Open Access Article
Open Access ArticleChondrogenic and chondroprotective response of composite collagen I/II-hyaluronic acid scaffolds within an inflammatory osteoarthritic environment†
Carly M.
Battistoni
 a,
Javier
Munoz Briones
a,
Javier
Munoz Briones
 bc,
Douglas K.
Brubaker
bc,
Douglas K.
Brubaker
 bde,
Alyssa
Panitch
bde,
Alyssa
Panitch
 f and
Julie C.
Liu
f and
Julie C.
Liu
 *ab
*ab
aDavidson School of Chemical Engineering, Purdue, University, West Lafayette, IN 47907, USA. E-mail: julieliu@purdue.edu; Tel: +765-494-1935
bWeldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907, USA
cPurdue Interdisciplinary Life Science Program, Purdue University, West Lafayette, IN 47907, USA
dCenter for Global Health and Diseases, Department of Pathology, Case Western Reserve University, Cleveland, OH 44016, USA
eBlood Heart Lung Immunology Research Center, University Hospitals, Cleveland, OH 44106, USA
fWallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, Georgia 30332, USA
First published on 21st April 2025
Abstract
Inflammation plays a key role in cartilage damage that occurs in osteoarthritis (OA). However, in vitro assessments of tissue-engineered constructs for cartilage regeneration generally do not consider their performance in the presence of inflammation. In this work, the chondrogenic differentiation potential of mesenchymal stromal cells (MSCs) was evaluated in the presence of both chondrogenic factors and inflammatory cytokines, and cartilage formation, degradative response, and inflammatory response were characterized. The addition of cytokines reduced cartilage production, increased cell proliferation, and resulted in an increase in inflammatory markers. Incorporation of hyaluronic acid (HA) had little impact on both collagen fibril microstructure and mechanical properties, two gel properties known to affect cell response, and thus allows the work to probe the biological impact of HA without the confounding effect of these gel properties. Regardless of in vitro environment, HA did not change cartilage production. The inflammatory response was similar with or without HA in terms of IL-6 and IL-10 secretion whereas IL-8 production exhibited some correlation with HA concentration as observed via a linear regression model. Additionally, in the presence of cytokines, inclusion of HA statistically decreased the gene- and protein-level expression of matrix metalloproteinase-13 (MMP-13). Thus, when exposed to both chondrogenic growth factors and inflammatory cytokines within a chondrogenic-promoting collagen I/II blended hydrogel, chondrogenic differentiation of MSCs was limited by the inflammatory environment. These findings emphasize the importance of understanding how biomaterials affect cell responses within disease-relevant inflammatory environments.
Introduction
Osteoarthritis (OA) is a disease of the joints that impacts greater than 500 million people globally.1 OA is characterized by synovial inflammation and cyclic degradation of articular cartilage.2In vivo, the few chondrocytes present cannot produce enough matrix to overcome the amount of matrix degradation that occurs.3 Meanwhile, chondrocytes release inflammatory molecules, cytokines, and chemokines that disrupt homeostasis and recruit immune cells to the diseased site.2,4 Currently, there are no clinically available treatments that successfully restore damaged articular cartilage. Instead, treatments are palliative and serve to alleviate pain.3,5 Tissue-engineered therapies offer the potential of regenerating damaged articular cartilage in patients with OA.Since cartilage damage during OA is driven by inflammation, the environment poses an additional challenge for potential tissue-engineered therapies.6 The inflammatory environment leads to difficulties with treatment implantation and sustained repair. In general, research has investigated promoting cartilage formation or reducing inflammation. Recently, researchers have begun to include anti-inflammatory treatments within tissue-engineered platforms through drug encapsulation or other inhibitors.7 However, the in vitro effect of inflammation on tissue-engineered constructs that have been designed to promote chondrogenesis has been minimally explored.
In vitro OA models include cartilage explants with different combinations of cytokines.8 The combination of 10 ng mL−1 of oncostatin-M (OSM) and 20 ng mL−1 of tumor necrosis factor-α (TNF-α) was verified in vitro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9,10 and in vivo
9,10 and in vivo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 to induce key OA characteristics, including upregulation of degradative enzymes and subsequent cartilage degradation. OSM is part of the interleukin-1 (IL-1) family and is upregulated in OA.12 Similarly, TNF-α is upregulated during OA and is therefore a natural choice to mimic OA in vitro.12 Other OA models include the use of mesenchymal stromal cells (MSCs), a popular cell choice for cartilage tissue engineering, in pellet culture using similar cytokine systems, such as IL-1β and TNF-α.13 However, the use of these OA models has not considered how MSCs within tissue-engineered constructs are affected by these cytokines while undergoing chondrogenic differentiation.
11 to induce key OA characteristics, including upregulation of degradative enzymes and subsequent cartilage degradation. OSM is part of the interleukin-1 (IL-1) family and is upregulated in OA.12 Similarly, TNF-α is upregulated during OA and is therefore a natural choice to mimic OA in vitro.12 Other OA models include the use of mesenchymal stromal cells (MSCs), a popular cell choice for cartilage tissue engineering, in pellet culture using similar cytokine systems, such as IL-1β and TNF-α.13 However, the use of these OA models has not considered how MSCs within tissue-engineered constructs are affected by these cytokines while undergoing chondrogenic differentiation.
Hyaluronic acid (HA), a glycosaminoglycan (GAG) found in healthy hyaline cartilage, improves chondrogenic differentiation of MSCs14 and can be incorporated into treatments to impart anti-inflammatory effects to counter inflammation found in OA.15 During OA, native HA is broken down into smaller molecular weight (MW) fragments and contributes to the degradation cycle of OA via HA-cell-receptor interactions.16 Thus, one palliative treatment for OA patients is viscosupplementation, or the injection of HA into the knee joint to increase lubrication and suppress inflammation.17 Higher MWs (>1 MDa) of HA are anti-inflammatory, whereas lower molecular weight fragments of HA elicit a pro-inflammatory response.15,18
Within cartilage tissue engineering applications, HA hydrogels have been created through various forms of crosslinking such as thiolation, hydrazine linkages, and methacrylation.18 Chemically modified HA has been included in composite gels with collagen I, collagen II, and collagen I/II to enhance recapitulation of the native cartilage environment.19,20 One major downside to crosslinking HA is that extensive modification reduces its bioactivity.21 For example, increased HA modification decreased HA binding to cell surface receptors and reduced MSC chondrogenesis within crosslinked HA gels.22 Alternatively, another study showed that, when HA was added to chondrogenic media, it promoted cartilage formation in chondrocyte-embedded collagen hydrogels.23 In contrast, this work encapsulates unmodified, high molecular weight (1.5 MDa) HA within a collagen I/II hydrogel to allow for access to all bioactive sites in HA molecules.
In this work, we focus on bridging the gap between cartilage regeneration and resisting inflammation by assessing cartilage formation within an in vitro inflammatory environment. Our lab previously developed a blended collagen (col) I/II construct with encapsulated MSCs that promoted cartilage regeneration within an in vivo rabbit model compared to col I alone.24 Here, we expanded on our system via incorporation of high MW (1.5 MDa) HA to impart anti-inflammatory properties to our blended col I/II hydrogel. To evaluate if the addition of HA reduces degradation and inflammation, hydrogels were studied within an in vitro OA model of OSM and TNF-α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9–11 to assess MSC chondrogenic differentiation within an inflammatory environment. Matrix deposition and chondrogenic gene expression were quantified after 4 weeks and 1 week in culture, respectively. The inflammatory response was assessed at both the gene and protein levels. Finally, degradation, an important attribute of OA, was evaluated.
9–11 to assess MSC chondrogenic differentiation within an inflammatory environment. Matrix deposition and chondrogenic gene expression were quantified after 4 weeks and 1 week in culture, respectively. The inflammatory response was assessed at both the gene and protein levels. Finally, degradation, an important attribute of OA, was evaluated.
Materials & methods
Materials
Unless otherwise noted, all materials were purchased from Sigma-Aldrich (St. Louis, MO).Hydrogel fabrication
The hydrogels were prepared using a protocol described in detail in Kilmer et al. with slight modification to include HA.24 Briefly, col II from lyophilized chicken sternum was prepared at the same concentration (9.44 mg mL−1) as the col I solution (Corning, Corning, NY) by reconstitution in 20 mM acetic acid and was sterile filtered for experiments involving cells. HA (MW of 1.5 MDa, Lifecore Biomedical, Chaska, MN) was reconstituted in sterile phosphate-buffered saline (PBS) (Gibco, ThermoFisher Scientific, Waltham, MA). A neutralization solution consisting of PBS and NaOH was used to adjust hydrogels to a physiological pH of 7.4. Neutralization solution was prepared fresh for all experiments and was mixed and kept on ice prior to use. For formulations with HA (“+HA”), HA dissolved in 1× PBS was added, whereas 1× PBS was added for hydrogels without HA (“−HA”). The final gel formulations consisted of 3 mg mL−1 col I, 1 mg mL−1 col II, 0.1 mg mL−1 HA, and 1.1× PBS. Hydrogels were polymerized at 25 °C for 3 hours for all experiments. Hydrogels were then moved to 37 °C after 1× PBS or media was added on top for hydration or cell feeding, respectively.Confocal microscopy
Ten μL hydrogels were prepared in Ibidi angiogenesis slides (Cat. No. 81507, Ibidi, Gräfelfing, Germany). After three hours of polymerization, 40 μL of media was added to the top of the hydrogel to simulate the environment during cell studies and to allow gels to reach an equilibrium state (48 h at 37 °C) before imaging. Reflectance images were taken with a Zeiss 900 microscope (Carl Zeiss Microscopy, White Plains, NY). Images were taken at a step size of 50 μm from the bottom of the well for a total of 4 images (bottom of the well, 50 μm, 100 μm, and 150 μm). Images taken 50 μm from the bottom of the well are presented in this work.Rheology
Frequency sweeps were performed on a Discovery HR-2 Rheometer (TA Instruments, New Castle, DE) using a 20 mm cone geometry and a constant strain of 0.1%. Hydrogels were polymerized on a Teflon-coated microscope slide (Tekdon, Myakka City, FL) with a volume of 500 μL. The Peltier plate was set to 5 °C prior to placing the Tekdon slide and was set to 37 °C before the start of the sweep. The Tekdon slide was held in place via a 3D-printed slide holder made to fit over the Peltier plate. A 10 second hold was implemented before the start of each frequency sweep to ensure temperature was reached and that the hydrogel-geometry contact was stable.MSC encapsulation
Human mesenchymal stromal cells (MSCs, Lonza, Walkersville, MD, Cat. No. PT-2501; one lot was used for all experiments) were expanded to passage four in low glucose Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum (Lonza, Walkersville, MD), 1% penicillin–streptomycin (Corning), and 10 ng mL−1 fibroblast growth factor 2 (PeproTech, Rocky Hill, NJ). MSCs were encapsulated within hydrogels at a cell density of 5 × 106 cells mL−1. Then, 50 μL gels were aliquoted into a 96-well tissue-culture plate. After polymerization for three hours in a humidified incubator with 5% CO2 at 25 °C, 200 μL of chondrogenic medium without (“−cyto”) or with cytokines (10 ng mL−1 OSM (PeproTech, Rocky Hill, NJ), 20 ng mL−1 TNF-α (PeproTech), “+cyto”) was added, and the plates were moved to 37 °C. Chondrogenic medium was composed of high glucose Dulbecco's Modified Eagle Medium supplemented with 1% ITS (insulin transferrin selenous) acid+ Premix (BD Biosciences, San Jose, CA), 1% penicillin–streptomycin (Corning), 50 μg mL−1L-ascorbic acid 2-phosphate, 0.1 μM dexamethasone, 1 mM sodium pyruvate, 50 μM proline, 4 mM L-glutamine (Corning), and 10 ng mL−1 transforming growth factor (TGF)-β3 (PeproTech). L-Ascorbic acid 2-phosphate, dexamethasone, TGF-β3, and cytokines were added fresh to the medium at each feeding. Medium was changed 3 times each week and collected in pre-weighed microcentrifuge tubes, weighed, and stored at −80 °C until future analysis. The naming convention used for medium analysis is by day collected (e.g., media collected on day 6 is called “Day 6”). Experimental gel groups were compared to a high-throughput pellet culture.25 A negative pellet control (chondrogenic medium without TGF-β3, “neg”) and a positive pellet control (chondrogenic medium with TGF-β3, “pos”) were made.Gel contraction
Gel contraction was monitored during each culture period. Plates were scanned after encapsulation before the first medium addition at 3 hours, after 1 day, and every time media was exchanged thereafter. Gel contraction was quantified with ImageJ (NIH) as the percentage of the original projected area, which was defined as the area after 3 hours of polymerization. When hydrogels were fragmented, the total projected areas were summed. Contraction was monitored in two separate experiments, and a representative data set is shown.Matrix quantification: glycosaminoglycan (GAG), col, and DNA quantification
Hydrogels with encapsulated human MSCs (prepared as described above) and neg and pos pellets were maintained for 28 days and harvested. Hydrogels and pellets were rinsed with 1× PBS and digested with 125 μg mL−1 of activated papain solution (papain in buffer consisting of: 5 mM ethylenediaminetetraacetic acid (EDTA), 5 mM L-cysteine (Alfa Aesar, Ward Hill, MA), and 100 mM NaH2PO4) at 60 °C for 24 h. After lyophilization, digested cell-hydrogel constructs were reconstituted in MilliQ water and characterized as described below.Sulfated GAG, within hydrogels and released into media, was quantified via a dimethyl methylene blue (DMMB) assay. DMMB solution at pH 3.0 was prepared by combining 3.04 g L−1 glycine, 2.37 g L−1 NaCl, 16 mg L−1 dimethyl-methylene blue, and 0.1 M HCl. Twenty μL of sample or the standard solutions, which were formulated with chondroitin sulfate (CS) from shark cartilage (Seikagaku, Tokyo, Japan), was combined with 250 μL of DMMB solution in a 96-well plate, and the absorbance was read immediately at 525 nm.
Col, in hydrogels and media, was determined using a modified hydroxyproline assay.26 Given that all gels start with the same mass of col, this assay allow us to measure relative changes in col content in the gels and media. Col samples and standard solutions (stock col I) were broken down overnight at 98 °C by combining equal volumes of the sample/standard with 5 N NaOH. Solutions were cooled to room temperature and neutralized using 5 N HCl. Chloramine-T solution (0.05 M chloramine-T, 0.629 M sodium hydroxide, 0.140 M citric acid, 0.453 M sodium acetate, 0.112 M glacial acetic acid in 74% DI water and 26% isopropanol) was added to each tube, and the tubes were vortexed and left at room temperature for 20 minutes. Ehlrich solution (1 M p-dimethylaminobenzaldehyde in 70% isopropanol and 30% concentrated HCl) was added, and the solutions were vortexed and incubated in a heat block at 65 °C for 15 minutes. The reaction was quenched in water, and the absorbance was read at 560 nm.
DNA was measured as previously described.27 For DNA quantification, 50 μL of standards, which were prepared from calf thymus DNA, and samples were added to a completely black 96-well plate, and 50 μL of Hoechst dye solution was added to each well. Fluorescence was measured at an excitation wavelength of 340 nm and an emission wavelength of 465 nm. GAG and col content were normalized to DNA content.
Col and GAG released into the media were quantified in two separate experiments over 1 week in culture, and a representative data set is shown. One of the two experiments continued for 4 weeks, and data from that experiment is shown.
Gene expression
After one week in culture, both pellets and gels were washed with PBS then homogenized in LS TRIzol (Ambion, Life Technologies, Carlsbad, CA) buffer using a homogenizer (ThermoFisher Scientific) followed by pipetting up and down with a positive displacement pipette to further break down the hydrogels. Two hydrogels were combined for each replicate (n = 7). Single negative control (−TGF-β3) and positive control (+TGF-β3) pellets were used for each replicate (n = 8). The Direct-zol RNA MiniPrep kit from Zymo Research (Irvine, CA) was used to isolate and purify RNA. A High Capacity cDNA Reverse Transcription kit from Applied Biosystems (Foster City, CA) was used. Relative expression levels were measured using qRT-PCR for cartilage-related genes: ACAN (aggrecan) and SOX9, degradative enzymes: matrix metalloproteinase-13 (MMP-13) and a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5), inflammatory markers: interleukin-10 (IL-10) and interleukin-6 (IL-6), and a housekeeping gene: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) with TaqMan probes (ThermoFisher Scientific Assay IDs: Hs00153936_m1, Hs00165814_m1, Hs00942584_m1, Hs01095518_m1, Hs00961622_m1, Hs00174131_m1, and Hs02758991_g1, respectively) and a QuantStudio 3 instrument (Applied Biosystems, Foster City, CA). The samples were heated for 20 s at 95 °C followed by 40 cycles for 1 s at 95 °C and 20 s at 60 °C. All values were normalized to GAPDH levels with an average Ct value of 22.5 cycles and a standard deviation of 0.86 cycles. All differences in gene expression were calculated relative to a control (positive pellet, “pos pel”) using the ΔΔCt method.28Cytokine quantification
Cytokine levels in the medium were quantified via Luminex (Bio-Plex 3D, Bio-Rad, Hercules, CA) and a Bio-Plex Pro Human Cytokine Assay 8-plex (Bio-Rad, #M50000007A). The cytokines quantified were granulocyte-macrophage colony-stimulating factor, interferon-gamma, IL-2, IL-4, IL-6, IL-8, IL-10, and TNF-α. Manufacturer protocols were followed. Briefly, diluent heterophilic binding (HB) buffer was used for dilutions of all standards, samples, and controls. Samples were assayed in duplicate by incubating 50 μL of 1× antibody-coupled magnetic beads in the 96-well plate for 1 h at room temperature (RT) with shaking at 850 rpm. The plate was washed three times with 100 μL of Bio-plex wash buffer using a HydroSpeed plate washer (Tecan, Männedorf, Switzerland). After the addition of 25 μL of 1× detection antibody, the plate was incubated for 30 min at RT with shaking at 850 rpm. Streptavidin-PE 1× was added to each well and incubated for 10 min at RT with shaking at 850 rpm. Next, 125 μL of assay buffer was added to each well and incubated at 850 rpm on the shaker for 30 s. Bio-Plex 3D xMAP technology was used to measure bound molecules on the beads by using a dual laser. Eight-point standard curves and blank medium were included for quantification and prepared according to manufacturer's instructions. Biological replicates (n = 7) were analyzed in duplicate. For each cytokine, 5-parameter logistic curves were fit to the standards (5-PL regression) using Bio-Plex Manager™ Software (Bio-Rad, Hercules, CA). Curves were used to calculate concentrations for each sample replicate. The comparison of mean between two groups was performed using Wilcoxon rank-sum test per cytokine concentration. To account for multiple testing, the Benjamini-Hochberg (BH) correction was applied to adjust the p-values, ensuring the controlled false discovery rate (FDR).Principal components analysis
Technical replicates were averaged, and each signal was mean-centered and variance-scaled. Principal Component analysis (PCA) was performed using the PCA function in R programming. PCA results are from normalized data.Modeling cytokine responses and linear models
For univariate cytokine analysis, we fit a generalized linear model for each cytokine with the concentration modeled as a function of experimental conditions (eqn (1)): | (1) |
HA represents hyaluronic acid dose and cyto represents the cytokine application. Models were constructed from normalized data. Models were computed using the glm function in R programming, and all code and data necessary to reproduce the results are deposited on the Brubaker Lab GitHub.29
MMP-13 and ADAMTS-5 quantification
MMP-13 release into media was quantified using a sandwich enzyme-linked immunosorbent assay (ELISA) kit from Invitrogen (Cat. No. EHMMP13, Fisher Scientific). The manufacturer's protocol was followed, and samples were diluted in assay buffer to fall within the standard curve.ADAMTS-5 release into the media was quantified via ELISA (Cat No. DY2198-05, R&D Systems, Minneapolis, MN). The manufacturer's protocol was followed.
Statistics
All acellular experiments were performed with 3–4 replicates. Matrix quantification experiments were performed with 4 replicates. Luminex and gene expression experiments were performed with 7–8 replicates. All data are shown as means with error bars representing one standard deviation. Statistical analysis was evaluated in Minitab (Minitab, LLC, State College, PA). Data was first tested for outliers using Grubb's test. Equal variances, tested via Levene's test, were assumed when appropriate. Two-tailed student's t-tests were evaluated with a critical value of α = 0.05 and compared ±cyto and ±HA independently. Residual normality was assessed via Anderson–Darling.Results & discussion
−HA and +HA col gels exhibited similar microstructure and mechanical properties
Because the microstructure and mechanical properties of col hydrogels impact cell response,30 we characterized our gels to determine whether there were differences when HA was added to the col I/II blended gel. The microstructures of –HA and +HA were qualitatively similar (Fig. 1A andB).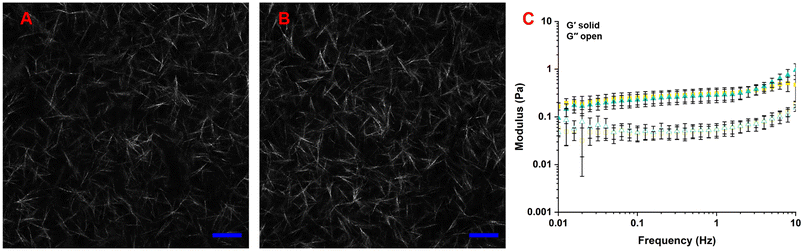 | ||
| Fig. 1 The addition of a low concentration of HA did not alter gel microstructure or mechanical properties. Representative confocal microscopy images of (A) −HA and (B) +HA. Images taken 50 μm into hydrogel. Scale bar represents 50 μm. (C) Frequency sweeps with constant strain of 0.1%. −HA in yellow and +HA in blue (n = 3). Replicate sweeps are shown in Fig. S1.† Closed symbols represent storage moduli (G′) and open symbols represent loss moduli (G′′). Data points represent mean ± standard deviation. | ||
Frequency sweeps revealed statistically similar storage moduli for −HA and +HA groups (Fig. 1C). Given the similar values, the influence of mechanical property differences on cell response was deemed negligible, and literature supports this assumption since large differences (i.e., order of magnitude differences) are needed to elicit major differences in cell response.31 The similar fibril formation and mechanical properties of −HA and +HA gels allow us to independently assess the impact of HA on cell response irrespective of gel properties.
Other studies have found similar results: low concentrations of HA have little impact on col microstructure and mechanical properties.30 For example, Matsiko et al. found no statistical differences in pore size or compressive moduli between 5 mg mL−1 col gels without or with 0.5 mg mL−1 HA.32 In col I gels without and with 0.1 mg mL−1 HA (1.52 MDa), there was no statistical change in col fibril volume fraction, but there were small statistical changes in fibril and pore diameters that the authors deemed negligible.33
Cytokines increased cell proliferation and suppressed cartilage differentiation but resulted in similar overall cartilage matrix levels
Prior to conducting cell studies, we verified the differentiation potential of the MSCs using negative and positive control pellets. As expected, pos exhibited statistically higher GAG/DNA compared to neg (Fig. S2†).Gel contraction is an early indicator of MSC chondrogenesis.34 As expected, treatments without cytokines (−cyto) contracted more quickly than gels with cytokines (+cyto) (Fig. S3A†). From days 4–8, in the presence of cytokines (+cyto), gels with HA (+HA) contracted more than gels without HA (−HA) and were statistically smaller after 6 and 8 days (Fig. S3A†). Additionally, although gels for all conditions looked similar and completely filled the well after polymerization, during the contraction process the gels for the −HA +cyto groups did not contract into one homogeneous hydrogel but rather smaller gel fragments (Fig. S3B†) that suggest a weaker, less connected matrix. Furthermore, the results for the −HA +cyto groups on days 8, 10, and 12 may be influenced by different cell and gel densities, ratios of surface area to volume, and contractile forces needed to contract smaller versus larger gels.
Chondrogenic genes, aggrecan (ACAN) and SOX9, were evaluated after one week (Fig. S4A†). Both aggrecan and SOX9 gene expression levels were affected by the presence of cytokines, and −cyto groups were statistically higher than their corresponding +cyto group (aside from −HA for ACAN expression, p = 0.076). When comparing gel types, no difference was observed for −HA compared to +HA.
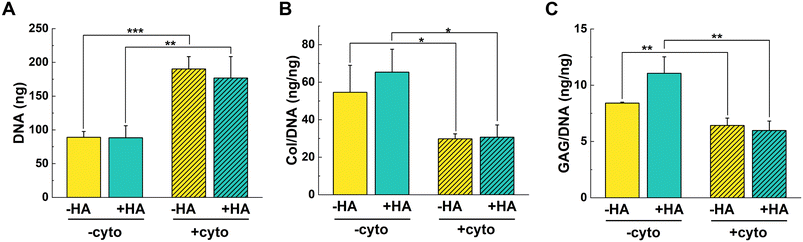
DNA was statistically higher for +cyto groups (Fig. 2A). These results suggest that the cells were proliferating instead of undergoing chondrogenic differentiation when exposed to the cytokine cocktail. A similar increase in DNA was observed in literature with chondrocyte-laden col gels under IL-1β-stimulation.35 DNA proliferation correlates with the limited contraction observed by +cyto gels at early time points. Thus, it is possible that the addition of cytokines delayed differentiation in favor of proliferation.
In terms of cartilage matrix, we expected that the addition of cytokines would reduce the col and GAG levels since TNF-α is known to reduce col production.3 When normalized to DNA, the results aligned with our expectations since both col/DNA and sulfated GAG/DNA were statistically higher for −cyto treatments compared to +cyto treatments (Fig. 2B and C). These results correlate with the lower chondrogenic gene expression in the presence of cytokines. Interestingly, when looking at overall matrix levels, there was no statistical difference for col and GAG amounts for +HA gels when cytokines were present or absent (Fig. S4B and C†). Furthermore, −HA gels had statistically higher col and GAG levels when cytokines were added compared to conditions without cytokines. It thus appears that, in response to cytokines, each cell produced less col and GAG, but since there were more cells in the cytokine treatment groups, the overall amount of matrix was similar or higher in groups with cytokines.
Given that prior studies examined the effect of adding this combination of cytokines to pre-existing cartilage matrix,9–11 it was unknown if the MSC response would be dominated by the inflammatory cytokines in the system or the chondrogenic growth factor TGF-β3 and cartilage-promoting biomolecules col II and HA. It is important to note that the effects of other cytokines, such as IL-1α36 and the combination of IL-1β and TNF-α,13 in combination with chondrogenic growth factors on MSCs have been explored in other systems. The cytokines TNF-α and IL-1β are known to inhibit col II gene expression.3 Similarly, the cytokine cocktail used in this study reduced chondrogenic gene and matrix expression per cell. On the other hand, literature has previously shown that even small amounts of HA stimulate cartilage matrix production by chondrocytes.23 In this study, however, HA did not dramatically alter the chondrogenic behavior of MSCs, and differences in cell response to the gel types were only observed in gel contraction. Finally, dexamethasone, a corticosteroid used as an anti-inflammatory treatment for OA,37 was administered to all treatment groups because it is an important component for stimulating MSC chondrogenic differentiation.
Immunomodulation: adding HA had little impact on IL-6 but correlated with IL-8
Inflammation is a key component of the sustained cycle of OA disease progression.8 Thus, the impact of HA on the inflammatory cycle was assessed via cytokine gene expression and protein-level production. Gene and protein expression for IL-6 and IL-10 were evaluated as representative pro- and anti-inflammatory cytokines, respectively, that are implicated in OA.2 Both IL-6 and IL-10 gene expression levels were statistically elevated at 7 days in +cyto treatment groups compared to the respective −cyto counterpart treatment (Fig. 3A and B). IL-6 protein expression at 6 days followed a trend similar to IL-6 gene expression but was not statistically significant (Fig. 3C). When cytokines were added, IL-6 gene expression levels (Fig. 3A) and IL-10 protein production (Fig. 3D) trended lower when HA was added to gels, but these trends were not statistically significant. Based on literature, we expected HA to have more of an effect on suppressing IL-6 and promoting IL-10. However, our experimental system differed from literature in that HA was incorporated in the gel rather than being administered orally38 or injected39,40 and because we promoted chondrogenic differentiation of MSCs and inflammation concurrently, and these distinctions may explain the discrepancy in HA impact.Protein-level expression of inflammatory markers was analyzed using PCA and then fit to a generalized linear model. PCA identified two principal components (PCs) that collectively explained 70.8% of the total variance in the system and resulted in outputs grouped by presence or absence of cytokines (Fig. 4A). Using a linear model with cytokine concentration as a function of experimental conditions (eqn (1) and Table S1†), associations were found between IL-6 and +cyto treatment (p < 0.001), IL-4 and +cyto (p < 0.001), and IL-8 and +HA (p < 0.1) (Fig. 4B). The correlations between adding cytokines (+cyto) and the production of IL-6 and IL-4 were expected as both are increased in OA.41 The observed increment of IL-8 under +HA is contradictory to several studies that demonstrate HA plays an anti-inflammatory role by decreasing the production of proinflammatory mediators (e.g., IL-640 and IL-840,42) and M1 macrophages.40 Additionally, it is worth noting that other literature shows that, similar to our results, HA induces an inflammatory response by upregulating IL-8; however, the literature results were associated with low MW HA43 and thus suggest that the HA in our hydrogels may have broken down into lower MW fragments. Given the association between IL-8 and HA in our linear model, it is likely that other concentrations or presentations of HA within this system would alter the resultant IL-8 response.15 Interestingly, the interaction between HA and cyto suggests a potential dampening effect on IL-8 levels compared to HA alone, but the evidence is not strong enough to confirm this effect statistically (p = 0.178). The lack of a significant interaction suggests that HA and cyto treatment may act through independent biological pathways.
Chondroprotection: adding HA reduced MMP-13 production and gene expression but had no effect on ADAMTS-5
Matrix degradation is a key component of the progression of OA and is exacerbated via the inflammatory environment of OA.17 Thus, the effect of the cytokine-induced inflammatory environment on matrix degradation was assessed. Overall, all conditions led to some col and GAG being released into the media (Fig. S5A and B†). Low levels of col and GAG were detected and generally suggest matrix turnover occurred in all treatments or that newly formed matrix components did not incorporate within the scaffold.Two degradative enzymes that are upregulated during OA are MMP-13 and ADAMTS-5, which degrade col and aggrecan, respectively.17 In the presence of cytokines, MMP-13 gene expression after 7 days was statistically lower for +HA compared to −HA (Fig. 5A). In the media harvested on day 6, MMP-13 protein production was statistically higher for +cyto treatments compared to −cyto treatments and, when cytokines were present, trended higher for −HA compared to +HA gels (Fig. 5B). By day 8, MMP-13 protein-level production was statistically higher for gels without HA compared to gels with HA (Fig. 5C). Other studies have similarly observed a decrease in MMP-13 production in the presence of high MW HA.15 By two weeks, MMP-13 production was similar, and lower, across all treatment groups (Fig. S5C†). TNF-α is known to increase MMP-13 expression,44 and thus it was unsurprising that +cyto treatments initially resulted in an increase in MMP-13 production and expression. The subsequent decrease in MMP-13 production may be similar to OA progression wherein MMP-13 production is upregulated in early diseased states and downregulated in later disease progression.45
ADAMTS-5 gene (Fig. 5D) and protein (Fig. 5E) levels were only affected by the addition of cytokines and were similar in −HA and +HA gels. Although GAG release within the first week in culture was largely not different across groups, after 4 weeks in culture there was statistically more GAG released for +cyto groups (Fig. S5B†). This difference at later time points could be explained by the statistical increase in ADAMTS-5 after ∼1 week in culture for +cyto groups. The increase in both MMP-13 and ADAMTS-5 for all +cyto treatments was expected and is indicative of an OA disease state.46 There are many pathways that can alter MMP-13 and ADAMTS-5 expression,2 and it was interesting that HA only statistically lowered MMP-13 within +cyto treatments and not ADAMTS-5. Different gene expression patterns for MMP-13 and ADAMTS-5 were observed in another GAG/col hydrogel system that was stimulated with IL-1β.35
Previously, the inflammatory cytokine model used in this study was validated in vitro using cartilage explants and in vivo.9–11 It was unclear if this cytokine model would have the same impact on differentiating MSCs within col hydrogels. It is apparent that the inflammatory cytokines elicited a pro-inflammatory state as evidenced by an increase in pro-inflammatory gene expression (Fig. 3A) and protein expression (Fig. 4B) and higher degradative enzyme production and gene expression (Fig. 5). Comparing these observations to cartilage explant models treated with the same concentrations of OSM and TNF-α, one study found that active forms of ADAMTS-4, MMP-9, and MMP-13 increased over time but at a different time scale from our experiment.9 Active ADAMTS-4, MMP-9, and MMP-13 were not detectable in the bovine explants until ∼14 days in culture, whereas in our work MMP-13 levels were much higher after 6–8 days compared to after 14 days. Furthermore, no active ADAMTS-5 was detected in the media from cartilage explants over 21 days. The discrepancy between these findings and our work may be due to differences between explants and MSCs in hydrogels. Another study with bovine explants and OSM and TNF-α treatment observed a statistical increase in MMP-13 gene expression compared to controls,10 and these results are similar to our findings of increased MMP-13 in +cyto treatments compared to −cyto treatments.
Since this cytokine system elicited an inflammatory response similar to that observed in OA,2 with increasing MMP-13, ADAMTS-5, IL-6, and IL-8, it serves as a promising OA model of cartilage regeneration and can be used to study hydrogel formulations intended to support cartilage tissue engineering. Furthermore, the ability of HA within hydrogels to decrease MMP-13 production could be vital for limiting the degradative cycle of OA as the degradation of col II is often viewed as a point of no return in OA.17
Conclusion
In this work, MSCs were encapsulated in blended col I/II hydrogels, which promote chondrogenesis, with and without HA and were exposed to an osteoarthritic, inflammatory environment. Although the hydrogels were simultaneously exposed to both pro-chondrogenic factors and inflammatory factors, the in vitro inflammatory environment of OSM and TNF-α dominated the cell response. The MSCs under pro-inflammatory conditions generated statistically lower col/DNA, GAG/DNA, and SOX9 gene expression compared to MSCs not under inflammatory conditions. However, hydrogels within the inflammatory environment resulted in statistically higher DNA amounts compared to their non-inflammatory counterparts. The inflammatory environment promoted an increase in IL-6 and IL-10 gene expression, and PCA revealed clustering based on the presence or absence of cytokines. The addition of HA in gels affected MSC response in a few ways: MMP-13 protein and gene expression levels after 7 days in culture with pro-inflammatory cytokines were statistically lower for gels with HA, and a linear model revealed an association between IL-8 protein expression and HA. Taken together, this work suggests that the effect of HA on the response of MSCs within the context of inflammation should be explored further, and this in vitro cytokine treatment model of OA can be extended to study other tissue-engineered therapies.Author contributions
C.M.B. conceptualized, performed experiments, analyzed data, wrote the original draft, and contributed to review and editing stages. J.M.B. performed and analyzed the results from the Luminex experiments and contributed to all relevant portions of the manuscript for the original draft and review stages. D.K.B. supervised Luminex experiments and edited the manuscript. A.P. conceptualized, supervised experiments, and edited the manuscript. J.C.L. conceptualized, supervised experiments, acquired funding, and edited the manuscript. All authors participated in discussions of the results, and all authors have revised the manuscript and have given approval of the final version of the manuscript.Data availability
The data files are available via the Purdue University Research Repository (https://doi.org/10.4231/BBS3-3542). Code for the linear model is available through the Brubaker Lab GitHub (https://github.com/Brubaker-Lab/CartilageGel-Univariate-Linear-Models).Conflicts of interest
The authors have no conflicts to declare.Acknowledgements
This work was supported by the Purdue Davidson School of Chemical Engineering and William P. and Amanda C. Madar. J. M. B. was partially supported by a Bottorff Fellowship from Purdue College of Engineering and the EMBRIO Institute, contract #2120200, a National Science Foundation (NSF) Biology Integration Institute. D. K. B. was supported by startup funds from the Weldon School of Biomedical Engineering at Purdue University. Graphical abstract was created in BioRender: Battistoni, C. (2025) https://BioRender.com/a96m827.References
- H. Long, Q. Liu, H. Yin, K. Wang, N. Diao, Y. Zhang, J. Lin and A. Guo, Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019, Arthritis Rheumatol., 2022, 74(7), 1172–1183 CrossRef PubMed.
- V. Molnar, V. Matišić, I. Kodvanj, R. Bjelica, Ž Jeleč, D. Hudetz, E. Rod, F. Čukelj, T. Vrdoljak, D. Vidović, M. Starešinić, S. Sabalić, B. Dobričić, T. Petrović, D. Antičević, I. Borić, R. Košir, U. Prosenc Zmrzljak and D. Primorac, Cytokines and chemokines involved in osteoarthritis pathogenesis, Int. J. Mol. Sci., 2021, 22, 9208 CrossRef CAS PubMed.
- M. B. Goldring, M. Otero, D. A. Plumb, C. Dragomir, M. Favero, K. El Hachem, K. Hashimoto, H. I. Roach, E. Olivotto, R. M. Borzì and K. B. Marcu, Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis, Eur. Cells Mater., 2011, 21, 202–220 CrossRef CAS PubMed.
- S. R. Goldring and M. B. Goldring, Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage bone crosstalk, Nat. Rev. Rheumatol., 2016, 12(11), 632–644 CrossRef PubMed.
- J. M. Patel, K. S. Saleh, J. A. Burdick and R. L. Mauck, Bioactive factors for cartilage repair and regeneration: Improving delivery, retention, and activity, Acta Biomater., 2019, 93, 222–238 CrossRef CAS PubMed.
- Y. Liu, G. Zhou and Y. Cao, Recent Progress in Cartilage Tissue Engineering—Our Experience and Future Directions, Engineering, 2017, 3(1), 28–35 CrossRef CAS.
- D. Yang, J. Xiao, B. Wang, L. Li, X. Kong and J. Liao, The immune reaction and degradation fate of scaffold in cartilage/bone tissue engineering, Mater. Sci. Eng., C, 2019, 104, 109927 CrossRef CAS PubMed.
- J. H. Teixeira, C. L. Pereira, M. I. Almeida, G. Q. Teixeira, R. M. Gonçalves, M. A. Barbosa and S. G. Santos, Articular Repair/Regeneration in Healthy and Inflammatory Conditions: From Advanced In Vitro to In Vivo Models, Adv. Funct. Mater., 2020, 30, 1909523 CrossRef CAS.
- Y. He, Q. Zheng, M. M. Jiang, S. Sun, T. G. Christiansen, M. Kassem, M. A. Karsdal and A. C. Bay-Jensen, The effect of protease inhibitors on the induction of osteoarthritis-related biomarkers in bovine full-depth cartilage explants, PLoS One, 2015, 10(4), 1–18 Search PubMed.
- P. Chen-An, K. V. Andreassen, K. Henriksen, M. A. Karsdal and A. C. Bay-Jensen, Investigation of chondrocyte hypertrophy and cartilage calcification in a full-depth articular cartilage explants model, Rheumatol. Int., 2013, 33(2), 401–411 CrossRef CAS PubMed.
- W. Hui, A. D. Rowan, C. D. Richards and T. E. Cawston, Oncostatin M in Combination with Tumor Necrosis Factor α Induces Cartilage Damage and Matrix Metalloproteinase Expression In Vitro and In Vivo, Arthritis Rheum., 2003, 48(12), 3404–3418 CrossRef CAS PubMed.
- C. J. Malemud, Biologic basis of osteoarthritis: State of the evidence, Curr. Opin. Rheumatol., 2015, 27(3), 289–294 CrossRef CAS PubMed.
- S. Pagani, F. Veronesi, G. Giavaresi, G. Filardo, T. Papio, I. Romandini and M. Fini, Autologous Protein Solution Effect on Chondrogenic Differentiation of Mesenchymal Stem Cells from Adipose Tissue and Bone Marrow in an Osteoarthritic Environment, Cartilage, 2021, 13(2), 225S–237S CrossRef CAS PubMed.
- E. Tsanaktsidou, O. Kammona and C. Kiparissides, Recent Developments in Hyaluronic Acid-Based Hydrogels for Cartilage Tissue Engineering Applications, Polymers, 2022, 14(4), 839 CrossRef CAS PubMed.
- R. Altman, A. Bedi, A. Manjoo, F. Niazi, P. Shaw and P. Mease, Anti-Inflammatory Effects of Intra-Articular Hyaluronic Acid: A Systematic Review, Cartilage, 2019, 10, 43–52 CrossRef CAS PubMed.
- A. Marinho, C. Nunes and S. Reis, Hyaluronic acid: A key ingredient in the therapy of inflammation, Biomolecules, 2021, 11, 1518 CrossRef CAS PubMed.
- C. Scotti, G. M. Peretti, A. Gobbi, G. Karnatzikos, I. Martin, K. Shimomura, J. G. Lane, G. M. Peretti and N. Nakamura, Cartilage Repair in the Inflamed Joint: Considerations for Biological Augmentation Toward Tissue Regeneration, Tissue Eng., Part B, 2016, 22(2), 149–159 CrossRef CAS PubMed.
- A. Fakhari and C. Berkland, Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment, Acta Biomater., 2013, 9, 7081–7092 CrossRef CAS PubMed.
- C. Intini, M. Lemoine, T. Hodgkinson, S. Casey, J. P. Gleeson and F. J. O'Brien, A highly porous type II collagen containing scaffold for the treatment of cartilage defects enhances MSC chondrogenesis and early cartilaginous matrix deposition, Biomater. Sci., 2022, 10(4), 970–983 RSC.
- D. G. O’Shea, T. Hodgkinson, C. M. Curtin and F. J. O’Brien, An injectable and 3D printable pro-chondrogenic hyaluronic acid and collagen type II composite hydrogel for the repair of articular cartilage defects, Biofabrication, 2024, 16(1), 015007 CrossRef PubMed.
- D. Eng, M. Caplan, M. Preul and A. Panitch, Hyaluronan scaffolds: A balance between backbone functionalization and bioactivity, Acta Biomater., 2010, 6(7), 2407–2414 CrossRef CAS PubMed.
- M. Y. Kwon, C. Wang, J. H. Galarraga, E. Puré, L. Han and J. A. Burdick, Influence of hyaluronic acid modification on CD44 binding towards the design of hydrogel biomaterials, Biomaterials, 2019, 222, 119451 CrossRef CAS PubMed.
- K. Kawasaki, M. Ochi, Y. Uchio, N. Adachi and M. Matsusaki, Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels, J. Cell Physiol., 1999, 179(2), 142–148 CrossRef CAS PubMed.
- C. E. Kilmer, C. M. Battistoni, A. Cox, G. J. Breur, A. Panitch and J. C. Liu, Collagen Type I and II Blend Hydrogel with Autologous Mesenchymal Stem Cells as a Scaffold for Articular Cartilage Defect Repair, ACS Biomater. Sci. Eng., 2020, 6(6), 3464–3476 CrossRef CAS PubMed.
- K. J. Penick, L. A. Solchaga and J. F. Welter, High-throughput aggregate culture system to assess the chondrogenic potential of mesenchymal stem cells, BioTechniques, 2005, 39(5), 687–691 CrossRef CAS PubMed.
- D. D. Cissell, J. M. Link, J. C. Hu and K. A. Athanasiou, A Modified Hydroxyproline Assay Based on Hydrochloric Acid in Ehrlich's Solution Accurately Measures Tissue Collagen Content, Tissue Eng., Part C, 2017, 23(4), 243–250 CrossRef CAS PubMed.
- Y. J. Kim, R. L. Y. Sah, J. Y. H. Doong and A. J. Grodzinsky, Fluorometric assay of DNA in cartilage explants using Hoechst 33258, Anal. Biochem., 1988, 174(1), 168–176 CrossRef CAS PubMed.
- A. L. Bookout, C. L. Cummins, D. J. Mangelsdorf, J. M. Pesola and M. F. Kramer, High–Throughput Real–Time Quantitative Reverse Transcription PCR, Curr. Protoc. Mol. Biol., 2006, 73(1), 1–28 Search PubMed.
- J. Munoz Briones, CartilageGel-Univariate-Linear-Models [Internet]. [cited 2024 Oct 21]. Available from: https://github.com/Brubaker-Lab/CartilageGel-Univariate-Linear-Models.
- Q. Xu, J. E. Torres, M. Hakim, P. M. Babiak, P. Pal, C. M. Battistoni, M. Nguyen, A. Panitch, L. Solorio and J. C. Liu, Collagen- and hyaluronic acid-based hydrogels and their biomedical applications, Mater. Sci. Eng., R, 2021, 146, 100641 CrossRef PubMed.
- S. R. Caliari and J. A. Burdick, A practical guide to hydrogels for cell culture, Nat. Methods, 2016, 13, 405–414 CrossRef CAS PubMed.
- A. Matsiko, T. J. Levingstone, F. J. O'Brien and J. P. Gleeson, Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering, J. Mech. Behav. Biomed. Mater., 2012, 11, 41–52 CrossRef CAS PubMed.
- M. H. Hakim, B. H. Jun, A. Ahmadzadegan, P. M. Babiak, Q. Xu, K. P. Buno, J. C. Liu, A. M. Ardekani, P. P. Vlachos and L. Solorio, Investigation of macromolecular transport through tunable collagen hyaluronic acid matrices, Colloids Surf., B, 2023, 222, 113123 CrossRef CAS PubMed.
- R. A. Somoza, J. F. Welter, D. Correa and A. I. Caplan, Chondrogenic differentiation of mesenchymal stem cells: Challenges and unfulfilled expectations, Tissue Eng., Part B, 2014, 20, 596–608 CrossRef PubMed.
- M. Nguyen, C. M. Battistoni, P. M. Babiak, J. C. Liu and A. Panitch, Chondroitin Sulfate/Hyaluronic Acid-Blended Hydrogels Suppress Chondrocyte Inflammation under Pro-Inflammatory Conditions, ACS Biomater. Sci. Eng., 2024, 10(5), 3242–3254 CrossRef CAS PubMed.
- P. H. Ousema, F. T. Moutos, B. T. Estes, A. I. Caplan, D. P. Lennon, F. Guilak and J. B. Weinberg, The inhibition by interleukin 1 of MSC chondrogenesis and the development of biomechanical properties in biomimetic 3D woven PCL scaffolds, Biomaterials, 2012, 33(35), 8967–8974 CrossRef CAS PubMed.
- V. S. Madamsetty, R. Mohammadinejad, I. Uzieliene, N. Nabavi, A. Dehshahri, J. García-Couce, S. Tavakol, S. Moghassemi, A. Dadashzadeh, P. Makvandi, A. Pardakhty, A. A. Afshar and A. Seyfoddin, Dexamethasone: Insights into Pharmacological Aspects, Therapeutic Mechanisms, and Delivery Systems, ACS Biomater. Sci. Eng., 2022, 8, 1763–1790 CrossRef CAS PubMed.
- A. Asari, T. Kanemitsu and H. Kurihara, Oral administration of high molecular weight hyaluronan (900 kDa) controls immune system via toll-like receptor 4 in the intestinal epithelium, J. Biol. Chem., 2010, 285(32), 24751–24758 CrossRef CAS PubMed.
- M. Sezgin, A.Ç Demirel, C. Karaca, Ö. Ortancıl, G. B. Ülkar, A. Kanık and A. Cakçi, Does hyaluronan affect inflammatory cytokines in knee osteoarthritis?, Rheumatol. Int., 2005, 25(4), 264–269 CrossRef CAS PubMed.
- L. Jin, K. Xu, Y. Liang, P. Du, S. Wan and C. Jiang, Effect of hyaluronic acid on cytokines and immune cells change in patients of knee osteoarthritis, BMC Musculoskelet Disord, 2022, 23(1), 812 CrossRef CAS PubMed.
- M. Kapoor, J. Martel-Pelletier, D. Lajeunesse, J. P. Pelletier and H. Fahmi, Role of proinflammatory cytokines in the pathophysiology of osteoarthritis, Nat. Rev. Rheumatol., 2011, 7, 33–42 CrossRef CAS PubMed.
- C. T. Wang, Y. T. Lin, B. L. Chiang, Y. H. Lin and S. M. Hou, High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis, Osteoarthr. Cartil., 2006, 14(12), 1237–1247 CrossRef PubMed.
- M. Litwiniuk, A. Krejner and T. Grzela, Hyaluronic Acid in Inflammation and Tissue Regeneration, Index Wounds, 2016, 28(3), 78–88 Search PubMed.
- E. Chisari, K. M. Yaghmour and W. S. Khan, The effects of TNF-alpha inhibition on cartilage: a systematic review of preclinical studies, Osteoarthr. Cartil., 2020, 28, 708–718 CrossRef CAS PubMed.
- H. Li, D. Wang, Y. Yuan and J. Min, New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis, Arthritis Res. Ther., 2017, 19(1), 1–12 CrossRef PubMed.
- L. Troeberg and H. Nagase, Proteases involved in cartilage matrix degradation in osteoarthritis, Biochim. Biophys. Acta, 2012, 1824(1), 133–145 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5bm00033e |
| This journal is © The Royal Society of Chemistry 2025 |