Shota Asai, Keisuke Tokushige and Takumi Abe
Chem. Commun., 2025, Advance Article
DOI:
10.1039/D5CC03064A,
Communication
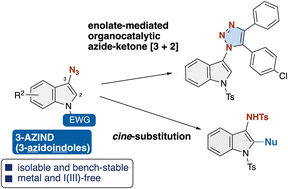
A concise protocol based on the E2 reaction of indoline hemiaminals for accessing 3-azidoindoles is reported. In contrast to previous methods that require in situ generation by hypervalent iodine reagents, our protocol allows for the isolation of a variety of 3-azidoindoles upon a mild reaction for a short reaction time at room temperature. The obtained 3-azidoindoles are reasonably reactive, bench-stable and easy to handle. These findings could be used as a starting point for various reactions, including Huisgen reaction, [3+2] cycloaddition, phosphoramidation, and cine-substitution with the release of N2.