Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and characterization of triazole-functionalized mixed-valent Si(I)–Si(III) and bis(germylene) compounds†
Madhusudan K.
Pandey
 a,
Zohreh
Hendi
a,
Xiaobai
Wang
a,
Shahila
Muhammed
c,
Arun
Kumar
a,
Mukesh K.
Singh
a,
Zohreh
Hendi
a,
Xiaobai
Wang
a,
Shahila
Muhammed
c,
Arun
Kumar
a,
Mukesh K.
Singh
 *b,
Regine
Herbst-Irmer
*b,
Regine
Herbst-Irmer
 a,
Dietmar
Stalke
a,
Dietmar
Stalke
 *a,
Pattiyil
Parameswaran
*a,
Pattiyil
Parameswaran
 *c and
Herbert W.
Roesky
*c and
Herbert W.
Roesky
 *a
*a
aInstitut für Anorganische Chemie, Georg-August-Universität Göttingen, Göttingen, 37077, Germany. E-mail: hroesky@gwdg.de; dstalke@chemie.uni-goettingen.de
bSchool of Chemistry, University of Edinburgh, Edinburgh, EH9 3FJ, UK
cNational Institute of Technology Calicut, Kozhikode 673601, India. E-mail: param@nitc.ac.in
First published on 28th February 2025
Abstract
The synthesis of mixed-valent main-group compounds is a challenging goal that has attracted significant interest recently. The reaction of 1-(2-bromophenyl)-4-phenyl-1H-1,2,3-triazole with nBuLi, followed by treatment with [(PhC(tBuN)2SiCl)], yielded a rare Si(I)–Si(III) compound (1), whereas treatment with [(PhC(tBuN)2GeCl)] produced a bis(germylene) compound (2).
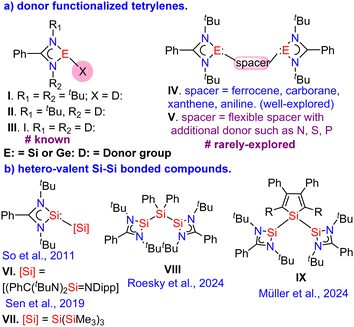
Over the past two decades, significant advancements have been made in understanding the chemistry of heavier carbene analogues, known as tetrylenes, including silylenes and germylenes.1 Notably, the tri-coordinated amidinato tetrylenes [PhC(NtBu)2ECl] (E = Si2 and Ge3), synthesized by Roesky and co-workers, have emerged as focal points of research. They contain divalent E(II) atoms (E = Si or Ge), with a lone pair of electrons and a vacant p orbital at the E(II) atoms. The past decade has seen growing interest in bi- or tridentate amidinato tetrylenes (ATs) (Fig. 1)4 due to the excellent catalytic properties demonstrated by many transition metal complexes, especially those utilizing silylenes.5 Amidinato tetrylenes (ATs) of types I and IV, where a donor or spacer replaces the Cl atom, are widely studied.4 ATs of types II and III, functionalized with a donor at the N atom of the amidinate backbone, are also explored but less common.4 In spacer-separated bis(tetrylenes) of type IV, two E(II) atoms are typically independent, with linkages providing sufficient space or orienting their lone pairs away from each other. Spacer-separated ATs of type V, with flexible spacers containing extra donor sites, can function as chelating multi-dentate ligands or it can bring E(II) atoms closer to show possible E⋯E (E = Si or Ge) interactions or E–E bond formation, are rarely investigated.4 In this context, Driess and co-workers demonstrated that the amidinato silylene forms a hypercoordinate Si
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si dimer when the two silicon atoms are forced to come closer.6 More recently, hetero-valent Si(IV)–Si(II) bonded silylenes have attracted significant interest due to their strong σ-donating properties, making them valuable synthons in main group and coordination chemistry.7
Si dimer when the two silicon atoms are forced to come closer.6 More recently, hetero-valent Si(IV)–Si(II) bonded silylenes have attracted significant interest due to their strong σ-donating properties, making them valuable synthons in main group and coordination chemistry.7
 | ||
| Fig. 1 (a) Selected types of known donor-functionalized tetrylenes. (b) Amidinate based hetero-valent Si–Si bonded systems. Dipp = 2,6-diisopropylphenyl. | ||
However, hetero-valent Si(I)–Si(III) bonded compounds remain scarce in the literature (Fig. 1b; VI). So and co-workers reported a Si(I)–Si(III) mixed-valent compound silaiminyl–silylene VI,8 which Roesky and co-workers utilized recently to demonstrate interesting stimuli-induced electromerism.9 Sen and co-workers synthesized the first amidinate-stabilized Si(II)–Si(IV) compound VII, featuring an unusually long Si(II)–Si(IV) bond distance of 2.4339(13) Å,10 which afforded unsymmetrical sp2–sp3 disilenes upon treatment with aliphatic chlorophosphines.11 More recently, Roesky and co-workers developed a unique bis(silylene) VIII with a Si(II)–Si(IV)–Si(II) bonding arrangement, exhibiting Si(II)–Si(IV) bond distances of 2.4212(8) Å and 2.4157(7) Å and explored its coordinating ability with Fe(0).12a Driess and co-workers employed VIII to activate the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O bonds of carbon monoxide under ambient conditions (1 atm, room temperature), leading to the formation of 1,3-disilacyclopentadiene.12b Müller and co-workers reported a bis(silylene)silole IX with Si(II)–Si(IV) bond distances of 2.4590(6) Å and 2.4635(5) Å and explored its reactivity with chalcogenide and organic azides.13 These examples underscore the significance of hetero-valent Si–Si bonded silylenes in both main group and coordination chemistry. Despite extensive research on functionalized triazoles with P, Se donors, their functionalization with tetrylene moieties remains unexplored.14 Incorporating heteroaryl groups with donor atoms like nitrogen, oxygen, or sulfur near tetrylene moieties can create versatile ligand systems with rich coordination chemistry, suitable for applications in homogeneous catalysis, molecular switches, logic gates, and sensors. We envisioned that the triazole functionalized E2 systems would not only provide a versatile ligand system but might also bring the E(II) atoms in close proximity to afford versatile mixed-valent E–E bonded compounds. Herein, we report the reaction of dilithiated triazole with amidinato tetrylenes [PhC(NtBu)2ECl] (E = Si and Ge) resulting in the formation of a rare Si(I)–Si(III) bonded compound 1 and a bis(germylene) compound 2, respectively.
O bonds of carbon monoxide under ambient conditions (1 atm, room temperature), leading to the formation of 1,3-disilacyclopentadiene.12b Müller and co-workers reported a bis(silylene)silole IX with Si(II)–Si(IV) bond distances of 2.4590(6) Å and 2.4635(5) Å and explored its reactivity with chalcogenide and organic azides.13 These examples underscore the significance of hetero-valent Si–Si bonded silylenes in both main group and coordination chemistry. Despite extensive research on functionalized triazoles with P, Se donors, their functionalization with tetrylene moieties remains unexplored.14 Incorporating heteroaryl groups with donor atoms like nitrogen, oxygen, or sulfur near tetrylene moieties can create versatile ligand systems with rich coordination chemistry, suitable for applications in homogeneous catalysis, molecular switches, logic gates, and sensors. We envisioned that the triazole functionalized E2 systems would not only provide a versatile ligand system but might also bring the E(II) atoms in close proximity to afford versatile mixed-valent E–E bonded compounds. Herein, we report the reaction of dilithiated triazole with amidinato tetrylenes [PhC(NtBu)2ECl] (E = Si and Ge) resulting in the formation of a rare Si(I)–Si(III) bonded compound 1 and a bis(germylene) compound 2, respectively.
The reaction of 1-(2-bromophenyl)-4-phenyl-1H-1,2,3-triazole14b with two equivalents of nBuLi at −80 °C in diethyl ether followed by treatment with two equivalents of [(PhC(tBuN)2SiCl)]2b resulted in the formation of a rare mixed valent Si(I)–Si(III) compound 1 as dark yellow crystalline solid in good yield, instead of the anticipated bis(silylene) compound 1′ (Scheme 1).14c,d Whereas, the reaction of 1-(2-bromophenyl)-4-phenyl-1H-1,2,3-triazole with two equivalents of nBuLi followed by treatment with two equivalents of amidinato germylene chloride [(PhC(tBuN)2GeCl)]3 afforded triazole fused bis(germylene) compound 2 as a colorless solid in good yield (Scheme 1). Both compounds were thoroughly characterized using various spectroscopic techniques and are highly stable in both solution and solid states for months without decomposition when stored under argon. The 29Si NMR spectrum of 1 in C6D6 showed two singlet resonances at 46.52 and −94.22 ppm, corresponding to Si(I) and Si(III) atoms (see ESI,† Fig. S3). The 29Si NMR resonance for the Si(I) atom in 1 is downfield shifted compared to the same in silaiminyl–silylene VI (δ = 31.8 ppm for Si(I) atom)9 and upfield shifted compared to [(PhC(tBuN)2Si)]2 (δ = 76.29 ppm).15 This suggests that the Si(III) fragment of silaiminyl–silylene VI is a better donor than the triazole and amidinate stabilized Si(III) fragment in 1, resulting in a downfield shift in the 29Si NMR spectrum of Si(I) atom in 1. The 1H NMR spectrum of 1 showed three distinct singlet resonances at 0.68, 0.78 and 1.22 ppm for tBu protons with an integral ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
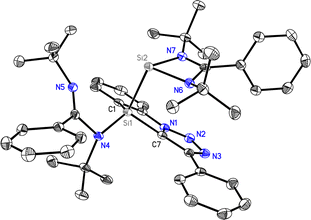
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, suggesting the formation of 1. Further, LIFDI mass spectrometry confirmed the molecular composition, which showed [M + H]+ molecular ion mass at 738.3. The 1H NMR spectrum of 2 suggested a bis(germylene) compound formation with two distinct singlet resonances for the tBu protons of the amidinate at 0.96 and 1.10 ppm. The aromatic protons appeared in the range of 6.99 to 8.32 ppm. Further, the high-resolution mass spectrometry showed a molecular ion peak at 828.3039 for [M + H]+ ion (calcd 828.3040), and the molecular structure was established using X-ray diffraction study.16 The molecular structures of 1 and 2 are shown in Fig. 2 and 3, and the selected bond lengths and bond angles are listed in the captions of the figure.
2, suggesting the formation of 1. Further, LIFDI mass spectrometry confirmed the molecular composition, which showed [M + H]+ molecular ion mass at 738.3. The 1H NMR spectrum of 2 suggested a bis(germylene) compound formation with two distinct singlet resonances for the tBu protons of the amidinate at 0.96 and 1.10 ppm. The aromatic protons appeared in the range of 6.99 to 8.32 ppm. Further, the high-resolution mass spectrometry showed a molecular ion peak at 828.3039 for [M + H]+ ion (calcd 828.3040), and the molecular structure was established using X-ray diffraction study.16 The molecular structures of 1 and 2 are shown in Fig. 2 and 3, and the selected bond lengths and bond angles are listed in the captions of the figure.
Compounds 1 and 2 crystallized in the monoclinic P21/n and triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space groups, respectively. The molecular structure of 1 confirms the formation of a rare Si(I)–Si(III) bonded mixed valent compound with a Si1–Si2 bond length of 2.4458(8) Å (Fig. 2). The Si(I) atom has a distorted trigonal pyramidal geometry with an amidinate ligand occupying two sites and the lone pair of electrons residing on the apex. The Si(III) atom has a four-coordinate tetrahedral arrangement with a bis-chelate triazole core occupying two coordination sites, forming a five-membered heterocyclic ring. The N and Si atoms of amidinate and silylene occupied the other two sites of the tetrahedron. The sum of the bond angles around the Si(1) atom (270.38°) and the angle between the centroid of the CN2Si plane and the Si(I)–Si(III) bond (104.2°) suggest the stronger s-character of the lone pair at Si(1). The Si1–Si2 bond length of 2.4458(8) Å in 1 is longer than the Si–Si bond length in interconnected amidinato bis(silylene) [PhC(NtBu)2Si]2 [2.413(2) Å], bis(silylene) VIII with Si(II)–Si(IV) bond distances of 2.4212(8) Å and 2.4157(7) Å and is slightly shorter in bis(silylene)silole IX with Si(II)–Si(IV) bond distances of 2.4590(6) and 2.4635(5) Å.12,13,15 The molecular structure of compound 2 reveals that the Ge atoms adopt a distorted trigonal pyramidal geometry, with bond angles summing to 268.63° (Ge1) and 258.1° (Ge2). A Ge⋯Ge distance of 4.205 Å in 2 also rules out any significant bonding interaction, which confirms the formation of a spacer-separated bis(germylene) compound (Fig. 3). Interestingly, the distances between Ge1⋯Ge2 and Ge1⋯N6 atoms are 4.205 and 3.355 Å, suggesting the bis(germylene) compounds might serve as chelating multidentate ligands (see ESI,† Fig. S9).
space groups, respectively. The molecular structure of 1 confirms the formation of a rare Si(I)–Si(III) bonded mixed valent compound with a Si1–Si2 bond length of 2.4458(8) Å (Fig. 2). The Si(I) atom has a distorted trigonal pyramidal geometry with an amidinate ligand occupying two sites and the lone pair of electrons residing on the apex. The Si(III) atom has a four-coordinate tetrahedral arrangement with a bis-chelate triazole core occupying two coordination sites, forming a five-membered heterocyclic ring. The N and Si atoms of amidinate and silylene occupied the other two sites of the tetrahedron. The sum of the bond angles around the Si(1) atom (270.38°) and the angle between the centroid of the CN2Si plane and the Si(I)–Si(III) bond (104.2°) suggest the stronger s-character of the lone pair at Si(1). The Si1–Si2 bond length of 2.4458(8) Å in 1 is longer than the Si–Si bond length in interconnected amidinato bis(silylene) [PhC(NtBu)2Si]2 [2.413(2) Å], bis(silylene) VIII with Si(II)–Si(IV) bond distances of 2.4212(8) Å and 2.4157(7) Å and is slightly shorter in bis(silylene)silole IX with Si(II)–Si(IV) bond distances of 2.4590(6) and 2.4635(5) Å.12,13,15 The molecular structure of compound 2 reveals that the Ge atoms adopt a distorted trigonal pyramidal geometry, with bond angles summing to 268.63° (Ge1) and 258.1° (Ge2). A Ge⋯Ge distance of 4.205 Å in 2 also rules out any significant bonding interaction, which confirms the formation of a spacer-separated bis(germylene) compound (Fig. 3). Interestingly, the distances between Ge1⋯Ge2 and Ge1⋯N6 atoms are 4.205 and 3.355 Å, suggesting the bis(germylene) compounds might serve as chelating multidentate ligands (see ESI,† Fig. S9).
We gained insight into the structural, bonding, and difference in reactivity aspects for both 1 and 2 through quantum chemical Density Functional Theory (DFT) calculations (please see ESI† for computational details). We hypothesize that the reaction of the dilithiated triazole with amidinato-silylene/germylene chlorides results in bis(silylene) (1′) or bis-germylene (2) (Fig. 4). However, we could not isolate bis(silylene) 1′ and always obtained a mixed-valent Si(I)–Si(III) bonded compound 1. The molecular orbital analysis (Fig. 4 and Fig. S12, ESI†) indicates that the highest occupied molecular orbital (HOMO) of bis-silylene (1′)/bis-germylene (2) are lone pair orbitals on the Group-14 element connected with the phenyl ring. Since the s-character of lone pair orbital on the germanium centre in 2 is more as compared to that of the silicon centre in 1′, the latter one is more reactive. This is corroborated by the eigenvalue of HOMO of 2 (−5.58 eV) and 1′ (−4.39 eV). On the other hand, the lowest unoccupied orbitals (LUMO) are the E–N σ* orbital in conjugation with the π-orbital of the triazole ring, which is slightly more stabilized for 1′ (−1.33 eV) as compared to that of 2 (−1.15 eV). Consequently, the intramolecular rearrangement by the coordination of lone pair orbital of heavier Group-14 centre connected to the phenyl ring with the E–N σ* orbital of the other Group-14 centre connected to the triazole ring leads to disilene int-1′-1 and digermene int-2-2′. Since the silicon lone pair and the Si−N σ* orbital is more reactive as compared to the germanium lone pair and the Ge–N σ* orbital, the formation of int-1′-1 (−10.5 kcal mol−1) is exergonic and the formation of int-2-2′ is endergonic (4.2 kcal mol−1). Note that Driess and coworkers isolated a similar derivative of disilene int-1′-1.6 The HOMO of the disilene int-1′-1 and the digermene int-2-2′ are pseudo-π-MO formed by the overlap of E−N σ* orbitals of tetrylene fragments having pseudo-π-symmetry. These pseudo-π-MOs (−4.05 eV for int-1′-1 and −5.15 eV for int-2-2′) lie higher in energy than the lone pair orbitals of 1′ and 2. The phenyl carbon bonded to the heavier Group-14 centre is more electrophilic (q = 0.05 e) than the triazole carbon (q = −0.29 e) bonded to the other heavier Group-14 centre. Hence, the pseudo-π-MO is susceptible to donate into the Si–C σ*-orbital leading to the migration of the phenyl ring accompanied by Si–N bond cleavage. The higher stability of 1 and 2 can be attributed to the higher stability of the lone pair compared to the pseudo-π-MO.
 | ||
| Fig. 4 Calculated reaction energetics for the formation of products from triazole as shown in Scheme 1 and the important molecular orbitals – (a) HOMO and LUMO of 1′, (b) HOMO and LUMO of 2, (c) HOMO of int-1′-1, (d) HOMO of int-2-2′. The calculations are carried out at M06/def2-TZVPP//BP86-D3(BJ)/def2-SVP level of theory. Surfaces are plotted at the iso-surface value of 0.03. | ||
Using Ahlrichs–Heinzmann population analysis,17 we found two types of Si atoms (Si(III) and Si(I)), with partial charge of 0.25 and 0.08, respectively. To better understand the Si–Si interaction in 1, we carried out natural bonding orbitals (NBO)18 and quantum theory of atoms in molecules (QTAIM)19 analysis. NBO-based bonding orbital shows a strong interaction between Si1(3s0.333pz0.67)0.61 and Si2(3s0.153pz0.85)0.39, with an occupancy of 1.79 (Fig. S14c, ESI†). Further, Wiberg bond index (WBI) calculation,20 which gives information about the bond order between two questioned atoms, suggests a bond between both Si atoms (0.83). The molecular graph plotted from DFT-QTAIM analysis displays a bond critical point (3,−1) between Si–Si with an electron density of ρ = 0.0771e Bohr−3, Laplacian of electron density (∇2ρ(r)) −0.1078e Bohr−5 and energy density (H(r)) −0.3501e Bohr−3 (Fig. S14d and e, ESI†).21 The large value of ρ and negative values for both ∇2ρ(r) and H(r) suggest a strong covalent bond between Si1⋯Si2. Additionally, the localized orbital locator (LOL) graph (Fig. S14f, ESI†) highlights a highly localized region between both Si centres, indicating the presence of the Si–Si bond.
In conclusion, we successfully synthesized and characterized two novel compounds: a rare mixed-valent Si(I)–Si(III) compound (1) and a bis(germylene) compound (2). In compound 2, the long interatomic distances (Ge1⋯Ge2 = 4.205 Å and Ge1⋯N6 = 3.355 Å) indicate the absence of significant Ge⋯Ge interaction, which maybe suggest its potential as a versatile multidentate ligand in coordination chemistry.
M. K. P.: conceptualization, data curation, formal analysis, investigation, writing – original draft, review & editing, visualization, Z. H.: data curation, formal analysis, writing – review & editing, X. W.: single-crystal measurement, S. M.: DFT calculations, writing – review & editing, A. K.: writing – review & editing, M. K. S.: NBO and QTAIM analysis, R. H.-I.: supervision, D. S.: supervision, writing – review & editing, P. P.: supervision, writing – review & editing, H. W. R.: writing – review & editing, supervision, funding acquisition.
Data availability
The NMR, mass and DFT calculation data that support the findings of this work have been included in the ESI.† Crystallographic data for 1 and 2 has been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition number 2376682 (1) and 2376683 (2) and can be obtained from https://www.ccdc.cam.ac.uk/structures/.Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) S. Nagendran and H. W. Roesky, Organometallics, 2008, 27, 457–492 CAS; (b) S. S. Sen, S. Khan, P. P. Samuel and H. W. Roesky, Chem. Sci., 2012, 3, 659–682 CAS; (c) L. Wang, Y. Li, Z. Li and M. Kira, Coord. Chem. Rev., 2022, 457, 214413 CAS; (d) Y. Mizuhata, T. Sasamori and N. Tokitoh, Chem. Rev., 2009, 109, 3479–3511 CAS; (e) M. S. Nechaev, Organometallics, 2021, 40, 3408–3423 CAS.
- (a) C.-W. So, H. W. Roesky, J. Magull and R. B. Oswald, Angew. Chem., Int. Ed., 2006, 45, 3948–3950 CAS; (b) S. S. Sen, H. W. Roesky, D. Stern, J. Henn and D. Stalke, J. Am. Chem. Soc., 2010, 132, 1123–1126 CAS.
- S. Nagendran, S. S. Sen, H. W. Roesky, D. Koley, H. Grubmüller, A. Pal and R. Herbst-Irmer, Organometallics, 2008, 27, 5459–5463 CrossRef CAS.
- (a) J. A. Cabeza and P. García-Álvarez, Chem. – Eur. J., 2024, 30, e202400786 CrossRef CAS PubMed; (b) Z. Hendi, M. K. Pandey, K. Rachuy, M. K. Singh, R. Herbst-Irmer, D. Stalke and H. W. Roesky, Chem. – Eur. J., 2024, 30, e202400389 CrossRef CAS PubMed; (c) M. K. Pandey, Z. Hendi, X. Wang, A. Bhandari, M. K. Singh, K. Rachuy, S. Kumar Kushvaha, R. Herbst-Irmer, D. Stalke and H. W. Roesky, Angew. Chem., Int. Ed., 2024, 63, e202317416 CrossRef CAS PubMed.
- (a) B. Blom, M. Stoelzel and M. Driess, Chem. – Eur. J., 2013, 19, 40–62 CrossRef CAS PubMed; (b) B. Blom, D. Gallego and M. Driess, Inorg. Chem. Front., 2014, 1, 134–148 RSC; (c) Z. Hendi, M. K. Pandey, S. K. Kushvaha and H. W. Roesky, Chem. Commun., 2024, 60, 9483–9512 RSC; (d) C. Shan, S. Yao and M. Driess, Chem. Soc. Rev., 2020, 49, 6733–6754 CAS; (e) M. Ghosh and S. Khan, Dalton Trans., 2021, 50, 10674–10688 CAS.
- A. Kostenko and M. Driess, J. Am. Chem. Soc., 2018, 140, 16962–16966 CAS.
- (a) A. V. Protchenko, A. D. Schwarz, M. P. Blake, C. Jones, N. Kaltsoyannis, P. Mountford and S. Aldridge, Angew. Chem., Int. Ed., 2013, 52, 568–571 CAS; (b) M. M. D. Roy, M. J. Ferguson, R. McDonald, Y. Zhou and E. Rivard, Chem. Sci., 2019, 10, 6476–6481 CAS; (c) D. Reiter, R. Holzner, A. Porzelt, P. J. Altmann, P. Frisch and S. Inoue, J. Am. Chem. Soc., 2019, 141, 13536–13546 CAS.
- S.-H. Zhang, H.-W. Xi, K. H. Lim, Q. Meng, M.-B. Huang and C.-W. So, Chem. – Eur. J., 2012, 18, 4258–4263 CrossRef CAS PubMed.
- R. Yadav, X. Sun, R. Köppe, M. T. Gamer, F. Weigend and P. W. Roesky, Angew. Chem., Int. Ed., 2022, 61, e202211115 CrossRef CAS PubMed.
- M. K. Bisai, V. S. V. S. N. Swamy, T. Das, K. Vanka, R. G. Gonnade and S. S. Sen, Inorg. Chem., 2019, 58, 10536–10542 CrossRef CAS PubMed.
- M. K. Bisai, T. Das, K. Vanka, R. G. Gonnade and S. S. Sen, Angew. Chem., Int. Ed., 2021, 60, 20706–20710 CrossRef CAS PubMed.
- (a) S. K. Kushvaha, P. Kallenbach, S. M. N. V. T. Gorantla, R. Herbst-Irmer, D. Stalke and H. W. Roesky, Chem. – Eur. J., 2024, 30, e202303113 CrossRef CAS PubMed; (b) S. Yao, M. S. Budde, X. Yang, Y. Xiong, L. Zhao and M. Driess, Angew. Chem., Int. Ed., 2024, e202414696 Search PubMed.
- C. Liu, M. Schmidtmann and T. Müller, Dalton Trans., 2024, 53, 10446–10452 RSC.
- (a) D. Mondal and M. S. Balakrishna, Eur. J. Inorg. Chem., 2020, 2392–2402 CrossRef CAS; (b) B. Choubey, L. Radhakrishna, J. T. Mague and M. S. Balakrishna, Inorg. Chem., 2016, 55, 8514–8526 CrossRef CAS PubMed; (c) F. E. Bugden, G. J. Clarkson and M. D. Greenhalgh, Eur. J. Org. Chem., 2023, e202201459 CrossRef CAS; (d) L. Radhakrishna, M. K. Pandey and M. S. Balakrishna, RSC Adv., 2018, 8, 25704–25718 RSC.
- S. S. Sen, A. Jana, H. W. Roesky and C. Schulzke, Angew. Chem., Int. Ed., 2009, 48, 8536–8538 CrossRef CAS PubMed.
- (a) Bruker AXS Inc., Madison 2016; (b) L. Krause, R. Herbst-Irmer, G. M. Sheldrick and D. Stalke, J. Appl. Crystallogr., 2015, 48, 3–10 Search PubMed; (c) G. M. Sheldrick, Acta Crystallogr., 2015, A71, 3–8 Search PubMed; (d) G. M. Sheldrick, Acta Crystallogr., 2015, C71, 3–8 Search PubMed; (e) C. B. Hübschle, G. M. Sheldrick and B. Dittrich, J. Appl. Crystallogr., 2011, 44, 1281–1284 CrossRef PubMed , CCDC no. 2376682 (1) and 2376683 (2)†.
- (a) E. R. Davidson, J. Chem. Phys., 1967, 46, 3320–3324 CrossRef CAS; (b) K. R. Roby, Mol. Phys., 1974, 27, 789–807 Search PubMed; (c) R. Heinzmann and R. Ahlrichs, Theoret. Chim. Acta, 1976, 42, 33–45 CrossRef CAS; (d) C. Erhardt and R. Ahlrichs, Theoret. Chim. Acta, 1985, 68, 231–245 CrossRef.
- J. P. Foster and F. Weinhold, J. Am. Chem. Soc., 1980, 102, 7211–7218 CAS.
- (a) T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed; (b) H. L. Schmider and A. D. Becke, J. Mol. Struc-THEOCHEM, 2000, 527, 51–61 CrossRef CAS.
- (a) K. B. Wiberg, Tetrahedron, 1968, 24, 1083–1096 CrossRef CAS; (b) L. K. Harper, A. L. Shoaf and C. A. Bayse, ChemPhysChem, 2015, 16, 3886–3892 CrossRef CAS PubMed; (c) I. Mayer, J. Comput. Chem., 2007, 28, 204–221 CrossRef CAS PubMed.
- (a) D. Kratzert, D. Leusser, J. J. Holstein, B. Dittrich, K. Abersfelder, D. Scheschkewitz and D. Stalke, Angew. Chem., Int. Ed., 2013, 52, 4478–4482 CrossRef CAS PubMed; (b) B. Niepötter and D. Stalke, in Organosilicon Compounds, ed. V. Y. Lee, Academic Press, 2017, pp. 3–58 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and spectral data are available. CCDC 2376682 and 2376683. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc06247g |
| This journal is © The Royal Society of Chemistry 2025 |