by contacting the fingertip with both white and red paper to ensure that one half of the mark sat on white paper and the other half on red paper, as illustrated in Fig. S1.†
These marks were then enhanced using 1,2-indandione and photographed as described in section 2.3.5. Of these marks, a subset of 3 was imaged either via DESI MSI on a Xevo-TQ-XS mass spectrometer or via MALD MSI on a SELECT SERIES MALDI MRT mass spectrometer.
The second set of marks consisted in 6 identically prepared fingerprints generated exclusively on red paper after dragging fingertips over a dried solution made out of 5 μL of 1 × 104 ng mL−1 risperidone and 5 μL of 1 × 104 ng mL−1 risperidone on a glass slide. This set of drug-contaminated fingerprints was enhanced with DMAC, as described in section 2.3.5, prior to splitting the marks and subjecting each of the halves of any one fingermark to DESI or MALDI MSI. In this instance, DESI MSI was carried out using a cIMS.
3. Results and discussion
Sexual Violence (SV), assisted by the covert administration of incapacitating drugs (DFSA), is an increasing global and societal concern due to the long-term impact on the victims and the cost of intervention to society.5,6 Proving DFSA is challenging and hampered by several factors, including, from an analytical perspective, the generally short half time of the drugs combined with late reporting, which make it very difficult, if not impossible, to detect/quantify these species from biological fluids.4,11–13 Fingermarks left by the victim, potentially containing the excreted “date rape drug” and/or its metabolite, could be an additional toxicological specimen of interest. Here we describe the development of a mass spectrometric-based approach for the detection of date rape drugs in fingermarks, using risperidone and its main and pharmacologically active metabolite 9-hydroxyrisperidone (paliperidone) as a case study.16,17–19 MALDI MSI and DESI MSI, in situ techniques preserving the integrity of the sample, and Chemical Reaction In Transfer, SICRIT®, (Plasmion GmbH, Augsburg, Germany) were employed in this study both as standalone techniques and in combination.3.1 Mapping of risperidone and paliperidone in fingermarks via MALDI MSI
A sensitivity imaging study was preliminarily conducted by spotting serial dilutions of either risperidone or paliperidone, in a concentration range between 1 × 103 ng mL−1 and 0.1 ng mL−1. These solutions were spotted on a natural fingerprint (Fig. S2†), generated without any prior cleaning or sebum enrichment of the fingertip. This experiment intended to assess the potential ion suppression exerted by the fingermark chemical composition on these two species and determine the working concentration of these two species for subsequent fingermark imaging analyses. It was found that the lowest concentration at which risperidone could be imaged in a mark was 1 ng mL−1 (0.5 μL of the drug mixed with 0.5, μL of α-CHCA, total of 0.5 ng of risperidone) (Fig. S2A†), with a S/N of >5. Although for both 10 ng mL−1 and 1 ng mL−1 only scattered pixels are visible (Fig. S2A,† inset (i)), the presence of risperidone at these concentrations was confirmed through mass spectra extracted from the region of interest (ROI) shown in Fig. S2B.† For paliperidone the LOD was higher, as it could only be detected and visualised at a concentration of 10 ng mL−1 (corresponding to 5 ng), (S/N 189) as confirmed by the ROI mass spectra in (Fig. S2D†) and by the S/N falling dramatically below 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.63), with the 1 ng mL−1 concentration. It is important to note that whilst other MALDI matrices were not tested, the α-CHCA composition and spray-coating conditions were preliminarily optimised. The selection of α-CHCA was due to the versatility of this matrix especially for small molecules in fingerprints so that a variety of different species could be detected; this allows for untargeted MALDI MSI analysis where casework is commissioned and molecular targets may not be known in advance.
1 (0.63), with the 1 ng mL−1 concentration. It is important to note that whilst other MALDI matrices were not tested, the α-CHCA composition and spray-coating conditions were preliminarily optimised. The selection of α-CHCA was due to the versatility of this matrix especially for small molecules in fingerprints so that a variety of different species could be detected; this allows for untargeted MALDI MSI analysis where casework is commissioned and molecular targets may not be known in advance.
Despite the LOD for risperidone and paliperidone at 1 ng mL−1 and 10 ng mL−1 respectively, the sensitivity when imaging a mark generated by dragging a fingertip over a 50 μL of a dried solution of the two species deposited on a glass slide, prior to touching the aluminium slide,29 was lower. The visualisation of these species in a mixture was only possible in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of a final concentration 5 × 103 ng mL−1, corresponding to a total amount of 250 ng per analyte (Fig. 1). The higher LOD in this instance is due to the chemical species spreading on a bigger area (the fingermark versus the spot) and dropping below the detection threshold of the mass spectrometer.
1 ratio of a final concentration 5 × 103 ng mL−1, corresponding to a total amount of 250 ng per analyte (Fig. 1). The higher LOD in this instance is due to the chemical species spreading on a bigger area (the fingermark versus the spot) and dropping below the detection threshold of the mass spectrometer.
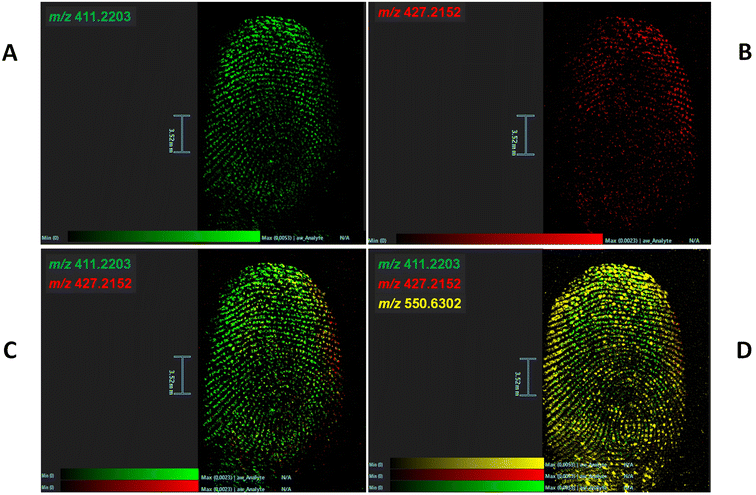
Fig. 1 shows the distributions of risperidone and paliperidone in a fingermark (using a fingertip-contaminating solution of the two species, each at a final concentration of 5 × 103 ng mL−1), providing chemical intelligence (Fig. 1A and B respectively), as well as in a combined image (Fig. 1C) to enhance the biometric information; in the latter instance, this overlay was also complemented by superimposing the image of distribution of a ubiquitous compound often found in fingermarks, namely dimethyldioctadecylammonium ion30–32 (Fig. 1D). This image overlay contributes to the provision of biometric intelligence, through improving the ridge pattern continuity. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 risperidone/paliperidone ratio was selected according to the average concentrations in blood for the two species in previous studies20,33 (with a ratio averaging 0.9 across stomach content, pericardial fluid, urine, kidney, cerebrum and liver tissues20). Whilst this MALDI MSI experiment has proven feasibility of the method, the approach needs to be further evaluated in fingerprints of individuals who have been administered with risperidone. Indeed, the amount of the two species in a fingerprint, just like in blood, may vary with the individual's metabolic rate, dosage, administration mode and sampling time. A study including a number of patients representative of the above conditions, would show in which instances, for example, only the metabolite is detectable, with the parent drug having undergone extensive metabolism and being no longer detectable.
1 risperidone/paliperidone ratio was selected according to the average concentrations in blood for the two species in previous studies20,33 (with a ratio averaging 0.9 across stomach content, pericardial fluid, urine, kidney, cerebrum and liver tissues20). Whilst this MALDI MSI experiment has proven feasibility of the method, the approach needs to be further evaluated in fingerprints of individuals who have been administered with risperidone. Indeed, the amount of the two species in a fingerprint, just like in blood, may vary with the individual's metabolic rate, dosage, administration mode and sampling time. A study including a number of patients representative of the above conditions, would show in which instances, for example, only the metabolite is detectable, with the parent drug having undergone extensive metabolism and being no longer detectable.
3.2 Mapping of risperidone and paliperidone in fingermarks via DESI MSI
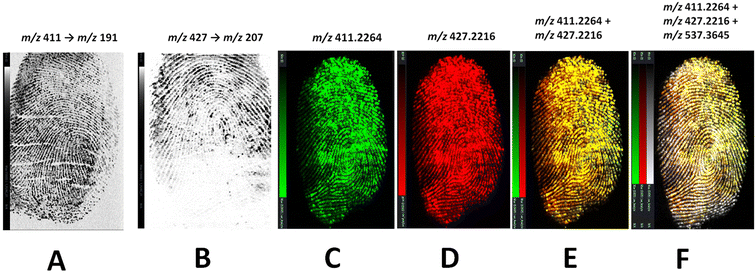
Risperidone and paliperidone were initially analysed as standard solutions and in a serial dilution to assess feasibility of DESI MS for the detection and mapping of these species. Two DESI systems were used, namely a tandem quadrupole (Xevo TQ-S) and the DESI cIMS, both mounting a DESI XS source. Initial LOD experiments were performed on the DESI – XEVO TQ-XS using the transitions 411 → 191 (risperidone) and 427 → 207 (paliperidone) for drug standards yielding an LOD for Risperidone of 5 ng mL−1 and a LOD for paliperidone of 500 ng mL−1. However, when a natural fingermark was spotted with a serial dilution of either risperidone or paliperidone and profiled, a higher concentration of fingertip contaminating solution of 5 × 103 ng mL−1 was taken forward to imaging experiments, due to the ion suppression exerted by the molecular composition of the fingermark. Under these concentration conditions, risperidone-, paliperidone- and risperidone/paliperidone contaminated-fingermarks were generated and analysed on either the DESI-Xevo TQ-XS mass spectrometer or the DESI-cIMS mass spectrometer. Using the grading scheme by Bandey,34 which assesses the quality of the fingermark from a biometric intelligence point of view, a grade 4 fingermark image was yielded when visualising risperidone using the transition 411 → 191 (Fig. 2B) and a grade 2 image was instead obtained for paliperidone using the transition 427 → 207 (Fig. 2B) on the DESI-Xevo TQ-XS mass spectrometer. Observing Fig. 2B, there is no technology reasoning to explain that the mass spectrometer did not visualise paliperidone in the bottom half of the mark; rather, it can be speculated that it could be due to either inhomogeneous rubbing of the fingertip on the dried paliperidone solution, or to important ion suppression exerted by molecules predominantly present in the bottom part of the (natural) mark, or to a combination of both factors. Performing DESI imaging experiments on a tandem quadrupole mass spectrometer has the benefit of increased sensitivity and speed of analysis with minimum effect on sensitivity, as specific ions are targeted through SRM or MRM experiments. However, the molecular target, and dominant fragment ions, must be known and there is a finite number of parent ions and ion fragments that can be selected in any one experiment. If one aims for the visualisation of forensically interesting substances onto the ridge pattern, and additionally the visualisation and superimposition of all those ions (whether known or unknown) that provide completeness of the ridge pattern in a combined image, then MS imaging, rather than MS/MS imaging is more appropriate. Fig. 2C–F show the DESI MS images obtained on a cIMS mass spectrometer in one experiment, where the fingermark was contaminated with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio solution of risperidone (m/z 411.2254) and paliperidone (m/z 427.2216). Here, it can be seen that it is possible to obtain separate images from risperidone, paliperidone and their superimposition. Whilst it is possible to obtain these images in MRM on the DESI TQ-XS, using MS imaging on the cIMS, it has been possible to superimpose a third ion (m/z 537.3645) (Fig. 2F), unknown and endogenous to the fingermark, in order to improve the ridge pattern continuity and increase the ridge flow/minutiae thus increasing the grade of the mark obtained. In a real case scenario, the presence of the metabolite or the drug and the metabolite together in a mark would indicate consumption of the drug, whereas the presence of the drug only may indicate drug handling. However, it is important to be mindful of the fact that there are other reasons for detection or lack thereof of the drug/metabolite, which may affect interpretation of the findings, not only for DESI MSI but for MALDI MSI and any other detection/imaging technique. For example, lack of detection of the parent drug in the case of consumption, could be due to extensive individual metabolism, though the detection of metabolite still may indicate consumption activity. However, there is still the possibility that the detection of the parent drug and/or the metabolite could be due to secondary transfer. Therefore whilst the data presented thus far and further in the manuscript prove feasibility of the approaches for the provision of both the chemical and biometric information from an alternative specimen such as fingerprints, interpretation of the source level information (why the species are there or absent) requires a framework and a quantitative/objective approach that are not currently available.
1 ratio solution of risperidone (m/z 411.2254) and paliperidone (m/z 427.2216). Here, it can be seen that it is possible to obtain separate images from risperidone, paliperidone and their superimposition. Whilst it is possible to obtain these images in MRM on the DESI TQ-XS, using MS imaging on the cIMS, it has been possible to superimpose a third ion (m/z 537.3645) (Fig. 2F), unknown and endogenous to the fingermark, in order to improve the ridge pattern continuity and increase the ridge flow/minutiae thus increasing the grade of the mark obtained. In a real case scenario, the presence of the metabolite or the drug and the metabolite together in a mark would indicate consumption of the drug, whereas the presence of the drug only may indicate drug handling. However, it is important to be mindful of the fact that there are other reasons for detection or lack thereof of the drug/metabolite, which may affect interpretation of the findings, not only for DESI MSI but for MALDI MSI and any other detection/imaging technique. For example, lack of detection of the parent drug in the case of consumption, could be due to extensive individual metabolism, though the detection of metabolite still may indicate consumption activity. However, there is still the possibility that the detection of the parent drug and/or the metabolite could be due to secondary transfer. Therefore whilst the data presented thus far and further in the manuscript prove feasibility of the approaches for the provision of both the chemical and biometric information from an alternative specimen such as fingerprints, interpretation of the source level information (why the species are there or absent) requires a framework and a quantitative/objective approach that are not currently available.
3.3 Mass spectrometry using SICRIT ionisation source
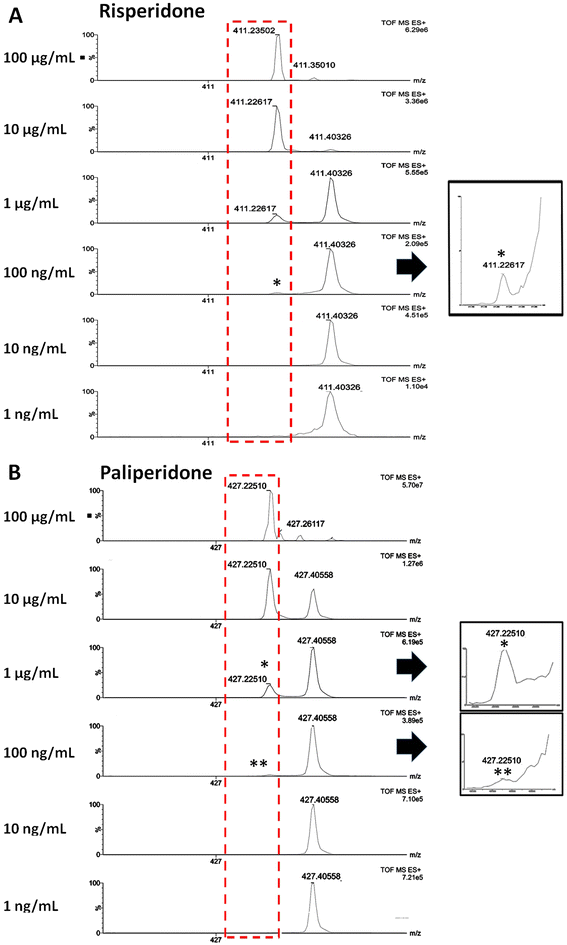
In the view of rapidly detecting risperidone and paliperidone in fingermarks, SICRIT ionisation was explored.Source parameters were optimised and both the amplitude and the temperature were observed to significantly impact ionisation. It was determined that an amplitude of 1600 V and a Temperature of 300 °C were optimal for analysis. Initially, standard solutions of either risperidone or paliperidone were analysed using the injection module to assess detectability and the limits of detection as described in section 2.3.3. In MS mode, an LOD of 100 ng mL−1 (absolute amount of 500 pg), for both the parent drug and paliperidone was observed. Compared to the LOD in DESI MS analysis on the Xevo-TQ-XS mass spectrometer, SICRIT ionisation showed to be slightly less sensitive for risperidone by a factor of 20 but slightly more sensitive for paliperidone by a factor of 5.
Risperidone and paliperidone were subsequently and separately spotted as 10 μL of a serial dilution from 1 × 105 ng mL−1 to 1 ng mL−1 on a natural fingermark, to assess the impact of any ion suppression caused by the molecular composition of the fingermark (Fig. 3). Risperidone and paliperidone were detected at m/z 411.223 and m/z 427.225 respectively, down to 100 ng mL−1 (absolute amount 1 ng), with a signal to noise of >167 for risperidone and >119 for paliperidone. Compared with the SICRIT analysis of these species as standards through direct infusion, there is only a factor of 2 decrease in the ability to detect risperidone and paliperdone when analysed in spots on fingermarks.
Again, compared to the spotted fingermark experiments in DESI MSI, SICRIT ionisation was more sensitive for risperidone by a factor of 50 and equally sensitive for paliperidone (better only by a factor of 2).
Compared to MALDI MSI fingermark spotting experiments, where risperidone could be detected/mapped down to 1 ng mL−1 for risperidone and to 10 ng mL−1 for paliperidone, SICRIT ionisation was less sensitive by a factor of 100 for risperidone and by a factor of 1000 for paliperidone (2 and 3 orders of magnitude respectively).
When employing SICRIT ionisation for risperidone and paliperidone in fingermarks, ion suppression can be invoked. Fig. 3 shows (i) the interference/ion suppression exerted by fingermark endogenous species at m/z 411.350 and m/z 411.403 (the latter likely squalene, theoretical m/z 411.399) being problematic for risperidone and (ii) the interference/ion suppression of the ions at m/z 427.261 and m/z 427.406 affecting the paliperidone detection; these ion interferences indicate that, for these particular species, to fully exploit the potential of SICRIT ionisation, this source should be coupled with a higher mass resolution mass spectrometer (as in the work by Conway et al.22) in order to improve the detection limits.
MS/MS analyses for both analytes were performed at a concentration of 100 μg mL−1 to investigate the formation of the most abundant ion fragments, which were observed to be at m/z 191.117 (C11H15N2O) and m/z 207.118 (C11H15N2O2) respectively (data not shown) confirming previous studies.35,36
3.4 Sequential imaging – SICRIT ionisation followed by mass spectrometry imaging
A multimodal approach was evaluated by employing SICRIT Ionisation as part of a sequential workflow. In one instance, SICRIT was followed by MALDI MSI and, in a subsequent experiment, it was followed by DESI MSI and then by MALDI MSI. The rationale behind trialling these approaches was that, provided that this ionisation source is coupled to HRMS (as in the work by Conway et al.22), these workflows would permit fast drug detection at a higher sensitivity, followed by the recovery of biometric intelligence using MSI.3.5 SICRIT → MALDI MSI
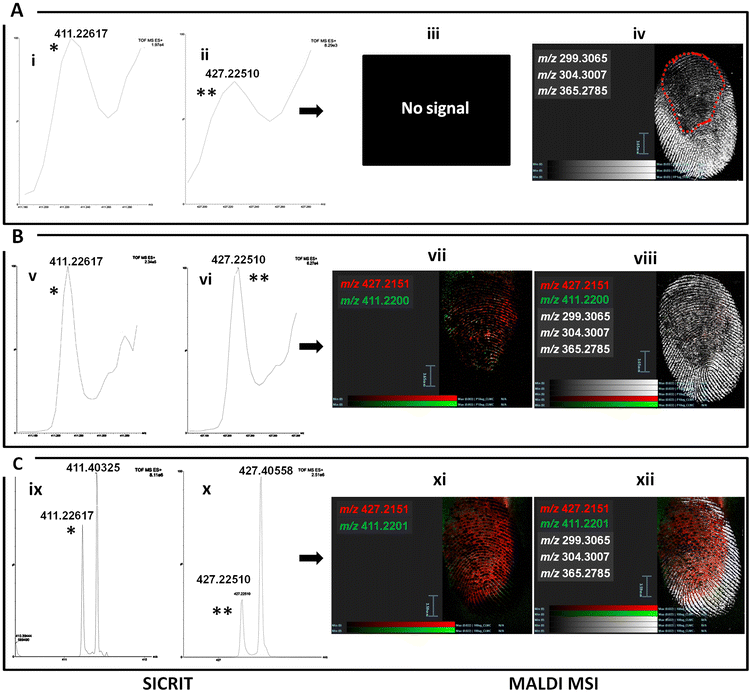
For this workflow, drug-contaminated fingermarks were generated after preliminarily spotting on a glass slide 50 μL of risperidone and paliperidone mixing 25 μL of each solution in equal concentrations varying between 1 × 105 ng mL−1 and 0.1 ng mL−1. Fig. 4 shows the MALDI MSI analyses for the drugs at an initial concentration of 1 × 103, 1 × 104 and 1 × 105 ng mL−1. In the case of the drugs concentrations at 1 × 103 ng mL−1, the SICRIT ionisation yielded an ion signal for risperidone at m/z 411.226 (i) (S/N 80) and paliperidone at m/z 427.225 (ii) (S/N 33) (absolute amount <5 ng). However, when subsequently imaged by MALDI MSI, none of the two species yielded a viable molecular image and this may be due to either presence below the detection limits, or because to the way in which the encoded frequency pushing algorithm works.37 It is also likely that the prior desorption of this drug via SICRIT ionisation made risperidone detection in fingermarks fall below the detection limits on the MALDI MRT. Nonetheless the fingermark ridge pattern could be reconstructed (Fig. 4A(iv)) through superimposition of the fingermark ions at m/z 299.30627, 304.30057 and 365.27905, thus showing the possibility to obtain both the chemical and the biometric information with a sequential workflow. Interestingly, Fig. 4A(iv) shows the region delimited in a discontinuous red line where most of the desorption of risperidone and other analytes in fingermarks appears to occur as a result of SICRIT ionisation prior to MALDI MSI. This region indicates an inhomogeneous SICRIT desorption process and perhaps the necessity to further engineer the source, to either enable a homogeneous desorption, or to confine the location of the desorption to a small region, in order to enhance the likelihood of both higher quality biometric information (more minutiae in a fingermark) and the recovery of chemical intelligence (in this case risperidone detection). When this experiment was repeated with a fingermark contaminated by mixing the two drugs’ solutions at 1 × 104 ng mL−1 each, both risperidone and paliperidone were detected at the same m/z as previously (Fig. 4B(v–vi)). This fingermark was then subjected to MALDI MSI and in this instance, the higher starting concentrations of the two species reduced the impact on MALDI MS visualisation by permitting both risperidone at m/z 411.21973 and paliperidone at m/z 427.21426 to be mapped in an overlaid fashion, onto the fingermark ridge pattern (Fig. 4B(vii)). The further superimposition with ions at m/z 299.30627, 304.30057 and 365.27905 enabled a much better ridge coverage with respect to the experiments with the drugs’ concentrations at 1 × 103 ng mL−1, although the “SICRIT desorption region” is still visible and superimposable with that of the experiment conducted with the two drugs at 1 × 103 ng mL−1. Finally the experiment was conducted with a fingermark generated by initially mixing the two drugs’ solutions at 100 μg mL−1 each; the higher concentrations of both drugs further improve both their ionisation and mass resolution using SICRIT, as well as the quality of the resulting MALDI MS image of the fingermarks reconstructed through the superimposition of these two ions (Fig. 4Cxi) and by superimposing further ions as in the previous examples (Fig. 4Cxii).3.6 SICRIT → DESI MSI → MALDI MSI
For this sequential workflow, two experiments were performed, one with a fingermark generated from fingertips contaminated with risperidone only at a concentration of 500 ng mL−1, and one with a fingermark generated from fingertips contaminated with risperidone and paliperidone in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio risperidone/paliperidone (each in a concentration of 1 × 104 ng mL−1). DESI MS and MALDI MS images were generated in sequence following SICRIT ionisation (Fig. 5).
1 ratio risperidone/paliperidone (each in a concentration of 1 × 104 ng mL−1). DESI MS and MALDI MS images were generated in sequence following SICRIT ionisation (Fig. 5).
In the first experiment, the risperidone-contaminated mark was preliminarily submitted to SICRIT Ionisation which did yield the risperidone ion signal at m/z 411.219. This detection was expected as detection of risperidone in spotted marks was possible down to a concentration of 100 ng mL−1 with no ion interference, as Fig. 3 reported. This mark was then subjected to DESI MSI on the Xevo-TQ-XS mass spectrometer.
As Fig. 5A shows, the fingermark ridge pattern could be reconstructed through the visualisation of risperidone using the transition m/z 411 → 191.
Previously, DESI MSI on the XEVO-TQ-XS yielded the images of distribution of risperidone in a drug-contaminated mark, using the same transition, with a fingertip contaminating solution of 500 ng mL−1. However, this time, despite some risperidone being desorbed during SICRIT ionisation, it was still possible to detect the drug and reconstruct the fingermark ridge pattern through visualising its MRM transition. The same mark was then submitted to MALDI MSI via the prior application and adaptation of the dry-wet method38,39 in the attempt to provide complementary biometric information; here the mark was preliminarily powdered with ball milled α-cyano 4 hydroxycinnamic acid average size of 10 μm using the planetary ball mill (Retsch, GmbH), following the method of Ferguson et al.38 and then it was spray coated with a 5 mg ml−1 α-CHCA solution in 70/30 ACN/0.1% TFAaq solution.39 The mark was then imaged by MALDI MSI on the SELECT SERIES MALDI MRT.
At this stage, risperidone had been desorbed first via SICRIT Ionisation and then through DESI MSI. The lack of MALDI ion signal and ion image for this drug is therefore expected, considering also that the minimum concentration that could be imaged in a single drug process workflow using MALDI MSI was 1 × 103 ng mL−1 (data not shown). However, the dry-wet method enabled the recovery of a combined MALDI MS ion image showing high quality level 1 and level 2 characteristics (ridge flow and local fingermark features, respectively), thus adding to the biometric information (Fig. 5B). The MALDI image has been obtained through the superimposition of several ions at m/z 221.97234, 265.96210, 476.98682 and 688.01215. In the second experiment, fingertips were contaminated with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixed solution of risperidone and paliperidone initially at a concentration of 0.5 μg mL−1, prior to contaminating a fingertip and depositing a drug/metabolite-contaminated fingermark on a glass slide. The glass slide was inserted in the heated cartridge and subjected to SICRIT ionisation and subsequently to DESI MSI on the XEVO TQ-XS mass spectrometer. After SICRIT Ionisation, the imaging analysis still yielded the reconstruction of the ridge pattern through the superimposition of the ion images from risperidone and paliperidone using the transitions m/z 411 → 191 and m/z 427 → 207 (Fig. 5C). However, this time, the DESI analysis shown was a second acquisition. Whilst it was observed that viable signal was still detected and mapped onto the ridge pattern in this second DESI MSI acquisition, the subsequent MALDI MSI experiment did not yield viable ridge pattern (Fig. 5D) as evidently, too much of the fingermark constituents had been already desorbed. A 3-techniques multi-modal workflow would only be recommended if the first MSI technique (1) fails to visualise the drug/metabolite onto the ridge pattern and/or (2) does not provide sufficient biometric information for submission to biometric identification technology such as AFIS, for fingermark comparison and match in a National Database.
1 mixed solution of risperidone and paliperidone initially at a concentration of 0.5 μg mL−1, prior to contaminating a fingertip and depositing a drug/metabolite-contaminated fingermark on a glass slide. The glass slide was inserted in the heated cartridge and subjected to SICRIT ionisation and subsequently to DESI MSI on the XEVO TQ-XS mass spectrometer. After SICRIT Ionisation, the imaging analysis still yielded the reconstruction of the ridge pattern through the superimposition of the ion images from risperidone and paliperidone using the transitions m/z 411 → 191 and m/z 427 → 207 (Fig. 5C). However, this time, the DESI analysis shown was a second acquisition. Whilst it was observed that viable signal was still detected and mapped onto the ridge pattern in this second DESI MSI acquisition, the subsequent MALDI MSI experiment did not yield viable ridge pattern (Fig. 5D) as evidently, too much of the fingermark constituents had been already desorbed. A 3-techniques multi-modal workflow would only be recommended if the first MSI technique (1) fails to visualise the drug/metabolite onto the ridge pattern and/or (2) does not provide sufficient biometric information for submission to biometric identification technology such as AFIS, for fingermark comparison and match in a National Database.
3.7 Chemical enhancement → MALDI MSI/DESI MSI of risperidone and paliperidone in fingermarks
Chemical enhancement with 1,2-indandione was applied to split contaminated fingermarks as shown in Fig. 6. Six samples were prepared and chemically enhanced, three for the subsequent application of DESI MSI and 3 for the subsequent application of MALDI MSI. For each set, a control mark (with no risperidone or paliperidone contamination), one contaminated with risperidone only and one with a mixture of risperidone and paliperidone in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.
1 ratio.
The application of 1,2-indandione and the visualisation through irradiation at 3 different wavelengths (Fig. 6A) yielded: (i) some level of visualisation on the white paper ranging for the two CNT marks and for only one of the risperidone only contaminated marks using blue light (420–470 nm); (ii) some level of visualisation ranging from grade 1 to 4 of the Home Office system for all the marks on white paper using blue/green light (445–510 nm) and (iii) same results as for the blue/green light were obtained using green light (480–560 nm), albeit the quality was slightly worse than the blue/green light. No visible fingermark on red paper could be observed for any of the wavelengths used or different types of fingermarks.
The subsequent application of DESI MSI did not yield any fingermark image (data not shown) and although MALDI MSI enabled visualisation of the fingermark through its endogenous content, neither risperidone nor raliperidone were detected; Fig. 6B shows the superimposed MALDI MS images of the endogenous species at m/z 249.98840, 265.96271, 299.30502 to reconstruct the ridge pattern reconstruction.
These observations demonstrated compatibility of MALDI MSI in the retrieval of biometric information after the application of this forensic enhancement technique, but not of the chemical intelligence, with respect to the drug and its metabolite. The absence of risperidone/paliperidone signal in the relevant marks on either white or red paper was ascribed to the use of a high impact chemical enhancement process requiring liquid immersion; furthermore this protocol is based on the Category A process formulation given in the UK Home Office Fingermark Visualisation Manual (FVM),23 but uses Petroleum ether as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 replacement for the recommended HFE7100 solvent (which ceases production in 2025); it is possible that the combination of liquid immersion and a solvent mixture in which both risperidone and paliperidone are soluble have led to these species being washed away, explaining absence of signal in both mass spectra and DESI/MALDI images. It is also interesting to note that from the chemical enhancement point of view, the use of 1,2-indandione produces the no or poor fingermark visualisation (on white paper) when Risperidone or Risperidone and Paliperidone are present. In consideration of the fact the red paper did not produce visualisation at any wavelength irradiation and that the hypothesised detrimental effect on MALDI MSI and DESI MSI signal caused by the liquid immersion process, another (lower impact) protocol was tested using a “drier” method consisting in the use of DMAC impregnated sheets (FVM, Cat B process23). The protocol was only applied to fingermarks on red paper to demonstrate the benefits of the subsequent application of mass spectrometry imaging techniques when the conventional forensic enhancement fails.
1 replacement for the recommended HFE7100 solvent (which ceases production in 2025); it is possible that the combination of liquid immersion and a solvent mixture in which both risperidone and paliperidone are soluble have led to these species being washed away, explaining absence of signal in both mass spectra and DESI/MALDI images. It is also interesting to note that from the chemical enhancement point of view, the use of 1,2-indandione produces the no or poor fingermark visualisation (on white paper) when Risperidone or Risperidone and Paliperidone are present. In consideration of the fact the red paper did not produce visualisation at any wavelength irradiation and that the hypothesised detrimental effect on MALDI MSI and DESI MSI signal caused by the liquid immersion process, another (lower impact) protocol was tested using a “drier” method consisting in the use of DMAC impregnated sheets (FVM, Cat B process23). The protocol was only applied to fingermarks on red paper to demonstrate the benefits of the subsequent application of mass spectrometry imaging techniques when the conventional forensic enhancement fails.
After enhancement and photography, the red paper was split and one-half mark was subjected to DESI MSI and the other half to MALDI MSI. Fig. 7 shows the chemical enhancement results with DMAC (Fig. 7A) and the subsequent visualisation using either DESI MSI or MALDI MSI (Fig. 7B).
This time, as ESI Fig. S3† shows with better clarity, the combination of DMAC enhancement and Blue light irradiation (420–470 nm) produced by and large the highest quality of visualisation (up to grade 3 for the control mark), though grade 1 marks could be observed with Blue/green wavelength (445–510 nm) but no mark at all was visible when using the green wavelength (480–560 nm), possibly due to the increased background fluorescence of the red paper at these wavelengths.
Following DMAC enhancement, all marks were split, and one half was imaged using DESI MSI on a cIMS mass spectrometer and the other half using MALDI MSI on the SELECT SERIES MALDI MRT (Fig. 7B). As a representative example, Fig. 7B shows 3 DESI/MALDI images of the first paper specimen from the left handside and ion images for both DESI MSI and MALDI MSI are shown for the ion at m/z 411.21973 (risperidone), m/z 427.21526 (paliperidone) and the combined image resulting from the superimposition of the images for these two ions. Different from the enhancement with 1,2-indandione, this time the fingermark ridge pattern could be reconstructed through visualising both drugs. This indicates that the immersion in a solvent in which the two species could be soluble, is too aggressive and supports the hypothesis that it may have washed risperidone and paliperidone away. The dry, vapour phase DMAC enhancement process will have preserved the presence of these two species, making possible the visualisation of their distribution through both DESI and MALDI. All the DESI MS and MALDI MS images were TIC normalised. It was observed that whilst, generally the relevant ion signals were more intense in the DESI images, the quality of the ridge detail was better in the MALDI images, despite nominally using the same spatial resolution.
4. Conclusions
In this paper we report an exploration of mass spectrometry imaging-based approaches as single processes or in combination for the detection and imaging of “date rape drugs” in fingerprints, using risperidone and its pharmacologically active metabolite paliperidone as a case study. Both MALDI MSI and DESI MSI provided contextual chemical information (drug and metabolite detection mapped on the ridges) and biometric information (reconstruction of the ridge pattern), thus expanding the capabilities of “molecular fingerprinting”, particularly those offered by MALDI MSI. DESI MSI was trialled both coupled with a tandem quadrupole and with a Cyclic Ion Mobility Mass Spectrometer, with the former offering naturally higher sensitivity, due to its targeted approach exploiting specific MRM transitions. A number of multi-modal approaches were tested, including the combination with Chemical Reaction In Transfer Ionisation via SICRIT® which had been shown by others to detect drugs of abuse in the picogram range in fingerprints. It was generally possible to achieve a quick drug detection in the fingerprint using SICRIT, subsequent risperidone/paliperidone mapping via DESI MSI and finally, additional biometric details using MALDI MSI in a sequential approach.The approach illustrated here is described as a means to corroborate the victim's statement through the detection and imaging of the drug and metabolite in the victim's fingermarks. However, the approach is equally important if applied to the suspect's fingermark; when not using needle spiking, in fact, the perpetrator would dissolve the drug in the drink and, as such, they may contaminate their fingertip and transfer the parent drug to their fingerprint upon contacting a surface. In this case too, it is possible to provide circumstantial evidence to be linked to an individual identity. In the case of the perpetrator, the amount of drug contaminating the fingertip is in the order of milligrams, and, as such, well within the detection limits afforded by MALDI MSI and DESI MSI using the cutting-edge instrumentation reported in this work. However, as for any detection/imaging analytical technique providing information at a source level, a word of caution is needed in relation to activity level propositions, especially with regards to the potential suspect. Secondary transfer could in fact be the reason why the drug is detecting in someone's fingerprints. Presently, the forensic and analytical community still generally lacks a solid framework to assess and distinguish quantitatively and objectively between different activity level propositions and, in this case, the cut off levels separating transfer from genuine handling of the substance in question; therefore the source level information must be treated with this in mind and evaluated in association with the intelligence gathered by classic police investigation and other obtained forensic evidence.
Finally, in order to show the possibility to integrate mass spectrometry-based imaging approaches in an operational workflow, drug/metabolite contaminated fingerprints were deposited on paper and enhanced with either 1,2-indandione or DMAC (conventional enhancement techniques applied to fingermarks on paper), prior to DESI or MALDI MSI. Whilst both enhancement techniques struggled to visualise fingermarks on red paper, the “dryer” process (DMAC) yielded, depending on irradiation wavelengths, some ridge detail, and was compatible with both DESI and MALDI MSI. Both MSI techniques provided much better biometric information in terms of clarity and number of fingerprint details (minutiae), through drug/metabolite mapping, demonstrating integrability of the mass spectrometry imaging techniques within a particular operational forensic workflow.
Data availability
Data for this article including mass spectrometry images and mass spectra are available in the Sheffield Hallam repository SHURDA at [URL – format https://shurda.shu.ac.uk/id/eprint/234].Conflicts of interest
Waters Corporation has matched-funded the PhD studentship awarded to Mr Rohith Krishna, enabling the generation of these data on Waters mass spectrometers. Notwithstanding there is no conflict of interest perceived.Acknowledgements
Plasmion GmbH, Augsburg, Germany, is gratefully acknowledged for letting the Centre for Mass Spectrometry Imaging, at Sheffield Hallam University, test and evaluate the SICRIT ionisation source and for assisting in the set up of the source. Sue Kennerley at KR Analytical Ltd, England, UK are also acknowledged for facilitating the free loan. Waters Corporations and Sheffield Hallam University are also gratefully acknowleged for funding the PhD studentship supporting Mr Rohith Krishna in the undertaking of this research.References
- Forensic analysis of drugs facilitating sexual assault and other criminal acts, accessed at https://www.unodc.org/documents/scientific/forensic_analys_of_drugs_facilitating_sexual_assault_and_other_criminal_acts.pdf on 28/01/2025.
- P. Prego-Meleiro, G. Montalvo, Ó. Quintela-Jorge and C. García-Ruiz, Forensic Sci. Int., 2020, 315, 110460 CrossRef CAS PubMed.
- M. A. Bellis and K. Hughes, Sex potions relationships between alcohol, drugs and sex, Adicciones, 2004, 16, 249–258 CrossRef.
- Policy paper Report: Understanding and tackling spiking (2024) accessed at https://www.gov.uk/government/publications/understanding-and-tackling-spiking/report-understanding-and-tackling-spiking-accessible on 28/1/2025.
- World Health Organization, World report on violence and health. Geneva, 2002, Accessed at https://apps.who.int/iris/bitstream/handle/10665/42495/9241545615_eng.pdf on 28/01/2025 Search PubMed.
- World Health Organisation, Interpersonal violence and Illicit Drugs, 2009. Accessed at https://www.who.int/docs/default-source/documents/child-maltreatment/interpersonal-violence-and-illicit-drug-use.pdf 2009 on 28/01/2025 Search PubMed.
- Sexual Violence-Victimisation Australia, Australian Bureau of Statistics, 2021. Accessed at https://www.abs.gov.au/articles/sexual-violence-victimisation#key-statistics on 28/01/2025 Search PubMed.
- D. G. Kilpatrick, H. S. Resnick, K. J. Ruggiero, L. M. Conoscenti and J. McCauley, 2007. Drug-facilitated, Incapacitated, and Forcible Rape: a National Study, National Criminal Justice Reference Service, Charleston. Accessed on 28/01/2025 at https://www.researchgate.net/profile/Dean-Kilpatrick/publication/224918954_National_Prevalence_of_Posttraumatic_Stress_Disorder_Among_Sexually_Revictimized_Adolescent_College_and_Adult_Household-Residing_Women/links/5891f0f8aca272f9a5582ebe/National-Prevalence-of-Posttraumatic-Stress-Disorder-Among-Sexually-Revictimized-Adolescent-College-and-Adult-Household-Residing-Women.pdf Search PubMed.
- K. C. Basile, S. G. Smith, Y. Liu, A. Lowe, A. K. Gilmore, S. Khatiwada and M. Kresnow, Drug Alcohol Depend., 2021, 226, 108839 CrossRef PubMed.
- M. Lynam, D. Keatley, G. Maker and J. Coumbaros, Forensic Sci. Int., 2024, 9, 100545 Search PubMed.
- L. J. Anderson, A. Flynn and J. L. Pilgrim, J. Forensic Leg. Med., 2017, 47, 46 CrossRef PubMed.
- A. Grela, L. Gautam and M. D. Cole, Forensic Sci. Int., 2018, 292, 50–60 CrossRef CAS PubMed.
- P. Adamowicz and M. Kała, Forensic Sci. Int., 2010, 198, 39–45 CrossRef CAS PubMed.
- M. J. Bailey, R. Bradshaw, S. Francese, T. L. Salter, C. Costa, M. Ismail, R. P. Webb, I. Bosman, K. Wolff and M. de Puit, Analyst, 2015, 140, 6254–6259 RSC.
- R. Bradshaw, N. Denison and S. Francese, Analyst, 2017, 142, 1581–1590 RSC.
- K. Skov, S. S. Johansen, K. Linnet and M. K. K. Nielsen, Eur. Rev. Med. Pharmacol. Sci., 2022, 26, 183–197 CAS.
- Drug Facilitated Sexual Assault (DFSA), Office of the Chief Medical Examiner City and County of San Francisco, 2021. Accessed at https://www.sf.gov/sites/default/files/2023-05/2021%20OCME%20DFSA%20Report.pdf on 28/01/2025 Search PubMed.
- S. M. R. Wille, K. Van Dijck, A. Van Assche, V. Di Fazio, M. D. M. Ramiréz-Fernandéz, V. Vanvooren and N. Samyn, Pharmaceuticals, 2021, 14, 432 CrossRef CAS PubMed.
- H. Poulsen, M.J. McCarthy, J. Baker, A. Verma, H. J. Moir, T. Brodie, B. Thatti, G. Trotter and B. Rooney, J. Anal. Toxicol., 2021, 45, 44–52 CrossRef CAS PubMed.
- H. Nozawa, K. Minakata, K. Hasegawa, I. Yamagishi, N. Miyoshi, K. Yuyama, M. Suzuki, T. Kitamoto, M. Kondo and O. Suzuki, Leg. Med., 2024, 69, 102340 CrossRef CAS PubMed.
- K. Longman, C. Frampas, H. Lewis, C. Costa, R. Nilforooshan, M. Chambers and M. Bailey, Front. Chem., 2023, 11, 1245089 CrossRef CAS PubMed.
- C. Conway, M. Weber, A. Ferranti, J.-C. Wolf and C. Haisch, Drug Test. Anal., 2024, 16, 1094–1101 CrossRef CAS PubMed.
- Fingermark Visualisation Manual Second Edition, ed. H. Bandey, Dstl, 2022, ISBN: 978-1-3999-0976-1 Search PubMed.
- S. Berdejo, M. Rowe and J. W. Bond, J. Forensic Sci., 2011, 211(57), 509–514 Search PubMed.
- S.-G. Cheng, C.-M. Chen, C.-L. Liu, S.-Q. Liu and S. K. Lim, Evaluation of Fingerprint Development Techniques Applied in Red Pockets and Its Subsequent Process, paper presented at International Fingerprint Research Group Meeting, Jerusalem, Israel, 2013.
- R. Krishna, J. Langridge, E. Claude, R. Bradshaw, L. Cole and S. Francese, Anal. Chim. Acta, 2025, 1354, 343998 CrossRef CAS PubMed.
- M. Strohalm, M. Hassman, B. Kosata and M. Kodicek, Rapid Commun. Mass Spectrom., 2008, 22, 905 CrossRef PubMed.
- M. Strohalm, D. Kavan, P. Novakand and V. Havlicek, Anal. Chem., 2010, 82, 4648 CrossRef CAS PubMed.
- G. Groeneveld, M. de Puit, S. Bleay, S. R. Bradshaw and S. Francese, Sci. Rep., 2015, 5, 11716 CrossRef CAS PubMed.
- M. L. Manier, D. S. Cornett, D. L. Hachey and R. M. Caprioli, J. Am. Soc. Mass Spectrom., 2008, 19, 666 CrossRef CAS PubMed.
- R. Wolstenholme, R. Bradshaw, M. R. Clench and S. Francese, Rapid Commun. Mass Spectrom., 2009, 23, 3031–3039 CrossRef CAS PubMed.
- R. Bradshaw, S. Bleay, R. Wolstenholme, M. R. Clench and S. Francese, Forensic Sci. Int., 2013, 232, 111–124 CrossRef CAS PubMed.
- E. Saar, D. Gerostamoulos, O. H. Drummer and J. Beyer, J. Mass Spectrom., 2010, 45, 915–925 CrossRef CAS PubMed.
- H. L. Bandey, Fingerprint Development and Imaging Newsletter: The Powders Process, Study 1, Police Scientific Development Branch, Home Office, Sandridge, 2004, p. 54/04 Search PubMed.
- E. Ezzeldin, M. Tammam and N. F. A. Talib, Int. J. Anal. Chem., 2017, 1271383, DOI:10.1155/2017/1271383.
- N. Vanwong, S. Prommas, A. Puangpetch, Y. Hongkaew, N. Nuntamool, C. N. Nakorn, N. Ngamsamut, P. Limsila and C. Sukasem, J. Clin. Lab. Anal., 2016, 30, 1236–1246 CrossRef CAS PubMed.
- P. Willis, J. Jaloszynski and V. Artaev, Int. J. Mass Spectrom., 2021, 459, 116467 CrossRef CAS.
- L. Ferguson, R. Bradshaw, R. Wolstenholme, M. R. Clench and S. Francese, Anal. Chem., 2011, 83, 5585–5591 CrossRef CAS PubMed.
- S. Francese and C. Heaton, Emerging Technologies: Use of Matrix Assisted Laser Desorption Ionisation Mass Spectrometry for the Analysis of Fingermark and Blood Evidence, in Applications of Mass Spectrometry for the Provision of Forensic Intelligence: State-of-the-art and Perspectives, ed. S. Francese and S. M. Bleay, 2023, pp. 159–183, ISBN: 978-1-83767-193-9 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5an00328h |
| This journal is © The Royal Society of Chemistry 2025 |