Ferromagnetic and plastically deformable organic–inorganic hybrid crystal: (C7H9NH3)2CuCl4†
Sotaro
Kusumoto
 *a,
Sakura
Nagasawa
a,
Ryo
Suzuki
*a,
Sakura
Nagasawa
a,
Ryo
Suzuki
 b,
Masaru
Tachibana
b,
Masaru
Tachibana
 b,
Yuta
Tsuji
b,
Yuta
Tsuji
 c,
Hikaru
Zenno
d,
Yuto
Nakashima
d,
Shinya
Hayami
c,
Hikaru
Zenno
d,
Yuto
Nakashima
d,
Shinya
Hayami
 de,
Yang
Kim
d and
Yoshihiro
Koide
*a
de,
Yang
Kim
d and
Yoshihiro
Koide
*a
aDepartment of Applied Chemistry, Faculty of Chemistry and Biochemistry, Kanagawa University, 3-27-1 Rokkakubashi, Kanagawa-ku, Yokohama 221-8686, Japan. E-mail: kusumoto@kanagawa-u.ac.jp
bDepartment of Materials System Science, Yokohama City University, 22-2 Seto, Kanazawa-ku, Yokohama, Kanagawa 236-0027, Japan
cFaculty of Engineering Sciences, Kyushu University, Kasuga, Fukuoka 816-8580, Japan
dDepartment of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
ePriority Organization for Innovation and Excellence, Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
First published on 6th June 2025
Abstract
We report a plastically deformable organic–inorganic hybrid crystal, (C7H9NH3)2CuCl4, exhibiting ferromagnetism below 8 K. Its 2D layered structure enables stress-induced plastic bending via van der Waals slip between alkyl chains. Nanoindentation reveals exceptional mechanical compliance, and magnetic studies confirm long-range ordering. This represents the first known example of a ferromagnetic crystal with plastic deformability.
Organic–inorganic hybrid metal halides (OIMHs) exhibit unique functionalities, including optical properties,1 electronic behavior,2 stability,3 and adsorption characteristics4 resulting from diverse combinations of organic cations and metal halides. These properties have attracted significant interest for applications in solar cells,5 light-emitting devices,6 sensors,7 and thermoelectric materials.8 Understanding the mechanical properties of OIMHs is essential for improving material durability and enabling practical implementation in flexible devices.9 Their crystal structure strongly influences the mechanical behavior of OIMHs. In three-dimensional (3D) frameworks, the contribution of organic cations is minimal, with the mechanical properties predominantly governed by the metal halide framework. For instance, nanoindentation studies on 3D OIMHs such as CH3NH3PbX3 (X = I−, Br−, Cl−) report elastic moduli of 10–20 GPa and hardness values of 0.25–0.5 GPa.10 In contrast, low-dimensional OIMHs—especially those with two-dimensional (2D) or lower structures—are characterized by weaker van der Waals interactions in the organic regions, resulting in reduced elastic modulus and hardness.11 OIMHs incorporating long-chain alkylammonium cations show exceptionally soft mechanical properties and tunability via the modulation of these van der Waals interactions.12 Although the relationship between structure and mechanical properties has been investigated, reports on macroscopic elastic13 or plastic deformation14 remain limited.
Recently, macroscopically flexible single crystals capable of significant mechanical deformation have gained attention for their potential applications in flexible electronics.15 These deformations are typically reversible (elastic)13 or irreversible (plastic).14 In plastically bendable crystals, weak interactions—particularly van der Waals forces—serve as slip planes, enabling permanent deformation by disrupting and reforming molecular packing.16 Although this behavior has been reported in crystals composed of organic molecules14 and metal complexes,17 its exploration in OIMHs remains limited, as the supramolecular interactions that contribute to the stabilization of the crystals do not provide a sufficient “buffer” to absorb external mechanical stimuli. Nevertheless, flexible OIMHs possess multiple functions, offering a promising platform for the design of multifunctional materials. In this study, we investigated the mechanical behavior of (C7H9NH3)2CuCl4 (C7-Cu), prepared from n-heptylamine and copper(II) chloride, and compared it with (C6H13NH3)2SnCl6 (C6-Sn), prepared from n-hexylamine and tin(II) chloride.
The C7-Cu compound was prepared from copper chloride and n-heptylamine in 6 M hydrochloric acid, yielding square plate-like crystals. Single-crystal X-ray analysis reveals that C7-Cu, with the formula (C7-NH3)2CuCl4, crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Table S1, ESI†). In its structure, two chloride ions are axially coordinated to each Cu2+ ion along the c-axis, while four additional chloride ions bridge to adjacent Cu(II) centers along the ab plane, forming a slightly zigzag-like 2D layer with octahedral geometry. n-Heptylammonium cations are intercalated between these layers, aligned parallel to the bc-plane (Fig. 1a). The crystal is stabilized by electrostatic interactions between organic and inorganic layers (Fig. S1, ESI†), along with CH⋯HC supramolecular interactions among the terminal methyl groups of adjacent alkyl chains (Fig. 1a). Attempts to prepare analogous crystals with C6 and C8 alkyl chains (C6-Cu and C8-Cu) were unsuccessful in yielding crystals large enough for bending tests. For comparison, C6-Sn crystals were prepared from n-hexylamine and tin chloride under similar acidic conditions (Fig. 1b and Fig. S3, ESI†). These crystals, which adopt a needle-like morphology, are suitable for bending experiments. We have also attempted to grow single crystals of C7-Sn and C8-Sn, but obtaining suitably sized crystals for bending tests has proven difficult. Single-crystal analysis confirms that C6-Sn also crystallizes in the P
(Table S1, ESI†). In its structure, two chloride ions are axially coordinated to each Cu2+ ion along the c-axis, while four additional chloride ions bridge to adjacent Cu(II) centers along the ab plane, forming a slightly zigzag-like 2D layer with octahedral geometry. n-Heptylammonium cations are intercalated between these layers, aligned parallel to the bc-plane (Fig. 1a). The crystal is stabilized by electrostatic interactions between organic and inorganic layers (Fig. S1, ESI†), along with CH⋯HC supramolecular interactions among the terminal methyl groups of adjacent alkyl chains (Fig. 1a). Attempts to prepare analogous crystals with C6 and C8 alkyl chains (C6-Cu and C8-Cu) were unsuccessful in yielding crystals large enough for bending tests. For comparison, C6-Sn crystals were prepared from n-hexylamine and tin chloride under similar acidic conditions (Fig. 1b and Fig. S3, ESI†). These crystals, which adopt a needle-like morphology, are suitable for bending experiments. We have also attempted to grow single crystals of C7-Sn and C8-Sn, but obtaining suitably sized crystals for bending tests has proven difficult. Single-crystal analysis confirms that C6-Sn also crystallizes in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, with the molecular formula (C6-NH3)2SnCl6 (Table S1, ESI†), where the alkyl chains are disordered (Fig. S2, ESI†). The discrete SnCl62− anions adopt 0D octahedral geometries arranged along the b-axis (Fig. 1b). The n-hexylammonium cations align along the ab-plane, engaging in electrostatics (Fig. S2, ESI†) and CH⋯HC supramolecular interactions between neighboring chains (Fig. 1b and Fig. S3, ESI†). Face-index analysis indicates that the C7-Cu crystal grows predominantly along the ab-plane, consistent with its 2D layered framework. Its shortest dimension corresponds to the c-axis, the stacking direction (Fig. S4, ESI†). In contrast, C6-Sn crystals exhibit a significant surface of (0−15) plane, inclined approximately 36° from the ab-plane (Fig. S4 and S5, ESI†).
space group, with the molecular formula (C6-NH3)2SnCl6 (Table S1, ESI†), where the alkyl chains are disordered (Fig. S2, ESI†). The discrete SnCl62− anions adopt 0D octahedral geometries arranged along the b-axis (Fig. 1b). The n-hexylammonium cations align along the ab-plane, engaging in electrostatics (Fig. S2, ESI†) and CH⋯HC supramolecular interactions between neighboring chains (Fig. 1b and Fig. S3, ESI†). Face-index analysis indicates that the C7-Cu crystal grows predominantly along the ab-plane, consistent with its 2D layered framework. Its shortest dimension corresponds to the c-axis, the stacking direction (Fig. S4, ESI†). In contrast, C6-Sn crystals exhibit a significant surface of (0−15) plane, inclined approximately 36° from the ab-plane (Fig. S4 and S5, ESI†).
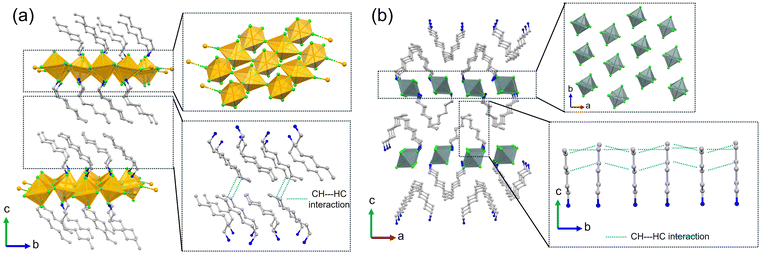 | ||
| Fig. 1 Crystal structures of C7-Cu (a) and C6-Sn (b), along with the molecular packing and interactions between alkyl chains in these compounds. | ||
Macroscopic mechanical tests on C7-Cu demonstrated its capacity for plastic deformation. When stress was applied perpendicular to the (100) face using tweezers, the crystal bent easily and retained the deformed shape after the stress was removed, indicating irreversible plastic deformation (Fig. 2a and Video S1, ESI†). This type of flexibility is unusual for OIMHs, particularly those with 2D coordination-based architectures. The deformation mechanism is attributed to the disruption and reformation of weak van der Waals interactions between adjacent alkyl chains (Fig. 1a and Fig. S6, ESI†).16 Further bending along the (110) or (1−10) faces using a scalpel produced similar deformation patterns, resembling wrinkles on a carpet (Fig. 2b and Video S2, ESI†)—again implicating interlayer slippage as the primary mechanism. Scanning electron microscopy (SEM) confirmed discontinuities along the crystal edges, consistent with the presence of stacked 2D layers (Fig. 2d). In contrast, the C6-Sn crystals fractured immediately upon application of stress to the (021) or (01−5) faces, demonstrating brittle behavior typical of conventional crystals (Fig. 2c). The zigzag packing of the alkyl chains in C6-Sn prevents the formation of slippable 2D planes. This is also supported by SEM images, which show smooth, fracture-free surfaces (Fig. 2e).
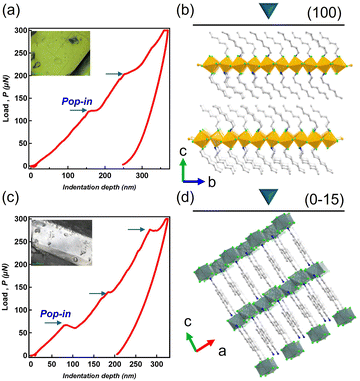
Nanoscale mechanical properties were investigated by performing nanoindentation tests on the crystal planes. As the load increases on the load-displacement (P–h) curve, multiple pop-ins are observed on the (100) face of C7-Cu, with a maximum indentation depth (hmax = 366 nm) occurring at the maximum load (Pmax = 300 μN) (Fig. 3a and b). After unloading, the crystal recovers 32%, leaving behind residual deformation. Additionally, a slip (pop-in) of approximately 20 nm was observed at depths around 150 nm and 250 nm, suggesting that the layered structure of 10 layers (each layer being about 20 Å thick) slides simultaneously. Depth-dependent elastic modulus (E) and hardness (H) exhibit variability, but in the 400–800 nm range, the average E and H values were determined to be 2.14 ± 0.58 GPa and 0.072 ± 0.031 GPa, respectively (Fig. S7, ESI†), indicating high compliance.18
In the C6-Sn crystal, multiple pop-ins (displacement bursts) corresponding to the sliding of various layers within the crystal are observed in the P–h curve on the (0−15) plane as the load increases (Fig. 3c and d). At maximum load (Pmax = 300 μN), the maximum indentation depth (hmax = 324 nm) is recorded. After unloading, the crystal recovers 37%; however, residual strain due to plastic deformation remains, suggesting that C6-Sn displays relatively higher elastic recovery despite its overall brittleness compared to the plastically deformable C7-Cu. Depth dependence is also noted in C6-Sn, with average values of E and H in the 400–800 nm range measuring 2.63 GPa and 0.13 GPa, respectively (Fig. S7, ESI†). From these results, the modulus of elasticity of the C6-Sn crystal is categorized as super compliant. Simultaneously, its hardness is classified as super-soft, indicating that this crystal displays soft mechanical properties.18 A comparison of the two crystals reveals that the plastic deformation of C7-Cu occurs more quickly and smoothly, a difference that appears to depend on the arrangement of the alkyl chains. The mechanical properties estimated by nanoindentation for the two compounds reflect the soft interactions between alkyl chains. Our previously reported elastic and plastic crystals of n-dodecyl-substituted tetrachlorophthalimide exhibited similar modulus and hardness, suggesting that the properties of the present system also arise from alkyl chain interactions rather than from the two-dimensional inorganic sheets.14b
Density functional theory (DFT) calculations were employed to quantify the interlayer interaction energies. The intermolecular interaction energy (Eint) was determined as the difference in structural energy between the bilayer system and the monolayer structure, achieved by removing one layer (Fig. S8, ESI†). The estimated Eint values are 0.877 (0.935) eV nm−2 for C7-Cu and 1.443 (1.516) eV nm−2 for C6-Sn, indicating that the interaction between the 2D sheets is predominantly governed by dispersion forces (values in brackets). Based on this data, the interaction of C6-Sn is approximately 1.6 times stronger than that of C7-Cu, which can be attributed to the increased overlap of long alkyl chains. The elevated interaction energy of C6-Sn inhibits interlayer sliding, resulting in brittle deformation behavior that ultimately enhances the mechanical strength of the crystal.
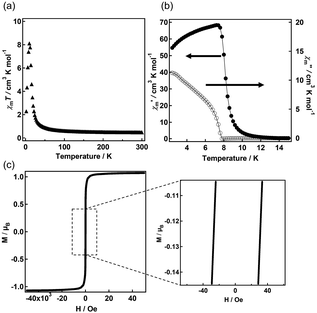
Two-dimensional copper halides have been widely reported as ferromagnets.19 Temperature-dependent data (Fig. 4a) on the magnetic susceptibility of C7-Cu show that the χmT value at 300 K is 0.4 cm3 K mole−1, consistent with the theoretical value for a single Cu2+ ion with S = 1/2.19,20 As the temperature decreases, χmT rapidly increases, indicating ferromagnetic coupling. The χmT value further decreases upon cooling, which is probably associated with weak antiferromagnetic interactions between the layers and magnetic saturation.19 AC magnetic susceptibility was conducted to confirm the presence of ferromagnetic ordering (Fig. 4b). The in-phase AC magnetic susceptibility (χ′) shows a maximum at approximately 8 K. In comparison, the out-of-phase AC magnetic susceptibility (χ′′) indicates the onset of ferromagnetic interaction at 8 K, suggesting the emergence of long-range magnetic ordering below this temperature. The field dependence of magnetization for C7-Cu at 2 K (Fig. 4c) shows a rapid increase in magnetization at low fields, resulting in a saturation magnetization of about 1μB. This value aligns with results obtained from the χmT–T plot and the expected value for the system with S = 1/2. The low-field hysteresis loop shows a coercive field of approximately 30 Oe, confirming soft magnetic behavior. This represents the first example of a flexible crystal exhibiting ferromagnetism.
In summary, we demonstrate that (C7H9NH3)2CuCl4 is a unique, organic–inorganic hybrid crystal that exhibits both plastic deformability and long-range ferromagnetic ordering. This rare combination arises from a 2D layered architecture allowing molecular slippage and cooperative magnetic interactions within the CuCl4 layers. Our comparative studies with a brittle analogue, (C6H13NH3)2SnCl6, underscore the crucial role of alkyl chain arrangement. This work opens avenues for designing multifunctional flexible materials integrating mechanical and magnetic properties for future device applications.
This work was supported by KAKENHI Grant Number JP22K14698. This work was also supported by the Grant-in-Aid for Transformative Research Areas (A) “Supra-ceramics” (JSPS KAKENHI Grant Number JP23H04636 and JP22H05146).
Author contributions
S. K. and Y. K. (Y. Koide) supervised the project. S. N. carried out the experimental work and data analysis. R. S. and M. T. conducted the nanoindentation experiments. Y. T. performed the theoretical calculations. H. Z., Y. N., and S. H. conducted the magnetic measurements. Y. K. (Y. Kim) contributed to scientific discussions. All authors contributed to the writing of the manuscript.Data availability
All data generated or analyzed during this study are included in this published article and its ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- R. Pardo, M. Zayat and D. Levy, Chem. Soc. Rev., 2011, 40, 672–687 RSC.
- Y. Zhao and K. Zhu, Chem. Soc. Rev., 2016, 45, 655–689 RSC.
- D. Li, P. Liao, X. Shai, W. Huang, S. Liu, H. Li, Y. Shen and M. Wang, RSC Adv., 2016, 6, 89356–89366 RSC.
- M.-L. Feng, D. Sarma, X.-H. Qi, K.-Z. Du, X.-Y. Huang and M. G. Kanatzidis, J. Am. Chem. Soc., 2016, 138, 12578–12587 CrossRef CAS PubMed.
- P. P. Boix, K. Nonomura, N. Mathews and S. G. Mhaisalkar, Mater. Today, 2014, 17, 16–23 CrossRef CAS.
- M. Sessolo and H. J. Bolink, Adv. Mater., 2011, 23, 1829–1845 CrossRef CAS PubMed.
- S. Wang, Y. Kang, L. Wang, H. Zhang, Y. Wang and Y. Wang, Sens. Actuators, B, 2013, 182, 467–481 CrossRef CAS.
- S. Yang, P. Qiu, L. Chen and X. Shi, Small Sci., 2021, 1, 2100005 CrossRef CAS PubMed.
- C. Gu and J.-S. Lee, ACS Nano, 2016, 10, 5413–5421 CrossRef CAS PubMed.
- (a) S. Sun, Y. Fang, G. Kieslich, T. J. White and A. K. Cheetham, J. Mater. Chem. A, 2015, 3, 18450–18455 RSC; (b) Z. Dai, M. C. Doyle, X. Liu, M. Hu, Q. Wang, C. E. Athanasiou, Y. Liu, B. W. Sheldon, H. Gao, S. Liu and N. P. Padture, Scr. Mater., 2023, 223, 115064 CrossRef CAS.
- S. Kusumoto, S. Masuda, R. Suzuki, M. Tachibana, M. Shimabukuro, M. Kobayashi, N. Ogiwara, S. Uchida, T. Fukui, Y. Tsuji, M. Okamura, S. Hikichi, Y. Kim and Y. Koide, J. Mater. Chem. C, 2025, 13, 8470–8478 RSC.
- Q. Tu, I. Spanopoulos, E. S. Vasileiadou, X. Li, M. G. Kanatzidis, G. S. Shekhawat and V. P. Dravid, ACS Appl. Mater. Interfaces, 2020, 12, 20440–20447 CrossRef CAS PubMed.
- (a) S. Kusumoto, Y. Kim and S. Hayami, Coord. Chem. Rev., 2023, 475, 214890 CrossRef CAS; (b) P. Naumov, S. Chizhik, M. K. Panda, N. K. Nath and E. Boldyreva, Chem. Rev., 2015, 115, 12440–12490 CrossRef CAS PubMed; (c) S. Hayashi, F. Ishiwari, T. Fukushima, S. Mikage, Y. Imamura, M. Tashiro and M. Katouda, Angew. Chem., Int. Ed., 2020, 59, 16195–16201 CrossRef CAS PubMed; (d) S. Kusumoto, A. Sugimoto, Y. Zhang, Y. Kim, M. Nakamura, L. F. Lindoy and S. Hayami, Inorg. Chem., 2021, 60, 1294–1298 CrossRef CAS PubMed; (e) W. M. Awad, D. W. Davies, D. Kitagawa, J. M. Halabi, M. B. Al-Handawi, I. Tahir, F. Tong, G. Campillo-Alvarado, A. G. Shtukenberg, T. Alkhidir, Y. Hagiwara, M. Almehairbi, L. Lan, S. Hasebe, D. P. Karothu, S. Mohamed, H. Koshima, S. Kobatake, Y. Diao, R. Chandrasekar, H. Zhang, C. C. Sun, C. Bardeen, R. O. Al-Kaysi, B. Kahr and P. Naumov, Chem. Soc. Rev., 2023, 52, 3098–3169 RSC.
- (a) P. Naumov, S. Chizhik, M. K. Panda, N. K. Nath and E. Boldyreva, Chem. Rev., 2015, 115, 12440–12490 CrossRef CAS PubMed; (b) S. Kusumoto, R. Suzuki, M. Tachibana, Y. Sekine, Y. Kim and S. Hayami, Chem. Commun., 2022, 58, 5411–5414 RSC; (c) Q. Di, M. B. Al-Handawi, L. Li, P. Naumov and H. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202403914 CrossRef CAS PubMed; (d) L. O. Alimi, P. Lama, V. J. Smith and L. J. Barbour, Chem. Commun., 2018, 54, 2994–2997 RSC; (e) S. Saha and G. R. Desiraju, J. Am. Chem. Soc., 2017, 139, 1975–1983 CrossRef CAS PubMed; (f) I. S. Divya, S. Kandasamy, S. Hasebe, T. Sasaki, H. Koshima, K. Woźniak and S. Varughese, Chem. Sci., 2022, 13, 8989–9003 RSC; (g) A. Worthy, A. Grosjean, M. C. Pfrunder, Y. Xu, C. Yan, G. Edwards, J. K. Clegg and J. C. McMurtrie, Nat. Chem., 2018, 10, 65–69 CrossRef CAS PubMed; (h) S. Hayashi, S. Yamamoto, D. Takeuchi, Y. Ie and K. Takagi, Angew. Chem., Int. Ed., 2018, 57, 17002–17008 CrossRef CAS PubMed.
- (a) C. Wei, L. Li, Y. Zheng, L. Wang, J. Ma, M. Xu, J. Lin, L. Xie, P. Naumov, X. Ding, Q. Feng and W. Huang, Chem. Soc. Rev., 2024, 53, 3687–3713 RSC; (b) L. Lan, L. Li, P. Naumov and H. Zhang, Chem. Mater., 2023, 35, 7363–7378 CrossRef CAS.
- M. K. Panda, S. Ghosh, N. Yasuda, T. Moriwaki, G. D. Mukherjee, C. M. Reddy and P. Naumov, Nat. Chem., 2015, 7, 65–72 CrossRef CAS PubMed.
- (a) L.-C. An, X. Li, Z.-G. Li, Q. Li, P. J. Beldon, F.-F. Gao, Z.-Y. Li, S. Zhu, L. Di, S. Zhao, J. Zhu, D. Comboni, I. Kupenko, W. Li, U. Ramamurty and X.-H. Bu, Nat. Commun., 2022, 13, 6645 CrossRef CAS PubMed; (b) B. Bhattacharya, A. A. L. Michalchuk, D. Silbernagl, M. Rautenberg, T. Schmid, T. Feiler, K. Reimann, A. Ghalgaoui, H. Sturm, B. Paulus and F. Emmerling, Angew. Chem., Int. Ed., 2020, 59, 5557–5561 CrossRef CAS PubMed; (c) M. Pisačić, I. Biljan, I. Kodrin, N. Popov, Ž. Soldin and M. Đaković, Chem. Mater., 2021, 33, 3660–3668 CrossRef.
- C. Wang and C. C. Sun, CrystEngComm, 2020, 22, 1149–1153 RSC.
- (a) G. V. Rubenacker, D. N. Haines and J. E. Drumheller, J. Magn. Magn. Mater., 1984, 43, 238–242 CrossRef CAS; (b) C. Han, J. A. McNulty, A. J. Bradford, A. M. Z. Slawin, F. D. Morrison, S. L. Lee and P. Lightfoot, Inorg. Chem., 2022, 61, 3230–3239 CrossRef CAS PubMed; (c) R. D. Willett, C. J. Gómez-García and B. Twamley, Eur. J. Inorg. Chem., 2012, 3342–3348 CrossRef CAS.
- A. Li, Y. Wang, H. Tian, A. Yalikun, Y. Journaux, M. Luo and H. Lu, Chin. Chem. Lett., 2024, 35, 108780 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2444578 and 2444579. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc02170g |
| This journal is © The Royal Society of Chemistry 2025 |