Open Access Article
Open Access ArticleResponsive deep eutectic solvents: mechanisms, applications and their role in sustainable chemistry
Filipa A.
Vicente
 *,
Nuša
Tkalec
and
Blaž
Likozar
*,
Nuša
Tkalec
and
Blaž
Likozar

Department of Catalysis and Chemical Reaction Engineering, National Institute of Chemistry, Hajdrihova 19, 1000 Ljubljana, Slovenia. E-mail: filipa.andre.vicente@ki.si
First published on 11th December 2024
Abstract
In an era so focused on sustainability, it is important to improve chemical processes by developing and using more environmentally friendly solvents and technologies. Deep eutectic solvents (DES) have proven to be a promising replacement for conventional solvents. In recent years, a new type of DES has emerged that responds to various stimuli. These responsive DES (RDES) may offer all the advantages of DES while allowing the recycling and reuse of solvents. As such, RDES can further contribute to a greener future. This review provides an overview of the diverse types of RDES, their switching mechanisms and their application in several fields. Lastly, it offers a critical perspective on current shortcomings and prospects.
1. Introduction
In today's world, tackling environmental challenges, such as pollution and waste reduction, transitioning from a linear to a circular economy, improving energy efficiency, ensuring the availability of food, and drinking water for all are among the most important tasks. These goals are crucial for the well-being of current and future generations. For this reason, concepts such as circular (bio)economy and sustainable development are being followed and discussed with great attention. A circular economy promotes the efficient use of resources and the reduction of waste through the reuse, recycling and regeneration of products and materials. The sustainable development outlined in the 2030 agenda aims to achieve a balance between economic growth, social inclusion and environmental protection. Integrating these concepts is an essential prerequisite for creating a resilient and sustainable future in which global challenges are addressed comprehensively and equitably. This requires the development of an initial framework for the circular economy and the sustainable development goals (SDGs) that considers the circular economy as a precursor and the SDGs as a consequence of implementing a circular economy.1 However, the main challenge is to create a circular economy that is designed to be restorative and regenerative so that products, components and materials always retain their highest utility and value rather than relying solely on end-of-life solutions. To this end, it is important to focus on the development of a circular chemistry that combines the principles of green chemistry with the circular economy approach to extend process sustainability to the entire life cycle of chemical products and create a circular chemical process for all solvents, materials and products.2 A step closer starts by replacing the use of toxic and environmentally hazardous solvents with greener alternatives, especially those that can be obtained from renewable sources and while using greener synthesis. Among the possibilities, biosolvents, ionic liquids and deep eutectic solvents (DES) have attracted considerable attention. DES share some of the ionic liquids’ interesting properties such as low vapor pressure, wide liquid range, high compounds solubility and designer solvent character; however, they present a more biocompatible and biodegradable nature, easier preparation and lower cost.3,4 DES are a mixture of two or more compounds, usually Lewis or Brønsted acids and bases, which act as hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs). Once the HBDs and HBAs interact, they form predominantly hydrogen bonds, resulting in a mixture whose eutectic point is well below the temperature of an ideal liquid mixture.5 Since the introduction of DES by Abbott et al.6 in 2003, there have been countless combinations of HBDs and HBAs that fall into one of the five DES classifications. Type I DES combines quaternary ammonium salts and metal chlorides, while type II DES replaces the metal chloride with a metal chloride hydrate. Type III DES is prepared by mixing a quaternary ammonium with an HBD, including alcohols, amides, carboxylic acids, sugars, etc. Type IV DES consists of a metal chloride and an HBD. Type V DES has been described recently and consists of non-ionic molecular HBDs and HBAs.3,7 Among them, type III DES is the most widely used because its properties can be more easily tuned by changing the HBD class, e.g. hydrophobicity/hydrophilicity, viscosity, biocompatibility and biodegradability. This type of DES is usually referred to as natural DES (NaDES), which is due to the origin of the HBD used. Therefore, these solvents have found immense potential in multiple fields as demonstrated by the large number of reviews on the topic, namely in downstream processes,8–10 biomass processing,4,11–14 drug engineering & delivery,15–17 renewable energy technologies,18–20 nanoengineering & nanobiotechnology,15,21 among others. To guarantee the sustainable and economic character and application of DES, a crucial factor is DES recycling and reuse. The current techniques studied and applied involve the use of anti-solvent addition, crystallization, membrane filtration, solid–liquid extraction, liquid–liquid extraction, short path distillation, supercritical fluid extraction and separation due to density and/or viscosity differences. However, the recovery process can be challenging, the yields are not always satisfactory, and certain techniques disrupt the integrity and performance of DES, especially after a few cycles.3,22,23 A potential solution is gaining attention through the development and use of switchable/responsive DES (RDES). RDES allow the reversible transition between monophasic and biphasic systems upon a stimulus, which not only facilitates their recycling but simultaneously enhances the separation, selectivity, and purification of compounds. This new class of DES is still in its early stages, as evidenced by the relatively low number of publications on the topic, with only two review articles available.24,25 Herein, we introduce and discuss the diverse types of RDES, their triggering mechanism as well as the myriads of applications of these solvents. Additionally, we have illustrated the different types of triggering mechanisms for easier interpretation. Lastly, a critical perspective is also presented on current shortcomings and future prospects.2. Responsive DES
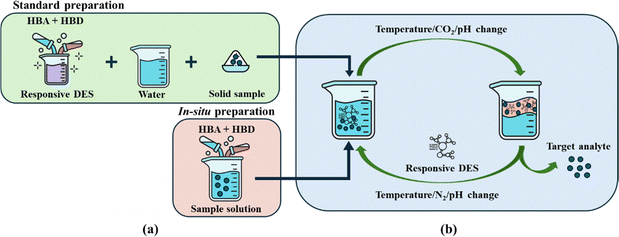
RDES represent an innovative class of solvents whose properties can be dynamically changed in response to external stimuli such as temperature, pH, or CO2 exposure. These stimuli-responsive DES are gaining attention as solvents in various fields due to their ability to undergo reversible changes, allowing for precise control over solvation properties, phase behaviour and reactivity. The mechanisms underlying these transformations typically involve reversible chemical reactions or physical processes, for instance the formation or breaking of hydrogen bonds, or ionization, depending on the nature of the stimulus.26 The phase transition mechanism is a solution to the challenge of separating components in a reaction mixture in liquid–liquid extractions. This facilitates the separation of the target compound from the solvent and at the same time allows the RDES to be recycled for further use. RDES are usually prepared using two different methods, as shown in Fig. 1a. In the first method, i.e. the standard method, the RDES is prepared by mixing the HBA and HBD in a chosen molar ratio and under specific conditions to obtain a liquid solvent. After the RDES is prepared, water and the sample containing the target analyte are added. In the second, less common method of preparing a RDES, the HBA and HBD are mixed directly with the sample solution and the conditions are adjusted to allow the RDES to form in situ. Regardless of the preparation technique chosen, the triggering mechanism is based on the application of an external stimulus, as shown in Fig. 1b.The composition of RDES has a fundamental impact on its role as a solvent in all application processes as well as on the switching mechanism. Thus, a significant amount of research on RDES is focused on understanding and carefully designing the solvent through the selection of HBAs and HBDs that can produce a DES that is responsive to stimuli for specific applications. Fundamental knowledge of the compounds properties as well as thorough research of already successfully produced RDES is key to the development of novel switchable DES for catalysis, downstream processes and other applications.26 A summary of the most selected RDES precursors reported in the literature, as well as the switching mechanism and the RDES research application is presented in Table 1. The HBAs that have so far proved efficient in the production of switchable DES include amines (secondary and tertiary), amidines, quaternary ammonium (salts), guanidine and nitrogenous bases, fatty acids, and phenols. HBDs include fatty acids, phenolic compounds, and alcohols.27–30 The preparation of RDES can be achieved by selecting and combining HBAs and HBDs in a simple and environmentally friendly process that produces no by-products.
| Driving factor | DES components (HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD) HBD) |
Molar ratio | Conditions | Applications | Ref. |
|---|---|---|---|---|---|
| a These studies are the most representative over the last 5 years. RT – room temperature. b In this study, several hydrophilic and hydrophobic DES were prepared and combined together with water, which were later applied for the extraction of different compounds from various sources, so the optimal molar ratio changes. | |||||
| CO2 | Diethylamine: 1,3-dibromopropane | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Stirring at 40 °C | Oil-solid separation | 31 |
Monoethanolamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4-methoxyphenol 4-methoxyphenol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at RT | Homogeneous liquid–liquid microextraction of chlorobenzenes | 32 | |
Thymol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octanoic acid octanoic acid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Stirring at 70 °C | Determination of fungicides contents in water, juice, wine, and vinegar | 33 | |
Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octanoat octanoat |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Stirring at 65 °C | Valorization of papaya peels for thrombolytic cysteine protease isolation | 34 | |
Imidazole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thylene glycol thylene glycol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 30 °C | Phase separation of DES/olive oil emulsion | 35 | |
Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octanoic acid octanoic acid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Stirring at 80 °C | Determination of anthraquinones in fried Cassiae semen tea infusions | 36 | |
4-Methoxyphenyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-amino-1-propanol 3-amino-1-propanol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 70 °C | Determination of enantiomers of the fungicide mefentrifluconazole in water, fruit juice, and fermented liquor samples | 37 | |
Tetramethylguanidine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol menthol |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 80 °C for 2 h | Extraction of lipids from Nannochloropsis sp. | 38 | |
Monoethanolamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) butanol butanol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at RT | Recovery and repurposing of waste isopropanol | 39 | |
Triethanolamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4-methoxyphenol 4-methoxyphenol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 25–80 °C | Extraction of hesperidin from orange peels | 40 | |
| Temperature | Lidocaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oleic acid oleic acid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 20 °C | Extraction of dyes from water | 30 |
Tetracaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lauric acid lauric acid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 80 °C | Extraction and separation of Lycium barbarum polysaccharides | 41 | |
Ethanolamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p-cresol p-cresol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at RT for 24 h | Extraction of polysaccharides from Ganoderma lucidum | 42 | |
Diethanolamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4-chlorophenol 4-chlorophenol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at RT | Selective separation of aromatic amino acids in water | 43 | |
Ethanolamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m-cresol m-cresol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at RT and drying in a vacuum dryer for 24 h | Flavonoid extraction from waste onion skins | 44 | |
Ethanolamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4- methoxyphenol 4- methoxyphenol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at RT | Extraction and separation of different polar active phytochemicals from Schisandra chinensis | 45 | |
Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) levulinic acid + octanoic acid levulinic acid + octanoic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lauric acid lauric acid |
Severalb | Stirring at RT | Extraction and separation of different of phytochemicals from different botanical sources | 46 | |
Ethanolamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) o-cresol o-cresol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at RT | Assessment of pyrethroid pesticides in surface soil samples | 47 | |
Lidocaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) heptanoic acid heptanoic acid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 60 °C | Determination of bisphenols in beverages | 48 | |
L-Menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phenyl salicylate phenyl salicylate |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 70 °C | Extraction of polysaccharides from wolfberry | 49 | |
| pH | Octylamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sulfonic acid sulfonic acid |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 80 °C | Extraction of sulfonamides from milk samples | 50 |
Thymol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanoic acid hexanoic acid |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 60 °C | Extraction of antibiotics from environmental water samples | 51 | |
L-Menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (1S)-(+)-camphor-10-sulfonic acid (1S)-(+)-camphor-10-sulfonic acid |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 40 °C for 20 min | Liquid–liquid extraction of abamectin and endosulfan from water and juice samples | 52 | |
Caprylic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) capric acid capric acid |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 50 °C | Recovery of β-carotene from pumpkin | 29 | |
Diethanolamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanoic acid hexanoic acid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 80 °C | Pre-concentration of liposoluble constituents in Salvia miltiorrhiza | 53 | |
Octanoic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) linalool linalool |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 80 °C | Extraction of polyphenols from millet | 54 | |
Thymol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octanol octanol |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 40 °C for 20 min | Analysis of herbicides in water and fruit juice samples | 55, 56 | |
Menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-octanoic acid n-octanoic acid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Stirring at 80 °C for 1 h | Preparation of pH-responsive nanoemulsions | 57 | |
Dodecaonic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octanoic acid octanoic acid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 40 °C | Extraction of polysaccharides from grape seeds by three-phase-partitioning | 56 | |
[N4,4,4,4]Br![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Triton X Triton X |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at 80 °C | Fermentation and purification of fibrinolytic protease from Bacillus subtilis | 58 | |
DL-Menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4-aminophenol 4-aminophenol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Stirring at 60 °C | Extraction of pyrethroid pesticides from milk | 59 | |
DL-Menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lauric acid lauric acid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at RT | Determination of phthalate esters in the packed milk samples | 60 | |
Ethyl maltol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octanoic acid octanoic acid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
Stirring at 60 °C | Extraction of oily waste from oily sawdust | 61 | |
Octylamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) succinic acid succinic acid |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stirring at RT | Extraction and determination of curcumin in water and food samples | 62 | |
In order to scientifically demonstrate the transformation of RDES during use and the change in behavior, HBA, HBD and prepared RDES need to be characterized. The characterization of RDES involves various analytical techniques to monitor these changes. Nuclear magnetic resonance (NMR) is widely used to monitor chemical shifts and confirm structural changes in the solvent. Fourier-transform infrared spectroscopy (FTIR) provides information on the changes in hydrogen bonding and molecular interactions.35,63 High-performance liquid chromatography (HPLC) is often used in the characterization of RDES to analyze the composition, purity, and stability of components in RDES, particularly in applications where these solvents are used for extraction and/or separation of target compounds.64,65 Additionally, techniques such as differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) are employed to evaluate thermal properties of the solvent.66,67 These characterization methods are critical to understanding the dynamics of RDES and optimizing them for specific applications.
2.1 CO2/N2 responsive DES
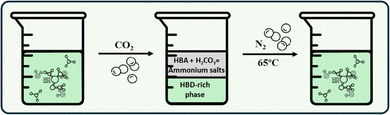
CO2/N2 trigger has been well studied as an external driving factor for switchable solvents, including RDES. The application of CO2/N2 during the extraction process with liquid solvents, such as RDES, alters their hydrophilicity/hydrophobicity, polarity and aggregation morphology, allowing phase separation, selective enrichment of the target analyte and subsequent recycling of RDES.68,69 For the preparation of CO2-RDES, the precursors, i.e. HBAs and HBDs, must be carefully selected, as the choice is closely related to the switching mechanism of the solvent and subsequently to its application. Alcohols, amines, polyamines, tertiary amines and quaternary ammonium salts are typical HBAs, while HBDs are usually phenols or alcohols; however, fatty acids can also be selected as both HBAs and HBDs for the preparation of CO2-RDES.70,71 It is important though that the CO2-RDES contain a suitable nitrogenous base to enable the switching mechanism. The introduction of CO2 into the reaction mixture by bubbling the gas into the aqueous solution leads to the formation of carbonic acid (H2CO3), which changes the acidity of the system and protonates the nitrogenous HBA. This reaction leads to the formation of ammonium salts, which break the hydrogen bonds between the HBA and the HBD. The increased ionic strength due to the formation of ammonium salts promotes phase separation, creating an ammonium salt-rich top phase and an HBD-rich bottom phase as a result of the salting-out effect. When CO2 is removed by introducing N2, the system returns to a monophasic state as the ammonium salts return to their original amine form. This is illustrated in Fig. 2.The possibility of preparing a DES that responds to CO2 in the medium and changes its physicochemical properties accordingly was first discovered by Sed and co-workers.28 In their study, a hydrophobic fatty acid-based NaDES was mixed with an amine solution and a hydrophilic homogeneous solution was formed by the formation of complexes between the two components. After CO2 was introduced to the solution, the fatty acid anions were reprotonated, restoring the hydrophobicity of the NaDES and causing the two phases to separate. Wan et al.68 prepared a series of CO2-RDES by combining alkanoamine compounds and phenols, resulting in hydrophilic DES that were stirred in a homogeneous solution with water. The addition of CO2 led to a shift in the acidity of the medium, resulting in the formation of an ammonium salt in the aqueous solution and the disruption of the hydrogen bonds between the HBA and HBD, promoting the phase separation, which was evident in the FTIR and 13C-NMR spectra. After the addition of N2, a shift back to the original monophasic state was observed, indicating the complete reversibility of the system. In another study, a switchable DES was prepared from alkanoamines and phenols for the extraction and preconcentration of chlorobenzenes.32 Purging CO2 led to a change in the nanostructure of the DES, resulting in the formation of two separate phases in the reaction mixture. Imidazole, a typical low-cost nitrogenous base, was also used as the HBA for the preparation of CO2-RDES, while polyols were evaluated as HDBs. The addition of olive oil to the DES resulted in the formation of an emulsion, which separated into two phases upon the addition of CO2 and the emulsion formed again upon the removal of CO2 by the introduction of N2.35
Overall, the use of CO2 as a trigger for switchable solvents, including DES, offers some key advantages, namely wide availability and ease of removal. However, this type of RDES has some shortcomings: most of the HBAs and HBDs used for RDES preparation present some toxicity; the DES network is completely disrupted, and during phase separation, the HBAs and HBDs migrate to different phases; not to mention that this process requires additional equipment for gas flow and its regulation. With regard to CO2 release, it should also be noted that the use of CO2 in this context does not contribute to the mitigation of global warming, as it is not permanently sequestered.
2.2 Thermo-responsive DES
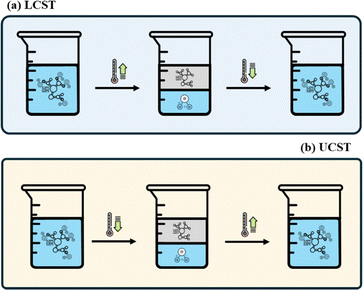
In contrast to the CO2/N2-RDES, where the injection of CO2 induces the phase change but, at the same time, represents an additional substance that can impair the separation and analysis of target samples, thermo-responsive DES respond to the temperature change in the system, eliminating the need for an additional component in the reaction mixture.For the design of thermo-RDES, HBAs and HBDs must be selected with knowledge of their behaviour, i.e., whether or not they exhibit phase behaviour and if so, whether they have a lower critical solution temperature (LCST) or upper critical solution temperature (UCST). For this reason, alkanolamines are used as HBAs and phenols as HBDs in thermo-RDES. Mixtures with an LCST are completely miscible with water at low temperatures due to favourable hydrophilic interactions such as hydrogen bonding between the DES components and water as well as within the DES itself. However, as the temperature increases and exceeds the LCST, these hydrogen bonds are disrupted by thermal energy, weakening the hydrophilic interactions, and strengthening the hydrophobic interactions, which leads to aggregation of the solute molecules and phase separation into two distinct phases. LCST behaviour is entropy-driven, with an increase in temperature causing the system to favour phase separation as a result of the prevalence of hydrophobic interactions.72 Although LCST behaviour is well-known, it is less common in thermo-RDES.30 UCST phase behaviour, on the other hand, involves phase separation at low temperatures owing to strong solute–solute interactions, such as hydrogen bonding, within the RDES. As the temperature increases, these interactions weaken, allowing the components to dissolve in the solvent and form a homogeneous, water-miscible phase.72,73 This is illustrated in Fig. 3.
A thermo-RDES with UCST phase behaviour was prepared by mixing various short-chain alkanolamines with phenols.43 After inducing the phase shift by increasing the temperature of the water–DES mixture, authors successfully extracted amino acids from water. FTIR and 13C-NMR were used to observe the binding behaviour within the network and confirm the changes in binding strengths between DES–DES and DES–water upon temperature shifts. Xiong et al.74 also designed UCST-based RDES but used only phenolic compounds, which served as both HBAs and HBDs. The prepared thermo-responsive DES were applied for the extraction of 5-hydroxymethylfurfural from the reaction medium, as its extraction and purification is usually challenging. The use of RDES enabled not only good recovery efficiency but also recycling of the DES without loss of efficiency. Some other studies have reported the formation of thermo-RDES with UCST phase behaviour,42,49,75 showing that the HBDs always contained an aromatic compound, namely o-, m- and/or p-cresol,47 guaiacol45 and methoxyphenol.45 However, these compounds have moderate to high toxicity, including ethanolamine, which is frequently used as HBA.46
Regarding the formation of LCST, Longeras et al.30 reported the first possibility of preparing a thermo-RDES that induces phase separation with the temperature increase. Authors mixed licodaine and oleic acid in different molar ratios and with the addition of different amounts of water and investigated the phase transition behaviour. This was initially done under a light microscope with precise temperature control and later with an FTIR spectrometer connected to a Hyperion microscope to allow more detailed characterization. This was only possible through the careful selection of the HBA and HBD. Lidocaine is an amphiphilic molecule and thus serves as a bridge between hydrophilic and hydrophobic interactions. This property is essential for facilitating the temperature-induced phase transition from a monophasic to a biphasic phase, as required for LCST-based RDES. On the other hand, oleic acid is a hydrophobic compound that can self-assemble when its concentration is above the critical micelle concentration. Therefore, their combination allowed the formation of a DES with temperature-dependent behaviour and confirmed the existence of an LCST with good extraction properties. Since then, these systems have been further explored, involving not only lidocaine, but also tetracaine and procaine76 as well as other carboxylic acids, namely pentanoic, hexanoic, heptanoic, octanoic, nonanoic, decanoic, dodecanoic, tetradecanoic and oleic acids.48,76 However, these HBAs exhibit moderate to high toxicity.46 To overcome this significant aspect, Cai and co-workers46 developed a novel temperature-dependent biphasic DES-based system for the extraction of phytochemicals without the need for amines and/or phenols. The authors prepared and mixed a hydrophilic and a hydrophobic DES, while also adding different amounts of water. The hydrophilic DES was choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) levulinic acid in a molar ratio of 1
levulinic acid in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 1
2 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, while the hydrophobic DES was either octanoic acid
3, while the hydrophobic DES was either octanoic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lauric acid in a molar ratio of 3
lauric acid in a molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or nonanoic acid
1 or nonanoic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) decanoic acid
decanoic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lauric acid in a molar ratio of 3
lauric acid in a molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. They then characterised the DES using thermogravimetric analysis and FTIR spectroscopy and investigated the switchability and phase behaviour of these systems as well as the polarity of the solvent. The RDES consisting of the ternary fatty acid mixture proved to be too hydrophobic, which considerably restricted and even impaired the monophasic region. Nevertheless, the use of the binary fatty acid mixture as a hydrophobic DES enabled the development of a well-functioning biphasic phase diagram. With this study, an efficient and more environmentally friendly option of LCST-based RDES for extraction purposes was presented.
1. They then characterised the DES using thermogravimetric analysis and FTIR spectroscopy and investigated the switchability and phase behaviour of these systems as well as the polarity of the solvent. The RDES consisting of the ternary fatty acid mixture proved to be too hydrophobic, which considerably restricted and even impaired the monophasic region. Nevertheless, the use of the binary fatty acid mixture as a hydrophobic DES enabled the development of a well-functioning biphasic phase diagram. With this study, an efficient and more environmentally friendly option of LCST-based RDES for extraction purposes was presented.
Overall, the operation of thermos-RDES is less complex than that of CO2-based RDES yet both present problems associated with the toxicity of the compounds used for the HBAs and HBDs. Besides, at lower temperatures, the system may be too viscous, which can hinder mass transfer.
2.3 pH responsive DES
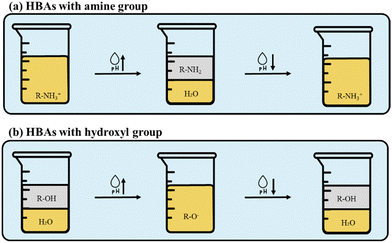
Adjusting DES properties by fine-tuning the pH of the solution with the introduction of appropriate proton donors and deprotonating agents provides a simple method for the extraction and separation of the target analyte without the need for additional equipment.25,28 In this method, pH-RDES are mainly prepared from amines, quaternary ammonium salts, medium-chain fatty acids, thymol and menthol as HBAs, while medium- and long-chain fatty acids play the role of HBDs. Alcohols and phenols can also be selected as HBDs, depending on the switching purpose of the RDES produced.25 The addition of an alkali or acid changes the physicochemical composition of a DES in aqueous solution. This depends on the pKa values and the ionization of the precursors of the RDES. When the pH of the medium is below the pKa value of the HBA and/or HBD, these DES precursors are protonated; when the pH of the medium is approximately at the pKa values of the compounds, 50% of the compounds are protonated and the other 50% are ionized/deprotonated; and when the pH of the medium is above the pKa value of the HBA and/or HBD, these precursors are ionized. Considering this, the switching mechanism of pH-RDES varies depending on the type of HBA, as shown in Fig. 4. When amines are used as HBA (Fig. 4a), the pKa refers to the respective conjugate of the amine, which also depends on the type of amine, i.e., primary, secondary, or tertiary amine, but is above pH 8. Therefore, when the RDES is prepared, the pH of the medium is below the pKa of the compound, so the RDES is protonated and soluble in the aqueous solution. For this reason, the solution is homogeneous. Upon the addition of a base, typically NaOH or KOH, but not exclusively, the HBA and possibly also the HBD are ionized, decreasing the RDES solubility and inducing phase separation. At this stage, a two-phase system is formed, which is reversible by the addition of an acid, usually HCl. When the acid is added to the system, the HBA and HBD are reprotonated and become miscible with the solution again.50 If the HBA has hydroxyl groups, such as the typically used menthol or thymol (Fig. 4b), the prepared RDES is protonated, so it is at its more hydrophobic character and is immiscible with the aqueous solution. In this case, the system is in a biphasic state. However, when a base is added, the HBA and possibly also the HBD are ionized/deprotonated, so the RDES takes on its hydrophilic character and becomes soluble in the solution. At this point, the system is a homogeneous solution that is reversible when an acid is added. At the moment the acid is added, the HBA and HBD are reprotonated and the RDES returns to its hydrophobic state and forms two phases again.77,78Jing et al.57 prepared a hydrophobic pH-RDES from menthol and octanoic acid. The prepared DES was mixed with water, and a surfactant-free microemulsion was formed. The emulsion was responsive due to the compounds used for the DESs preparation and was converted into a nanoemulsion after the addition of HCl. Furthermore, the authors showed that the nanoemulsion reverted back to a microemulsion after the addition of NaOH in response to pH changes. In another study, five hydrophobic pH-RDES were prepared by combining a terpenoid, namely thymol, and fatty acids (heptanoic to decanoic acid).50 All RDES were prepared in situ by changing the acidity of the aqueous solution to induce mixing of the precursors. This was possible due to the characteristics of both precursors, which in an alkaline solution, are present in anionic hydrophilic forms, thus being water soluble at high pH values. By decreasing the pH value of the media, the hydrophilic forms are protonated, and the precursors’ molecular form dominates, inducing a phase separation and formation of DES droplets in the solution. After inducing a shift of pH to acidic environment, the RDES formed a hydrophilic organic phase, which was stable in acidic media. Further studies on the production of efficient pH-RDES were conducted by Salamat and Soylat62 who developed a simple and fast extraction method for microextraction procedures. For this purpose, an RDES composed of octylamine: succinic acid was prepared. The RDES was hydrophilic under neutral conditions and therefore miscible with water. After the addition of NaCl, which led to a change in the aqueous environment to an alkaline state, a heterogeneous solution was formed. This phase shift of the RDES was attributed to the choice of HBAs and HBDs. Short-chain fatty acids, such as succinic acid, have hydrophilic properties. As far as octylamine is concerned, increasing the hydrophobic alkyl portion in the amines increases the molar mass of the amines, which leads to a reduction in their water solubility. When mixed with succinic acid to form a DES, the resulting product is hydrophilic but can become hydrophobic by changing the environment and deprotonating the amine groups. The resulting DES was analysed by 1H NMR spectroscopy for its ability to undergo a phase transition with NaOH and HCl.
The development of pH-controlled RDES systems with highly efficient extraction and separation capabilities offers a promising solution to the challenges associated with current switchable phase transition processes, such as high temperature requirements, high energy consumption and the need for specialised equipment. In contrast to other approaches, this method does not require heating of the extraction mixture or the application of additional energy sources to achieve the switch and can therefore also be used in the extraction of temperature-sensitive analytes.50 Additionally, this type of RDES is associated with lower toxicity due to the use of less hazardous precursors.
3. Applications
RDES represent a further development of conventional DES and offer a considerable improvement in functionality and versatility in several areas. Unlike traditional DES, RDES exhibit dynamic behaviour in response to external stimuli such as temperature, CO2/N2 and pH variations. This responsiveness allows for more precise control of solubility, viscosity, and catalytic activity, making them particularly suitable for a broad range of applications. Simultaneously, RDES offer better adaptability and functionality in the development of smart and responsive systems across diverse fields such as biomedicine, green chemistry, materials science, and environmental sustainability. In biomedicine, for example, RDES can be used for targeted drug delivery systems that release therapeutic agents in response to specific biological signals. In green chemistry, their tunable properties enable more efficient and environmentally friendly downstream processes by facilitating solvent recycling. In addition, RDES are being explored in materials science for the synthesis of advanced materials with customizable properties, while their ability to modulate solubility and reactivity under different conditions holds great promise for environmental sustainability, e.g. in pollution management.79–81 This section highlights several novel and significant applications of RDES in the aforementioned fields.3.1 Downstream processes & agri-food waste valorization
The increasing demand for resources is accompanied by an increased volume of waste from biomass processing in several industries. This includes primary industries such as agriculture, the oil and gas industry, fisheries, and aquaculture as well as the secondary sector with the manufacturing, chemical, pharmaceutical and food industries. Proper waste management is crucial, while at the same time the reuse and recycling of waste materials to obtain high-added value products and bioactive compounds is in line with the principles of sustainability. This approach promotes the transition from conventional practices to more environmentally conscious methods, fostering resource efficiency and reducing environmental impacts through the implementation of circular economy practices. DES have attracted considerable attention in the field of waste valorisation due to their unique physico-chemical properties and environmental benefits. The typical characteristics of a DES-based system, namely non-volatility, biocompatibility, biodegradability, and lower toxicity, represent an advantage over traditional solvents for downstream processes. In addition, DES offer high solubility for a wide range of natural products, can be easily tailored to specific compounds and operate under mild conditions, preserving the integrity and bioactivity of sensitive molecules. Characterised by their adaptability and precise tunability in response to stimuli, RDES represent an even more attractive alternative to conventional solvents. The responsiveness of these solvents not only enables analyte-specific applications, but also makes the extraction process simpler and faster, often eliminating the need for additional equipment or procedures to obtain different compounds, not to mention it allows the solvent recycling and reuse. As a result, the use of RDES instead of established solvents and non-switching DES has become a hot topic and has been successfully introduced for the recovery of many compounds, including bioactive molecules, polysaccharides, and pigments.Carotenoids, such as β-carotene, lutein, and zeaxanthin, are high-value compounds found in many agricultural products and have positive effects on the human body. β-Carotene is specifically hard to recover due to its properties, namely poor water solubility and sensitivity to light, heat and oxygen and is thus often extracted using hazardous solvents. A significant improvement in β-carotene extraction was achieved using pH-RDES.29 By using a fatty acid-based switchable solvent, high amounts of β-carotene were extracted (90 μg mL−1) from pumpkin and simultaneously separated from the pumpkin extract without the need for additional separation processes and solvents. Similarly to carotenoids, plant polysaccharides exhibit pharmacological properties, such as antioxidant, anti-aging, and anti-tumor, but their recovery is difficult as certain methods and solvents commonly used can impair their biological activity. To overcome the challenging polysaccharide recovery, thermo-RDES have been applied for polysaccharides extraction from Lycium barbarum.41 The extraction was performed at room temperature in the monophasic region and the system was then subjected to temperature to promote the phase separation, namely a hydrophobic DES-rich phase and an aqueous phase containing the polysaccharides. This not only allowed the polysaccharides extraction but also the recycling and reuse of DES for five cycles. The extraction yields of polysaccharides were ∼460 and ∼427 mg g−1 of biomass in the first and fifth cycle, respectively. Simultaneously, the RDES recovery efficiencies were 82.5% and 80.2% of the first and fifth cycle, respectively. Thus, proving an efficient use, recycling, and reusing of this thermo-RDES. Another study also reported high polysaccharides extraction yields from Ganoderma lucidum while using thermo-RDES, but this time using a UCST-based system with ethanolamine and p-cresol composing the RDES.42 Results showed it was possible to recover 88% and 79% of the polysaccharides in the aqueous phase after the first and fifth extraction cycle, respectively. Lastly, the authors added CO2 to the DES-rich phase to promote another phase separation and in this case separate the DES components. The top phase was the HBA salt-rich phase whereas the bottom phase corresponded to the HBD-rich phase. The former was further treated with N2 to recover the HBA in the original state, and the HBD was dried, achieving around 90% recovery of p-cresol. Continuing the polysaccharides extraction, Chen et al.56 prepared a series of pH-RDES that were included in a three-phase partitioning to extract and purify polysaccharides from grape seeds and achieved high extraction yields of up to 98.04 mg g−1 under optimized conditions. This represents an almost complete extraction yield on the first cycle. The RDES was later reused in the study during 25 cycles and led to a small yield decrease of 14% in the last cycle.
Moreover, RDES have also been used for the extraction of phytochemicals from plants. Attempting to extract nine different phytochemicals from Roxa roxburghii, a hydrophobic CO2-RDES was prepared and manipulated through the addition of CO2 and N2 to the system.82 The process of alternating the state of the DES in the aqueous solution enabled simultaneous extraction and enrichment of all nine hydrophilic and hydrophobic phytochemicals from R. roxburghii. At the same time, RDES was recycled during the extraction process, offering a new sustainable approach for phytochemical recovery from plants. In a similar study, researchers aimed to develop both highly efficient as well as environmentally friendly methods for flavonoid extraction from waste onion peels.44 For this purpose, UCST-based thermo-RDES were prepared and applied over the microwave-assisted extraction of quercetin, kaempferol, luteolin and quercetin-3-O-β-D-glucoside. Under the optimized conditions, the total flavonoid yields reached ∼48 mg g−1, which was more efficient than traditional organic solvents. However, in order to obtain crude flavonoid extracts, the authors used the macroporous resin HPD 600, allowing recovery rates of >90% for all of the four extracted flavonoid compounds. A similar observation considering the performance of RDES versus other solvents was reported by Zhang and coworkers.83 A pH-RDES with polarity-switching characteristics, namely N,N-dimethylbenzylamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octanoic acid (1
octanoic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) with 3 wt% β-cyclodextrin and 30 wt% water, outperformed pure DES (without β-cyclodextrin) and ethanol in total flavonoid recovery: ∼158 mg g−1 using RDES in comparison with 135 and 105 mg g−1 using pure DES and 60% ethanol, respectively. However, in this study, a macroporous AB-8 resin had to be used to recover the flavonoids and recycle the solvent. After 5 cycles, the extraction efficiency still remained >90%. Lastly, authors performed the cytotoxicity analysis of the RDES, and the results showed an exceptionally low or even negligible cytotoxicity with the EC50 > 2000 mg L−1. Wang et al.53 used a hydrophilic switchable DES for the pre-concentration of liposoluble constituents from Salvia mitiorrhiza. The RDES (diethanolamine
2 molar ratio) with 3 wt% β-cyclodextrin and 30 wt% water, outperformed pure DES (without β-cyclodextrin) and ethanol in total flavonoid recovery: ∼158 mg g−1 using RDES in comparison with 135 and 105 mg g−1 using pure DES and 60% ethanol, respectively. However, in this study, a macroporous AB-8 resin had to be used to recover the flavonoids and recycle the solvent. After 5 cycles, the extraction efficiency still remained >90%. Lastly, authors performed the cytotoxicity analysis of the RDES, and the results showed an exceptionally low or even negligible cytotoxicity with the EC50 > 2000 mg L−1. Wang et al.53 used a hydrophilic switchable DES for the pre-concentration of liposoluble constituents from Salvia mitiorrhiza. The RDES (diethanolamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanoic acid, 1
hexanoic acid, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) was prepared and added to the extractant phase in a homogeneous phase. Then, authors added HCl to switch the DES polarity, the RDES became hydrophobic and immiscible with water, hence leading to a biphasic system. This allowed concentration factors of diterpenoid quinones ranging from 59 to 274, which was considerably better than hexanoic acid alone.
1 molar ratio) was prepared and added to the extractant phase in a homogeneous phase. Then, authors added HCl to switch the DES polarity, the RDES became hydrophobic and immiscible with water, hence leading to a biphasic system. This allowed concentration factors of diterpenoid quinones ranging from 59 to 274, which was considerably better than hexanoic acid alone.
Pochivalov and co-workers50 went one step further in terms of using RDES for downstream processing by using RDES for the determination of antibacterial agents in milk samples. The RDES was prepared in situ from a terpenoid and a carboxylic acid after changing the acidity of the aqueous mixture, thus eliminating the need for additional energy sources and heating normally used for DES preparation. This approach proved successful for the determination of sulfamethazine, sulfamethoxazole, sulfaquinoxaline and sulfadiazine in milk samples, with pre-concentration factors between 22 and 103, which increase with increasing hydrophobicity of the analyte.
To summarise, the application of RDES in downstream processes has shown not only high extraction efficiency and selectivity, but also the possibility of solvent recycling and reuse with minimal performance loss.
3.2 Biomedicine
In medicine, solvents play a crucial role in numerous applications, including drug formulation, drug delivery and diagnostics. Conventional solvents often pose problems in terms of toxicity, volatility, and environmental impact, necessitating the search for safer and more sustainable alternatives. DES represent innovative solvents for different biomedical applications, in particular as drug delivery systems, as an alternative to traditional solvents in pharmacokinetic studies, for the production of biomedical materials, antiviral agents and as stabilizers for pharmaceutical formulations.81,84,85 Moreover, DES can be used to prepare the so-called “eutectogels”, which are dynamic gels that are stable, conductive and stretchable and can be used in wound dressings and tissue engineering.86 This is only possible due to the properties of DES, particularly their lower toxicity, improved solubility87 and stability of active pharmaceutical ingredients,88 as well as their versatility, which allows different formulations for multiple delivery purposes.65,89,90 As RDES gain increasing attention, their applications in biomedicine are also being explored. Their unique adaptability enables precise control over drug formulation, improving solubility, targeting, and selectivity in response to specific external stimuli.Thorough research into the pharmacokinetics and pharmacodynamics of drugs is an important part of drug development. For this purpose, the biological samples must be carefully pre-treated, which is traditionally done with hazardous solvents and time-consuming procedures. In an attempt to find an alternative, more environmentally friendly and faster sample pre-treatment method for post-application drug monitoring, Yang et al.91 focused on establishing a liquid–liquid microextraction (LLME) method based on a CO2-RDES. A switchable DES was prepared using 4-methoxyphenol and monoethanolamine as HBD and HBA, respectively, in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio. After mixing the biological samples with the prepared RDES, LLME was applied to remove proteins and lipids, and the alkaloids in the samples were pre-concentrated simultaneously. The injection of CO2 into the reaction mixture changed the RDES properties and led to the two-phase formation, i.e., an aqueous-rich phase and DES-rich phase containing the target analytes. The results provide a new insight into the biological behaviour of drugs in vivo in a simple, fast, and efficient way and demonstrate the possibility of using RDES in pharmacokinetic and pharmacodynamic studies. Similarly, LLME extraction in combination with a RDES was proposed as a new method for the separation and quantification of an anticancer drug daunorubicin in human plasma samples.65 Authors prepared several pH-RDES, with the most efficient one being composed of L-menthol and (1S)-(+)-camphor-10-sulfonic acid in a 5
2 molar ratio. After mixing the biological samples with the prepared RDES, LLME was applied to remove proteins and lipids, and the alkaloids in the samples were pre-concentrated simultaneously. The injection of CO2 into the reaction mixture changed the RDES properties and led to the two-phase formation, i.e., an aqueous-rich phase and DES-rich phase containing the target analytes. The results provide a new insight into the biological behaviour of drugs in vivo in a simple, fast, and efficient way and demonstrate the possibility of using RDES in pharmacokinetic and pharmacodynamic studies. Similarly, LLME extraction in combination with a RDES was proposed as a new method for the separation and quantification of an anticancer drug daunorubicin in human plasma samples.65 Authors prepared several pH-RDES, with the most efficient one being composed of L-menthol and (1S)-(+)-camphor-10-sulfonic acid in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The results showed that daunorubicin was not found in the blank sample, but it was detected in all four real samples in a concentration ranging between of 58.7–188.3 μg L−1. The results showed that the relative recoveries of daunorubicin in plasma samples were in the range of 91.0–107.8%, with an RSD < 7. This proved to be an efficient and robust technique for drug analysis in biological samples.
1 molar ratio. The results showed that daunorubicin was not found in the blank sample, but it was detected in all four real samples in a concentration ranging between of 58.7–188.3 μg L−1. The results showed that the relative recoveries of daunorubicin in plasma samples were in the range of 91.0–107.8%, with an RSD < 7. This proved to be an efficient and robust technique for drug analysis in biological samples.
At this stage, these studies have shown the potential of using RDES in biomedicine but have also made it clear that their application has so far been limited mainly to analytical purposes.
3.3 Environmental sustainability
Meeting current needs without jeopardizing the ability of future generations to meet their needs is one of the environmental priorities. The concept of environmental sustainability focuses on reducing pollution, improving resource efficiency, and minimizing waste. DES play a significant role in environmental sustainability as they are a more environmentally friendly alternative to traditional solvents commonly applied in the chemical sector. The use of RDES further contributes to environmental sustainability as they facilitate solvent recycling and reuse.Pollutants, including chemical waste, household waste, heavy metals, and bioactive compounds, have a negative impact on the environment and human health. Tackling the pollution crisis requires both the detection and elimination of pollutants, and RDES are emerging as a tool for this transformation. A switchable DES consisting of thymol and octanoic acid was assessed for the determination of strobilurin fungicides in water, juice, wine, and vinegar samples.33 The method has shown favourable linearity, precision, detection limit and recovery of fungicides with pH adjustment and manipulation of RDES. Furthermore, the method did not require additional solvents and equipment. Another application of RDES to prevent environmental pollution is the extraction of isopropanol from wastewater.39 Herein, several CO2-RDES were analysed, with monoethanolamine: butanol showing the most efficient recovery of isopropanol due to its hydrogen bonding with butanol. Once the CO2 was purged in the system, the RDES dissociated and resulted in the two-phase separation, with HBA salts forming the top phase and a butanol-rich bottom phase containing isopropanol. In this study, the RDES was not recycled as the butanol/isopropanol mixture can now be directly applied in the preparation of alternative fuels.
The presence of high concentrations of estrogenic hormones in environmental samples can have a negative impact on human health and must therefore be closely monitored. Extraction and pre-concentration are crucial steps for analysing these hormones in the samples, which are usually performed using non-environmentally friendly solvents and techniques. In this sense, Fattahi et al.92 developed a fast, simple, and environmentally friendly pre-treatment method using a pH-RDES. The prepared pH-RDES containing L-menthol and (1S)-(+)-camphor-10-sulfonic acid was manipulated by adding KOH to the solution. After changing the polarity, the RDES became miscible with water and a homogeneous mixture was formed, promoting a better interaction of the RDES and the steroids being extracted. Then, HCl was added to the system, restoring RDES hydrophobic state and promoting again the phase separation. The steroid hormones were concentrated in the RDES-rich phase and were then quantified. The sample analysis showed good linearity, precision, and sensitivity of the method as well as ease of use, which emphasizes the potential of RDES for monitoring environmental samples. Ma and co-workers,51 on the other hand, prepared a few pH-RDES using thymol as the HBA and medium-chain fatty acids as the HBDs. RDES were used as solvent in the liquid–liquid extraction of two antibiotics, namely levofloxacin and ciprofloxacin, that most commonly accumulate in environmental water samples, affect the human body and lead to drug resistance in pathogenic bacteria. The extraction of the target analytes was achieved by changing the medium acidity through the addition of HCl, which led to a change in the polarity of the RDES and its miscibility with water. The method showed high extraction efficiency and good repeatability, making it a promising approach for similar pre-concentration and extraction procedures. Likewise, Lu and coworkers93 utilized fatty acid-based RDES to concentrate/extract UV filters from river, lake, sea, and bathing water samples. To achieve this, a dispersive liquid–liquid microextraction was employed, and a switchable solvent, namely a pH-RDES, served two purposes by acting as a dispersive solvent and an extraction solvent, depending on the pH of the solution and thus the solvent's polarity. The prepared octanoic acid-based RDES extracted all target analytes in a short amount of time (within 9 minutes) and the method exhibited low detection limits coupled with a wide linear range, making it a practical and easy approach for water sample monitoring.
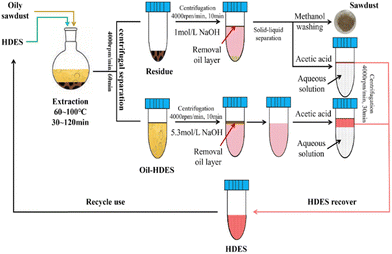
Regarding waste management, an ethyl maltol-fatty acid-based pH-RDES was applied for the oil removal from oily sawdust with a ∼67% oil content.61 The process includes multiple steps to recover the oil from the solid and liquid fractions, as shown in Fig. 5. Considering the liquid fraction, i.e., the oil-RDES fraction, the homogeneous phase was disturbed by the addition of NaOH, which deprotonated the RDES and increased its hydrophilicity, thus forcing the phase separation with an oil-rich top phase and aqueous RDES-rich bottom phase. After collecting the oil phase, acetic acid was added to the system, reprotonating the RDES and returning its hydrophobic character, which in turn promoted a new phase separation. This allowed the recycling and reuse of the RDES for 5 cycles with an efficiency loss of ∼10% from the first cycle (∼86%) to the second, remaining stable in the consequent cycles (∼76%). Additionally, authors also studied heavy metals extraction from the same sample and under the optimal conditions, the RDES showed extraction rates of >60% for all explored heavy metals except iron.
The use of RDES in the field of environmental sustainability is far more advanced than in biomedicine, with most studies focusing on the application of RDES to concentrate and extract contaminants from different types of samples either as a monitoring measure or for remediation purposes.
4. Critical analysis and future perspective
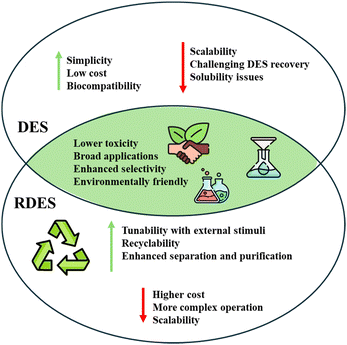
As aforementioned, RDES have emerged as interesting alternative solvents with applications in multiple fields, for instance downstream processing, environmental monitoring and even medicine. Understanding the advantages and limitations of solvents is essential for optimizing experimental outcomes, enhancing reaction rates, and ensuring environmental and health safety. This section explores the key benefits as well as the drawbacks associated with RDES, as summarized in Fig. 6, and offers critical feedback on the path forward.RDES offer the possibility to change solvent properties such as polarity, viscosity, and solubility in response to external stimuli such as CO2, pH, or temperature. This adaptability makes RDES highly versatile for applications, for instance extraction and separation processes.32,43 Their switchable nature contributes to improved selectivity of target compounds, enabling simultaneous extraction of multiple compounds with different chemical properties and leading to better overall performance. In addition, the general adaptability of RDES simplifies separation processes, enhancing the sustainability of industrial processes by reducing solvent consumption and costs. In this context, recyclability is another important feature of RDES. The tunable properties of RDES mean easy and fast solvent recycling, and as shown in several studies, the recycling process hardly affects the effectiveness of the RDES, especially forthermo- and pH-RDES, which is of great importance for sustainable chemistry.41,94 Moreover, RDES are simply a particular type of DES, so their designer solvent character remains one of the most intriguing and useful properties of these solvents. Thus, a wide range of possible green and biocompatible precursors is available, depending only on the switching mechanism and the target application. This is in line with the principles of green chemistry and points to a promising change in chemistry, but also in other areas where the need for better, more efficient, and environmentally friendly solvents and innovations is constantly growing.88
However, despite their great advantages, RDES also face several challenges. The first obstacle is the complexity of some operations, which can lead to higher costs and require additional equipment. CO2-RDES require additional equipment and regulations for their use, as well as special amines and phenols, which are more expensive HBAs and HBDs. Similarly, thermo-RDES require heating to promote phase switching, and in the case of LCST-based RDES, temperatures can be relatively high. This complexity can also hinder the transition to a larger scale. As the name “responsive” implies, these solvents respond to external stimuli. However, to fine-tune the solvent, external stimuli must be precisely controlled, which can complicate some industrial applications.
As far as the recyclability of RDES is concerned, all studies state that it is easy and possible to recover and recycle DES. However, this is not entirely clear for CO2-RDES, especially since CO2 injection leads to the formation of carbonic acid, which in turn reacts with the HBA and induces the formation of an ammonium salt-rich top phase and an HBD-rich bottom phase. At this stage, the HBA and HBD are in separate phases regardless of the location of the analyte, so a further technique is required to separate the DES precursor and analyte before the two phases can be combined and exposed to the N2 stream to restore the HBA character and thus allow the “regeneration” of the RDES. So far, most studies lack a clear explanation on how the analyte is recovered and on how the RDES is recycled and reused. Often, the authors mention that the analyte has preferentially migrated to one of the phases and present its quantification but continue with the discussion of the N2 flux to induce the monophasic state. When the CO2-RDES is recycled and reused, the authors mentioned the number of cycles performed and the extraction efficiency, but without explaining how this was done. In this sense, readers have considerable difficulty understanding the procedures performed in this type of RDES, so future studies should begin to address this shortcoming. This is not to say that only thermo- and pH-RDES enable DES recycling, but these tend to be much clearer, not to mention that both HBA and HBD remain in the same phase during phase separation, which facilitates the RDES recycling. In addition, some studies have reported the use of resins to separate the target compound from DES, allowing the solvents to be reused.44,83
From the point of view of sustainability and biocompatibility, the current RDES are not as biocompatible as the regular DES, which is due to the choice of HBAs and HBDs. To change their properties in response to a stimulus, RDES require precursors with specific properties, and until now, researchers have focused mainly on these properties rather than on the toxicity of these compounds. This is particularly true for HBAs and HBDs that are used in most CO2- and thermo-RDES and generally exhibit moderate to high toxicity.46 In contrast, pH-RDES tend to use more natural compounds such as thymol, menthol, and fatty acids, which could then be labelled as natural RDES. This large current gap offers room for improvement and should encourage scientists to search for more natural compounds with comparable properties to those needed in CO2- and thermo-RDES. In addition, this would further enhance the possibilities and applications of RDES in the biomedical field.
Finally, up to date and to the best of our knowledge, there is not a single study that assesses the environmental footprint of this type of DES (i.e., RDES) and RDES-based processes, nor has anyone conducted a techno-economic analysis of these processes. This clearly shows that the development, understanding and application of RDES in various fields is still in its infancy, especially when compared to the progress made so far with regular DES. Nevertheless, this review has also highlighted the enormous potential of RDES, particularly in downstream processing and in biomedical and environmental applications.
5. Conclusions
In a world that is battling climate change and pollution with the aim to shift towards a sustainable future, green chemistry plays a key role, especially when it regards to the development and use of greener solvents and technologies that are more environmentally friendly, efficient, and adaptable. Among the different possibilities, the use of DES stands out, as they offer numerous advantages over conventional solvents. Unlike the latter that can be hazardous and non-recyclable while also presenting high operational costs and complex and time-consuming processing, DES are more biocompatible, versatile, and capable of solubilizing a wide range of compounds. In recent years, responsive or switchable DES have emerged as an upgrade of regular DES, as they combine the advantages of DES with the additional ability to respond and switch physical properties when exposed to specific stimuli (pH, temperature, CO2). This not only contributes to better efficiency and selectivity of the solvent, but simultaneously also allows solvent recycling. The ability to precisely control the behaviour of the solvent depending on the conditions of the medium can optimise both industrial processes and their environmental footprint. All the advantages of using RDES have led to this type of solvent being successfully used in the extraction of various compounds and their valorisation, in the monitoring of environmental samples and in the pre-treatment of biological samples for pharmaceutical studies. Despite recent advancements, several limitations still restrict the broader use of RDES. Future developments in the areas of scalability, system complexity and comprehensive health and toxicological assessments need to be addressed in order to enable a broad application of RDES in various academic and industrial settings. In addition, the integration of RDES into green chemistry methods will depend on the development of more sustainable and environmentally friendly RDES formulations. With ongoing research, RDES could play a significant role in various industries by providing tailored solvent systems that respond to external stimuli, increasing productivity and decreasing waste. Considering all that, the future of RDES is promising, with potential to transform various sectors through innovation and sustainability, align with the sustainable development goals and green chemistry practices, and pave the way for a more sustainable future.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Slovenian Research Agency under research core funding P2-0152 and research projects NC-0024 and NC-24002.References
- D. Gallardo-Vázquez, S. Scarpellini, A. Aranda-Usón and C. Fernández-Bandera, Humanit. Soc. Sci. Commun., 2024, 11(1), 1–18 CrossRef.
- T. Keijer, V. Bakker and J. C. Slootweg, Nat. Chem., 2019, 11(3), 190–195 CrossRef CAS PubMed.
- F. H. B. Sosa, J. A. P. Coutinho and A. M. da C. Lopes, Deep eutectic solvents vs. ionic liquids: Similarities and differences, Elsevier, 2022 Search PubMed.
- F. A. Vicente, V. Urbančič, B. Likozar and J. F. B. Pereira, in Deep Eutectic Solvents: Properties, Applications and Toxicity, ed. E. S. dos Santos, C. E. de Araújo Padilha, F. C. de Sousa Júnior and N. S. Rios, Nova Science Publishers, Inc., 2022, p. 287 Search PubMed.
- D. O. Abranches and J. A. P. Coutinho, Annu. Rev. Chem. Biomol. Eng., 2023, 14, 141–163 CrossRef CAS PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- A. Prabhune and R. Dey, J. Mol. Liq., 2023, 379, 121676 CrossRef CAS.
- B. A. Pereira, C. T. Matos, L. Costa, L. M. Ferreira, J. G. Crespo and C. Brazinha, Sep. Purif. Technol., 2025, 353, 128510 CrossRef CAS.
- S. Kaoui, B. Chebli, K. Basaid and Y. Mir, Sustainable Chem. Pharm., 2023, 31, 100937 CrossRef CAS.
- M. Marchel, M. P. Rayaroth, C. Wang, L. Kong, J. A. Khan and G. Boczkaj, Chem. Eng. J., 2023, 475, 144971 CrossRef CAS.
- C. L. Yiin, Z. Y. Lai, B. L. F. Chin, S. S. M. Lock, K. W. Cheah, M. J. Taylor, A. Al-Gailani, B. W. Kolosz and Y. H. Chan, J. Cleaner Prod., 2024, 470, 143248 CrossRef CAS.
- M. del Mar Contreras-Gámez, Á. Galán-Martín, N. Seixas, A. M. da Costa Lopes, A. Silvestre and E. Castro, Bioresour. Technol., 2023, 369, 128396 CrossRef.
- K. T. T. Amesho, Y. C. Lin, S. V. Mohan, S. Halder, V. K. Ponnusamy and S. R. Jhang, Environ. Chem. Lett., 2022, 21(1), 183–230 CrossRef.
- Á. Lobato-Rodríguez, B. Gullón, A. Romaní, P. Ferreira-Santos, G. Garrote and P. G. Del-Río, Bioresour. Technol., 2023, 388, 129744 CrossRef.
- Y. Ma, Y. Yang, T. Li, S. Hussain and M. Zhu, Green Chem., 2024, 26, 3627–3669 RSC.
- N. V. P. Veríssimo, C. U. Mussagy, H. B. S. Bento, J. F. B. Pereira and V. de C. Santos-Ebinuma, Biotechnol. Adv., 2024, 71, 108316 CrossRef.
- F. Oyoun, A. Toncheva, L. C. Henríquez, R. Grougnet, F. Laoutid, N. Mignet, K. Alhareth and Y. Corvis, ChemSusChem, 2023, 16, e202300669 CrossRef CAS PubMed.
- Y. Fan, Y. Kong, P. Jiang, G. Zhang, J. Cong, X. Shi, Y. Liu, P. Zhang, R. Zhang and Y. Huang, Chem. Eng. J., 2023, 463, 142278 CrossRef CAS.
- J. Wang, Y. Lyu, R. Zeng, S. Zhang, K. Davey, J. Mao and Z. Guo, Energy Environ. Sci., 2024, 17, 867–884 RSC.
- R. Al-Farsi and M. Hayyan, Renewable Sustainable Energy Rev., 2023, 184, 113505 CrossRef CAS.
- A. Azzouz and M. Hayyan, Chem. Eng. J., 2023, 468, 143563 CrossRef CAS.
- X. Liang, Y. Fu and J. Chang, Sep. Purif. Technol., 2019, 210, 409–416 CrossRef CAS.
- A. Isci and M. Kaltschmitt, Biomass Convers. Biorefin., 2022, 12, 197–226 CrossRef.
- J. Zhang, S. Li, L. Yao, Y. Yi, L. Shen, Z. Li and H. Qiu, Chin. Chem. Lett., 2023, 34, 107750 CrossRef CAS.
- M. Zhang, Z. Zhang, Z. Gul, M. Tian, J. Wang, K. Zheng, C. Zhao and C. Li, J. Sep. Sci., 2023, 46, 2300098 CrossRef CAS.
- P. Pollet, C. A. Eckertabc and C. L. Liotta, Chem. Sci., 2011, 2, 609–614 RSC.
- A. Mero, S. Koutsoumpos, P. Giannios, I. Stavrakas, K. Moutzouris, A. Mezzetta and L. Guazzelli, J. Mol. Liq., 2023, 377, 121563 CrossRef CAS.
- G. Sed, A. Cicci, P. G. Jessop and M. Bravi, RSC Adv., 2018, 8(65), 37092–37097 RSC.
- A. Stupar, V. Šeregelj, B. D. Ribeiro, L. Pezo, A. Cvetanović, A. Mišan and I. Marrucho, Ultrason. Sonochem., 2021, 76, 105638 CrossRef CAS.
- O. Longeras, A. Gautier, K. Ballerat-Busserolles and J. M. Andanson, ACS Sustainable Chem. Eng., 2020, 8, 12516–12520 CrossRef CAS.
- X. Li, Z. Yang, H. Sui, A. Jain and L. He, Fuel, 2018, 221, 303–310 CrossRef CAS.
- M. Nazraz, Y. Yamini, A. M. Ramezani and Z. Dinmohammadpour, J. Chromatogr. A, 2021, 1636, 461756 CrossRef CAS PubMed.
- L. Jia, X. Huang, W. Zhao, H. Wang and X. Jing, Food Chem., 2020, 317, 126424 CrossRef CAS PubMed.
- H. Babu Balaraman, G. Viswanathan, R. Muniasamy, T. Gayatri and S. Kumar Rathnasamy, Microchem. J., 2022, 175, 107118 CrossRef CAS.
- F. Liu, Z. Xue, X. Lan, Z. Liu and T. Mu, Chem. Commun., 2021, 57, 627–630 RSC.
- Z. Shi, X. Li, Y. Tian, Y. Fan, J. Liu and H. Zhang, Anal. Methods, 2021, 13, 4739–4746 RSC.
- H. Jiang, X. Huang, H. Xue, M. Wang, Y. Qi, L. Jia and X. Jing, Chirality, 2022, 34, 968–976 CrossRef CAS.
- C. Cai, X. Chen, F. Li and Z. Tan, Sep. Purif. Technol., 2021, 279, 119685 CrossRef CAS.
- T. W. Lee, Y. H. Su and C. Chen, Sci. Total Environ, 2023, 896, 165053 CrossRef CAS PubMed.
- S. Wang, T. Lei, L. Liu and Z. Tan, Food Chem., 2024, 432, 137255 CrossRef CAS PubMed.
- Z. Tang, Y. Xu, C. Cai and Z. Tan, J. Mol. Liq., 2023, 383, 122063 CrossRef CAS.
- C. Cai, Y. Wang, W. Yu, C. Wang, F. Li and Z. Tan, J. Cleaner Prod., 2020, 274, 123047 CrossRef CAS.
- D. Xiong, Q. Zhang, W. Ma, Y. Wang, W. Wan, Y. Shi and J. Wang, Sep. Purif. Technol., 2021, 265, 118479 CrossRef CAS.
- X. C. Shang, Y. Q. Zhang, Y. F. Zheng and Y. Li, Biomass Convers. Biorefin., 2024, 14, 3729–3738 CrossRef CAS.
- X. Y. Yan, Z. H. Cai, P. Q. Zhao, J. D. Wang, L. N. Fu, Q. Gu and Y. J. Fu, Food Res. Int., 2023, 165, 112541 CrossRef CAS.
- Z. H. Cai, X. Y. Dong, L. T. Wang, W. M. Zhou, Y. N. Wang, J. Yang and Y. J. Fu, Chem. Eng. J., 2024, 499, 156012 CrossRef CAS.
- B. Shiquan, R. X. Sun, P. Zhou, Y. Q. Li and X. C. Shang, Microchem. J., 2022, 181, 107733 CrossRef.
- P. Godunov, A. Gerasimova, A. Shishov and A. Bulatov, J. Food Compos. Anal., 2024, 135, 106569 CrossRef CAS.
- Y. Cao, H. Wang, Y. Jian, G. Hao, D. Di and J. Liu, J. Mol. Liq., 2024, 398, 124352 CrossRef CAS.
- A. Pochivalov, K. Cherkashina, A. Shishov and A. Bulatov, J. Mol. Liq., 2021, 339, 116827 CrossRef CAS.
- W. Ma and K. H. Row, Microchem. J., 2021, 160, 105642 CrossRef CAS.
- M. Hassanpour, M. Shamsipur, N. Babajani, F. Shiri, B. Hashemi and N. Fattahi, Microchem. J., 2023, 187, 108391 CrossRef CAS.
- X. Ping Wang, R. Quin Wang, X. Yu Pan, R. Rong Xing, L. Yang, X. Chen and S. Hu, J. Chromatogr. A, 2022, 1666, 462858 CrossRef.
- H. Zhang, W. Zhao, T. Bai, L. Fu, Z. Chen, X. Jing and X. Wang, LWT, 2022, 170, 114082 CrossRef CAS.
- N. Fattahi, P. Zohrabi, F. Shiri, F. Hobi Bordón Sosa and B. Hashemi, Sep. Purif. Technol., 2024, 339, 126607 CrossRef CAS.
- R. Wang and Z. Tan, Food Chem., 2023, 412, 135557 CrossRef.
- J. Jing, X. Li, Y. Zhang, Y. Liu, H. Lu, J. Wang and Y. Wu, Langmuir, 2022, 38, 7898–7905 CrossRef CAS PubMed.
- R. Muniasamy, B. S. Balamurugan, D. Rajamahendran and S. Rathnasamy, Sci. Rep., 2022, 12, 903 CrossRef CAS PubMed.
- A. Niroumandpassand, A. Javadi and M. R. Afshar Mogaddam, Anal. Methods, 2021, 13, 1747–1756 RSC.
- X. L. Wang, Y. Lu, L. Shi, D. Yang and Y. Yang, Microchem. J., 2020, 159, 105332 CrossRef CAS.
- N. Gao, Y. Wang, H. Luo, Y. Xu, J. Liu and Y. Chen, Chem. Eng. J., 2024, 495, 153339 CrossRef CAS.
- Salamat Q. and Solyak M., Talanta, 2024, 269, 125401 CrossRef.
- X. Li, Z. Yang, H. Sui, A. Jain and L. He., Fuel, 2018, 221, 303–310 CrossRef CAS.
- L. Cao and Y. Li, J. Sep. Sci., 2024, 47, 2300776 CrossRef CAS PubMed.
- R. Akramipour, H. Babaei, F. Castru-Cayllaha, M. R. Golpayegani, N. Fattahi and F. Fattahi, Heliyon, 2024, 10, e23532 CrossRef CAS PubMed.
- X. Zhang, X. Yan, Z. Cai, L. Fu, X. Dong, J. Cui, H. Zheng, M. Xu and Y. Fu, Ind. Crops Prod., 2024, 221, 119324 CrossRef CAS.
- S. H. Jung, G. Choi, S. Jeong, J. Park, H. Yoon, J. J. Park and H. Kim, ACS Sustainable Chem. Eng., 2022, 10, 13816–13824 CrossRef CAS.
- W. Wan, D. Xiong, Q. Zhang and J. Wang, ACS Sustainable Chem. Eng., 2019, 7, 17882–17887 CrossRef CAS.
- T. W. Lee, Y. H. Su and C. Chen, Sci. Total Environ., 2023, 896, 165053 CrossRef CAS PubMed.
- J. R. Vanderveen, J. Durelle and P. G. Jessop, Green Chem., 2014, 16, 1187–1197 RSC.
- P. G. Jessop, L. Kozycz, Z. G. Rahami, D. Schoenmakers, A. R. Boyd, D. Wechsler and A. M. Holland, Green Chem., 2011, 13, 619–623 RSC.
- S. Li, L. Feng, H. Lu and S. Feng, New J. Chem., 2017, 41, 1997–2003 RSC.
- E. A. Clark and J. E. G. Lipson, Polymer (Guildf), 2012, 53, 536–545 CrossRef CAS.
- D. Xiong, Y. Wang, H. Ma, L. Lu, Q. Zhang, Y. Shi, Y. Zhao and J. Wang, ACS Sustainable Chem. Eng., 2023, 11, 399–406 CrossRef CAS.
- X. Y. Yan, Z. H. Cai, P. Q. Zhao, J. D. Wang, L. N. Fu, Q. Gu and Y. J. Fu, Food Res. Int., 2023, 165, 112541 CrossRef CAS PubMed.
- Z. Tang, Y. Xu, C. Cai and Z. Tan, J. Mol. Liq., 2023, 383, 122063 CrossRef CAS.
- N. F. A. Van Der Vegt and D. Nayar, J. Phys. Chem. B, 2017, 121, 9986–9998 CrossRef CAS.
- J. N. Israelachvili, Intermolecular and Surface Forces, 3rd edn, 2011, pp. 1–676 Search PubMed.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- Y. P. Mbous, M. Hayyan, A. Hayyan, W. F. Wong, M. A. Hashim and C. Y. Looi, Biotechnol. Adv., 2017, 35, 105–134 CrossRef CAS PubMed.
- P. A. Shah, V. Chavda, D. Hirpara, V. S. Sharma, P. S. Shrivastav and S. Kumar, J. Mol. Liq., 2023, 390, 123171 CrossRef CAS.
- J. D. Wang, Q. Gu, Z. H. Cai, G. S. Liu, Q. Zhou, L. N. Fu, S. L. Yang, S. Zhang and Y. Fu, ACS Sustainable Chem. Eng., 2023, 11, 18051–18063 CrossRef CAS.
- Y. Zhang, H. Li, X. Hai, X. Guo and X. Di, J. Chromatogr. A, 2024, 1730, 465084 CrossRef CAS.
- M. M. Abdelquader, S. Li, G. P. Andrews and D. S. Jones, Eur. J. Pharm. Biopharm., 2023, 186, 85–104 CrossRef CAS.
- T. Swebocki, A. Barras, A. Abderrahmani, K. Haddadi and R. Boukherroub, Int. J. Mol. Sci., 2023, 24, 8492 CrossRef CAS PubMed.
- F. Xu, C. Dawson, M. Lamb, E. Mueller, E. Stefanek, M. Akbari and T. Hoare, Front. Bioeng. Biotechnol., 2022, 10, 849831 CrossRef.
- C. Lu, J. Cao, N. Wang and E. Su, MedChemComm, 2016, 7, 955–959 RSC.
- B. Olivares, F. Martínez, L. Rivas, C. Calderón, J. M. Munita and P. R. Campodonico, Sci. Rep., 2018, 8, 1–12 CAS.
- A. M. Curreri, M. Dunne, M. G. Bibbey, N. Kapate, J. Kim and S. Mitragotri, Adv. Healthcare Mater., 2024, 2400327 CrossRef CAS PubMed.
- J. Kim, Y. Shi, C. J. Kwon, Y. Gao and S. Mitragotri, Adv. Healthcare Mater., 2021, 10, 2100585 CrossRef CAS.
- L. Yang, X. Yang, E. Wang, B. Lu and L. Duan, Anal. Chim. Acta, 2024, 1307, 342620 CrossRef CAS PubMed.
- N. Fattahi, M. Shamsipur, Z. Nematifar, N. Babajani, M. Moradi, S. Soltani and S. Akbari, RSC Adv., 2022, 12, 14467–14476 RSC.
- Y. Lu, X. Wang, H. Gu and M. Gao, Microchem. J., 2021, 169, 106626 CrossRef CAS.
- Y. Liu, J. Li, R. Fu, L. Zhang, D. Wang and S. Wang, Ind. Crops Prod., 2019, 140, 111620 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |