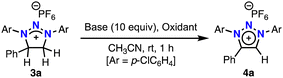Open Access Article
Open Access ArticleConversion of simple alkenes into 1H-1,2,3-triazolium salts by oxidative cycloaddition and subsequent dehydrogenation†
Kazuhito
Yamakawa
a,
Yuki
Obara
a,
Yosuke
Sumiya
 b,
Tianzi
Wang
a,
Shoki
Ogata
c and
Ryosuke
Haraguchi
b,
Tianzi
Wang
a,
Shoki
Ogata
c and
Ryosuke
Haraguchi
 *a
*a
aDepartment of Applied Chemistry, Graduate School of Engineering, Chiba Institute of Technology, 2-17-1 Tsudanuma, Narashino, Chiba 275-0016, Japan. E-mail: haraguchi.ryosuke@p.chibakoudai.jp
bDepartment of Applied Chemistry, Yamaguchi Universi-ty, Tokiwadai 2-16-1, Ube 755-8611, Japan
cDepartment of Applied Chemistry, Faculty of Engineering, Chiba Institute of Technology, 2-17-1 Tsudanuma, Narashino, Chiba 275-0016, Japan
First published on 5th May 2025
Abstract
A new method for the synthesis of 1H-1,2,3-triazolium salts from triazenes and simple alkenes has been developed. The oxidative [3+2] cycloaddition of triazenes with alkenes affords 4,5-dihydro-1H-1,2,3-triazolium salts, which undergo dehydrogenative aromatization under remarkably mild conditions (using potassium bicarbonate in air at room temperature) to provide 1H-1,2,3-triazolium salts. This method exhibits broad functional group tolerance and enables the synthesis of a triazolium-based diol, which serves as a cationic diol monomer for cationic polymer synthesis.
1H-1,2,3-Triazolium salts have been used as cationic scaffolds across various fields, including materials science,1 supramolecular chemistry,2 and organocatalysis.3 Moreover, deprotonation of 1H-1,2,3-triazolium salts provides 1,2,3-triazol-5-ylidenes,4 which possess superior electron-donating properties compared to conventional N-heterocyclic carbenes such as imidazol-2-ylidenes. Consequently, extensive efforts have been directed towards developing methods for the synthesis of 1H-1,2,3-triazolium salts. The most common approach involves the N-alkylation of preformed 1H-1,2,3-triazoles, which are readily synthesized via the copper-catalyzed [3+2] cycloaddition of azides and alkynes (click reaction).5 The remarkable efficiency and high functional-group tolerance of the click reaction have enabled the synthesis of structurally diverse 1H-1,2,3-triazolium salts. Heterocyclic frameworks of triazolium salts have also been constructed through the oxidative [3+2] cycloaddition of triazenes and alkynes (Scheme 1a).6 Notably, this one-step method provides more direct access to triazolium salts than the previously described cycloaddition-alkylation sequence, can be performed in air, and exhibits highly functional group tolerance. Moreover, haloalkenes can be used as reaction partners instead of alkynes in triazolium salt syntheses.6b,7 In this case, oxidative cycloaddition initially yields 4,5-dihydro-1H-1,2,3-triazolium salts, which undergo dehydrohalogenation to afford 1H-1,2,3-triazolium salts (Scheme 1b). Since a halogen substituent is required for the aromatization step, simple alkenes cannot be used in the synthesis of 1H-1,2,3-triazolium salts. Given that the number of commercially available simple alkenes is significantly higher than that of alkynes, developing a synthetic method for triazolium salts using alkenes would be expected to greatly expand their structural diversity. Herein, we report that the cycloaddition of triazenes with alkenes, followed by base-promoted dehydrogenative aromatization, affords 1H-1,2,3-triazolium salts (Scheme 1c).
Dehydrogenative aromatization of neutral heterocycles in the presence of an oxidant is widely reported,8 but no studies have explored its application to cationic heterocycles. We first examined various oxidants for the dehydrogenation of dihydro-1H-1,2,3-triazolium salt 3a, which was prepared by the oxidative [3+2] cycloaddition of triazene 1a and alkene 2a. Treatment of 3a with DDQ, a common oxidant for dehydrogenative aromatization of neutral heterocycles, at room temperature for 18 h resulted in no formation of the desired product 4a (Scheme 2a). The reaction did not proceed even at 90 °C. Other oxidants, including molecular iodine and potassium peroxodisulfate, were also ineffective in this reaction. The low reactivity of 3a toward oxidants may be attributed to its electron-deficient nature. However, we unexpectedly obtained 4a in 4% yield when the reaction of 1a and triazene 2a was quenched with water (Scheme 2b). Surprisingly, using potassium bicarbonate instead of water in the quenching process increased the product yield up to 65%. Encouraged by this result, we performed the reaction of 3a with potassium bicarbonate at 30 °C in air, affording 4a in high yield (Scheme 2c). While several examples of base-promoted aerobic heterocycle dehydrogenation have been reported, they typically require harsh conditions, such as the use of t-BuOK at 140 °C.9 In contrast, our reaction conditions—potassium bicarbonate at 30 °C—are exceptionally mild for aerobic heterocycle dehydrogenation.
 | ||
| Scheme 2 (a) Examination of oxidants. (b) Unexpected formation of triazolium salt 4a. (c) Base-promoted dehydrogenation of dihydrotriazolium salt 3a. | ||
Next, we investigated the effects of solvents and bases on the oxidative cycloaddition of 1a and 2a, followed by the subsequent dehydrogenative aromatization (Table 1). The reaction of 1a and 2a in the presence of t-butyl hypochlorite and potassium hexafluorophosphate in ethyl acetate at 0 °C for 1 h, followed by the addition of potassium bicarbonate and stirring at room temperature for 18 h, afforded triazolium salt 4a in 94% yield. While other solvents, such as acetone, acetonitrile, tetrahydrofuran, and dichloromethane, also promoted the reaction (entries 2–5), ethyl acetate proved to be the most effective (entry 1). When the dehydrogenative aromatization was performed using sodium bicarbonate, the product yield decreased to 35% (entry 6). Potassium carbonate and potassium hydroxide also gave 4a in high yields, though small amounts of unidentified byproducts were observed. Among the organic bases screened, triethylamine proved to be the most efficient for the dehydrogenative aromatization (entries 9–11).
| Entry | Variation from the standard conditions | Yieldb (%) |
|---|---|---|
| a Standard conditions: 1a (0.20 mmol), 2a (1.5 equiv.), t-BuOCl (2.3 equiv.), KPF6 (2.3 equiv.) in AcOEt (1.0 mL) at 0 °C for 1 h; KHCO3 (10 equiv.) at room temperature for 18 h. b Determined by 1H NMR analysis of the crude reaction mixture using dibromomethane as the internal standard. | ||
| 1 | None | 94 |
| 2 | Acetone, instead of AcOEt | 77 |
| 3 | CH3CN, instead of AcOEt | 83 |
| 4 | THF, instead of AcOEt | 61 |
| 5 | CH2Cl2, instead of AcOEt | 58 |
| 6 | NaHCO3, instead of KHCO3 | 35 |
| 7 | K2CO3, instead of KHCO3 | 87 |
| 8 | KOH, instead of KHCO3 | 81 |
| 9 | Pyridine, instead of KHCO3 | 9 |
| 10 | Et3N, instead of KHCO3 | 81 |
| 11 | DBU, instead of KHCO3 | 42 |
With the optimized reaction conditions in hand, the scope of alkenes in the triazolium salt synthesis was examined (Scheme 3). Notably, all triazolium products 4 were purified only by washing with diethyl ether. Aryl-substituted alkenes with electron-donating and electron-withdrawing groups were successfully converted into the corresponding 1H-1,2,3-triazolium salts 4a–d in moderate to good yields. Halogen and silyl groups were well tolerated in the reaction (4e–h), and neither steric hindrance (4i) nor Lewis-basic functionality (4j) hindered the reaction. Alkyl-substituted alkenes provided the corresponding products 4k and 4l, while various internal disubstituted alkenes also participated successfully (4m–p). Moreover, cyclic alkenes, such as cyclopentene and cyclohexene, produced triazolium-fused carbocycles 4q and 4r. We next investigated the scope of triazenes in the reaction. Triazenes bearing both electron-withdrawing and electron-donating substituents on aromatic rings yielded the corresponding products 4s–v in good yields. Sterically hindered aromatic substituents did not negatively affect the reaction (4w and 4x).
To gain mechanistic insights into the dehydrogenative aromatization of dihydrotriazolium salts, we performed several control experiments (Table 2). Under a nitrogen atmosphere and in the presence of potassium bicarbonate, 4a was obtained in only 6% yield (entry 1). In contrast, the yield increased to 42% when the reaction was performed in air (entry 2), and an oxygen atmosphere allowed the reaction to proceed quantitatively (entry 3). Notably, no product formation was observed without a base (entry 4). Moreover, other oxidants commonly used for the dehydrogenative aromatization of neutral heterocycles were ineffective in this reaction (entries 5 and 6). These results highlight the essential role of both molecular oxygen and base in dehydrogenative aromatization. The plausible reaction mechanism is shown in Scheme S1 (ESI†).
| Entry | Oxidant | Base | Conversionb (%) | Yieldb (%) |
|---|---|---|---|---|
| a Standard conditions: 3a (0.50 mmol), base (10.0 equiv.) in CH3CN (2.5 mL) at room temperature for 1 h. b Determined by 1H NMR analysis of the crude reaction mixture using dibromomethane as the internal standard. c Oxidant (2.0 equiv.) | ||||
| 1 | — | KHCO3 | 9 | 6 |
| 2 | Air | KHCO3 | 50 | 42 |
| 3 | O2 balloon | KHCO3 | 100 | 98 |
| 4 | O2 balloon | — | 3 | 0 |
| 5 | K2S2O8 | KHCO3 | 11 | 7 |
| 6c | I2 | KHCO3 | 3 | 0 |
To demonstrate the practicality of the method, we performed the reaction of 1a and 2a on a 20 mmol scale, affording product 4a in 49% yield (5.4 g) (Scheme S2, ESI†). We then explored the synthesis of bistriazolium salt 4z, which contains two different substituents on the triazolium nitrogen atoms (Scheme 4a). This approach was motivated by DFT calculations, which revealed that the activation barrier for the oxidative [3+2] cycloaddition of triazenes and alkenes is lower than that for alkynes (Fig. S1, ESI†). Indeed, when aryl-substituted alkene 1s, featuring an alkynyl functionality, was reacted with triazene 2a at −90 °C, followed by a dehydrogenative process, the alkynyl-functionalized triazolium salt 4y was obtained in good yield. Subsequent treatment of 4y with diisopropylphenyl-substituted triazene 2g afforded 4z in high yield. This compound might be used to prepare heterobimetallic NHC complexes via sequential deprotonative metalation because of the difference in steric environments between these deprotonative protons. Furthermore, this method enabled the synthesis of triazolium-based diol 4aa, which is challenging to prepare using alkynes as starting materials due to the lower reactivity of alkyne 1u compared to alkene 1t (Scheme 4b). As a preliminary result, this diol 4aa was successfully applied for the polycondensation with diacyl chloride 5, affording triazolium-based cationic polyester 6 (Scheme 4c). In addition, the oxidative cycloaddition–dehydrogenation strategy enabled the transformation of polybutadiene into triazolium-based polymer 7 (Scheme 4d). As cationic polymers have been widely used in various fields, including medicinal chemistry10 and materials science,11 the properties and applications of these cationic polymers will be investigated in the future work.
In conclusion, we developed a method for the preparation of 1H-1,2,3,-triazolium salts via the oxidative [3+2] cycloaddition of triazenes and alkenes, followed by dehydrogenation. Notably, the dehydrogenative aromatization proceeded under milder reaction conditions compared to those for neutral heterocycles probably due to the distinct electronic properties of dihydrotriazolium salts. This method has a broad substrate scope and could be applied to cationic polymer synthesis.
This work was supported by the Institute for Quantum Chemical Exploration (IQCE).
Data availability
The data underlying this study are available in the published article and its ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) M. M. Obadia and E. Drockenmuller, Chem. Commun., 2016, 52, 2433–2450 RSC; (b) J. M. Aizpurua, R. M. Fratila, Z. Monasterio, N. Pérez-Esnaola, E. Andreieff, A. Irastorza and M. Sagartzazu-Aizpurua, New J. Chem., 2014, 38, 474–480 RSC.
- (a) M. S. Shad, P. V. Santhini and W. Dehaen, Beilstein J. Org. Chem., 2019, 15, 2142–2155 CrossRef CAS; (b) B. Schulze and U. S. Schubert, Chem. Soc. Rev., 2014, 43, 2522–2571 RSC.
- (a) W. Liu, A. Vianna, Z. Zhang, S. Huang, L. Huang, M. Melaimi, G. Bertrand and X. Yan, Chem. Catal., 2021, 1, 196–206 CrossRef CAS; (b) D. Ranolia, I. Avigdori, K. Singh, A. Koronatov, N. Fridman and M. Gandelman, Org. Lett., 2022, 24, 3915–3919 CrossRef CAS; (c) K. Torita, R. Haraguchi, Y. Morita, S. Kemmochi, T. Komatsu and S.-I. Fukuzawa, Chem. Commun., 2020, 56, 9715–9718 RSC; (d) K. Ohmatsu, R. Suzuki, Y. Furukawa, M. Sato and T. Ooi, ACS Catal., 2020, 10, 2627–2632 CrossRef CAS; (e) S.-K. Chen, W.-Q. Ma, Z.-B. Yan, F.-M. Zhang, S.-H. Wang, Y.-Q. Tu, X.-M. Zhang and J.-M. Tian, J. Am. Chem. Soc., 2018, 140, 10099–10103 CrossRef CAS; (f) K. Ohmatsu, M. Kiyokawa and T. Ooi, J. Am. Chem. Soc., 2011, 133, 1307–1309 CrossRef CAS PubMed.
- (a) R. Maity and B. Sarkar, JACS Au, 2022, 2, 22–57 CrossRef CAS; (b) K. O. Marichev, S. A. Patil and A. Bugarin, Tetrahedron, 2018, 74, 2523–2546 CrossRef CAS; (c) G. Guisado-Barrios, J. Bouffard, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2010, 49, 4759–4762 CrossRef CAS.
- (a) M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952–3015 CrossRef CAS PubMed; (b) H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- (a) D. Sawaguchi, S. Hayakawa, M. Sakuma, K. Niitsuma, D. Kase, S. Michii, M. Ozawa, Y. Sakai, K. Sakamaki, K. Ueyama and R. Haraguchi, Asian J. Org. Chem., 2021, 10, 901–905 CrossRef CAS; (b) J. Bouffard, B. K. Keitz, R. Tonner, G. Guisado-Barrios, G. Frenking, R. H. Grubbs and G. Bertrand, Organometallics, 2011, 30, 2617–2627 CrossRef CAS PubMed; (c) W. Wirschun, M. Winkler, K. Lutz and J. C. Jochims, J. Chem. Soc. Perkin 1, 1998, 1755–1762 RSC.
- (a) T. Mitsuhashi, D. Kase, A. Tomatsuri, T. Takada and R. Haraguchi, Tetrahedron Lett., 2024, 139, 154993 CrossRef; (b) X. Xu, Z. Zhang, S. Huang, L. Cao, W. Liu and X. Yan, Dalton Trans., 2019, 48, 6931–6941 RSC.
- (a) Y. Kawashita and M. Hayashi, Molecules, 2009, 14, 3073–3093 CrossRef PubMed; (b) A. Bera, S. Bera and D. Banerjee, Chem. Commun., 2021, 57, 13042–13058 RSC.
- (a) R. Yang, S. Yue, W. Tan, Y. Xie and H. Cai, J. Org. Chem., 2020, 85, 7501–7509 CrossRef; (b) T. Liu, K. Wu, L. Wang and Z. Yu, Adv. Synth. Catal., 2019, 361, 3958–3964 CrossRef.
- (a) H. Lv, S. Zhang, B. Wang, S. Cui and J. Yan, J. Control. Release, 2006, 114, 100–109 CrossRef PubMed; (b) S. K. Samal, M. Dash, S. Van Vlierberghe, D. L. Kaplan, E. Chiellini, C. van Blitterswijk, L. Moroni and P. Dubruel, Chem. Soc. Rev., 2012, 41, 7147–7194 RSC; (c) M. Farshbaf, S. Davaran, A. Zarebkohan, N. Annabi, A. Akbarzadeh and R. Salehi, Artif. Cells Nanomed. Biotechnol., 2018, 46, 1872–1891 Search PubMed.
- (a) N. Du, C. Roy, R. Peach, M. Turnbull, S. Thiele and C. Bock, Chem. Rev., 2022, 122, 11830–11895 CrossRef PubMed; (b) Y.-J. Wang, J. Qiao, R. Baker and J. Zhang, Chem. Soc. Rev., 2013, 42, 5768–5787 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc02068a |
| This journal is © The Royal Society of Chemistry 2025 |




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif)