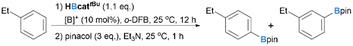Open Access Article
Open Access ArticleBorenium-catalysed para-selective borylation of alkylarenes†
Xinyue
Tan
 and
Huadong
Wang
and
Huadong
Wang
 *
*
Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Department of Chemistry, Fudan University, Songhu Road 2005, Shanghai, 200438, China. E-mail: huadongwang@fudan.edu.cn
First published on 20th February 2025
Abstract
A borenium-based catalytic system for para-selective borylation of mono-alkylbenzenes has been developed using 4-tert-butyl-catecholborane (HBcattBu) as the borylation reagent and (p-tol)OBcattBu as a Brønsted base additive. This study highlights the complementary selectivity of borenium-based systems compared to transition-metal catalysts and provides a straightforward approach to accessing para-selective arylboron compounds.
Arylboron compounds are synthetically versatile building blocks in both organic synthesis1,2 and materials chemistry.3 One of the most efficient ways to access arylboron compounds is catalytic C–H borylation of arenes, an area long dominated by transition-metal catalysts.4–8 Since the regioselectivity of transition-metal-based catalytic systems is largely determined by steric factors, mono-substituted arenes, in the absence of directing groups, typically afford a mixture of meta- and para-borylated products in statistical distribution.9–11 To overcome this limitation, taking advantage of ligand–substrate interactions, a number of strategies have been developed,8,12–14 in which iridium catalysts with elegantly designed ligands allow selective meta- or para-borylation of aryl C–H bonds. To ensure reasonable ligand–substrate interactions, arene substrates with steric bulky15–21 or heteroatom-containing substituents22–28 are generally required. For mono-alkyl arenes (such as ethylbenzene), there is only one example of catalytic regioselective C–H borylations known. Asako, Ilies, and co-workers employed a bulky spirobipyridine ligated Ir complex as a catalyst to selectively borylate meta-C–H bonds of toluene and ethylbenzene with meta/para ratios of 5.0
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 7.3
1 and 7.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively.17 Despite these advancements, catalytic para-selective C–H borylation of mono-alkyl arenes still remains an unmet challenge.29
1, respectively.17 Despite these advancements, catalytic para-selective C–H borylation of mono-alkyl arenes still remains an unmet challenge.29
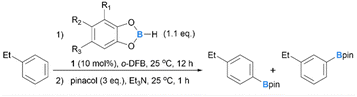
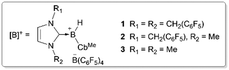
Main-group-element-catalysed electrophilic C–H borylation of arenes represents an alternative approach to access arylboron compounds.30–35 These metal-free systems typically proceed via a SEAr pathway with their regioselectivity determined by electronic factors, thus complementary to metal-based systems and offering a potential solution to regioselective C–H borylations of mono-alkyl arenes. Very recently, our group reported the C–H borylation of arenes using [IBnF-B(H)-CbMe][B(C6F5)4] (1, IBnF = 1,3-bis(2,3,4,5,6-pentafluorobenzyl)imidazol-2-ylidene, CbMe = 2-methyl-o-carboran-1-yl) as a catalyst with 4-chloro-catechol borane (HBcatCl) as a borylation reagent.36 While excellent para-selectivity was achieved for the arenes with strong electron-donating groups such as amino and phenoxyl groups, mono-alkyl arenes gave a roughly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of para- and meta-borylated products. In this study, we investigated how tuning the electronic factors of the borylation reagent, a borenium catalyst, and the addition of bases can enhance the para-selectivity of aromatic C–H borylations. We discovered that with a new borenium catalyst [IBnFMe-B(H)-CbMe][B(C6F5)4] (2, IBnFMe = 1-methyl-3-(2,3,4,5,6-pentafluorobenzyl)imidazol-2-ylidene), mono-alkyl arenes can be borylated with para/meta (p/m) ratios up to 10
1 mixture of para- and meta-borylated products. In this study, we investigated how tuning the electronic factors of the borylation reagent, a borenium catalyst, and the addition of bases can enhance the para-selectivity of aromatic C–H borylations. We discovered that with a new borenium catalyst [IBnFMe-B(H)-CbMe][B(C6F5)4] (2, IBnFMe = 1-methyl-3-(2,3,4,5,6-pentafluorobenzyl)imidazol-2-ylidene), mono-alkyl arenes can be borylated with para/meta (p/m) ratios up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using 4-tert-butyl-catechol borane (HBcattBu) as a borylation reagent and (p-tol)OBcattBu as an additive.
1 using 4-tert-butyl-catechol borane (HBcattBu) as a borylation reagent and (p-tol)OBcattBu as an additive.
Our previous work has shown that in the borenium 1 catalysed C–H borylation system,36 the B–H bond of HBcatCl is synergistically activated by the arene substrate and 1, leading to the formation of a boryl-substituted Wheland intermediate (WI) and a neutral N-heterocyclic carbene (NHC)-stabilised hydroborane (IBnF-B(H)2-CbMe, 1-H). Subsequently, the rate-determining deprotonation of WI with 1-H affords the borylation product and H2 accompanied by the regeneration of the borenium catalyst. Although a SEAr process involving mono-alkyl arenes typically favours electron-rich para-sites over meta-ones, the catalytic system based on 1 and HBcatCl showed a very moderate preference of the para-C–H bonds for the borylation of mono-alkyl arenes. For example, toluene, ethylbenzene, and cumene, gave the corresponding arylboronates with a roughly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of p/m-isomers. We speculated that the low regioselectivity could be due to facile 1,2-boryl migration prior to the product-determining deprotonation step (Scheme 1). A similar explanation was also invoked for the low p/m selectivity in the electrophilic borylation system reported by Ingleson and co-workers.37
1 mixture of p/m-isomers. We speculated that the low regioselectivity could be due to facile 1,2-boryl migration prior to the product-determining deprotonation step (Scheme 1). A similar explanation was also invoked for the low p/m selectivity in the electrophilic borylation system reported by Ingleson and co-workers.37
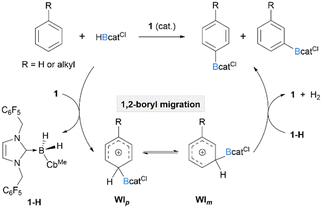 | ||
| Scheme 1 Proposed reaction pathway involving a 1,2-boryl migration ([B(C6F5)4]− counterion omitted for clarity). | ||
Since the intramolecular 1,2-boryl migration likely involves the interactions between the π orbitals of the arene substrates and the vacant p orbital of the boron centre, we hypothesized that reducing electrophilicity of the catechol-ligated boron centre could weaken such interaction, thus mitigating the undesired 1,2-boryl migration and improving the p/m ratios. Therefore, we started our investigation by exploring how tuning the electrophilicity of the borylation reagent would affect the regioselectivity. A series of borylation reagents with variant catechol ligands (HBcatR), including 3,5-di(tert-butyl)-catechol borane (HBcattBu2), HBcattBu, 4-methyl-catechol borane (HBcatMe), 4-fluoro-catechol borane (HBcatF), and HBcatCl, were synthesized from their corresponding catechol precursors (22 to 74% yield). Ethylbenzene was chosen as the model substrate with 1 (10 mol%) as the catalyst (Table 1). The moisture-sensitive BcatR moiety was converted to a Bpin moiety by treating with pinacol and Et3N after the reaction. The p/m ratio of Et-C6H4-Bpin was determined by 1H-NMR analysis. As we expected, the para-selectivity of ethylbenzene steadily increases with decreasing electrophilicity of the borylation reagent. Borylation reagents containing electron-withdrawing groups (EWGs), such as fluoro or chloro substituents, led to high efficiency yet poor regioselectivity (p/m = 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in both cases, Table 1, entries 1 and 2). The selectivity was marginally improved (p/m = 1.0
1 in both cases, Table 1, entries 1 and 2). The selectivity was marginally improved (p/m = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Table 1, entry 3) with moderately electron-donating methyl groups. When better electron-donating tert-butyl groups were introduced to the catechol ligand, the regioselectivity was improved further with p/m ratios of 1.1
1, Table 1, entry 3) with moderately electron-donating methyl groups. When better electron-donating tert-butyl groups were introduced to the catechol ligand, the regioselectivity was improved further with p/m ratios of 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for HBcattBu and 1.4
1 for HBcattBu and 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for HBcattBu2, respectively (Table 1, entries 4 and 5). However, the yield was low (29%) for HBcattBu2 which was probably due to the insufficient electrophilicity of BcattBu2 in the initial SEAr process. Therefore, HBcattBu was chosen as the borylation reagent for further optimizations.
1 for HBcattBu2, respectively (Table 1, entries 4 and 5). However, the yield was low (29%) for HBcattBu2 which was probably due to the insufficient electrophilicity of BcattBu2 in the initial SEAr process. Therefore, HBcattBu was chosen as the borylation reagent for further optimizations.
| Entry | R1 | R2 | R3 | Yield (%) | p/m ratio |
|---|---|---|---|---|---|
| 1 (ref. 36) | H | Cl | H | 86 | 0.8 |
| 2 | F | H | H | 92 | 0.8 |
| 3 | H | Me | H | 22 | 1.0 |
| 4 | H | tBu | H | 63 | 1.1 |
| 5 | tBu | H | tBu | 29 | 1.4 |
Besides the modification of the borylation reagents, another approach to enhance para-selectivity is to accelerate the intermolecular deprotonation of WIp, allowing it to outcompete the intramolecular 1,2-boryl migration. One possible way to facilitate deprotonation involves increasing the hydricity of B–H bonds in neutral hydroborane species, which would promote effective dehydrocoupling with the Brønsted acidic WIp. To probe the effects of the NHC ligand of borenium catalysts 4 regioselectivity, we synthesized two new borenium ions [IBnFMe-B(H)-CbMe][B(C6F5)4] (2, IBnFMe = 1-methyl-3-(2,3,4,5,6-pentafluorobenzyl)imidazol-2-ylidene) and [IMe2-B(H)-CbMe][B(C6F5)4] (3, IMe2 = 1,3-dimethyl-imidazol-2-ylidene) and examined their catalytic performance. For borenium 2, a p/m ratio of 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and borylation yield of 70% were observed (Table 2, entry 2). Borenium 3, which contains a more electron-donating IMe2 ligand, gave a higher para-selectivity (p/m = 4.4
1 and borylation yield of 70% were observed (Table 2, entry 2). Borenium 3, which contains a more electron-donating IMe2 ligand, gave a higher para-selectivity (p/m = 4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) yet unsatisfactory yield (24%). Although increasing the reaction temperature to 60 °C can improve the borylation efficiency to 42% yield, the selectivity dropped to 1.5
1) yet unsatisfactory yield (24%). Although increasing the reaction temperature to 60 °C can improve the borylation efficiency to 42% yield, the selectivity dropped to 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 2, entries 3 and 4). 2 was thus chosen as the borenium catalyst for further optimizations.
1 (Table 2, entries 3 and 4). 2 was thus chosen as the borenium catalyst for further optimizations.
Subsequently, we examined if addition of exogenous Brønsted bases could further improve the regioselectivity. 2,6-Dibromopyridine was evaluated first as the addition of pyridine derivatives was well-documented to promote C–H borylation catalysis via an FLP-type mechanism.38–41 However, 2,6-dibromopyridine (10 mol%) completely shut down the borylation of ethylbenzene with borenium 2 as the catalyst and HBcattBu as the borylation reagent (Table 3, entry 2). Using P(C6F5)3 as an exogenous base gave borylation products with an increased p/m ratio of 2.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and p-tolyl ether further improved the p/m ratio to 5.0
1, and p-tolyl ether further improved the p/m ratio to 5.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 3, entries 3 and 4). Encouraged by the performance of this O-based additive, we then examined phenoxyl boronates as additives due to the following reasons: (1) phenoxyl boronates can be readily accessed via in situ dehydrocoupling of phenol and the borylation reagent; (2) the electronic properties of phenoxyl boronates could be easily tuned by varying the substituents on the phenyl ring. One-pot reaction was carried out by pre-mixing 10 mol% phenol and 1.1 equiv. of HBcattBu, followed by the addition of ethylbenzene (1.0 equiv.) and catalyst 2 (10 mol%). Subsequent stirring at room temperature for 12 h afforded the borylation product in a p/m ratio of 5.1
1 (Table 3, entries 3 and 4). Encouraged by the performance of this O-based additive, we then examined phenoxyl boronates as additives due to the following reasons: (1) phenoxyl boronates can be readily accessed via in situ dehydrocoupling of phenol and the borylation reagent; (2) the electronic properties of phenoxyl boronates could be easily tuned by varying the substituents on the phenyl ring. One-pot reaction was carried out by pre-mixing 10 mol% phenol and 1.1 equiv. of HBcattBu, followed by the addition of ethylbenzene (1.0 equiv.) and catalyst 2 (10 mol%). Subsequent stirring at room temperature for 12 h afforded the borylation product in a p/m ratio of 5.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with 38% yield after workup (Table 3, entry 5). Switching phenol to p-cresol gave the best para-selectivity obtained so far (p/m = 7.1
1 with 38% yield after workup (Table 3, entry 5). Switching phenol to p-cresol gave the best para-selectivity obtained so far (p/m = 7.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), albeit with a relatively low borylation yield of 59% (Table 3, entry 7). Running the reactions at 60 °C can improve the borylation efficiency (74% yield, Table 3, entry 8) while maintaining the same para-selectivity. A similar result was observed when isolated (p-tol)OBcattBu was applied as an additive (Table 3, entry 12). Phenols containing a chloro group (Table 3, entry 6) or other alkyl groups (Table 3, entries 9 and 10) all showed inferior regioselectivities. The addition of alkoxyl boronates such as tBuOBcattBu also gave lower para-selectivity (Table 3, entry 11). When the reaction was carried in a 5 mmol scale, the borylation product was obtained with 69% yield and a p/m ratio of 7.1
1), albeit with a relatively low borylation yield of 59% (Table 3, entry 7). Running the reactions at 60 °C can improve the borylation efficiency (74% yield, Table 3, entry 8) while maintaining the same para-selectivity. A similar result was observed when isolated (p-tol)OBcattBu was applied as an additive (Table 3, entry 12). Phenols containing a chloro group (Table 3, entry 6) or other alkyl groups (Table 3, entries 9 and 10) all showed inferior regioselectivities. The addition of alkoxyl boronates such as tBuOBcattBu also gave lower para-selectivity (Table 3, entry 11). When the reaction was carried in a 5 mmol scale, the borylation product was obtained with 69% yield and a p/m ratio of 7.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
| Entry | Additive | Yield (%) | p/m ratio |
|---|---|---|---|
| a Phenoxyl boronates generated in situ by dehydrocoupling of HBcattBu and corresponding phenols. b 60 °C. | |||
| 1 | None | 70 | 1.5 |
| 2 | 2,6-Br2-py | NR | — |
| 3 | P(C6F5)3 | 50 | 2.9 |
| 4 | (p-tol)O(p-tol) | 32 | 5.0 |
| 5a | C6H5OH | 38 | 5.1 |
| 6a,b | (p-Cl)C6H4OH | 44 | 2.3 |
| 7a | p-Cresol | 59 | 7.1 |
| 8a,b | p-Cresol | 74 | 7.4 |
| 9ab | (p-Et)C6H4OH | 53 | 5.0 |
| 10a,b | (p-iPr)C6H4OH | 49 | 4.9 |
| 11b | tBuOBcattBu | 32 | 3.9 |
| 12b | (p-tol)OBcattBu | 72 | 6.7 |
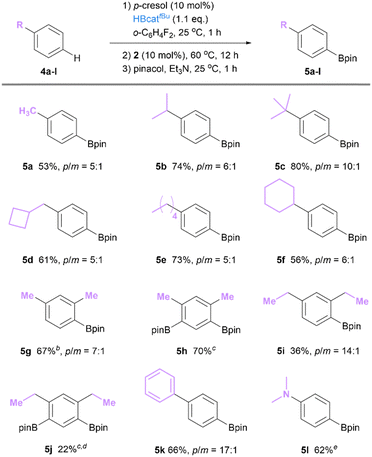
With the optimal reaction conditions in hand, we examined the substrate scope of our catalytic system. A comparable para-selectivity was achieved with toluene (4a), (cyclobutylmethyl)benzene (4d) and n-amylbenzene (4e), providing the corresponding borylation products in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of para- and meta-isomers. Mono-alkyl arenes bearing a secondary alkyl group such as isopropyl (4b) and cyclohexyl (4f) gave a higher p/m ratio of 6
1 mixture of para- and meta-isomers. Mono-alkyl arenes bearing a secondary alkyl group such as isopropyl (4b) and cyclohexyl (4f) gave a higher p/m ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Notably the para-selectivity reached 10
1. Notably the para-selectivity reached 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with tert-butylbenzene (4c). 1,3-Disubstituted arenes were also explored. Such substitution patterns, in iridium catalysis systems, typically give 5-borylated products as the exclusive regioisomer.4 With our catalytic platform, m-xylene (4g) was converted to a mixture of 4- and 5-borylated isomers in a ratio of 7
1 with tert-butylbenzene (4c). 1,3-Disubstituted arenes were also explored. Such substitution patterns, in iridium catalysis systems, typically give 5-borylated products as the exclusive regioisomer.4 With our catalytic platform, m-xylene (4g) was converted to a mixture of 4- and 5-borylated isomers in a ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, showcasing complementary regioselectivity compared to transition-metal catalysis.9 The 4,6-diborylated product was also obtained as a minor product in 9% yield. When 2.5 equiv. of HBcattBu was applied, the yield of 4,6-diborylated product 5h can be enhanced to 70%, thus providing a straightforward route for the preparation of meta-diboryl benzenes, useful building blocks for covalent organic frameworks.42 Additionally, diphenyl can be borylated with para-selectivity of 17
1, showcasing complementary regioselectivity compared to transition-metal catalysis.9 The 4,6-diborylated product was also obtained as a minor product in 9% yield. When 2.5 equiv. of HBcattBu was applied, the yield of 4,6-diborylated product 5h can be enhanced to 70%, thus providing a straightforward route for the preparation of meta-diboryl benzenes, useful building blocks for covalent organic frameworks.42 Additionally, diphenyl can be borylated with para-selectivity of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, substantially higher compared to our previous system based on 1 and HBcatCl (2.5
1, substantially higher compared to our previous system based on 1 and HBcatCl (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).36 For substrates containing heteroatom substituents, exclusive para-borylation products can be obtained without a (p-tol)OBcattBu additive (Scheme 2).
1).36 For substrates containing heteroatom substituents, exclusive para-borylation products can be obtained without a (p-tol)OBcattBu additive (Scheme 2).
Furthermore, we investigated the activity of our system in the borylation of polystyrene, as the boryl moiety can provide a valuable linchpin for the modification of the bulk and surface properties of polystyrene.4,43 To the best of our knowledge, the state of the art polystyrene borylation was reported by Bae and co-workers, where an iridium complex was employed as a catalyst at 150 °C with a p/m selectivity of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.44 In our study, syndiotactic polystyrene (Mn = 9.39 × 104 g mol−1, PDI = 2.06) was chosen as the substrate. Under standard conditions (10 mol% catalyst based on styrene unit), 19% of the phenyl ring was borylated with a para-selectivity of 1.6
4.44 In our study, syndiotactic polystyrene (Mn = 9.39 × 104 g mol−1, PDI = 2.06) was chosen as the substrate. Under standard conditions (10 mol% catalyst based on styrene unit), 19% of the phenyl ring was borylated with a para-selectivity of 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The Mn of the resulting polymer slightly increased to 1.46 × 105 g mol−1 with an almost unchanged PDI (1.58), revealing little alteration of the polystyrene main chain. When the borylation reaction was repeated without (p-tol)OBcattBu additive, nearly identical polymer was obtained, implying that the additive has little effect on the regioselectivity of borylation. This could be due to the difficulty associated with diffusion45 of the additive in the medium containing the polymer, which might hinder the deprotonation process.
1. The Mn of the resulting polymer slightly increased to 1.46 × 105 g mol−1 with an almost unchanged PDI (1.58), revealing little alteration of the polystyrene main chain. When the borylation reaction was repeated without (p-tol)OBcattBu additive, nearly identical polymer was obtained, implying that the additive has little effect on the regioselectivity of borylation. This could be due to the difficulty associated with diffusion45 of the additive in the medium containing the polymer, which might hinder the deprotonation process.
In this study, we investigated the influence of borylation reagents, borenium catalysts and Brønsted base additives on the para-selectivity of borylation of mono-alkylbenzenes. It was found that an electron-rich catecholborane derivative HBcattBu as a borylation reagent, a moderate electrophilic borenium 2 and (p-tol)OBcattBu as an additive can lead to the borylation of mono-alkylbenzenes with p/m ratios up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Furthermore, this catalytic system can be readily applied to the borylation of polystyrene, albeit with moderate p/m selectivity of 1.6
1. Furthermore, this catalytic system can be readily applied to the borylation of polystyrene, albeit with moderate p/m selectivity of 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. These results showcased the complementary selectivity of the borenium catalytic system compared to transition-metal ones. Exploring the application of the borenium catalytic system in other C–H functionalizations is currently underway in our laboratory.
1. These results showcased the complementary selectivity of the borenium catalytic system compared to transition-metal ones. Exploring the application of the borenium catalytic system in other C–H functionalizations is currently underway in our laboratory.
This work was supported by the National Natural Science Foundation of China (22071027, 22271050, 22471039).
Data availability
The data supporting this article were available within the article and the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS.
- D. Hall, Boronic acids: preparation and applications in organic synthesis, medicine and materials, Wiley-VCH, 2011 Search PubMed.
- S. K. Mellerup and S. Wang, Chem. Soc. Rev., 2019, 48, 3537 RSC.
- I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, Chem. Rev., 2010, 110, 890 CrossRef CAS PubMed.
- J. F. Hartwig, Acc. Chem. Res., 2012, 45, 864 CrossRef CAS PubMed.
- A. Ros, R. Fernandez and J. M. Lassaletta, Chem. Soc. Rev., 2014, 43, 3229 RSC.
- L. Xu, G. Wang, S. Zhang, H. Wang, L. Wang, L. Liu, J. Jiao and P. Li, Tetrahedron, 2017, 73, 7123 CrossRef CAS.
- R. Bisht, C. Haldar, M. M. M. Hassan, E. M. Hoque, J. Jagriti and B. Chattopadhyay, Chem. Soc. Rev., 2022, 51, 5042 RSC.
- J. F. Hartwig, Chem. Soc. Rev., 2011, 40, 1992 Search PubMed.
- M. A. Larsen and J. F. Hartwig, J. Am. Chem. Soc., 2014, 136, 4287 CrossRef CAS PubMed.
- J. S. Wright, P. J. J. Scott and P. G. Steel, Angew. Chem., Int. Ed., 2021, 60, 2796 CrossRef CAS PubMed.
- Y. Kuroda and Y. Nakao, Chem. Lett., 2019, 48, 1092 CrossRef CAS.
- C. Haldar, E. Hoque, J. Chaturvedi, M. M. M. Hassan and B. Chattopadhyay, Chem. Commun., 2021, 57, 13059 RSC.
- M. M. M. Hassan, S. Guria, S. Dey, J. Das and B. Chattopadhyay, Sci. Adv., 2023, 9, eadg3311 Search PubMed.
- Y. Saito, Y. Segawa and K. Itami, J. Am. Chem. Soc., 2015, 137, 5193 CrossRef CAS PubMed.
- H. J. Davis, M. T. Mihai and R. J. Phipps, J. Am. Chem. Soc., 2016, 138, 12759 CrossRef CAS PubMed.
- B. Ramadoss, Y. Jin, S. Asako and L. Ilies, Science, 2022, 375, 658 Search PubMed.
- L. Yang, K. Semba and Y. Nakao, Angew. Chem., Int. Ed., 2017, 56, 4853 Search PubMed.
- M. T. Mihai, B. D. Williams and R. J. Phipps, J. Am. Chem. Soc., 2019, 141, 15477 CrossRef CAS PubMed.
- J. R. M. Bastidas, T. J. Oleskey, S. L. Miller, M. R. Smith and R. E. Maleczka, J. Am. Chem. Soc., 2019, 141, 15483 CrossRef PubMed.
- S. Lu, T. Zheng, J. Ma, Z. Deng, S. Qin, Y. Chen and Y. Liang, Angew. Chem., Int. Ed., 2022, 134, e202201285 CrossRef.
- Y. Kuninobu, H. Ida, M. Nishi and M. Kanai, Nat. Chem., 2015, 7, 712 CrossRef CAS PubMed.
- R. Bisht, M. E. Hoque and B. Chattopadhyay, Angew. Chem., Int. Ed., 2018, 57, 15762 CrossRef CAS PubMed.
- J. Chaturvedi, C. Haldar, R. Bisht, G. Pandey and B. Chattopadhyay, J. Am. Chem. Soc., 2021, 143, 7604 CrossRef CAS PubMed.
- W. Chang, Y. Chen, S. Lu, H. Jiao, Y. Wang, T. Zheng, Z. Shi, Y. Han, Y. Lu, Y. Wang, Y. Pan, J.-Q. Yu, K. N. Houk, F. Liu and Y. Liang, Chemistry, 2022, 8, 1775 CrossRef CAS.
- M. E. Hoque, R. Bisht, C. Haldar and B. Chattopadhyay, J. Am. Chem. Soc., 2017, 139, 7745 Search PubMed.
- J. R.-M. Bastidas, A. Chhabra, Y. Feng, T. J. Oleskey, M. R. Smith and R. E. Maleczka, ACS Catal., 2022, 12, 2694 CrossRef PubMed.
- S. Guria, M. M. M. Hassan, S. Dey, K. N. Singh and B. Chattopadhyay, Angew. Chem., Int. Ed., 2024, 63, e202409010 Search PubMed.
- For para-selective electrophilic C–H borylation, see: S. Kim, J. W. Treacy, Y. A. Nelson, J. A. M. Gonzalez, M. Gembicky, K. N. Houk and A. M. Spokoyny, Nat. Commun., 2023, 14, 1671 CrossRef CAS PubMed.
- M. J. Ingleson, Synlett, 2012, 1411 CrossRef CAS.
- S. Bähr and M. Oestreich, Pure Appl. Chem., 2018, 90, 723 Search PubMed.
- Y. Ma, S.-J. Lou and Z. Hou, Chem. Soc. Rev., 2021, 50, 1945 Search PubMed.
- F.-G. Fontaine and V. Desrosiers, Synthesis, 2021, 4599 Search PubMed.
- X. Tan and H. Wang, Chem. Soc. Rev., 2022, 51, 2583 RSC.
- X. Tan and H. Wang, ChemCatChem, 2023, 15, e202300734 CrossRef CAS.
- X. Tan, X. Wang, Z.-H. Li and H. Wang, J. Am. Chem. Soc., 2022, 144, 23286 CrossRef CAS PubMed.
- V. Bagutski, A. D. Grosso, J. A. Carrillo, I. A. Cade, M. D. Helm, J. R. Lawson, P. J. Singleton, S. A. Solomon, T. Marcelli and M. J. Ingleson, J. Am. Chem. Soc., 2013, 135, 474 CrossRef CAS PubMed.
- D. W. Stephan and G. Erker, Angew. Chem., Int. Ed., 2015, 54, 6400 CrossRef CAS PubMed.
- D. W. Stephan, Science, 2016, 354, aaf7229 CrossRef PubMed.
- S. Tanaka, Y. Saito, T. Yamamoto and T. Hattori, Org. Lett., 2018, 20, 1828 CrossRef CAS PubMed.
- M. E. Grund, L. Sotorrios, M. K. Bisai, K. Yuan, S. A. Macgregor and M. J. Ingleson, ACS Catal., 2023, 13, 2286 CrossRef PubMed.
- C.-P. Hou, J. Yang, L. Zhang, Z.-H. Ma, Q. Li, J.-F. Xiang and H.-Y. Gong, Chem. – Eur. J., 2020, 26, 9466 CrossRef CAS PubMed.
- F. Jäkle. Borylated Polystyrenes as Versatile Functional Materials, Springer, 2019 Search PubMed.
- J. Shin, S. M. Jensen, J. Ju, S. Lee, Z. Xue, S. K. Noh and C. Bae, Macromolecules, 2007, 40, 8600 CrossRef CAS.
- O. J. Karlsson, J. M. Stubbs, L. E. Karlsson and D. C. Sundberg, Polymer, 2001, 42, 4915 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc06488g |
| This journal is © The Royal Society of Chemistry 2025 |