Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An ionic ultramicroporous polymer with engineered nanopores enables enhanced acetylene/carbon dioxide separation†
Asif
Raza‡
 a,
Sousa Javan
Nikkhah‡
a,
Sousa Javan
Nikkhah‡
 a,
Lilia
Croitor
a,
Ahmed Gamal
Attallah
a,
Lilia
Croitor
a,
Ahmed Gamal
Attallah
 bc,
Eric
Hirschmann
bc,
Eric
Hirschmann
 b,
Matthias
Vandichel
b,
Matthias
Vandichel
 *a and
Soumya
Mukherjee
*a and
Soumya
Mukherjee
 *a
*a
aDepartment of Chemical Sciences, Bernal Institute and Research Ireland Centre for Pharmaceuticals (SSPC), University of Limerick, Limerick V94 T9PX, Ireland. E-mail: Matthias.Vandichel@ul.ie; Soumya.Mukherjee@ul.ie
bHelmholtz-Zentrum Dresden – Rossendorf, Institute of Radiation Physics, Bautzner Landstraße 400, 01328, Dresden, Germany
cPhysics Department, Faculty of Science, Minia University, Minia 61519, Egypt
First published on 27th March 2025
Abstract
A nanopore engineering approach enhances acetylene (C2H2) over carbon dioxide (CO2) selectivity in ionic ultramicroporous polymers (IUPs), an understudied class of sorbents. Extending the cationic arm of a prototypical IUP nearly doubles its C2H2/CO2 selectivity from 4.9 to 8.5 (at 298 K, 1 bar), underpinned by further observations from dynamic separation experiments and bespoke computational insights.
Acetylene (C2H2) is a key industrial feedstock used in the production of plastics, pharmaceuticals, fuels, and other raw chemicals.1 Traditionally, C2H2 is produced via methane combustion or petroleum cracking, processes that generate carbon dioxide (CO2) as the primary impurity. The separation of these two gases is key to obtaining polymer-grade C2H2 (≥99.5%), but their similar molecular sizes (kinetic diameters ∼ 3.3 Å), linear shapes, and close boiling points (189.2 K for C2H2 and 194.7 K for CO2) make their separation highly challenging.2 State-of-the-art processes still rely upon traditional purification methods, such as solvent extraction and cryogenic distillation, which are energy-intensive and inefficient, underscoring the need for more sustainable alternatives.3–5
Adsorptive separation, particularly physisorption, offers clear advantages because of its minimal energy demand for sorbent recycling (i.e., regeneration).6 Physisorbents that offer strong, selective binding for C2H2 over CO2 appear particularly suited for the target C2H2/CO2 separation.6,7 Reticular crystalline physisorbents like porous coordination networks (PCNs), such as metal–organic frameworks (MOFs)8 and hybrid ultramicroporous materials (HUMs),9,10 and hydrogen bonded organic frameworks (HOFs)11,12 have lately been developed for C2H2/CO2 separation. Whereas high selectivity has been realised by a handful of such crystalline reticular sorbents, and the total number of crystalline CNs in the Cambridge Structural Database (CSD) is soaring above 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000,13 the labile nature of the metal–organic bonds/presence of metals have handicapped the performance benchmarks. With regard to chemical separations and/or purifications, such as light hydrocarbon separations, limited water stability has particularly kept the “spectrum of performance parameters” elusive for MOF physisorbents thus far.6,14 Conversely, porous organic polymers (POPs), constructed from covalent bonds, tend to be stable under aqueous conditions, their physisorptive separation performances are poor because of two key factors that drive purifications in ultramicroporous PCNs, especially HUMs: (a) wrong pore size to enable tight binding, i.e., ultramicroporosity (<7 Å); (b) inappropriate pore chemistry for selective binding between the adsorbent and target molecule. Crystalline POPs, as exemplified by covalent organic frameworks (COFs) suffer from these weaknesses despite their well-defined structures. For example, NKCOF-12, though capable of high acetylene (C2H2) uptake at 298 K, exhibits a modest C2H2/CO2 selectivity (<5), limiting its applicability.15 Amorphous POPs, such as porous aromatic frameworks (PAFs), offer high stability, scalability, and processability for membranes or monoliths, etc., but fail to offer densely packed physisorption sites, i.e., optimal combinations of pore size and chemistry key to strong and selective binding.
000,13 the labile nature of the metal–organic bonds/presence of metals have handicapped the performance benchmarks. With regard to chemical separations and/or purifications, such as light hydrocarbon separations, limited water stability has particularly kept the “spectrum of performance parameters” elusive for MOF physisorbents thus far.6,14 Conversely, porous organic polymers (POPs), constructed from covalent bonds, tend to be stable under aqueous conditions, their physisorptive separation performances are poor because of two key factors that drive purifications in ultramicroporous PCNs, especially HUMs: (a) wrong pore size to enable tight binding, i.e., ultramicroporosity (<7 Å); (b) inappropriate pore chemistry for selective binding between the adsorbent and target molecule. Crystalline POPs, as exemplified by covalent organic frameworks (COFs) suffer from these weaknesses despite their well-defined structures. For example, NKCOF-12, though capable of high acetylene (C2H2) uptake at 298 K, exhibits a modest C2H2/CO2 selectivity (<5), limiting its applicability.15 Amorphous POPs, such as porous aromatic frameworks (PAFs), offer high stability, scalability, and processability for membranes or monoliths, etc., but fail to offer densely packed physisorption sites, i.e., optimal combinations of pore size and chemistry key to strong and selective binding.
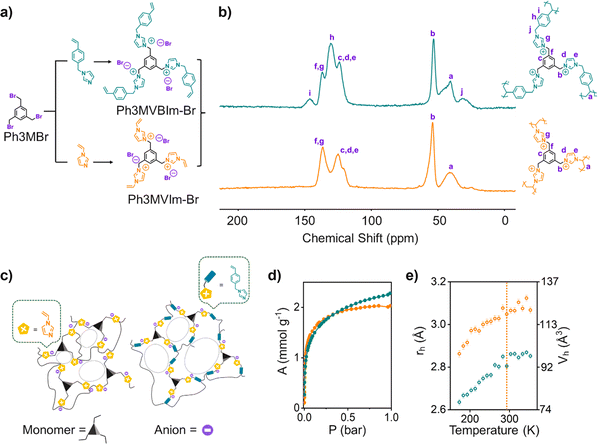
To overcome these oft-encountered limitations, ionic ultramicroporous polymers (IUPs), an advanced form of polyionic liquids (PILs)16,17 that feature strong electrostatic functionalities and high stability have recently come to the fore.5 However, the field is still in its infancy, with only a few reported examples and limited design principles for tailoring binding sites to selectively capture gases like C2H2, that could potentially afford binary C2H2/CO2 separation performances. Among the handful of known IUPs, hitherto only one strategy, namely copolymerisation has been shown to improve the C2H2/CO2 separation,18 thanks to the authors controlling pore structure and functionality in P(Ph3Im-Br-nDVB) (Ph = phenyl, Im = vinylimidazolium cation, Br = bromide anion, DVB = divinylbenzene crosslinker, n = molar ratio of monomer and crosslinker). P(Ph3Im-Br-0.5DVB) registered a C2H2/CO2 selectivity of 17.9 at 1 bar and 298 K, highlighting the necessity for further iteration of the IUP platform. Such compositional control in IUPs is likely to enable an improved, high-density distribution of the desired C2H2 binding sites. Herein, we report a nanopore engineering strategy by looking at IUPs as a tripartite composition, i.e., a neutral central core (usually aromatic ring), cationic arm (having quaternized nitrogen) and counteranion. In this report, we have modulated the cationic arm by extending it with addition of an aromatic benzyl ring (Fig. 1a). This extended arm helped in almost doubling the selectivity from 4.9 for P(Ph3MVIm-Br) (Ph = phenyl, VIm = vinylimidazole, Br = bromide anion) to 8.5 for P(Ph3MVBIm-Br) (Ph = phenyl, VBIm = vinylbenzylimidazole, Br = bromide anion) at 1 bar and 298 K for equimolar mixtures of C2H2/CO2.
IUPs were synthesized using a free radical polymerization technique, with detailed procedures provided in the ESI† (Fig. S1–S7). As shown in Fig. 1c, P(Ph3MVBIm-Br) features VBIm as its cationic arm, an extended version of VIm found in P(Ph3MVIm-Br). During polymerization, the vinyl groups crosslinks, forming a porous structure. Solid-state NMR highlights differences between the polymers. P(Ph3MVBIm-Br) exhibits additional aliphatic signals at ∼30 ppm (CH2 groups) and ∼43 ppm (covalently linked vinyl carbons). Peaks at ∼54 ppm correspond to methylene linkers, while ∼124 ppm and ∼138 ppm can be attributed to aromatic carbons in the phenyl core and substituted imidazolium carbons, respectively. Unique signals in the aromatic region (h) and (i) confirm the presence of the benzene ring in the cationic arm of P(Ph3MVBIm-Br) (Fig. 1b). FT-IR spectra indicate successful polymerization, as the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching band at 1650 cm−1 diminishes in the polymers (Fig. S6 and S7, ESI†).
C stretching band at 1650 cm−1 diminishes in the polymers (Fig. S6 and S7, ESI†).
The textural properties of the polymers were assessed via CO2 adsorption isotherms at 195 K (Fig. 1d), with structural parameters summarized in Table S1 (Fig. S8–S11, ESI†). Horvath Kawazoe analysis (Fig. S8 and S10 inset, ESI†) indicated pore widths of 4–7 Å. Positron annihilation lifetime spectroscopy (PALS) provided further insights, showing average pore radii of 2.6–2.8 Å for P(Ph3MVBIm-Br) and 2.8–3.1 Å for P(Ph3MVIm-Br) (Fig. 1e), with ortho-positronium (o-Ps) lifetimes of 2.16 ns and 1.93 ns at 298 K, respectively (Fig. S12, ESI†). More details about PALS are provided in the ESI.† Thermogravimetric analysis (TGA) further demonstrated the excellent thermal stability of these materials, with an initial decomposition temperature of 290 °C under a nitrogen atmosphere (Fig. S13 and S14, ESI†). The surface chemical properties of P(Ph3MVBIm-Br) were analysed using X-ray photoelectron spectroscopy (XPS) (Fig. S15, ESI†). XPS detected carbon, nitrogen, oxygen, and bromine, with their percentage abundances detailed in Table S2 (Fig. S15a, ESI†). High-resolution spectra revealed three distinct C 1s peaks at 284.5 eV (aromatic and cross-linked carbons), 285.6 eV (C–N bonds in imidazole rings), and 286.6 eV (C–O bonds, can be attributed to the presence of methanol solvent) (Fig. S15c, ESI†). N 1s spectra showed peaks at 399.3 eV (non-quaternized nitrogen) and 401.4 eV (quaternized nitrogen), confirming the polymer's cationic backbone (Fig. S15b, ESI†). Br 3d signals at 67.2 and 68.2 eV validated the presence of bromide anions as charge-balancing counterions (Fig. S15d, ESI†).
To evaluate the properties of IUPs in acetylene purification, adsorption isotherms of C2H2 and CO2 were determined, revealing superior C2H2 uptake of 1.4 mmol g−1P(Ph3MVIm-Br) and 1.0 mmol g−1P(Ph3MVBIm-Br) at 298 K and 1 bar, with CO2 uptakes significantly lower at 0.65 mmol g−1 and 0.4 mmol g−1 (Fig. 2a and b). The IAST selectivity for C2H2/CO2 mixtures improved from 4.96 to 8.49 upon extending the cationic arm (Fig. 2c and Fig. S16, S17, ESI†), surpassing many porous organic polymers e.g., ZJUT-3 (3.2),19 NKCOF-12 (4.0),15 and 3D pts COF (3.8)20 (Fig. 2d and Table S3, ESI†). Adsorption data at 273 K and 283 K showed higher C2H2 uptake and a stronger binding affinity (Qst = 46.7 kJ mol−1 for C2H2vs. 39.7 kJ mol−1 for CO2), (Fig. S18–S22 and Table S4, ESI†) affirming effective separation. The higher SAC for P(Ph3MVBIm-Br)vs.P(Ph3MVBIm-Br) (Fig. 2c) is found to be in contrast with the significantly higher C2H2 uptake registered by the latter (1.4 mmol g−1) over the former (1.0 mmol g−1). Upon closely looking at the CO2 isotherms’ differences, particularly under low pressures, the weaker CO2 affinity for P(Ph3MVBIm-Br) turns out to be the key behind its higher SAC.
The realization of P(Ph3VBIm-Br) for challenging C2H2/CO2 separation highlights the potential of IUPs in industrial applications. We further assessed its performance under ambient conditions by conducting laboratory-scale dynamic column breakthrough (DCB) experiments. Equimolar C2H2/CO2 mixtures were passed through a packed column of activated P(Ph3MVBIm-Br) at 1 bar and 298 K. This showed delayed C2H2 breakthrough (24.33 min g−1) compared to CO2 (13.54 min g−1), yielding a separation factor of 4.07 and a CO2 purity of 99.12% during separation (Fig. 2e). Temperature-programmed desorption (TPD) confirmed facile regeneration at 60 °C, with high-purity fuel-grade C2H2 (>98%) eluted over 2.55–21.49 min g−1 and laboratory-grade C2H2 (>99%) eluted over 3.06–19.96 min g−1 (Fig. S23, ESI†). The peak C2H2 purity was 99.57%, with productivity reaching 1.53 L kg−1 for fuel-grade and 1.34 L kg−1 for lab-grade C2H2, demonstrating the material's industrial potential.
To gain insight into the mechanism underlying the separation performance, we conducted Canonical Monte Carlo (CMC) simulations using the crystallographic structure of the Ph3MVIm-Br monomer as the basis (Table S5, ESI†). For molecular models of P(Ph3MVIm-Br) and P(Ph3MVBIm-Br), the CMC simulations at 298 K revealed that the C2H2 and CO2 binding sites are located on the polymer surfaces (Fig. S32 and S33, ESI†). This surface-reliant binding is attributed to the morphology and high density of the polymers (Table S8, ESI†). Br− ions afford a shielding layer on the surface (Fig. S30, ESI†), eliciting an electronegative environment that strongly interacts with C2H2 and CO2, both polarizable in nature. Conversely, the Br− ions limit diffusion into the dense interior (Fig. S30 and S31, ESI†).
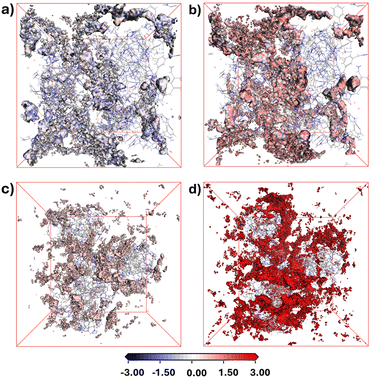
Isosurface analyses, colour-mapped along potential energy (Fig. 3), revealed that C2H2 has the most favourable binding site on the surface, indicated by its lowest potential energy (Fig. 3a and c). This is driven by C2H2's high polarizability, electron-rich triple bond, and acetylenic hydrogens with pKa ∼25, enabling strong electrostatic and dipole interactions with the cationic backbone and the anionic Br−. In contrast, CO2 (Fig. 3c and d), with lower polarizability and no hydrogens, interacts weakly with the polymer surface, resulting in weak binding. Adsorbate binding density analysis revealed that P(Ph3MVIm-Br) features a higher density of binding sites for both C2H2 and CO2 (Fig. S32, ESI†) vs. those in P(Ph3MVBIm-Br) (Fig. S33, ESI†), which leads to a higher uptake of both gases in P(Ph3MVIm-Br). This is likely due to its compact structure, which may enhance adsorption through stronger dipole-quadrupole interactions between the closely packed imidazolium groups and the sorbate molecules. In P(Ph3MVBIm-Br), an interplay of electrostatic interactions, steric hindrance from additional bulky phenyl groups, and the compact internal structure of the polymers further limits CO2 adsorption (Fig. 3d), enhancing the selectivity for C2H2/CO2.
Our findings demonstrate that tweaking the cationic arm of a prototypal IUP doubles the C2H2/CO2 selectivity by limiting the CO2 coadsorption. A detailed computational study reinforced the latter and further elucidated the importance of ionic surfaces in fostering strong binding sites, vs. the often-emphasised impact of pore voids and channels. It is therefore highly likely that next generation IUP sorbents will exhibit a high-density of ionic sites and abundant ultramicroporosity to address challenging separations.
Research Ireland awards (21/PATH-S/9454; 23/FFP-A/12221), SSPC Research Funding Award AzAds, the Irish Center for High-End Computing (ICHEC), Enterprise Ireland, and the European Union's H2020 research and innovation programme under Marie Skłodowska-Curie grant agreement 847402 (ID: MF20210297) are acknowledged.
Data availability
The data supporting this article are included in the ESI.† Crystallographic data for Ph3MVIm-Br can be accessed from the Cambridge Crystallographic Data Centre (CCDC) as deposition number 2418964.† Modelled polymerizations are included in the Videos entitled Ph3MVlm-Br.mp4, and Ph3MVBlm-Br.mp4.Conflicts of interest
The authors have no conflicts to declare.Notes and references
- P. Pässler, et al., Chemistry, Wiley, 2011 Search PubMed.
- M. L. Foo, et al., J. Am. Chem. Soc., 2016, 138, 3022–3030 CrossRef CAS PubMed.
- G. Verma, et al., Eur. J. Inorg. Chem., 2021, 4498–4507 CrossRef CAS.
- R. S. Haszeldine, Science, 2009, 325, 1647–1652 CAS.
- X. Suo, et al., Adv. Mater., 2020, 32, 1907601 CAS.
- S. Mukherjee, et al., Chem. Commun., 2020, 56, 10419–10441 CAS.
- Y. Peng, et al., Angew. Chem., Int. Ed., 2018, 57, 10971–10975 CAS.
- S. Kitagawa, et al., Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CAS.
- S. Mukherjee, et al., Angew. Chem., Int. Ed., 2021, 60, 10902–10909 CAS.
- X. Cui, et al., Science, 2016, 353, 141–144 CrossRef CAS.
- Z. Bao, et al., J. Am. Chem. Soc., 2018, 140, 4596–4603 CrossRef CAS PubMed.
- X. Chen, et al., Chem. – Eur. J., 2024, 30, e202303580 CrossRef CAS.
- C. R. Groom, et al., Acta Crystallogr., Sect. B:Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS.
- T. He, et al., Acc. Chem. Res., 2021, 54, 3083–3094 CrossRef CAS PubMed.
- Z. Zhang, et al., J. Am. Chem. Soc., 2022, 144, 14992–14996 CrossRef CAS PubMed.
- W. Zhang, et al., ACS Nano, 2019, 13, 10261–10271 CrossRef CAS PubMed.
- Y. Shao, et al., ACS Nano, 2018, 12, 11704–11710 CrossRef CAS PubMed.
- H. Pan, et al., Adv. Funct. Mater., 2023, 33, 2214887 CAS.
- C. Gong, et al., Angew. Chem., Int. Ed., 2022, 61, e202204899 CrossRef CAS.
- L. Chen, et al., J. Am. Chem. Soc., 2021, 143, 10243–10249 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2418964. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc01092f |
| ‡ A. R. and S. J. N. contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |