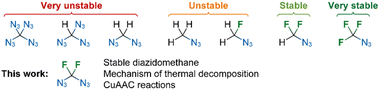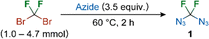Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
From boom to bloom: synthesis of diazidodifluoromethane, its stability and applicability in the ‘click’ reaction†
Mykyta
Ziabko
 ab,
Sergeii
Suikov
ab,
Sergeii
Suikov
 a,
Josef
Filgas
a,
Josef
Filgas
 c,
Petr
Slavíček
c,
Petr
Slavíček
 c,
Michaela
Gazdurová
a,
Lucie
Bednárová
c,
Michaela
Gazdurová
a,
Lucie
Bednárová
 a,
Robert
Matyáš
d,
Blanka
Klepetářová
a,
Robert
Matyáš
d,
Blanka
Klepetářová
 a,
Tomáš
David
a,
Tomáš
David
 a and
Petr
Beier
a and
Petr
Beier
 *a
*a
aInstitute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo nám. 2, 160 00 Prague, Czechia. E-mail: beier@uochb.cas.cz
bDepartment of Organic Chemistry, Faculty of Science, Charles University, Hlavova 2030/8, 166 28 Prague, Czechia
cDepartment of Physical Chemistry, University of Chemistry and Technology, Technická 5, 166 28 Prague, Czechia
dInstitute of Energetic Materials, Faculty of Chemical Technology, University of Pardubice, Doubravice 41, 532 10 Pardubice, Czechia
First published on 20th November 2024
Abstract
Diazidodifluoromethane was prepared from dibromodifluoromethane, sodium azide and an alkanethiolate initiator. It represents the first example of a diazidomethane that is stable enough to be used in synthesis. The stability of (poly)azidomethanes was explored with ab initio calculations. Copper(I)-catalysed azide–alkyne cycloaddition of the title azide with alkynes afforded difluoromethylene-containing bis(1,2,3-triazoles)amenable to Rh(II)-catalysed transannulation with nitriles to difluoromethylene bis(imidazoles).
In recent years, α-fluorinated azidoalkanes have become the focus of considerable attention1,2 as stable yet reactive precursors of trifluoromethyldiazonium amines,3 trifluoromethylnitrene for N-trifluoromethylaziridines,4 and N-fluoroalkylated 1,2,3-triazoles.5–8 These unique triazoles have proven invaluable in the synthesis of a variety of N-fluoroalkylated nitrogen heterocycles via Rh(II)-catalysed transannulation9–11 or acid-mediated denitrogenation reactions leading to new N-alkenyl compounds, such as enamides,12 imidoyl halides,13 and ketimines.14
Monofluoro-, difluoro- and trifluoroazidomethanes are known,1,2 but diazidodifluoromethane has not been reported before. Its non-fluorinated analogue diazidomethane is a highly sensitive compound that can be generated from dichloromethane or dibromomethane and nucleophilic azide salts or a polymeric azide reagent.15,16 The intermediate azidochloromethane or azidobromomethane17 could not be detected in these reactions, even with substoichiometric amounts of azide salts, suggesting that the second halide displacement proceeds much faster than the first. Astonishingly, the whole family of azidomethanes from mono- to tetra-azidomethane is known; however, polyazidomethanes display explosive character (Fig. 1).18
Azidotrifluoromethane is a much more stable compound than azidomethane. Whereas azidomethane is a sensitive compound at room temperature, azidotrifluoromethane is safe to use in solution at temperatures below 150 °C.5 It has been reported to decompose at 330 °C (ref. 19) (or above 1120 K in another report).20 This is reflected in large differences between decomposition lifetimes ranging from a few hours (experimental value for CH3N3) to several years (calculated value for CF3N3) at 200 °C.21 The reasons for this increased stability are not fully understood; nevertheless, activation barriers for the decomposition of azidomethanes into nitrenes are much higher for CF3N3 than for CH3N3.
Taking these facts into account, we set out to synthesize diazidodifluoromethane and investigate its stability and reactivity. The target compound is a new fluorinated one-carbon azide, potentially more stable and safer-to-use derivative of diazidomethane, which is unusable in synthesis.
At first, the synthetic strategy for obtaining diazidodifluoromethane based on the nucleophilic displacement of a leaving group, for example, from commercial dibromodifluoromethane with azide salts, seemed unfeasible. Fully substituted (per)fluoroalkyl halides do not undergo nucleophilic substitution. However, the azide anion is highly nucleophilic and sterically undemanding, and the substitution of the bromine atom in bromodifluoromethylated aromatics (ArCF2Br) with sodium azide to form ArCF2N3 is known to proceed under mild conditions (50 °C in DMF), probably via an SRN1 mechanism rather than an SN2 or SN1 mechanism.22
The reaction of dibromodifluoromethane with an excess of sodium azide under elevated temperature in DMF did not lead to the target azide 1 (Table 1, entry 1). The same results were obtained when the reaction was performed in water, THF, MeCN or 1,2-dimethoxyethane solvents. When tetrabutylammonium azide was used instead of sodium azide in DMF or NMP, low product yields were observed (entries 2 and 3). A breakthrough was achieved when the additive sodium dodecylthiolate was used in a catalytic amount (entry 4). The addition of a low-boiling solvent (THF) followed by distillation afforded a solution of 1 in good yield. However, the product was contaminated with unreacted dibromodifluoromethane. The preparation of 1 was therefore scaled-up to 1 g (4.7 mmol) of the starting dibromodifluoromethane with the same efficiency. Pure, neat 1 was obtained by distillation of the reaction mixture without the addition of a low-boiling solvent.
| Entry | Azide | Additive (10 mol%) | Solvent | Yield of 1b (%) |
|---|---|---|---|---|
| a See the ESI for more extensive optimization table. b 19F NMR yield of 1 in the crude reaction mixture. c 19F NMR yield of a solution of 1 obtained after the addition and distillation of THF (30 ml). | ||||
| 1 | NaN3 | — | DMF | 0 |
| 2 | (n-Bu4N)N3 | — | DMF | 20 |
| 3 | (n-Bu4N)N3 | — | NMP | 17 |
| 4 | (n-Bu4N)N3 | n-C12H25SNa | DMF | 39 |
| 5 | [n-Bu3P(n-C16H33)]N3 | n-C12H25SNa | DMF | 27 |
| 6 | NaN3 | n-C12H25SNa | NMP | 15 |
| 7 | NaN3 | n-C12H25SNa | DMF | 67c |
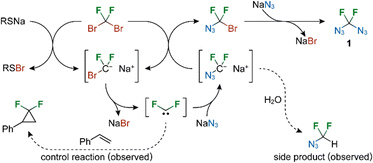
Considering the mechanism by which 1 is formed, the fact that the additive sodium dodecylthiolate was required for the efficient formation of 1 suggested the occurrence of bromophilic attack and that the reaction proceeds via difluorocarbene (Scheme 1). The presumed intermediate azidobromodifluoromethane was never identified, suggesting that the second bromine substitution is a fast process. Radical intermediates are most probably not involved as control experiments with equimolar amounts of TEMPO or 1,1-diphenylethylene did not decrease the yield of 1 and did not show significant amounts of new fluorinated products. Further indirect support for the proposed reaction mechanism was provided by the 1H and 19F NMR observation of a small amount of N3CF2H as a side-product in a reaction conducted in wet DMF. This azide resulted from the protonation of unstable carbanion intermediate N3CF2Na. The addition of styrene led to the formation of a difluorocyclopropane product (detected by 19F NMR), proving the formation of a difluorocarbene intermediate.
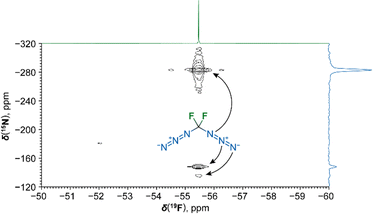
Compound 1 was characterized by 19F, 13C, 15N NMR and infrared (ν(N3) 2154 cm−1) spectroscopy and high-resolution mass spectrometry of [CF2N3 + H]+ and [CF2N]+ fragments of 1 (no molecular signal was observed). An 19F and 15N gradient-enhanced HMBC NMR experiment revealed the topology of the diazidomethane molecule (Fig. 2).
The possible low stability and potentially explosive nature of 1 was of concern. However, we never observed any violent decomposition when handling any solution of 1. A fall-hammer test (sensitivity to impact) at energy level of 50 J of a THF solution of 1 (0.1 M) turned out negative, which means that this solution can be considered not sensitive to impact according to UN and EU legislation. However, on one occasion a drop of neat 1 violently decomposed upon contact with a rough glass surface, so care should be taken even when handling a solution of 1, similarly when handling a diazomethane solution. It can be expected that compound 1 is sensitive to excessive heat.
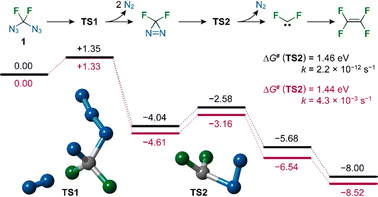
We calculated activation Gibbs free energies (ΔG#), rate constants (k) and lifetimes for the decomposition of all possible fluorinated and non-fluorinated (poly)azidomethanes in the gas phase and in THF solution at 300 K and 473.15 K at the ab initio DLPNO-CCSD(T)/aug-cc-pVQZ level23,24 with thermal corrections from PBE0/6-31+g* calculations.25 Geometries were optimized at the PBE0/6-31+g* level. For each (poly)azidomethane, the ΔG# value includes all the transition states, corresponding to all the azide groups present (see the ESI† for details). The rate constants were evaluated using the Eyring–Polanyi equation. The data for the gas phase and solutions represented by a dielectric continuum were very similar. It was observed that starting from azidomethane, the formal substitution of a hydrogen atom for an azido group or fluorine atom resulted in highly destabilized azides (Fig. 3, second row). Interestingly, further formal substitutions of hydrogens for N3 or F stabilized the azides, with CF3N3 standing out as the most stable member of the family (Fig. 3, third and fourth rows). The indicative stabilities of known azides roughly correspond to these computed values. Furthermore, the calculations make it possible to predict that the unknown fluorinated polyazidomethanes CHF(N3)2 and CF(N3)3 are relatively unstable compounds, albeit more stable than CH2(N3)2. Although the lifetime of 1 is predicted to be 13 s at 200 °C, it is actually about 400 years at room temperature.
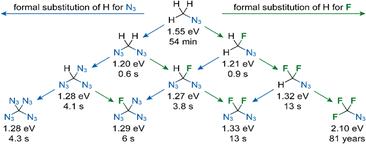 | ||
| Fig. 3 Calculated activation Gibbs free energies (ΔG#) in eV and lifetimes for the decomposition of (poly)azidomethanes in the gas phase at 200 °C. | ||
The reaction mechanism of the decomposition of diazidodifluoromethane was revealed by ab initio computation in the gas phase using the same method. After losing a dinitrogen molecule the nitrene product N3CF2N undergoes a barrier-less decomposition forming difluorodiazirine,26 a compound previously reported to be prepared by a multistep synthesis and known to quickly decompose into difluorocarbene, which can dimerize to form tetrafluoroethylene. Calculated activation Gibbs free energies and rate constants are presented in Fig. 4.
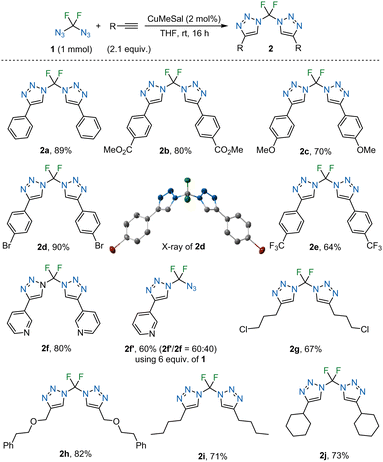
The prepared diazidodifluoromethane (1) was used in copper(I)-catalysed azide–alkyne cycloaddition (CuAAC) to afford bis(1,2,3-triazoles) 2 with a difluoromethylene linking unit (Table 2). The reaction proceeded with terminal alkynes and catalytic copper(I) 3-methylsalicylate (CuMeSal) as a THF-soluble catalyst at ambient temperature.
Aromatic acetylenes with electron-neutral, -withdrawing, or -donating groups participated efficiently in the reaction, as did m-pyridyl, alkyl and cycloalkyl acetylenes. Diverse functional groups, such as alkoxy, alkoxycarbonyl, halogen, trifluoromethyl and heterocyclic groups were well tolerated. The products (2) are stable solids.
The crystal structure of 2d (CCDC 2382300†) confirmed the bis(triazole) structure and revealed the conformation in the solid state where the nitrogen atoms of the triazole rings point to the same side of the molecule.
Using an excess of azide 1, N-azidodifluoromethyl-containing triazole 2f′ was prepared and isolated from side-product bis(triazole) 2f. A similar N-azidodifluoromethyl triazole was prepared by us in three steps from PhSO2CF2N3.27
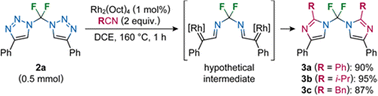
Differential scanning calorimetry measurement of 2a revealed two endotherms, one at 157 °C and the other at 159 °C, that correspond to a mesophase transition and melting, respectively. A large exotherm with an onset at 163 °C and a maximum at 184 °C, corresponding to exothermic decomposition followed. We exploited this thermal decomposition behaviour by microwave heating of 2a with benzonitrile, isobutyronitrile and phenylacetonitrile in the presence of an Rh(II) catalyst in double transannulation reactions affording bis(imidazoles) 3a–3c in excellent yields (Scheme 2).
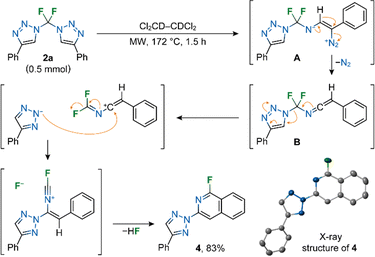
On the other hand, microwave heating of 2a in the absence of a catalyst or nitrile led to the formation of derivative 1-fluoroisoquinoline 4 in high yield (Scheme 3). The reaction most probably proceeds through the formation of vinyl diazonium A and ketenimine B, which undergoes a triazole ring shift and SEAr cyclization to 4. Compound 4 (CCDC 2382301†) is the first example of a 1-fluoro-3-(2H-1,2,3-triazol-2yl)isoquinoline structure.
In conclusion, a new diazidomethane CF2(N3)2 was prepared from dibromodifluoromethane by halogen substitution with nucleophilic azide salts in the presence of a thiolate additive which enables the reaction by acting as a bromophilic initiator. In contrast to other known polyazidomethanes, the new azide product is stable in solution. An ab initio study revealed trends in the stabilities of all possible fluorinated and non-fluorinated (poly)azidomethanes showing that diazidodifluoromethane is much more stable than diazidomethane. The computed reaction mechanism of the decomposition of the title azide suggests that it can proceed via difluorodiazirine and difluorocarbene intermediates. CuAAC of the title azide with terminal alkynes provided symmetrical difluorinated bis(1,2,3-triazoles) with high efficiency. The bis(triazoles) are amenable to Rh(II)-catalysed transannulation with nitriles to bis(imidazoles) or thermally decompose in an unprecedented way into a new 1-fluoroisoquinoline. This work demonstrates that diazidodifluoromethane can be obtained in a straightforward way and that it is stable enough to be used in synthesis to access new selectively fluorinated nitrogen heterocycles.
This work was financially supported by the Czech Academy of Sciences (Research Plan RVO: 61388963) and by the Czech Science Foundation (Projects 23-04659S and 24-11466S). We thank Dr Václav Matoušek from CF Plus Chemicals s.r.o. for his suggestions and discussion.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- O. Bakhanovich and P. Beier, Chem. – Eur. J., 2020, 26, 773 CrossRef CAS PubMed.
- A. Markos, V. Matoušek and P. Beier, Aldrichimica Acta, 2022, 55, 37 CAS.
- T. Saal, Z. E. Blastik, R. Haiges, A. Nirmalchandar, A. F. Baxter, K. O. Christe, M. Vasiliu, D. A. Dixon, P. Beier and G. K. S. Prakash, Angew. Chem., Int. Ed., 2020, 59, 12520 CrossRef CAS PubMed.
- N. Baris, M. Dračínský, J. Tarábek, J. Filgas, P. Slavíček, L. Ludvíková, S. Boháčová, T. Slanina, B. Klepetářová and P. Beier, Angew. Chem., Int. Ed., 2024, 63, e202315162 CrossRef CAS PubMed.
- Z. E. Blastik, S. Voltrová, V. Matoušek, B. Jurásek, D. W. Manley, B. Klepetářová and P. Beier, Angew. Chem., Int. Ed., 2017, 56, 346 CrossRef CAS PubMed.
- Y. Cai, H. Jiang and C. Zhu, Adv. Synth. Catal., 2023, 365, 342 CrossRef CAS.
- X. Wang, S. Liu, S. Xu, S. Wu, J. Wu and F. Wu, Org. Chem. Front., 2024, 11, 2054 RSC.
- C. Barrett, A. Alagaratnam, A. Knieb, C. J. Koch and G. K. S. Prakash, Org. Lett., 2024, 26, 6385 CrossRef CAS PubMed.
- V. Motornov, A. Markos and P. Beier, Chem. Commun., 2018, 54, 3258 RSC.
- V. Motornov and P. Beier, J. Org. Chem., 2018, 83, 15195 CrossRef CAS PubMed.
- O. Bakhanovich, B. Klepetářová and P. Beier, Org. Biomol. Chem., 2023, 21, 7924 RSC.
- A. Markos, S. Voltrová, V. Motornov, D. Tichý, B. Klepetářová and P. Beier, Chem. – Eur. J., 2019, 25, 7640 CrossRef CAS PubMed.
- A. Markos, L. Janecký, T. Chvojka, T. Martinek, H. Martinez-Seara, B. Klepetářová and P. Beier, Adv. Synth. Catal., 2021, 363, 3258 CrossRef CAS.
- A. Kubíčková, A. Markos, S. Voltrová, A. Marková, J. Filgas, B. Klepetářová, P. Slavíček and P. Beier, Org. Chem. Front., 2023, 10, 3201 RSC.
- A. Hassner and M. Stern, Angew. Chem., Int. Ed. Engl., 1986, 25, 478 CrossRef.
- A. Hassner, M. Stern, H. E. Gottlieb and F. J. Frolow, J. Org. Chem., 1990, 55, 2304 CrossRef CAS.
- K. Banert, Y.-H. Joo, T. Rüffer, B. Walfort and H. Lang, Tetrahedron Lett., 2010, 51, 2880 CrossRef CAS.
- K. Banert, Y.-H. Joo, T. Rüffer, B. Walfort and H. Lang, Angew. Chem., Int. Ed., 2007, 46, 1168 CrossRef CAS.
- S. P. Makarov, A. Y. Yakubovich, A. S. Filatov, M. A. Englin and T. Y. Nikiforova, Zh. Obshch. Khim., 1968, 38, 709 CAS.
- H. Bock, R. Dammel and D. D. Desmarteau, Z. Naturforsch. B, 1987, 42, 308 CrossRef CAS.
- S. Voltrová, J. Filgas, P. Slavíček and P. Beier, Org. Chem. Front., 2020, 7, 10 RSC.
- A. Haas, M. Spitzer and M. Lieb, Chem. Ber., 1988, 121, 1329 CrossRef CAS.
- C. Riplinger and F. Neese, J. Chem. Phys., 2013, 138, 034106 CrossRef.
- F. Neese, F. Wennmohs, U. Becker and C. Riplinger, J. Chem. Phys., 2020, 152, 224108 CrossRef CAS PubMed.
- C. Adamo, M. Cossi and V. Barone, J. Mol. Struct.:THEOCHEM, 1999, 493, 145 CrossRef CAS.
- R. A. Moss, L. Wang and K. Krogh-Jespersen, J. Am. Chem. Soc., 2009, 131, 2128 CrossRef CAS PubMed.
- M. Ziabko, B. Klepetářová and P. Beier, J. Org. Chem., 2023, 88, 6939 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC2382300 and 2382301. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc05128a |
| This journal is © The Royal Society of Chemistry 2025 |