 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of highly efficient and selective palladium catalysts for telomerization of 1,3-butadiene with alcohols†
Edson Leonardo Scarpa de Souza‡
a,
Sebastian Ahrens‡ a,
Anke Spannenberga,
Helfried Neumann
a,
Anke Spannenberga,
Helfried Neumann a,
Kathrin Junge
a,
Kathrin Junge a,
Carlos Roque Duarte Correia
a,
Carlos Roque Duarte Correia b,
Ralf Jackstell*a and
Matthias Beller
b,
Ralf Jackstell*a and
Matthias Beller *a
*a
aLeibniz-Institut für Katalyse e.V., Albert-Einstein-Str. 29a, 18059 Rostock, Germany. E-mail: matthias.beller@catalysis.de
bDepartment of Organic Chemistry, Institute of Chemistry, University of Campinas, Rua Josué de Castro, 10384-612, Campinas, São Paulo, Brazil
First published on 2nd May 2025
Abstract
Novel phosphine ligands based on (benzo)furylphosphines, enable the efficient palladium-catalyzed telomerization of 1,3-butadiene with methanol. The synthesis of industrially relevant 1-methoxy-2,7-octadiene (1-MODE) occurs in quantitative yields and excellent productivity (TON = 95.000), even at ambient temperatures.
1-Octene plays a pivotal role in industrial chemistry, with a multi-100
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-ton scale utilization as a monomer in the production of linear low-density polyethylene. Its significance extends to its role as a precursor for plasticizers, alcohols, and solvents, as well as in the production of fine chemicals, including corrosion inhibitors and herbicides.1,2 Consequently, the synthesis of 1-octene has been a subject of interest for decades, and numerous methods have been developed to achieve this objective, including oligomerization of ethylene.3,4 Among the industrial processes that have been applied, telomerization of 1,3-butadiene is noteworthy for its selectivity and environmental friendliness, exhibiting 100% atomic efficiency.1,5–7
000-ton scale utilization as a monomer in the production of linear low-density polyethylene. Its significance extends to its role as a precursor for plasticizers, alcohols, and solvents, as well as in the production of fine chemicals, including corrosion inhibitors and herbicides.1,2 Consequently, the synthesis of 1-octene has been a subject of interest for decades, and numerous methods have been developed to achieve this objective, including oligomerization of ethylene.3,4 Among the industrial processes that have been applied, telomerization of 1,3-butadiene is noteworthy for its selectivity and environmental friendliness, exhibiting 100% atomic efficiency.1,5–7
The telomerization of 1,3-butadiene with methanol was first reported in 1967 independently by Smutny at Shell8 and Takahashi at Osaka University.9 In general, the telomerization can selectively afford 1-octene from inexpensive feedstocks through a three-step process (Scheme 1). Initially, the telomer 1-methoxy-2,7-octadiene (1-MODE, 2) is produced via a palladium-catalyzed reaction. It is noteworthy that this step, apart from yielding the desired linear product (1-MODE), can also result in the formation of the branched product (3-MODE) and other byproducts. In the second step, hydrogenation of 1-MODE 2 furnishes 1-methoxyoctane 3, which subsequently gives 1-octene 4 and methanol by thermal cracking.1 The subsequent steps, namely hydrogenation and thermal cracking, are well-established processes that yield their respective products in a quantitative yield. Therefore, the efficiency of the overall process is determined by the telomerization reaction. Consequently, numerous researchers in both industry and academia have demonstrated a persistent interest in this transformation, with the objective of enhancing the yield, catalyst productivity, and selectivity for 1-MODE 2 synthesis.10–17 To achieve high catalyst activity and regio- and chemoselectivity, a multitude of ligands, particularly mono- and bidentate phosphines, have been developed and evaluated in this transformation over the years.
As an example, in the presence of PPh3, 1-MODE 2 is obtained in moderate to good yield, but poor linear/branched selectivity.18–21 Derivatives of PPh3 containing electron-donating methoxy substituents, especially in the para position, have been shown to yield superior results.22–24 Intriguingly, ligands possessing bulky xanthene backbones have been observed to exhibit remarkable activity at low temperatures, and their oxygen coordination properties have been postulated to contribute to their enhanced performance.25–27
In addition, several trialkylphosphines were applied in the conversion of 1,3-butadiene into 1-MODE. It was observed that PEt3 gave higher selectivity than PPh3, while PCy3 tended to give mainly undesired 1,3,7-octatriene instead of 1-MODE 2.23,28 Compared to PPh3, bidentate phosphines showed better selectivity in the telomerization of 1,3-butadiene at lower temperature. Finally, it is worth mentioning that in the past two decades N-heterocyclic carbenes (NHCs) were introduced and studied in detail. These Pd–NHC-complexes afforded 1-MODE 2 in high selectivity in the presence of only ppm amounts of Pd (Scheme 1).2
While most prior research has been conducted with in situ generated Pd catalysts, some molecularly defined Pd complexes have also been examined in the telomerization reaction. Despite the necessity for additional synthesis steps and the potential increased sensitivity of the corresponding complexes, well-defined complex structures offer a distinct advantage in terms of enhanced catalyst activity. For instance, PPh3–Pd–(η2–η2-allylether) was shown to be active even at −10 °C.2,29,30
Recently, our group prepared novel electron-rich furyl and benzofuryl phosphines for cobalt-catalyzed isomerization reactions. Building upon the above-mentioned previous works in the field of telomerization, we have postulated that the corresponding palladium complexes should be active in this transformation. Consequently, we have initiated a project to evaluate their performance. To enable proper activity evaluation and potential applications, all catalytic experiments were performed at low temperature (room temperature) and low Pd loading (0.001 mol%). Mechanistic studies showed that the active Pd species contains only one coordinated phosphine.19,31,32 However, to ensure proper stabilization of the active metal species at low metal concentration, a metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratio of 1
ligand ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was applied. Utilizing PPh3 as a ligand under these conditions gave 1-MODE 2 in 50% yield. In comparison, the use of tri(furan-2-yl)phosphine (TFP) L1, a commercially available ligand, resulted in 1-MODE 2, albeit with a slightly lower yield (Table 1). It is also noteworthy that the majority of the previously examined monodentate ligands are symmetrical in nature. Interestingly, non-symmetric phosphine, obtained through the substitution of one or two phenyl groups with furyl groups, resulted in the telomerization product 2 in higher yields (L2, 60% and L4, 63%, respectively). In contrast, the more electron-rich TFP derivatives L5 and L6 provided 1-MODE 2 with yields of 49% and 60%, respectively. The evaluation of different tribenzofurylphosphines (L7–L9) demonstrated that the resulting orientation of the furan and benzofuran ring plays a crucial role in the coordination to the metal center, which is decisive for the catalytic activity.
3 was applied. Utilizing PPh3 as a ligand under these conditions gave 1-MODE 2 in 50% yield. In comparison, the use of tri(furan-2-yl)phosphine (TFP) L1, a commercially available ligand, resulted in 1-MODE 2, albeit with a slightly lower yield (Table 1). It is also noteworthy that the majority of the previously examined monodentate ligands are symmetrical in nature. Interestingly, non-symmetric phosphine, obtained through the substitution of one or two phenyl groups with furyl groups, resulted in the telomerization product 2 in higher yields (L2, 60% and L4, 63%, respectively). In contrast, the more electron-rich TFP derivatives L5 and L6 provided 1-MODE 2 with yields of 49% and 60%, respectively. The evaluation of different tribenzofurylphosphines (L7–L9) demonstrated that the resulting orientation of the furan and benzofuran ring plays a crucial role in the coordination to the metal center, which is decisive for the catalytic activity.
| a Reaction conditions: Pd(OAc)2 (0.001 mol%), ligand (0.003 mol%), NaOH (1 mol%), MeOH (1.5 mL/1.0 g 1,3-butadiene), condensed 1,3-butadiene, Ar, 25 °C, stirring at 750 rpm, 19 h. Yields calculated from GC-FID slope with isooctane as ISTD. |
|---|
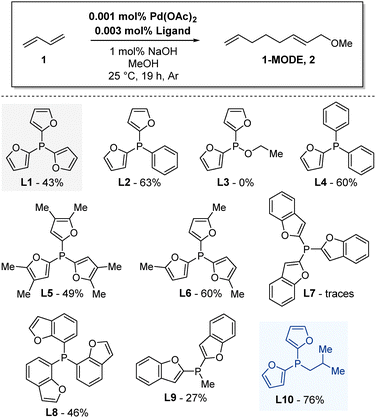 |
While only traces of the desired product were observed with tri-2-benzofuranephosphine L7, tri-8-benzofuranephosphine L8 gave a yield of 46% of 1-MODE 2. Furthermore, we tested alkyl-substituted furyl and benzofurylphosphines and difuryl-isobutylphosphine L10 provided the desired 1-MODE 2 in 76% yield. It is noteworthy that most of the catalytic experiments were performed at least twice and exhibited a very good degree of reproducibility. Considering the favorable outcome observed with L10, a systematic variation of the alkyl substituent on the difuryl phosphines was employed to enhance the activity of the respective palladium catalyst. A total of eight distinct alkyl difuryl phosphines were synthesized and utilized in the benchmark reaction (see Table 2 for details). The catalysis with the methyl-substituted phosphine L11 furnished 1-MODE 2 in 53% yield, while the ethyl-substituted ligand L12 yielded 70% of the product. Notably, the n-propyl-substituted difuryl phosphine L13 achieved a remarkable 95% yield. Conversely, when iso-butyl- (L10) and n-butyl- (L14) substituted phosphines were utilized, the yields were found to be considerably lower, at 76% and 63%, respectively. Ligands bearing alkyl groups with increased steric hindrance, such as tert-butyl L15 and neopentyl L16, yielded 1-MODE in 52% and 47% yield, respectively. In the possession of the optimal ligand (L13), we proceeded to synthesize L17, which possess a single furyl group and two n-propyl chains attached to the phosphorus atom. When the telomerization reaction was performed with L18, the desired product was obtained in 41% yield, indicating the crucial role of the two furyl motifs and the propyl chain for the activity of the Pd catalyst.
| a Reaction conditions: Pd(OAc)2 (0.001 mol%), ligand (0.003 mol%), NaOH (1 mol%), MeOH (1.5 mL/1.0 g butadiene), condensed 1,3-butadiene, Ar, 25 °C, stirring at 750 rpm, 19 h. Yields calculated from GC-FID slope with isooctane as ISTD. |
|---|
 |
As demonstrated in Table 2, a clear correlation exists between the size of the linear alkyl chain in the ligand and the yield of 1-MODE 2, which attains an optimal level with n-propyl chains. Further increases in chain length (>n-propyl) and/or the bulkiness of the alkyl chains result in a decline in the desired product yield. It is noteworthy that the reaction in the presence of difuryl-alkyl-phosphine ligands proceeds with low catalyst loadings at room temperature towards the desired product with high selectivity, yielding exclusively the Z-isomer of 1-MODE 2.
Nevertheless, even more active ligands could be expected by the proper combination of phosphine substituents, e.g. L13 with methyl-furyl units.33 Even though the yield of 1-MODE 2 was nearly quantitative with L13 (95%, TON 95.000), further investigation was conducted into the role of the ligand (Table 3). In the absence of a ligand, no product was formed (Table 3, entry 1). Subsequent assessment of the ligand-to-metal ratio (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M ratio) revealed a decline in yield for 1-MODE 2 when the L
M ratio) revealed a decline in yield for 1-MODE 2 when the L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M ratio was 1
M ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 5
1 or 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 3, entries 2 and 3). Furthermore, the effect of temperature on the telomerization reaction was investigated, and the catalytic system demonstrated optimal efficiency at 25 °C (Table 3, entry 5). Conversely, at 0 °C, the reaction yield was 10% (Table 3, entry 4). At 50 °C, the catalytic system exhibited a 93% yield. However, at elevated temperatures of 70 °C or 90 °C, the yield of 1-MODE 2 decreased to 52% and 58%, respectively (Table 3, entries 6–8). Notably, utilizing only 0.0005 mol% of Pd(OAc)2 produced the desired product in a moderate yield of 35%, as shown in Table 3, entry 9. Additionally, we investigated the air stability of L13 and, to our surprise, the electron-rich phosphine L13 (31P NMR: −62.0 ppm) is considerably stable under aerobic conditions. More specifically, only 6% of the corresponding phosphine oxide L18 (31P NMR: 12.2 ppm, 1.0
1 (Table 3, entries 2 and 3). Furthermore, the effect of temperature on the telomerization reaction was investigated, and the catalytic system demonstrated optimal efficiency at 25 °C (Table 3, entry 5). Conversely, at 0 °C, the reaction yield was 10% (Table 3, entry 4). At 50 °C, the catalytic system exhibited a 93% yield. However, at elevated temperatures of 70 °C or 90 °C, the yield of 1-MODE 2 decreased to 52% and 58%, respectively (Table 3, entries 6–8). Notably, utilizing only 0.0005 mol% of Pd(OAc)2 produced the desired product in a moderate yield of 35%, as shown in Table 3, entry 9. Additionally, we investigated the air stability of L13 and, to our surprise, the electron-rich phosphine L13 (31P NMR: −62.0 ppm) is considerably stable under aerobic conditions. More specifically, only 6% of the corresponding phosphine oxide L18 (31P NMR: 12.2 ppm, 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06 ligand
0.06 ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand oxide ratio) was detected in a 31P NMR spectrum after exposing L13 to air at room temperature for an overnight period.
ligand oxide ratio) was detected in a 31P NMR spectrum after exposing L13 to air at room temperature for an overnight period.
| Entry | Changes from standard conditions | 1-MODE (%) |
|---|---|---|
| a Reaction conditions: Pd(OAc)2 (0.001 mol%), L13 (0.003 mol%), NaOH (1 mol%), MeOH (1.5 mL/1.0 g 1,3-butadiene), condensed 1,3-butadiene, Ar, 25 °C, stirring at 750 rpm, 19 h. Yields calculated from GC-FID slope with isooctane as ISTD.b Reaction performed at 90 °C.c Ligand: 0.0015 mol% L13. | ||
| 1b | Without L13 | 0 |
| 2 | 0.001 mol% L13 | 50 |
| 3 | 0.005 mol% L13 | 42 |
| 4 | 0 °C | 10 |
| 5 | 25 °C | 95 |
| 6 | 50 °C | 93 |
| 7 | 70 °C | 52 |
| 8 | 90 °C | 58 |
| 9c | 0.0005 mol% Pd(OAc)2 | 35 |
Following a week of storage in ambient air, the presence of approximately 21% phosphine oxide L18 (with a ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand oxide ratio of 1.0
ligand oxide ratio of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.27) was ascertained through 31P NMR analysis. The complete oxidation of L13 to L18 was only achieved using hydrogen peroxide as the oxidizing agent (for the crystal structure of L18 see Fig. S4, ESI†). Notably, negligible amounts of 1-MODE 2 were observed with L18.
0.27) was ascertained through 31P NMR analysis. The complete oxidation of L13 to L18 was only achieved using hydrogen peroxide as the oxidizing agent (for the crystal structure of L18 see Fig. S4, ESI†). Notably, negligible amounts of 1-MODE 2 were observed with L18.
In the previous studies of telomerization reactions, the reactivity of the alcohol was often neglected. To examine the activity of this newly developed catalytic system towards different alcohols, the telomerization reaction was performed in a mixture of two alcohols with very similar structure and properties, namely methanol and ethanol. Notably, under standard conditions (Table 3), the catalytic system demonstrated a high degree of selectivity for methanol, yielding the products 1-MODE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-EtODE in a 10
1-EtODE in a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (ESI†). We assume that this selectivity is a result of the preferred protonation of the Pd–(η3,η1-octadienediyl)-complex, formed by the oxidative addition of 1,3-butadiene to the Pd(0)-complex. Notably, even when the percentage of ethanol in the solvent mixture was increased to a 1
1 ratio (ESI†). We assume that this selectivity is a result of the preferred protonation of the Pd–(η3,η1-octadienediyl)-complex, formed by the oxidative addition of 1,3-butadiene to the Pd(0)-complex. Notably, even when the percentage of ethanol in the solvent mixture was increased to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of methanol
1 ratio of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol, the reaction remained selective towards 1-MODE (ESI†). This observation led to the subsequent evaluation of the selectivity of the novel catalytic system for methanol in the presence of n-butanol. The resultant 1-MODE
ethanol, the reaction remained selective towards 1-MODE (ESI†). This observation led to the subsequent evaluation of the selectivity of the novel catalytic system for methanol in the presence of n-butanol. The resultant 1-MODE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-nBuODE ratio of 9
1-nBuODE ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ESI†) indicates the high degree of chemoselectivity of this system, which might be used for selective telomerizations of alcohol mixtures and substrates with more than one hydroxyl group. Finally, the mechanism of this reaction is believed to operate in a manner consistent with the findings of the original studies conducted by Jolly and colleagues31 and subsequent investigations.32,34–36
1 (ESI†) indicates the high degree of chemoselectivity of this system, which might be used for selective telomerizations of alcohol mixtures and substrates with more than one hydroxyl group. Finally, the mechanism of this reaction is believed to operate in a manner consistent with the findings of the original studies conducted by Jolly and colleagues31 and subsequent investigations.32,34–36
In summary, we have synthesized and applied novel difuryl-alkyl-phosphines for the Pd-catalyzed telomerization reaction of 1,3-butadiene. Notably, the n-propyl derivative (L13) was identified as the optimal ligand for the synthesis of 1-MODE 2. The newly developed catalytic system, as outlined in this report, enables the efficient synthesis of 1-MODE 2 with a high degree of purity using only 0.001 mol% of Pd at ambient temperature. Furthermore, experiments employing mixtures of alcohols in the telomerization reaction demonstrated a high degree of chemoselectivity for the optimal catalyst system.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Recker, C. M. Gordon, A. Peace, C. Redepenning and W. Marquardt, Chem. Ing. Tech., 2017, 89, 1479–1489 CrossRef CAS.
- R. Jackstell, M. G. Andreu, A. Frisch, K. Selvakumar, A. Zapt, H. Klein, A. Spannenberg, D. Röttger, O. Briel, R. Karch and M. Beller, Angew. Chem., Int. Ed., 2002, 41, 986–988 CrossRef CAS PubMed.
- J. Yang, P. Wang, H. Neumann, R. Jackstell and M. Beller, Ind. Chem. Mater., 2023, 1, 155–174 RSC.
- T. A. Faßbach, A. J. Vorholt and W. Leitner, ChemCatChem, 2019, 11, 1153–1166 CrossRef.
- Y. Zong, C. Wang and Y. Zhang, Polymers, 2023, 15, 1044 Search PubMed.
- M. Sharif, R. Jackstell, S. Dastgir, B. Al-Shihi and M. Beller, ChemCatChem, 2017, 9, 542–546 Search PubMed.
- D. Peruzzo, M. Drelon, C. Dumont, A. Mortreux, I. Suisse and M. Sauthier, Mol. Catal., 2021, 502, 111369 CrossRef CAS.
- E. J. Smutny, J. Am. Chem. Soc., 1967, 89, 6793–6794 CrossRef CAS.
- S. Takahashi, T. Shibano and N. Hagihara, Tetrahedron Lett., 1967, 2451–2453 CrossRef CAS.
- H. Zhang, C. Shen, Z. Xu, X. Tian and K. Dong, Mol. Catal., 2021, 515, 111883 CrossRef CAS.
- M. A. Topchiy, S. A. Rzhevskiy, L. I. Minaeva and A. F. Asachenko, Pet. Chem., 2022, 62, 1235–1241 CrossRef CAS.
- D. Peruzzo, M. Drelon, C. Dumont, A. Mortreux, I. Suisse and M. Sauthier, Mol. Catal., 2021, 502, 1–6 Search PubMed.
- D. S. Suslov, M. V. Bykov, M. V. Pakhomova, T. S. Orlov, Z. D. Abramov, A. V. Suchkova, A. A. Pavlova and P. A. Abramov, J. Struct. Chem., 2023, 64, 2235–2254 CrossRef CAS.
- S. Recker, C. M. Gordon, A. Peace and C. Redepenning, Chem. Ing. Tech., 2017, 1479–1489 CrossRef CAS.
- Z. Wang, S. Hasegawa, K. Motokura, S. Kuang and Y. Yang, Ind. Eng. Chem. Res., 2023, 62, 3151–3156 CrossRef CAS.
- S. A. Rzhevskiy, M. A. Topchiy, V. N. Bogachev, A. A. Ageshina, L. I. Minaeva, G. K. Sterligov, M. S. Nechaev and A. F. Asachenko, Mendeleev Commun., 2021, 31, 478–480 CrossRef CAS.
- J. Colavida, J. A. Lleberia, A. Salom-Catala, A. Gual, A. Collado, E. Zangrando, J. M. Ricart, C. Godard, C. Claver, J. J. Carbo and S. Castillon, ACS Catal., 2020, 10, 11458–11465 CrossRef CAS.
- B. Estrine, S. Bouquillon, F. Hénin and J. Muzart, Eur. J. Org. Chem., 2004, 2914–2922 CrossRef CAS.
- F. Vollmüller, J. Krause, S. Klein, W. Mägerlein and M. Beller, Eur. J. Inorg. Chem., 2000, 1825–1832 CrossRef.
- M. Drelon, D. S. Mérel, A. Mortreux, I. Suisse and M. Sauthier, ChemCatChem, 2019, 11, 1742–1746 Search PubMed.
- J. Wang, X. Feng, J. Song, Z. Gao and W. Wang, ChemistrySelect, 2020, 5, 9404–9408 CrossRef CAS.
- J. L. Klinkenberg and K. P. Lawry, Org. Process Res. Dev., 2019, 23, 1654–1658 CrossRef CAS.
- F. Benvenuti, C. Carlini, M. Lami, M. Marchionna, R. Patrini, A. M. Raspolli Galletti and G. Sbrana, J. Mol. Catal. A: Chem., 1999, 144, 27–40 CrossRef CAS.
- R. Palkovits, I. Nieddu, C. A. Kruithof, R. J. M. K. Gebbink and B. M. Weckhuysen, Chem. – Eur. J., 2008, 14, 8995–9005 CrossRef CAS PubMed.
- T. E. Barder, S. D. Walker, J. R. Martinelli and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 4685–4696 Search PubMed.
- M. J. L. Tschan, H. Launay, H. Hagen, J. Benet-Buchholz and P. W. N. M. Van Leeuwen, Chem. – Eur. J., 2011, 17, 8922–8928 CrossRef CAS PubMed.
- M. J. L. Tschan, E. J. García-Suárez, Z. Freixa, H. Launay, H. Hagen, J. Benet-Buchholz and P. W. N. M. Van Leeuwen, J. Am. Chem. Soc., 2010, 132, 6463–6473 CrossRef CAS PubMed.
- F. Benvenuti, C. Carlini, M. Marchionna, R. Patrini, A. M. Raspolli Galletti and G. Sbrana, J. Mol. Catal. A: Chem., 1999, 140, 139–155 CrossRef CAS.
- J. Krause, G. Cestaric, K. J. Haack, K. Seevogel, W. Storm and K. R. Pörschke, J. Am. Chem. Soc., 1999, 121, 9807–9823 CrossRef CAS.
- A. Grotevendt, R. Jackstell, D. Michalik, M. Gomez and M. Beller, ChemSusChem, 2009, 2, 63–70 CrossRef CAS PubMed.
- P. W. Jolly, Angew. Chem., Int. Ed. Engl., 1985, 24, 283–295 Search PubMed.
- C. F. Huo, R. Jackstell, M. Beller and H. Jiao, J. Mol. Model., 2010, 16, 431–436 CrossRef CAS PubMed.
- We thank one of the referees for pointing out this aspect.
- F. Vollmüller, W. Ma, S. Klein, J. Krause and M. Beller, Adv. Synth. Catal., 2001, 29–33 CrossRef.
- N. D. Clement, L. Routaboul, A. Grotevendt and R. Jackstell, Chem. – Eur. J., 2008, 14, 7408–7420 CrossRef CAS PubMed.
- F. Vollmüller, J. Krause, S. Klein, W. Mägerlein and M. Beller, Eur. J. Inorg. Chem., 2000, 1–7 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: CCDC 2339359. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc01036e |
| ‡ These authors contributed equally to the development of this work. |
| This journal is © The Royal Society of Chemistry 2025 |


