Open Access Article
Open Access ArticleSolution triggered facile ion-exchange and phase transformation of ternary cesium-copper halide pseudo-perovskites†
Cintia Hajdua,
Tamás Sándor Zsigmonda,
Bence Kutus c,
Ditta Ungorab,
Edit Csapóab,
Csaba Janáky
c,
Ditta Ungorab,
Edit Csapóab,
Csaba Janáky *a and
Gergely Ferenc Samu
*a and
Gergely Ferenc Samu *c
*c
aDepartment of Physical Chemistry and Materials Science, Interdisciplinary Excellence Centre, University of Szeged, Aradi Square 1, Szeged, H-6720, Hungary. E-mail: janaky@chem.u-szeged.hu
bMTA-SZTE Lendület “Momentum” Noble Metal Nanostructures Research Group, University of Szeged, Rerrich Béla Square 1, Szeged, H-6720, Hungary
cDepartment of Molecular and Analytical Chemistry, University of Szeged, Dóm Square 7-8, Szeged, H-6721, Hungary. E-mail: samugf@chem.u-szeged.hu
First published on 28th January 2025
Abstract
Ternary cesium-copper halide pseudo-perovskites are an emerging class of semiconductors in the field of optoelectronics. Similarly to metal-halide perovskites, by controlling the halide-composition, their emission properties can be fine-tuned. Here, a post-synthetic halide exchange method was employed to alter the halide-composition and thus the emission properties of polycrystalline Cs3Cu2Br5 layers.
The crystal structure of Cs3Cu2X5 and CsCu2X3 (X = Br, I, Cl) pseudo-perovskites consists of anionic metal-halides with intercalated monovalent cations.1 They have gained significant attention as light-emitting diodes,2–4 photodetectors,5,6 charged-particle or X-ray scintillators,7,8 and even as anti-counterfeiting materials.9 These compounds have the advantages of good thermal and chemical stability,10 and low toxicity,11 coupled with simple and cost-effective preparation methods.12 Their soft lattice can easily undergo lattice distortions after excitation,13 which results in the formation of self-trapped exciton (STE) states. Light emission through STE states is shifted to notably longer wavelengths compared to the absorption onset (large Stokes shift), eliminating reabsorption completely.14,15 Furthermore, the large detrapping barrier bestows high quantum efficiencies to STE emission processes.15
Composition engineering targeting the halide content can be used to fine-tune the light emission properties (e.g., Stokes shift, photoluminescence quantum yield and lifetime) of pseudo-perovskites.16–24 This is the result of the large contribution of the np orbital of the halide anions to the band structure.12,18
The halide composition of metal halide perovskites can be fine-tuned either during synthesis25 or by post-synthetic halide exchange methods.24,26,27 The former method additionally influences the crystallization kinetics28 of the films (even without halide incorporation)29 or passivates surface trap states30 impacting device properties. The latter approach can either preserve the original crystal structure of the starting material21 or result in a different crystal structure, which can give further control over the optoelectronic properties.23 The exchange can be performed in the gas,31 liquid22–24,32 or solid phase,33 but in the latter case achieving complete exchange is difficult and often poorly controllable.13 In the case of films, the use of harsh reaction conditions (e.g., elevated temperatures >70 °C), and long reaction times (few days) are often necessary to achieve complete exchange. In one example, bromide-to-iodide halide exchange was studied in a 75 nm thick CsPbBr3 film, at 75 °C for 40 minutes. If the layer thickness was increased to 350 nm, the solution temperature had to be increased to 120 °C, and the immersion time extended to 480 min.34 Mechanistic studies revealed that the exchange is kinetically driven by ion diffusion within the layers and the surrounding environment.13,35,36 From the layer side, the presence of crystal defects plays a key role in the halide exchange process, as they govern ion migration pathways within the material, and reduce the activation energy of diffusion. Similarly, temperature also plays a role according to the Arrhenius equation.13,37 Applying electrical bias can facilitate ion migration38,39 and the created vacancies can further accelerate this process. From the environment (solution) side, the difference in halide solubility has a detrimental effect on the rate of halide exchange.13,40
So far, halide exchange in pseudo-perovskites is an unexplored area. To the best of our knowledge, only Cl− to I− post-synthetic halide exchange has been demonstrated on Cs3Cu2Cl5 single crystals.41 The process was performed with hydrogen-iodide injection at 60 °C in a phosphorous acid containing ethanol solution. Additionally, a 0D–1D phase transition (Cs3Cu2I5–CsCu2I3) was shown for these materials by immersing them in solvents with different polarity.9,42,43
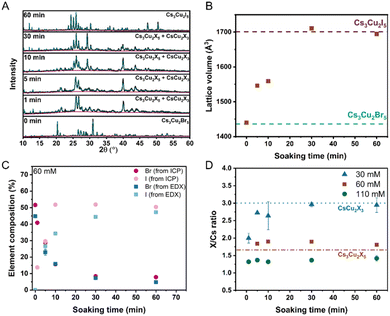
In this work, we studied the bromide-to-iodide halide exchange process in a Cs3Cu2Br5 polycrystalline film, with 10 μm thickness (details of the preparation can be found in the ESI†). We performed the halide exchange in the liquid phase, by immersing the Cs3Cu2Br5 layers in CsI-containing methanol solutions at room temperature (Fig. S1, ESI†). The effect of the CsI concentration and soaking time on the crystal structure, elemental composition, and luminescence properties was studied. XRD (X-ray diffraction) patterns were recorded to monitor the effect of halide exchange on the crystal structure of the Cs3Cu2Br5 layers (Fig. 1A and Fig. S2, ESI†). After soaking the layers in 60 mM CsI solution (Fig. 1A), a complex transformation occurred. The emergence of new reflections, and shifts of existing reflections to lower 2 theta values were both observed. Le Bail fitting44 of the XRD patterns revealed that after 5–30 min of soaking, two crystal forms were coexisting. Apart from mixed Cs3Cu2Br5−xIx (Pnma) phases, the emergence of CsCu2X3 (Cmcm) could be observed. After 60 min of immersion the transformation of the film was complete, and only reflections from the Cs3Cu2I5 phase could be detected. The individual lattice parameters (Fig. S3A, ESI†) and unit cell volume (Fig. 1B) of the halide-exchanged Cs3Cu2Br5−xIx layers were extracted from the fits of the patterns. The gradual increase in the lattice volume over time suggests the exchange of the smaller bromide (r = 196 pm) with larger iodide (r = 220 pm) in the lattice. The determined lattice volume (Fig. 1B) stabilized after 30 min, which signals the completion of the halide exchange process (at least in the probing depth of the XRD measurements). These results were also corroborated by the composition analysis of the exchanged layers both with ICP-MS (inductively coupled plasma mass spectrometry) (Fig. 1C and Fig. S4, Tables S1–S3, ESI†) and EDX (energy dispersive X-ray spectroscopy) (Fig. 1C and Table S4, ESI†) measurements. After 30 min almost all bromide was exchanged to iodide in the layers. Interestingly, in the case of 30 mM CsI solutions, the XRD patterns (Fig. S2A, ESI†) revealed a slower transformation of the soaked layers, which stopped at the formation of iodide-rich Cs3Cu2X3 films. In stark contrast, when using 110 mM (saturated) CsI solutions, the crystal structure of the starting Cs3Cu2Br5 was retained (Fig. S2B, ESI†) and no lattice volume change was observed (Fig. S5A, ESI†), while the formation of Cs3Cu2I5 phase was prevalent throughout the experiment as corroborated by ICP-MS and EDX measurements (Tables S3 and S6, ESI†). In the case of the 30 mM CsI solution, the iodide incorporation, at the expense of bromide, was confirmed (Tables S1 and S5, ESI†). Interestingly, a lower iodide content was achieved with the 110 mM CsI soaking solution (Tables S3 and S6, ESI†). This, together with the XRD results, points toward the formation of a layered structure, where iodide incorporation is confined to the outmost regions of the films. Detailed discussion of the ICP-MS results can be found in the ESI.†
To quantify the change in the surface halide composition of the layers, we calculated the overall halide/cesium ratio of the films (Fig. 1D) from EDX. When concentrated solutions (60 mM and 110 mM) were used, the halide/Cs ratio retained its value (which was close to the expected 1.67 value in Cs3Cu2X5 compounds). In the case of the dilute 30 mM CsI solution, the halide/Cs ratio shifted to higher values (close to the expected 3.0 value in the CsCu2X3 compounds). These results are in line with XRD measurements, supporting the change of crystal structure in the 30 mM CsI case.
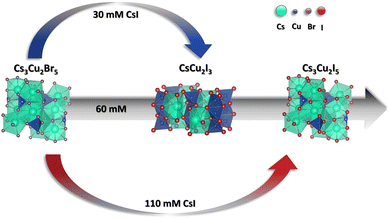
The schematic representation of the proposed mechanism of the halide exchange process is summarized in Fig. 2. In dilute CsI solution the halide exchange is coupled with a 0D–1D phase transition between Cs3Cu2Br5 and Cs3Cu2I3. The reason for this compositional/structural change can be the CsBr leaching from the layers to the solution phase (as revealed by ICP-MS). This is supported by the negligible weight loss of the films after immersion in the 30 mM CsI solution (Table S7, ESI†). As the iodide/bromide exchange should increase the weight of the films, a slight dissolution seems necessary to induce the 0D–1D transformation process. When a more concentrated CsI soaking solution is employed, the transition can progress gradually through a mixed phase of CsCu2I3 and Cs3Cu2I5 to phase pure Cs3Cu2I5. In contrast, when saturated CsI solution is used, no phase change occurs, however examining the unit cell dimensions, Cs3Cu2Br5 and surface Cs3Cu2I5 can be identified.
To observe the morphological changes accompanying the halide exchange process, top-down SEM (scanning electron microscopy) images were recorded. Before the soaking procedure, granular Cs3Cu2Br5 layers were observed (Fig. S6A, ESI†). Upon immersion in 60 mM exchange solution for 10 minutes of soaking time, a mixture of grains and rods can be observed (Fig. S6B, ESI†). The rod-like morphology is characteristic of the 1D CsCu2X3 phase,12 signalling that a mixed Cs3Cu2Br5−xIx/CsCu2I3 composition was obtained. After prolonged soaking time, the rods disappeared from the surface and only grains can be observed (Fig. S7, ESI†). In the case of the less concentrated CsI solution (30 mM), only rods were present (Fig. S6C, ESI†), throughout the entirety of the process (Fig. S7, ESI†). Interestingly, by examining the stack of rods, the original grains of the parent layers can still be recognized. In a similar fashion, when the saturated 110 mM CsI solution was used, only the surface of the layers was exchanged, preserving the granular structure of the parent Cs3Cu2X5 material (Fig. S6D and S7, ESI†).
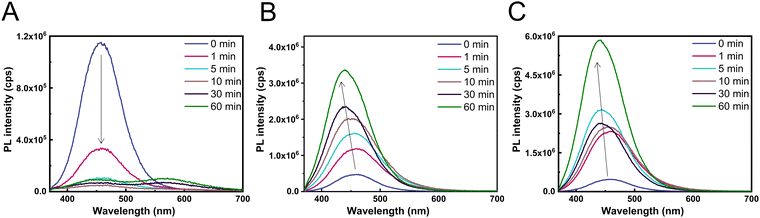
To probe the effect of the halide exchange process on the light emission properties of the layers, steady state PL (photoluminescence) spectra were recorded (Fig. 3). The initial Cs3Cu2Br5 layers had a PL peak maximum position of 460 nm and a PLQY of ∼14 ± 3%. After the layers were immersed in the 30 mM CsI solution, a rapid decrease of the PL intensity could be observed (Fig. 3A) at the characteristic wavelength of the parent Cs3Cu2Br5. This was accompanied with an increase in PL intensity at 570 nm, which corresponds to the formation of CsCu2I3. The difference in the PL intensity is the result of the low PLQY of the forming CsCu2I3 (∼2.2%). In stark contrast, when performing the halide exchange with concentrated exchange solutions (60 mM and 110 mM) a rapid increase in the PL intensity (Fig. 3B and C) could be observed, together with a shift of the PL maximum to lower wavelengths (∼440 nm), characteristic of the formation of Cs3Cu2I5 (high PLQY ∼72%).
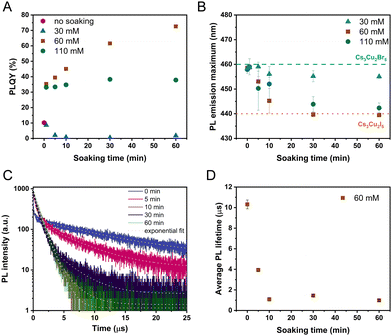
To quantify the change in the light emission properties, we integrated the PL peaks (Fig. S8, ESI†) and determined the absolute PLQY (Fig. 4A and Fig. S9, ESI†) of the layers. Both measurements revealed that the highest PLQY (73%) belongs to the samples treated with the 60 mM soaking solution, where Cs3Cu2I5 was obtained (deduced from materials characterization). This value is comparable to a pure Cs3Cu2I5 reference layer (78%), prepared by the spray-coating method. In a similar manner, the halide exchange carried out in the saturated 110 mM CsI solution improves the PLQY to 38%. Interestingly, the initial period of soaking (1 minute) in the concentrated solutions (60 mM and 110 mM) preserves the peak position of Cs3Cu2Br5 (Fig. 4B), while drastically increasing the PLQY to ∼35%. One possible reason can be the trap state passivation of the Cs3Cu2Br5 layer by the surface exchange to Cs3Cu2I5. Ultimately, however, the PL maximum shifts to 440 nm in these cases as seen in Fig. 4B. The halide exchange process influences the decay of the PL response as well (Fig. 4C). We fitted the decay traces by a multiexponential function (Table S8, ESI†) and calculated the average PL lifetime (Fig. 4D, Table S9, ESI†), which is independent of the model used. This approach is justified by the multiphasic nature of the samples, which makes identifying separate processes unreliable. The initial Cs3Cu2Br5 layers have the longest average lifetime (10.7 ± 0.2 μs). After performing the halide exchange in the 60 mM CsI solution, the decay of the PL signal gradually accelerates. The shortest lifetime of 1.13 ± 0.02 μs was determined for the sample after 60 min soaking. This value is in good correlation with literature data for phase pure Cs3Cu2I5 further signalling complete halide exchange.
To summarize our work, we successfully prepared Cs3Cu2Br5 pseudo-perovskite layers and demonstrated bromide/iodide exchange with a simple room temperature solution phase approach. Depending on the concentration of the CsI exchange solution, both simple halide exchange and a 0D–1D transition were achieved. The halide exchange could be either complete or confined to the Cs3Cu2Br5 surface. In all cases, the formed Cs3Cu2I5 improved the PLQY of the layers compared to the parent Cs3Cu2Br5. When full halide exchange was performed, a high PLQY of 73% was achieved, which is comparable to a reference Cs3Cu2I5 sample. The halide exchange has a long-lasting effect as the increased PLQY still persists even after 1 year of storage (Table S10, ESI†). As an outlook this simple, fast method could be used to improve and precisely control the optoelectronic properties of Cs3Cu2Br5 layers. Notably, the method can form layered structures that could be used in different light/radiation detection applications.
The authors thank Ádám Balog for SEM/EDX measurements. This work was supported by TKP-2021-NVA-19 financed by the National Research, Development and Innovation Fund of the Ministry of Culture and Innovation of Hungary and by the National Research, Development and Innovation Office (NKFIH) through the FK-138888 project and the ERA-NET COFUND/EJP COFUND Program with co-funding from the European Union Horizon 2020 research and innovation program and the NKFIH (2019-2.1.7-ERA-NET-2021-00025).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. Nah, D. Solanki and D. H. Kim, Cell Rep. Phys. Sci., 2022, 3, 101171 CrossRef CAS.
- T. Jun, K. Sim, S. Iimura, M. Sasase, H. Kamioka, J. Kim and H. Hosono, Adv. Mater., 2018, 30, 1804547 CrossRef PubMed.
- L. Wang, Z. Shi, Z. Ma, D. Yang, F. Zhang, X. Ji, M. Wang, X. Chen, G. Na, S. Chen, D. Wu, Y. Zhang, X. Li, L. Zhang and C. Shan, Nano Lett., 2020, 20, 3568–3576 CrossRef CAS PubMed.
- X. Liu, Y. Yu, F. Yuan, C. Zhao, H. Dong, B. Jiao and Z. Wu, ACS Appl. Mater. Interfaces, 2020, 12, 52967–52975 CrossRef CAS PubMed.
- Z. X. Zhang, C. Li, Y. Lu, X. W. Tong, F. X. Liang, X. Y. Zhao, D. Wu, C. Xie and L. B. Luo, J. Phys. Chem. Lett., 2019, 10, 5343–5350 CrossRef CAS PubMed.
- Y. Li, Z. Shi, L. Wang, Y. Chen, W. Liang, D. Wu, X. Li, Y. Zhang, C. Shana and X. Fang, Mater. Horiz., 2020, 7, 1613–1622 RSC.
- M. Hunyadi, G. F. Samu, L. Csige, A. Csík, C. Buga and C. Janáky, Adv. Funct. Mater., 2022, 32, 2206645 CrossRef CAS.
- L. Lian, M. Zheng, W. Zhang, L. Yin, X. Du, P. Zhang, X. Zhang, J. Gao, D. Zhang, L. Gao, G. Niu, H. Song, R. Chen, X. Lan, J. Tang and J. Zhang, Adv. Sci., 2020, 7, 2000195 CrossRef CAS PubMed.
- X. Zhang, B. Zhou, X. Chen and W. W. Yu, Inorg. Chem., 2022, 61, 399–405 CrossRef CAS PubMed.
- F. Zeng, Y. Guo, W. Hu, Y. Tan, X. Zhang, J. Yang, Q. Lin, Y. Peng, X. Tang, Z. Liu and Z. Yao, J. Lumin., 2020, 223, 117178 CrossRef CAS.
- T. D. Creason, T. M. McWhorter, Z. Bell, M. H. Du and B. Saparov, Chem. Mater., 2020, 32, 6197–6205 CrossRef CAS.
- Z. Guo, J. Li, R. Pan, J. Cheng, R. Chen and T. He, Nanoscale, 2020, 12, 15560–15576 RSC.
- H. Jiang, S. Cui, Y. Chen and H. Zhong, Nano Sel., 2021, 2, 2040–2060 CrossRef CAS.
- S. Li, J. Luo, J. Liu and J. Tang, J. Phys. Chem. Lett., 2019, 10, 1999–2007 CrossRef CAS PubMed.
- B. Zhang, X. Wu, S. Zhou, G. Liang and Q. Hu, Front. Optoelectron., 2021, 14, 459–472 CrossRef PubMed.
- R. Roccanova, A. Yangui, H. Nhalil, H. Shi, M. H. Du and B. Saparov, ACS Appl. Electron. Mater., 2019, 1, 269–274 CrossRef CAS.
- G. F. Samu, T. S. Zsigmond, C. Hajdu, M. Hunyadi, L. Csige, A. Csík, J. Kopniczky, B. Hopp and C. Janáky, Adv. Opt. Mater., 2023, 11, 2300825 CrossRef CAS.
- J. S. Manser, J. A. Christians and P. V. Kamat, Chem. Rev., 2016, 116, 12956–13008 CrossRef CAS PubMed.
- D. Zhang, Y. Yang, Y. Bekenstein, Y. Yu, N. A. Gibson, A. B. Wong, S. W. Eaton, N. Kornienko, Q. Kong and M. Lai, J. Am. Chem. Soc., 2016, 138, 7236–7239 CrossRef CAS PubMed.
- L. Han, B. Sun, C. Guo, G. Peng, H. Chen, Z. Yang, N. Li, Z. Ci and Z. Jin, Adv. Opt. Mater., 2022, 10, 1–9 Search PubMed.
- G. Nedelcu, L. Protesescu, S. Yakunin, M. I. Bodnarchuk, M. J. Grotevent and M. V. Kovalenko, Nano Lett., 2015, 15, 5635–5640 CrossRef CAS PubMed.
- M. Imran, V. Caligiuri, M. Wang, L. Goldoni, M. Prato, R. Krahne, L. D. Trizio and L. Manna, J. Am. Chem. Soc., 2018, 140, 2656–2664 CrossRef CAS PubMed.
- N. Pellet, J. Teuscher, J. Maier and M. Grätzel, Chem. Mater., 2015, 27, 2181–2188 CrossRef CAS.
- Q. A. Akkerman, V. D’Innocenzo, S. Accornero, A. Scarpellini, A. Petrozza, M. Prato and L. Manna, J. Am. Chem. Soc., 2015, 137, 10276–10281 CrossRef CAS PubMed.
- S. Ye, M. Zhao, J. Song and J. Qu, Nano Res., 2018, 11, 4654–4663 CrossRef CAS.
- P. Ramasamy, D. H. Lim, B. Kim, S. H. Lee, M. S. Lee and J. S. Lee, Chem. Commun., 2016, 52, 2067–2070 RSC.
- X. He, P. Liu, S. Wu, Q. Liao, J. Yao and H. Fu, J. Mater. Chem. C, 2017, 5, 12707–12713 RSC.
- J. Kim, S. Park, Y. Lee, H. Hosono, B. Park and J. Kim, SusMat, 2023, 3, 821–833 CrossRef CAS.
- J. Qiu, et al., SusMat, 2023, 3, 894–908 CrossRef CAS.
- Y. Xia, M. Zhu, L. Qin, C. Zhao, D. Hong, Y. Tian, W. Yan and Z. Jin, Energy Mater., 2023, 3, 300004 CrossRef CAS.
- D. Solis-Ibarra, I. C. Smith and H. I. Karunadasa, Chem. Sci., 2015, 6, 4054–4059 RSC.
- V. Thampy and K. H. Stone, Inorg. Chem., 2020, 59, 13364–13370 CrossRef CAS PubMed.
- Y. Liu, F. Li, Q. Li, K. Yang, T. Guo, X. Li and H. Zeng, ACS Photonics, 2018, 5, 4504–4512 CrossRef CAS.
- J. B. Hoffman, A. L. Schleper and P. V. Kamat, J. Am. Chem. Soc., 2016, 138, 8603–8611 CrossRef CAS PubMed.
- C. Eames, J. M. Frost, P. R. F. Barnes, B. C. O’Regan, A. Walsh and M. S. Islam, Nat. Commun., 2015, 6, 2–9 Search PubMed.
- A. Walsh, D. O. Scanlon, S. Chen, X. G. Gong and S. Wei, Angew. Chem., 2015, 127, 1811–1814 CrossRef.
- P. V. Kamat and M. Kuno, Acc. Chem. Res., 2021, 54, 520–531 CrossRef CAS PubMed.
- G. F. Samu, A. Balog, F. De Angelis, D. Meggiolaro, P. V. Kamat and C. Janaky, J. Am. Chem. Soc., 2019, 141, 10812–10820 CrossRef CAS PubMed.
- Z. Xu, R. A. Kerner, J. J. Berry and B. P. Rand, Adv. Funct. Mater., 2022, 32, 2203432 CrossRef CAS.
- G. D. Moon, S. Ko, Y. Xia and U. Jeong, ACS Nano, 2010, 4, 2307–2319 CrossRef CAS PubMed.
- D. Shen, X. Wang, X. Zhang, Y. Liu, Y. Shi, X. Li, X. Chen and Y. Zhang, ACS Appl. Opt. Mater., 2023, 1, 435–441 CrossRef CAS.
- S. Liu, Y. Yue, X. Zhang, C. Wang, G. Yang and D. Zhu, J. Mater. Chem. C, 2020, 8, 8374–8379 RSC.
- W. Cui, J. Zhao, L. Wang, P. Lv, X. Li, Z. Yin, C. Yang and A. Tang, J. Phys. Chem. Lett., 2022, 13, 4856–4863 CrossRef CAS PubMed.
- B. H. Toby and R. B. Von Dreele, J. Appl. Crystallogr., 2013, 46, 544–549 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc06335j |
| This journal is © The Royal Society of Chemistry 2025 |